Abstract
The isocitrate dehydrogenases (ICDs) catalyse the oxidative decarboxylation of isocitrate to alpha-ketoglutarate and can use either NAD+ or NADP+ as a cofactor. Recent studies demonstrate that the NADP+-dependent isocitrate dehydrogenase, as a source of electrons for cellular antioxidants, is important for protection against oxidative damage. ICD, however, is susceptible to oxidative inactivation, which in turn compromises cellular antioxidant defense. This study investigates the effect of a superoxide dismutase (SOD) mimic, MnTM-2-PyP5+, on the inactivation of NADP+-dependent ICD in SOD-deficient Escherichia coli and in diabetic rats. The findings show that E. coli ICD is inactivated by superoxide, but the inactivated enzyme is replaced by de novo protein synthesis. Statistically significant decrease of ICD activity was found in the hearts of diabetic rats. MnTM-2-PyP5+ protected ICD in both models.
Keywords: Isocitrate dehydrogenase inactivation, manganese porphyrin, MnTM-2-PyP, SOD mimic, diabetes
Introduction
Isocitrate dehydrogenases (ICDs) encompass a family of enzymes that catalyse the conversion of isocitrate to alpha-ketoglutarate in the Krebs cycle [1]. Eukaryotic cells contain two different types of ICDs that depend on NAD+ or NADP+ as cofactors and serve different biological functions. NADP+-linked ICD is found both in mitochondria and cytosol [2,3] and as a source of NADPH for the regeneration of cellular antioxidants is shown to protect against oxidative damage [4–6], against singlet oxygen- [7] and heat shock-induced apoptosis [8,9] and against cadmium toxicity [10]. Recent studies demonstrated that the antioxidant function of ICD is crucial for defense against ionizing radiation [11–13]. At the same time the NADP+-linked ICD itself is susceptible to oxidative inactivation. Nitric oxide [14], peroxynitrite [15], ROS [16] and lipid peroxidation products [17,18] inactivate the enzyme, presumably by modifying essential thiol groups. In vitro experiments with purified enzyme demonstrate that it loses activity if exposed to H2O2, superoxide radical, hydroxyl radical and to photochemically generated singlet oxygen [16]. These findings suggest that in vivo, under conditions of oxidative stress, ICD would be a sensitive ROS/RNS target. Inactivation of ICD would further contribute to perturbation of the balance between oxidants and antioxidants and therefore will exacerbate oxidative/nitrosative stress. This assumption, however, is based mainly on in vitro experiments where ICD has been exposed to concentrations of ROS exceeding biologically relevant values.
Our previous experiments have shown that SOD-deficient E. coli is a useful tool for identifying ROS-sensitive targets [19–21]. It has been determined that the electron transport chain of exponentially growing E. coli generates ~3 µm of superoxide per second [22]. In the absence of SOD, O2• − produced at such a rate is enough to inactivate critical metabolic enzymes [23]. In contrast to eukaryotes, E. coli contains only one ICD, which is NADP+-dependent and is regulated by phosphorylation/dephosphorylation [24]. Reportedly, E. coli ICD can be oxidatively inactivated by iron/ascorbate [25].
In this study we used SOD-deficient E. coli to investigate the effect of endogenously generated superoxide on the activity of the NADP+-linked ICD and its protection by a Mn-porphyrin SOD mimic, MnTM-2-PyP [26]. We then used a streptozotocin-diabetic rat model to test if MnTM-2-PyP can prevent the inactivation of the NADP+-dependent ICD in vivo.Mn porphyrins are potent catalytic O2• − scavengers. The most efficacious of them are those that bear positively charged N-alkylpyridyl groups that allow both thermodynamic and electrostatic facilitation for the approach of superoxide. Such a compound is MnTM-2-PyP, with log kcat = 7.79 (log kcat for SOD enzymes is 8.84–9.30 [27–29]). We have previously shown that MnTM-2-PyP was able to substitute for SOD in SOD-deficient E. coli [26] and extended the life-span of streptozotocin diabetic rats [30].
Materials and methods
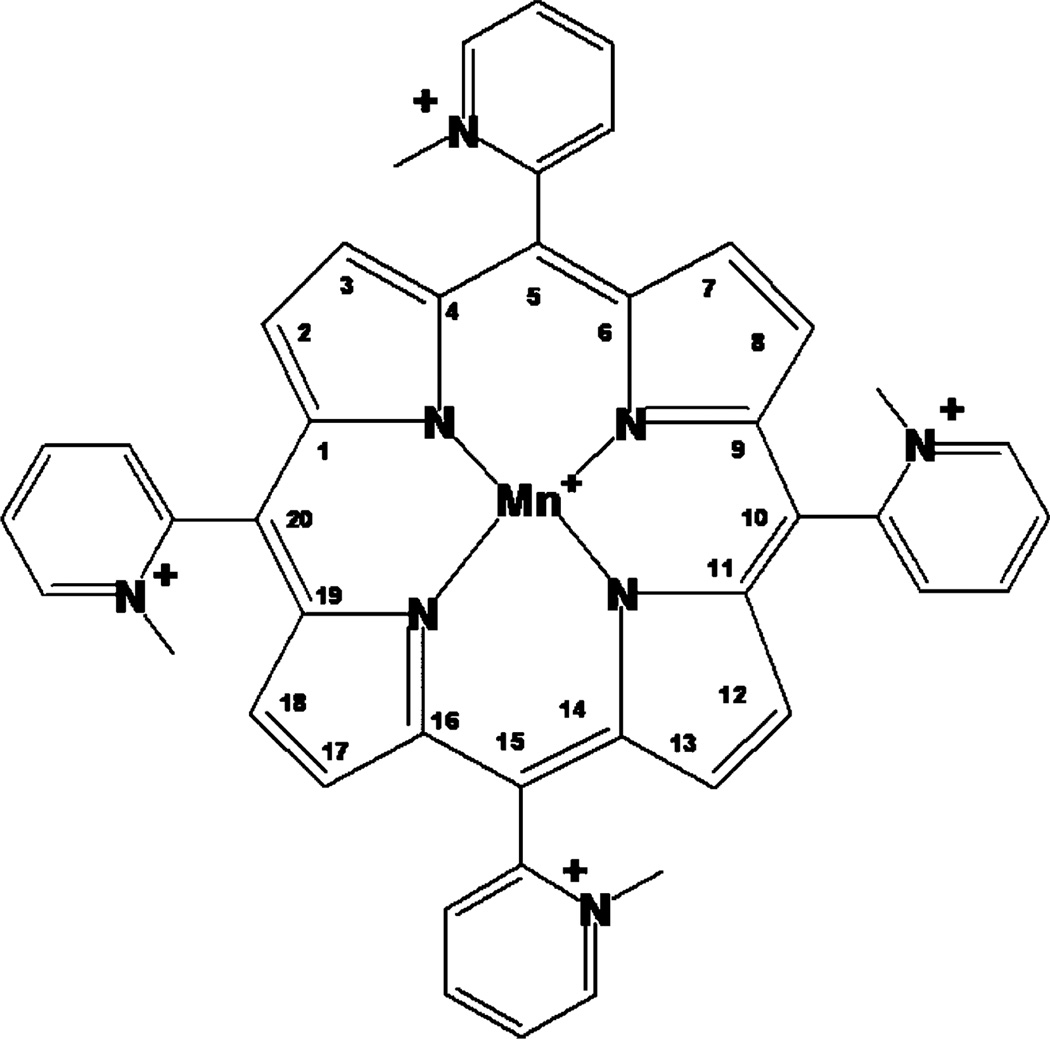
The 5,10,15,20-tetrakis(2-pyridyl)porphyrin (H2T-2-PyP4+) was supplied by MidCentury Chemicals (Chicago, IL). The N-methylation and metal incorporation to obtain MnTM-2-PyP was accomplished as previously described [26]. The structure of the SOD mimic is shown in Figure 1.
Figure 1.
MnTM-2-PyP5+, Mn(III) tetrakis(N-methylpyridinium-2-yl)porphyrin.
All other chemicals were obtained from standard sources.
The strains of E. coli used were: GC4468 = parental; QC1799 = GC4468 Δ sodA3 Δ sodB-kan [31,32]. These were prepared by D. Touati (Institute Jacques Monod, CNRS, University Paris, France). Additional strains were: AB1157 parental; JI132 = AB1157 plus (sodA:: MmdPR13) 25 (sodB-kan) 1-Δ 2 [33]; These were provided by J. Imlay (University of Illinois, Champaign-Urbana, Urbana, IL). Although results are presented for GC4468 and QC1799, similar results were also obtained with AB1157 and JI132. The sodA sodB strains are referred to as SOD-nulls even though they retain the periplasmic Cu, ZnSOD, which is present in only very small amounts.
Starter cultures were grown overnight at 37°C in Luria-Bertoni (LB) medium and were then diluted 200-fold into LB or M9CA medium. M9CA medium consisted of minimal A salts [34], 0.2% casamino acids, 0.2% glucose, 3 mg pantothenate and 5 mg of thiamine per litre. Growth was followed at 600 nm. Unless otherwise indicated, cultures intended for enzyme assays were harvested when A600nm = 0.6–1.0. The cells were thrice washed and then lysed by sonication in 50 mm TRIS (pH 7.4), containing 1.0 mm cysteine, 1.0 mm citrate and 0.5 mm MnCl2 [35] for assay of aconitase. Debris were removed by centrifugation at 16 000 × g for 10 min. The cell extracts thus obtained were assayed for aconitase [36] and ICD [37]. The aconitase activity was assayed spectrophotometrically at 340 nm in a mixture containing 50 mm Tris-Cl buffer, pH 7.4, 30 mm sodium citrate, 0.5 mm MnCl2, 0.2 mm NADP+, 2.0 units/ml of isocitrate dehydrogenase, and cell extract, in a total volume of 1.0 ml. To obtain 100% aconitase activity the enzyme was reactivated by anaerobic incubation with 0.5 mm dithiothreitol, 20 mm ferrous ammonium sulphate and 20 mm Na2S [35].
ICD activity was assayed as described by Fatania et al. [37], by monitoring the production of NADPH at 340 nm. The 1.0 ml reaction mixture contained 33 mm Tris-EDTA buffer (pH 7.4), 1.33 mm MnCl2, 1.3 mm DL-Isocitrate and 0.1 mm NADP+. One unit of the enzyme activity is defined as production of 1 µmol of NADPH per minute.
In vitro, superoxide was generated using xanthine oxidase (XO) with hypoxanthine as a substrate. Hypoxanthine (500 µm) and XO were added to cell extracts to give an initial superoxide generation rate of 5 µm/min.
NADP+/NADPH ratio was determined as described by Micheli et al. [38].
All E. coli experiments were repeated three times with 3–5 replicates.
Diabetes in male Wistar rats was induced by a single (60 mg/kg) intraperitoneal injection of STZ. Induction of diabetes was confirmed by the presence of glucosuria within 24 h. Rats which maintained blood glucose concentrations above 15 mm were randomly divided into two groups designated as ‘Diabetic’ and ‘Diabetic treated’. The animals in the second group received subcutaneous injection of sterile MnTM-2-PyP solution, 1 mg/kg/day for 2 months, for 5 days per week, with 2 days rest after each 5-day cycle. This drug administration scheme was adopted to avoid side-effects of MnTM-2-PyP administration, probably due to blood pressure drop [39].
Sixty days after beginning of the treatment, the animals were anaesthetized after 12 h of fasting and killed by decapitation. Hearts were perfused with cold saline, snap-frozen in liquid nitrogen and stored at −80°C until analysis. The samples were homogenized immediately before assays and homogenates were used for determination of ICD [40], aconitase [41] and fumarase [42,43] activities. One unit of fumarase was taken to be the activity that converted 1 µmol/min of L-malate to fumarate using ε250nm = 1.62 mm−1 cm−1. The initial concentration of L-malate was 50 mm and the assay buffer was 50 mm sodium phosphate, pH 7.3 at 25°C.
Diabetes experiments were repeated twice with 7–12 animals per group.
Mean value and standard error, one-way analysis of variance and the Student-Neumann-Keuls test were used for the statistical analysis of the data. The 0.05 level of probability was used as the criterion of significance. In the figures significance is indicated as *p < 0.05 compared to control or non-treated group.
Results and discussion
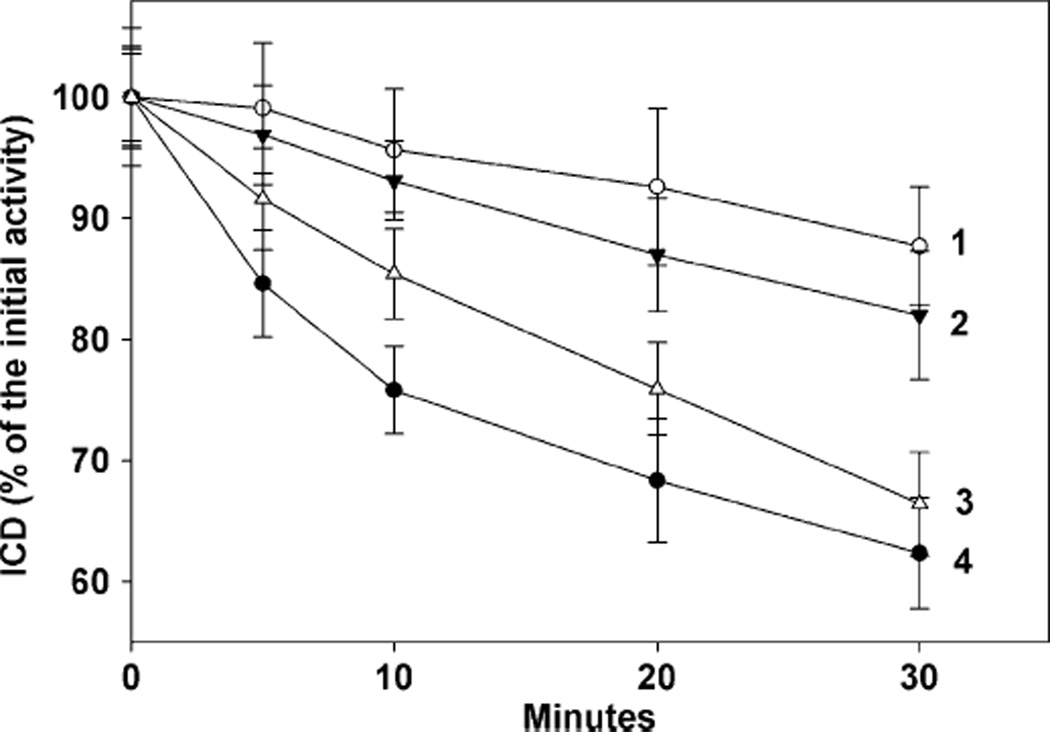
The aim of our initial experiments was to check if E. coli ICD is susceptible to inactivation by superoxide in vitro. A system containing xanthine oxidase with hypoxanthine as a substrate was used as a source of O2• −. Xanthine oxidase alone did not have any appreciable effect on ICD, while the incubation with hypoxanthine alone caused a slight decrease in ICD activity (not shown). Results presented in Figure 2 demonstrate that the complete system, xanthine oxidase, plus hypoxanthine, inactivated ICD in the SOD-deficient cell extracts faster (line 4) than in cell extracts of parental cells (line 1). It is interesting to note that when SOD-deficient cells were grown in the presence of 20 µm of the SOD mimic, MnTM-2-PyP, and then disrupted, such cell extracts demonstrated low inactivation rates similar to those in the parental cell extracts (Figure 2, line 2). However, when the SOD-deficient cells were grown in the absence of the SOD mimic and the mimic was added at final concentrations of 20 µm to the homogenate after the disruption of the cells, the effect was negligible (Figure 2, line 3).
Figure 2.
Inactivation of ICD by enzymatically generated superoxide. E. coli cell extracts (~1.0 mg protein/ml) were incubated at 37°C in the presence of 500 µm hypoxanthine and xanthine oxidase to give an initial superoxide generation rate of 5 µm/min. Experiments were repeated three times with 3–5 replicates. Means±SE are presented. Line 1, parental; Line 2, cell extract of sodAsodB cells grown with 20 µm of MnTM-2-PyP; Line 3, sodAsodB cell extracts plus 20 µm MnTM-2-PyP; Line 4, sodAsodB cell extracts.
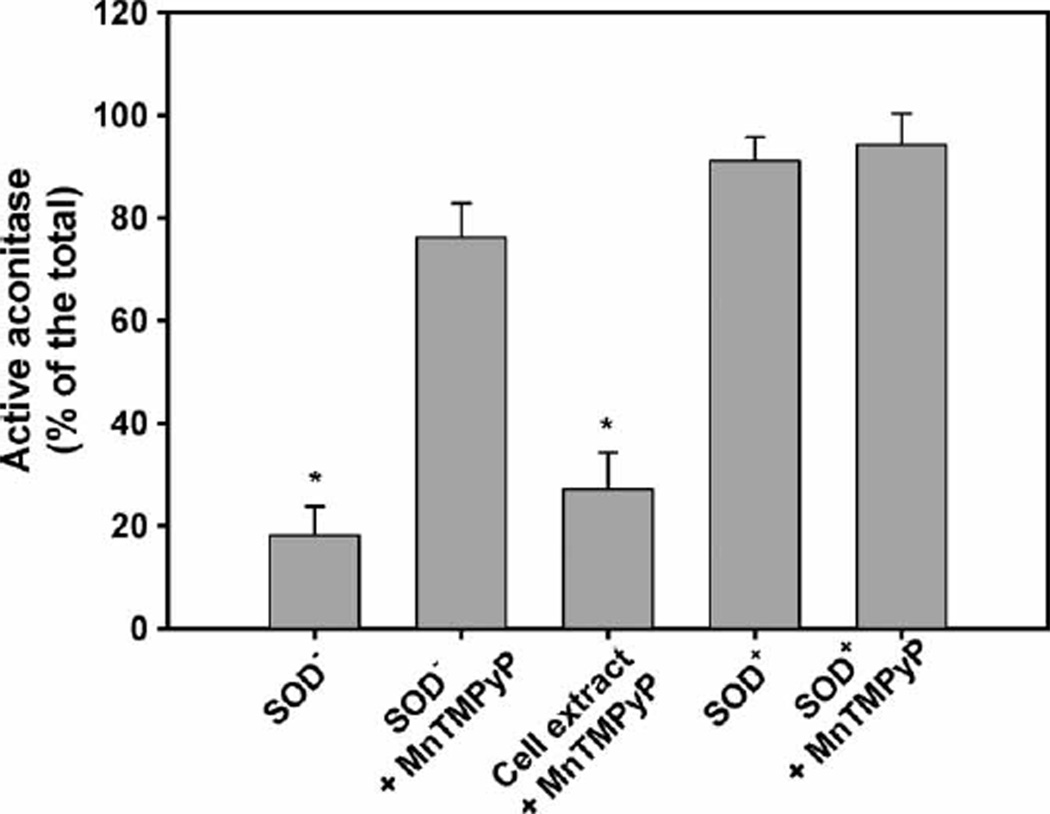
Near its substrate-binding site ICD contains -SH groups whose modification renders the enzyme inactive [37,44–49]. It has been shown that modification of these -SH groups is responsible for the inactivation of the enzyme by nitric oxide [14], ONOO− [15] and 4-hydroxynonenal [17]. Superoxide radical, however, has a relatively low reactivity. The estimated rate constant for O2• − reaction with thiols is in the range of 30–1000 m−1 s−1 [50] and superoxide practically does not react with other amino acid residues of proteins. Therefore, the inactivation of ICD will not result from direct enzyme–superoxide interaction, but from interactions with secondary, highly reactive ROS derived from O2• −, e.g. HO•. Production of HO• by the Fenton reaction requires catalytic ‘free’ Fe2+ and it has been shown that in SOD-deficient E. coli superoxide liberates Fe2+ by attacking Fe-S clusters [51–54]. It has been estimated that in aerobically grown SOD-deficient E. coli the content of ‘free’ Fe increases ~8-fold, reaching 80 µm [52]. In in vitro experiments, treatment of E. coli cell extracts with 10 µm FeSO4 plus ascorbate inactivated ICD [25]. Affinity cleavage experiments revealed that iron coordinated to Asp283 and Asp307, catalysing site specific HO• production, was responsible for the inactivation [25]. Judging by the inhibition of the O2• − driven aconitase inactivation (Figure 3, bars 1 and 2), when the SOD-null cells were grown in the presence of MnTM-2-PyP, the SOD mimic protected the Fe-S clusters and hence prevented the release of Fe2+. Addition of MnTM-2-PyP after disruption of the cells did not protect aconitase (Figure 3, third bar) and, therefore, did not prevent the release of ‘free’ iron. We further tried to prove that liberation of iron is responsible for the inactivation of ICD in SOD-deficient extracts by using metal chelators. ICD, however, requires metal ions for its activity [55] and chelators that bind Fe (desferroxamine, DETAPAC) inactivated the enzyme.
Figure 3.
Aconitase activity in parental and SOD-deficient cells grown with or without MnTM-2-PyP. E. coli cultures were grown to a density of A600nm 0.7–0.8 with or without 20 µm of MnTM-2-PyP. The cells were washed, disrupted by sonication and aconitase was assayed. The assay mixture contained 50 mm Tris-Cl buffer, pH 7.4, 30 mm sodium citrate, 0.5 mm MnCl2, 0.2 mm NADP+, 2.0 units/ml of isocitrate dehydrogenase and cell extract. Results are presented as a percentage of the total aconitase activity obtained after reactivation of the enzyme. Experiments were repeated three times with 3–5 replicates. Bars represent means±SE.
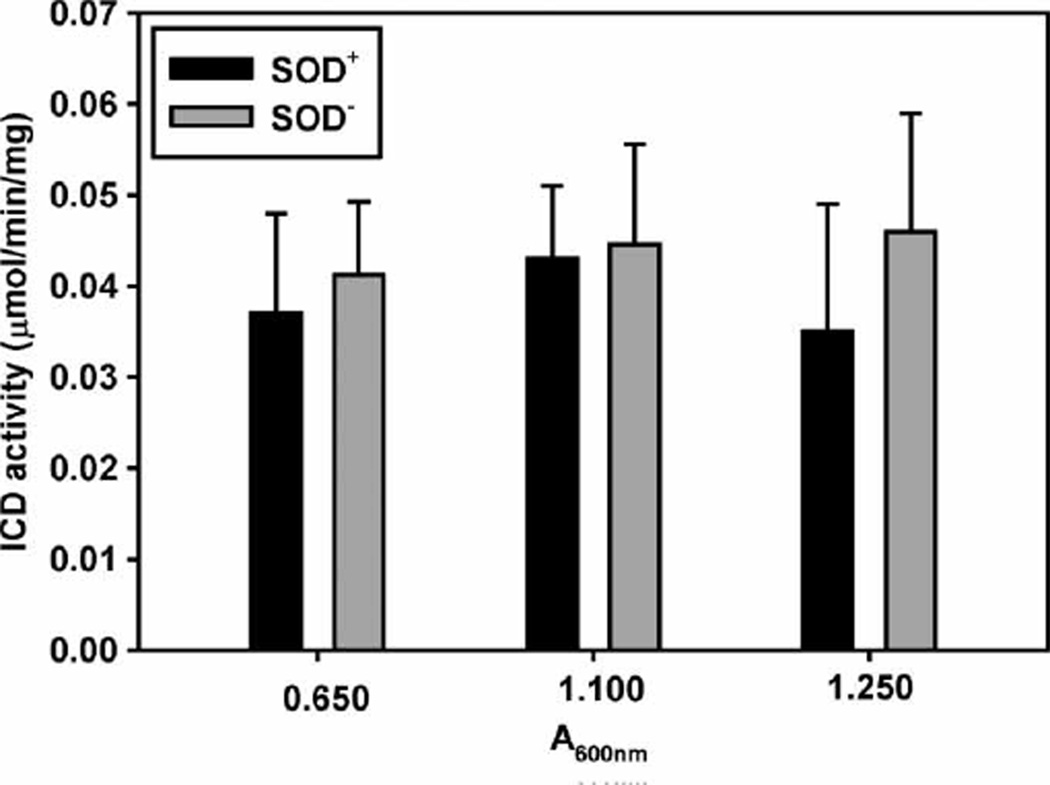
The fact that an external source of O2• − inactivated ICD much faster in the SOD− cell extracts suggests that ICD activity would be lower in the SOD-deficient cells, where the steady state [O2• −] is higher than in the parental cells. Comparison of the ICD activity in exponentially growing SOD− and SOD+ cells revealed, however, that contrary to the expectations, ICD activity was not lower in the SOD− cells. Since ICD expression and activity depend on the availability of nutrients and growth conditions [56,57], the strains were grown in two different media, LB and M9CA, and ICD activity was assayed at different time intervals. No loss of ICD activity was observed in the exponentially growing SOD-null cultures. For the entire period of growth in LB medium, the specific activity of ICD in the SOD− remained slightly higher than in the SOD+ strain (Figure 4). Similar results were obtained when cultures were grown in M9CA medium (not shown), but in that case the activity of ICD in the SOD− cells remained slightly lower than in the parental cells. This result was unexpected and indicated that either ICD is not inactivated or that the enzyme is inactivated, but the inactive protein is reactivated or continuously replaced by de novo protein synthesis.
Figure 4.
Isocitrate dehydrogenase activity in exponentially growing E. coli cultures. E. coli parental and SOD-deficient strains were grown in LB medium to the indicated densities. The cells were washed, disrupted by sonication and ICD activity was measured in 1.0 ml reaction mixture contained 33 mm Tris-EDTA buffer (pH 7.4), 1.33 mm MnCl2, 1.3 mm DL-Isocitrate and 0.1 mm NADP+. Experiments were repeated three times with 3–5 replicates. Bars represent means±SE.
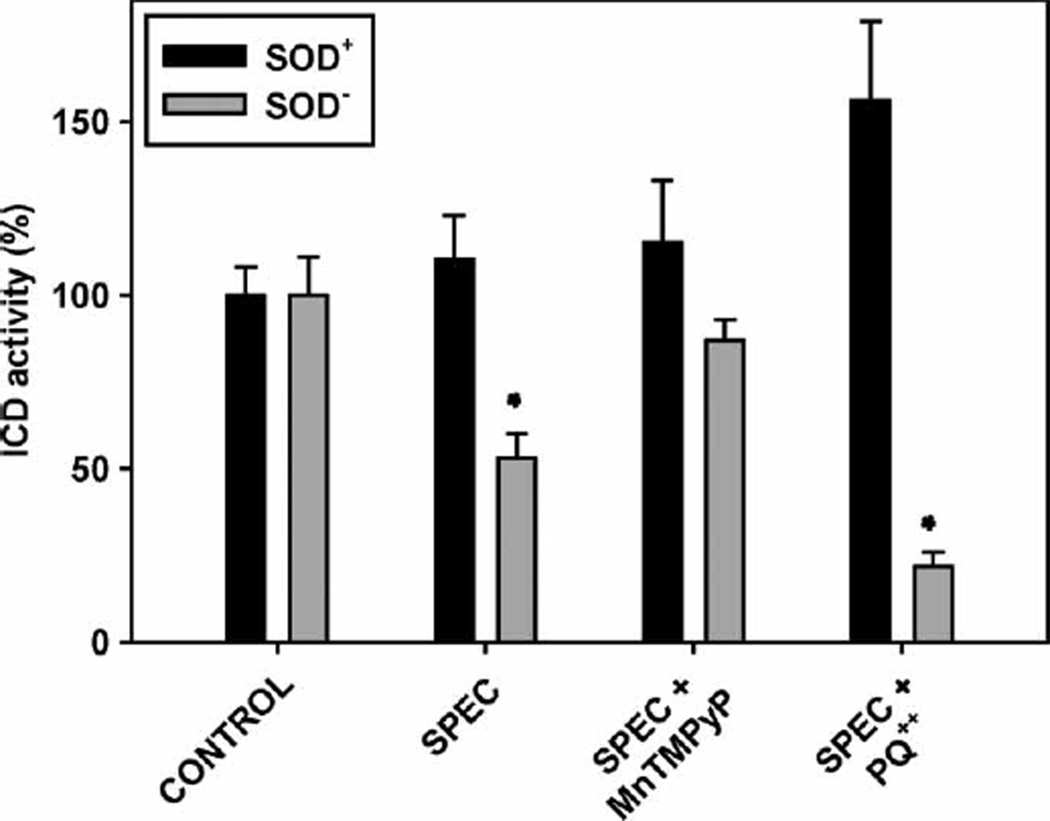
To test the later possibility, spectinomycin (500 µg/ml) was added to the E. coli culture to block protein synthesis [58]. No growth was observed after the addition of the antibiotic, as judged by A600nm. Sixty minutes after the addition of the antibiotic, the cells were disrupted and ICD activity was assayed. Results are summarized in Figure 5. This shows that in SOD− cells where protein synthesis had been stopped, the ICD activity dropped almost 50%, while in the SOD+ cells it remained practically unchanged. To prove that ROS/O2• − are responsible for the inactivation of the enzyme, in parallel experiments the antibiotic was added to cells grown with 20 µm of the SOD mimic MnTM-2-PyP. As seen (Figure 5), the SOD mimic protected ICD against inactivation.
Figure 5.
Effect of spectinomycin and paraquat on isocitrate dehydrogenase activity. E. coli parental and SOD-deficient strains were grown in LB medium to a density of A600nm 0.6. Spectinomycin was added to 500 µm, followed 15 min later by addition of paraquat to 50 µm. One hour after the addition of paraquat the cells were washed, disrupted by sonication and ICD activity was measured. Experiments were repeated three times with 3–5 replicates. Bars represent means±SE.
In further experiments a redox cycling agent, paraquat (PQ++), was added to increase superoxide production. Cells were pre-incubated for 15 min with spectinomycin before the addition of paraquat. It is interesting to note that PQ++ caused increase in the ICD activity in the parental strain, even though protein synthesis had been blocked (Figure 5). The ICD of E. coli is tightly regulated and is acting as a switch, directing substrates either to the Krebs cycle or to the glyoxylate cycle [24]. Inactivation and reactivation of ICD is achieved by phosphorylation and dephosphorylation of a critical Ser residue, which is catalysed by a bifunctional enzyme, ICD kinase/phosphatase [24]. A possible reason for the activation of ICD is dephosphorylation of the enzyme. Since PQ++ is a redox-cycling agent which uses NADPH, a decline of the NADPH/NADP+ ratio could be a signal, which triggers the activation of the NADPH-producing enzymes in order to restore the NADPH/NADP+ ratio [59]. When NADPH/NADP+ ratio was measured, it was found that it decreased from 5.1±1.6 (at zero time) to 1.3±0.5 (at 60 min after the addition of PQ++).
In contrast to the increase of ICD activity in the parental cells, addition of PQ++ to the SOD-deficient cells caused a sharp decrease of the ICD activity (Figure 5). The NADPH/NADP+ ratio in the SOD-deficient cells decreased (from 3.8±0.7 to 0.5±0.4), but this did not lead to activation of ICD. It therefore appears that ICD is inactivated by endogenously produced superoxide. The damaged enzyme, however, is replaced by de novo protein synthesis. As mentioned before, in E. coli ICD activity determines the flux of isocitrate between the Krebs’ cycle and the glyoxalate bypass [24]. The precise balance of the activities of the two pathways is so important that a specific, highly sophisticated mechanism of ICD regulation has evolved. In addition, ICD gene expression is a subject of complex regulation by rpoS, arcA and fnr [56,57]. It is tempting to speculate that uncontrolled drop of ICD activity triggers compensatory mechanisms inducing ICD gene expression. At the same time, the damaged protein is recognized and degraded by a special proteolytic system [60], both events contributing to faster ICD protein turnover.
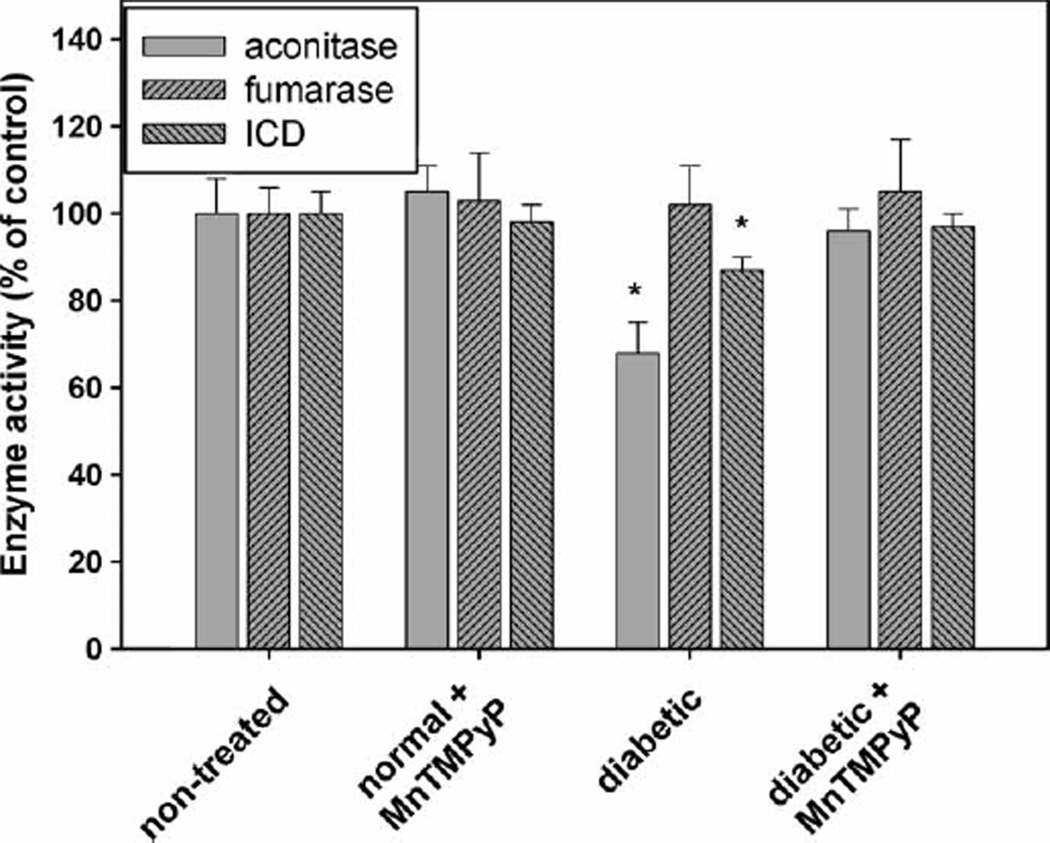
Increased ROS/RNS production has been clearly documented in diabetes [61–63]. In addition to inactivation by ROS/RNS, the NADP+-dependent ICD is highly susceptible to inactivation by glycation, both in vitro and in vivo [64]. Therefore, in the diabetic rats ICD is expected to be exposed simultaneously to ROS/RNS and high glucose, both causing inactivation of the enzyme. For example, in the kidney of streptozotocin diabetic rats the mitochondrial ICD activity was found to be ~30% of the activity found in healthy animals [64]. When we measured the total NADP+-linked ICD in diabetic rat hearts, however, it was only 13% lower than the activity found in controls (Figure 6). Even though statistically significant, such a small decrease would barely have any effect on cardiomyocyte metabolism and NADPH production. Again we used aconitase inactivation to monitor superoxide production and Fe-S clusters disruption in the heart [41]. To verify that aconitase is selectively inactivated by superoxide, fumarase activity was measured in parallel. As seen (Figure 6), aconitase activity in the diabetic harts was ~30% lower compared to the normal animals, while fumarase remained unchanged. Treatment with the SOD mimic, MnTM-2-PyP, restored both aconitase and ICD activities to the control level (Figure 6). These results show that superoxide/ONOO− production is increased in diabetic hearts, yet ICD is almost unaffected. Such a finding either indicates that in the heart ICD is less accessible or Fe released from the Fe-S clusters is sequestered by other chelators or because of its importance the enzyme is a subject of replacement/reactivation. NADP+-dependent ICD activity is considered crucial for maintaining cardiomyocyte energy and redox status [17] and inactivation of the enzyme might be life incompatible. It seems reasonable to expect that cardiomyocytes use various means to keep it active and our data reflect a steady-state ICD activity resulting from two opposing processes—inactivation by ROS/RNS/glycation and replacement/reactivation. The current study, however, does not provide sufficient data to clarify this point.
Figure 6.
Activities of isocitrate dehydrogenase, aconitase and fumarase in rat hearts. The enzyme activities were measured in heart homogenates of control, non-diabetic MnTM-2-PyP-treated, diabetic and diabetic MnTM-2-PyP-treated rats. This experiment was repeated twice with 7–12 animals per group. Bars represent means±SE. * p < 0.05 compared to non-treated group.
In conclusion, our experiments demonstrate that the SOD mimic, MnTM-2-PyP, protects ROS/RNS sensitive enzymes such as NADP+-dependent ICD and aconitase against inactivation. This, together with effects on redox-based signalling pathways [65,66] and other, so far unidentified mechanisms, must be among the reasons for the beneficial effects of the SOD mimic in diabetes [30].
Acknowledgements
The excellent technical assistance of Fatima Sequeira and Milini Thomas is acknowledged. This work was supported by grant MB 07/04 from Kuwait University and HSC Research Core Facility grant GM01/01. IBH acknowledges the support from NIH U19 AI67798-01 and NIH-IR21-ESO/3682. We are grateful to Dr H. R. Fatania for helpful discussions.
Abbreviations
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- ICD
isocitrate dehydrogenase
- SOD
superoxide dismutase
- XO
xanthine oxidase
- MnTM-2-PyP
manganese(III) 5,10,15,20-tetrakis(N-methylpyridinium-2-yl)porphyrin (MnTM-2-PyP5+ (charges omitted throughout text for clarity), AEOL-10112)
- LB
Luria-Bertoni
- PQ++
paraquat
- DETAPAC
diethylenetriaminepentaacetic acid
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Xu X, Zhao J, Xu Z, Peng B, Huang Q, Arnold E, Ding J. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004;279:33946–33957. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- 2.Haselbeck RJ, McAlister-Henn L. Function and expression of yeast mitochondrial NAD- and NADP-specific isocitrate dehydrogenases. J Biol Chem. 1993;268:12116–12122. [PubMed] [Google Scholar]
- 3.Jennings GT, Sechi S, Stevenson PM, Tuckey RC, Parmelee D, McAlister-Henn L. Cytosolic NADP(+)-dependent isocitrate dehydrogenase. Isolation of rat cDNA and study of tissue-specific and developmental expression of mRNA. J Biol Chem. 1994;269:23128–23134. [PubMed] [Google Scholar]
- 4.Jo SH, Son MK, Koh HJ, Lee SM, Song IH, Kim YO, Lee YS, Jeong KS, Kim WB, Park JW, Song BJ, Huh TL. Control of mitochondrial redox balance and cellular defense against oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. J Biol Chem. 2001;276:16168–16176. doi: 10.1074/jbc.M010120200. [DOI] [PubMed] [Google Scholar]
- 5.Lee SM, Koh HJ, Park DC, Song BJ, Huh TL, Park JW. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med. 2002;32:1185–1196. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Park JW. Cellular defense against singlet oxygeninduced oxidative damage by cytosolic NADP+-dependent isocitrate dehydrogenase. Free Radic Res. 2003;37:309–316. doi: 10.1080/1071576021000050429. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Lee SM, Tak JK, Choi KS, Kwon TK, Park JW. Regulation of singlet oxygen-induced apoptosis by cytosolic NADP+-dependent isocitrate dehydrogenase. Molec Cell Biochem. 2007;302:27–34. doi: 10.1007/s11010-007-9421-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Park JW. Oxalomalate, a competitive inhibitor of NADP+-dependent isocitrate dehydrogenase, regulates heat shock-induced apoptosis. Biochem Biophys Res Commun. 2005;337:685–691. doi: 10.1016/j.bbrc.2005.09.104. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Kang BS, Park JW. Cellular defense against heat shock-induced oxidative damage by mitochondrial NADP+-dependent isocitrate dehydrogenase. Free Radic Res. 2005;39:441–448. doi: 10.1080/10715760500066265. [DOI] [PubMed] [Google Scholar]
- 10.Kil IS, Shin SW, Yeo HS, Lee YS, Park JW. Mitochondrial NADP+-dependent isocitrate dehydrogenase protects cadmium-induced apoptosis. Molec Pharmacol. 2006;70:1053–1061. doi: 10.1124/mol.106.023515. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Park JW. Oxalomalate regulates ionizing radiationinduced apoptosis in mice. Free Radic Biol Med. 2007;42:44–51. doi: 10.1016/j.freeradbiomed.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Jo SH, Lee SM, Koh HJ, Song H, Park JW, Lee WH, Huh TL. Role of NADP+-dependent isocitrate dehydrogenase (NADP+-ICDH) on cellular defence against oxidative injury by gamma-rays. Int J Radiat Biol. 2004;80:635–642. doi: 10.1080/09553000400007680. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Kim SY, Kil IS, Park JW. Regulation of ionizing radiation-induced apoptosis by mitochondrial NADP+- dependent isocitrate dehydrogenase. J Biol Chem. 2007;282:13385–13394. doi: 10.1074/jbc.M700303200. [DOI] [PubMed] [Google Scholar]
- 14.Yang ES, Richter C, Chun JS, Huh TL, Kang SS, Park JW. Inactivation of NADP(+)-dependent isocitrate dehydrogenase by nitric oxide. Free Radic Biol Med. 2002;33:927–937. doi: 10.1016/s0891-5849(02)00981-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Yang ES, Park JW. Inactivation of NADP+- dependent isocitrate dehydrogenase by peroxynitrite. Implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 16.Lee SM, Huh TL, Park JW. Inactivation of NADP(+)- dependent isocitrate dehydrogenase by reactive oxygen species. Biochimie. 2001;83:1057–1065. doi: 10.1016/s0300-9084(01)01351-7. [DOI] [PubMed] [Google Scholar]
- 17.Benderdour M, Charron G, DeBlois D, Comte B, Des Rosiers C. Cardiac mitochondrial NADP+-isocitrate dehydrogenase is inactivated through 4-hydroxynonenal adduct formation: an event that precedes hypertrophy development. J Biol Chem. 2003;278:45154–45159. doi: 10.1074/jbc.M306285200. [DOI] [PubMed] [Google Scholar]
- 18.Yang JH, Yang ES, Park JW. Inactivation of NADP+- dependent isocitrate dehydrogenase by lipid peroxidation products. Free Radic Res. 2004;38:241–249. doi: 10.1080/10715760310001657712. [DOI] [PubMed] [Google Scholar]
- 19.Benov L, Fridovich I. Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli. The transketolase connection. J Biol Chem. 1999;274:4202–4206. doi: 10.1074/jbc.274.7.4202. [DOI] [PubMed] [Google Scholar]
- 20.Benov L, Al-Ibraheem J. Glycerol metabolism in superoxide dismutase-deficient Escherichia coli. Free Radic Res. 2001;35:867–872. doi: 10.1080/10715760100301361. [DOI] [PubMed] [Google Scholar]
- 21.Benov L, Kredich NM, Fridovich I. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J Biol Chem. 1996;271:21037–21040. doi: 10.1074/jbc.271.35.21037. [DOI] [PubMed] [Google Scholar]
- 22.Imlay JA, Fridovich I. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem. 1991;266:6957–6965. [PubMed] [Google Scholar]
- 23.Gort AS, Imlay JA. Balance between endogenous superoxide stress and antioxidant defenses. J Bacteriol. 1998;180:1402–1410. doi: 10.1128/jb.180.6.1402-1410.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaPorte DC. The isocitrate dehydrogenase phosphorylation cycle: regulation and enzymology. J Cell Biochem. 1993;51:14–18. doi: 10.1002/jcb.240510104. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Tsubouchi R, Fukayama M, Ogawa T, Yoshino M. Oxidative inactivation of reduced NADP-generating enzymes in E. coli: iron-dependent inactivation with affinity cleavage of NADP-isocitrate dehydrogenase. Arch Microbiol. 2006;186:385–392. doi: 10.1007/s00203-006-0153-1. [DOI] [PubMed] [Google Scholar]
- 26.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium- 2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 27.Vance CK, Miller A. A simple proposal that can explain the inactivity of metal-substituted superoxide dismutases. J Am Chem Soc. 1998;120:461–467. [Google Scholar]
- 28.Michel E, Nauser T, Sutter B, Bounds PL, Koppenol WH. Kinetics properties of Cu,Zn-superoxide dismutase as a function of metal content. Arch Biochem Biophys. 2005;439:234–240. doi: 10.1016/j.abb.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein S, Fridovich I, Czapski G. Kinetic properties of Cu,Zn-superoxide dismutase as a function of metal content-order restored. Free Radic Biol Med. 2006;41:937–941. doi: 10.1016/j.freeradbiomed.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 30.Benov L, Batinic-Haberle I. A manganese porphyrin suppresses oxidative stress and extends the life span of streptozotocin- diabetic rats. Free Radic Res. 2005;39:81–88. doi: 10.1080/10715760400022368. [DOI] [PubMed] [Google Scholar]
- 31.Touati D. Investigating phenotypes resulting from a lack of superoxide dismutase in bacterial null mutants. Meth Enzymol. 2002;349:145–154. doi: 10.1016/s0076-6879(02)49330-5. [DOI] [PubMed] [Google Scholar]
- 32.Touati D, Farr SB. Elevated mutagenesis in bacterial mutants lacking superoxide dismutase. Meth Enzymol. 1990;186:646–651. doi: 10.1016/0076-6879(90)86160-w. [DOI] [PubMed] [Google Scholar]
- 33.Imlay JA, Fridovich I. Isolation and genetic analysis of a mutation that suppresses the auxotrophies of superoxide dismutase-deficient Escherichia coli K12. Molec Gen Genet. 1991;228:410–416. doi: 10.1007/BF00260634. [DOI] [PubMed] [Google Scholar]
- 34.Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: A laboratory manual. NY: Cold Spring Harbor Laboratory, Cold Spring Harbor; 1982. [Google Scholar]
- 35.Hausladen A, Fridovich I. Measuring nitric oxide and and superoxide: rate constants for aconitase reactivity. Meth Enzymol. 1996;269:37–41. doi: 10.1016/s0076-6879(96)69007-7. [DOI] [PubMed] [Google Scholar]
- 36.Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli aconitase. J Biol Chem. 1991;266:19328–19333. [PubMed] [Google Scholar]
- 37.Fatania HR, al-Nassar KE, Thomas N. Chemical modification of rat liver cytosolic NADP(+)-linked isocitrate dehydrogenase by N-ethylmaleimide. Evidence for essential sulphydryl groups. FEBS Lett. 1993;322:245–248. doi: 10.1016/0014-5793(93)81579-o. [DOI] [PubMed] [Google Scholar]
- 38.Micheli V, Simmonds HA, Bari M, Pompucci G. HPLC determination of oxidized and reduced pyridine coenzymes in human erythrocytes. Clin Chim Acta. 1993;220:1–17. doi: 10.1016/0009-8981(93)90002-l. [DOI] [PubMed] [Google Scholar]
- 39.Ross AD, Sheng H, Warner DS, Piantadosi CA, Batinic- Haberle I, Day BJ, Crapo JD. Hemodynamic effects of metalloporphyrin catalytic antioxidants: structure-activity relationships and species specificity. Free Radic Biol Med. 2002;33:1657–1669. doi: 10.1016/s0891-5849(02)01140-1. [DOI] [PubMed] [Google Scholar]
- 40.Fatania H, al-Nassar KE, Sidhan V. Purification and partial characterisation of NADP(+)-linked isocitrate dehydrogenase from rat liver cytosol. FEBS Lett. 1993;320:57–60. doi: 10.1016/0014-5793(93)81657-l. [DOI] [PubMed] [Google Scholar]
- 41.Gardner PR. Aconitase: sensitive target and measure of superoxide. Meth Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 42.Liochev SI, Fridovich I. Modulation of the fumarases of Escherichia coli in response to oxidative stress. Arch Biochem Biophys. 1993;301:379–384. doi: 10.1006/abbi.1993.1159. [DOI] [PubMed] [Google Scholar]
- 43.Liochev SI, Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci USA. 1992;89:5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colman RF. The role of sulfhydryl groups in the catalytic function of isocitrate dehydrogenase. I. Reaction with 5,5′-dithiobis(2-nitrobenzoic acid) Biochemistry. 1969;8:888–898. doi: 10.1021/bi00831a019. [DOI] [PubMed] [Google Scholar]
- 45.Colman RF, Chu R. The role of sulfhydryl groups in the catalytic function of isocitrate dehydrogenase. 3. Effect of N-ethylmaleimide on chemical and physical properties. J Biol Chem. 1970;245:608–615. [PubMed] [Google Scholar]
- 46.Colman RF, Chu R. The role of sulfhydryl groups in the catalytic function of isocitrate dehydrogenase. II. Effect of N-ethylmaleimide on kinetic properties. Jf Biol Chem. 1970;245:601–607. [PubMed] [Google Scholar]
- 47.Little C, Holland P. Sulfhydril groups and the concerted inhibition of NADP+-linked isocitrate dehydrogenase. Can J Biochem. 1972;50:1109–1113. doi: 10.1139/o72-151. [DOI] [PubMed] [Google Scholar]
- 48.Panchenko LF, Olfer’ev AM, Loktaeva TD, Ivkov NN, Komissarova T. Effect of thiol-oxidizing agents on several enzymes in rat liver mitochondria. Ukr Biokhim Zhurnal. 1976;48:455–459. [PubMed] [Google Scholar]
- 49.Kil IS, Park JW. Regulation of mitochondrial NADP+- dependent isocitrate dehydrogenase activity by glutathionylation. J Biol Chem. 2005;280:10846–10854. doi: 10.1074/jbc.M411306200. [DOI] [PubMed] [Google Scholar]
- 50.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27:322–328. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 51.Imlay JA. Iron-sulphur clusters and the problem with oxygen. Molec Microbiol. 2006;59:1073–1082. doi: 10.1111/j.1365-2958.2006.05028.x. [DOI] [PubMed] [Google Scholar]
- 52.Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc Natl Acad Sci USA. 1996;93:13635–13640. doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woodmansee AN, Imlay JA. Quantitation of intracellular free iron by electron paramagnetic resonance spectroscopy. Meth Enzymol. 2002;349:3–9. doi: 10.1016/s0076-6879(02)49316-0. [DOI] [PubMed] [Google Scholar]
- 54.Liochev SI, Fridovich I. Superoxide and iron: partners in crime. IUBMB Life. 1999;48:157–161. doi: 10.1080/713803492. [DOI] [PubMed] [Google Scholar]
- 55.Colman RF. Role of metal ions in reactions catalyzed by pig heart triphosphopyridine nucleotide-dependent isocitrate dehydrogenase. II. Effect on catalytic properties and reactivity of amino acid residues. J Biol Chem. 1972;247:215–223. [PubMed] [Google Scholar]
- 56.Chao G, Shen J, Tseng CP, Park SJ, Gunsalus RP. Aerobic regulation of isocitrate dehydrogenase gene (icd) expression in Escherichia coli by the arcA and fnr gene products. J Bacteriol. 1997;179:4299–4304. doi: 10.1128/jb.179.13.4299-4304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung IL, Kim SK, Kim IG. The RpoS-mediated regulation of isocitrate dehydrogenase gene expression in Escherichia coli. Curr Microbiol. 2006;52:21–26. doi: 10.1007/s00284-005-8006-8. [DOI] [PubMed] [Google Scholar]
- 58.Djaman O, Outten FW, Imlay JA. Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem. 2004;279:44590–44599. doi: 10.1074/jbc.M406487200. [DOI] [PubMed] [Google Scholar]
- 59.Bautista J, Satrustegui J, Machado A. Evidence suggesting that the NADPH/NADP ratio modulates the splitting of the isocitrate flux between the glyoxylic and tricarboxylic acid cycles, in Escherichia coli. FEBS Lett. 1979;105:333–336. doi: 10.1016/0014-5793(79)80642-0. [DOI] [PubMed] [Google Scholar]
- 60.Davies KJ, Lin SW. Oxidatively denatured proteins are degraded by an ATP-independent proteolytic pathway in Escherichia coli. Free Radic Biol Med. 1988;5:225–236. doi: 10.1016/0891-5849(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 61.Ceriello A. Oxidative stress and diabetes-associated complications. Endocrine Practice. 2006;1:60–62. doi: 10.4158/EP.12.S1.60. [DOI] [PubMed] [Google Scholar]
- 62.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 64.Kil IS, Lee JH, Shin AH, Park JW. Glycation-induced inactivation of NADP(+)-dependent isocitrate dehydrogenase: implications for diabetes and aging. Free Radic Biol Med. 2004;37:1765–1778. doi: 10.1016/j.freeradbiomed.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 65.Tse HM,Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 66.Bottino R, Balamurugan AN, Tse H, Thirunavukkarasu C, Ge X, Profozich J, Milton M, Ziegenfuss A, Trucco M, Piganelli JD. Response of human islets to isolation stress and the effect of antioxidant treatment. Diabetes. 2004;53:2559–2568. doi: 10.2337/diabetes.53.10.2559. [DOI] [PubMed] [Google Scholar]