Abstract
Background
Streptococcus infantarius subsp. infantarius (Sii) belongs to the Streptococcus bovis/Streptococcus equinus complex associated with several human and animal infections. Sii is a predominant bacterium in spontaneously fermented milk products in Africa. The genome sequence of Sii strain CJ18 was compared with that of other Streptococcus species to identify dairy adaptations including genome decay such as in Streptococcus thermophilus, traits for its competitiveness in spontaneous milk fermentation and to assess potential health risks for consumers.
Results
The genome of Sii CJ18 harbors several unique regions in comparison to Sii ATCC BAA-102T, among others an enlarged exo- and capsular polysaccharide operon; Streptococcus thermophilus-associated genes; a region containing metabolic and hypothetical genes mostly unique to CJ18 and the dairy isolate Streptococcus gallolyticus subsp. macedonicus; and a second oligopeptide transport operon. Dairy adaptations in CJ18 are reflected by a high percentage of pseudogenes (4.9%) representing genome decay which includes the inactivation of the lactose phosphotransferase system (lacIIABC) by multiple transposases integration. The presence of lacS and lacZ genes is the major dairy adaptation affecting lactose metabolism pathways also due to the disruption of lacIIABC.
We constructed mutant strains of lacS, lacZ and lacIIABC and analyzed the resulting strains of CJ18 to confirm the redirection of lactose metabolism via LacS and LacZ.
Natural competence genes are conserved in both Sii strains, but CJ18 contains a lower number of CRISPR spacers which indicates a reduced defense capability against alien DNA. No classical streptococcal virulence factors were detected in both Sii strains apart from those involved in adhesion which should be considered niche factors. Sii-specific virulence factors are not described. Several Sii-specific regions encoding uncharacterized proteins provide new leads for virulence analyses and investigation of the unclear association of dairy and clinical Sii with human diseases.
Conclusions
The genome of the African dairy isolate Sii CJ18 clearly differs from the human isolate ATCC BAA-102T. CJ18 possesses a high natural competence predisposition likely explaining the enlarged genome. Metabolic adaptations to the dairy environment are evident and especially lactose uptake corresponds to S. thermophilus. Genome decay is not as advanced as in S. thermophilus (10-19%) possibly due to a shorter history in dairy fermentations.
Keywords: Streptococcus infantarius, Streptococcus bovis/Streptococcus equinus complex, Streptococcus thermophilus, Streptococcus gallolyticus subsp. macedonicus, Dairy fermentation, Lactose metabolism, Africa, Camel, Health risk, Streptococcus virulence factors
Background
The putative pathogen Streptococcus infantarius subsp. infantarius (Sii) is a lactic acid bacterium (LAB) commonly associated with the gastrointestinal tract of animals and humans [1]. Additionally, Sii has been isolated from dairy products, feces (including the type strain ATCC BAA-102T and isogenetic strain CCUG 43820T), human blood (n = 3) and human endocarditis (n = 3) [2-5]. Recently, it was identified as the predominant species in several spontaneously fermented African dairy products such as suusac, gariss and fènè[2,5-7] and in the Mexican fermented maize beverage pozol[8]. Sii belongs to the Lancefield group D Streptococcus bovis/Streptococcus equinus complex (SBSEC) which comprises the species S. bovis, S. equinus, Streptococcus lutetiensis (known as Streptococcus infantarius subsp. coli), Streptococcus gallolyticus subsp. gallolyticus (formerly S. bovis biotype I), Streptococcus gallolyticus subsp. macedonicus, Streptococcus gallolyticus subsp. pasteurianus and Streptococcus alactolyticus[3,4,9].
The SBSEC is commonly associated with many infectious diseases such as bacteremia, endocarditis and bloat [1]. Moreover, some members of the group, especially S. gallolyticus subsp. gallolyticus, are suspected to play a role in colonic cancer development [10,11], partly associated to increasing mRNA levels of IL-1, IL-8 and COX-2 in colorectal tissue, which contribute to inflammation caused tumor development [12]. Because of the high risk association of mainly S. gallolyticus subsp. gallolyticus with infectious diseases and cancer, research on virulence within the SBSEC group has largely focused on this species [12-16]. Virulence factors such as fibrinogen binding factor FimB, glucosyltransferase Gtf and pilus subunit B PilB have been identified in several SBSEC members [16-19]. Additionally, potential virulence factors such as adhesion proteins have been shown e.g. the surface protein histone-like protein A (HlpA), the “adhesion to collagen of the S. bovis group” (Acb) and “S. bovis group surface protein” (Sbs) [14,20]. However, many of these factors seem to be necessary for survival of SBSEC in the gastrointestinal tract and should therefore be considered as niche factors [21].
The pathogenicity of Sii is less elucidated. Potential pro-inflammatory proteins were detected in Sii and the species is also associated with non-colonic cancer [22,23]. In parallel to S. gallolyticus subsp. gallolyticus, a Sii strain isolated from feces of an infected baby was able to translocate across a polarized epithelial monolayer of Caco-2 cells, a property which potentially facilitates infection [24]. This ability was so far only demonstrated for a single Sii strain of clinical and not of food origin. In a recent and broad clinical study on 58 S. bovis strains, only the subspecies S. infantarius subsp. coli (n = 17), but not Sii, was isolated from blood of infected patients among 29 S. gallolyticus subsp. gallolyticus and 12 S. gallolyticus subsp. pasteurianus[10]. This suggests only a minor role of Sii in infectious diseases. Nevertheless, the predominance of Sii in African food fermentations [2-5] and, as a consequence, the ingestion of high amounts of viable cells of this species by the consumer demands further research to elucidate any potential pathogenic traits of this SBSEC member and possibly diverge dairy from clinical isolates.
Streptococcus thermophilus is the only streptococcal species recommended by the qualified presumption of safety (QPS) for use in fermented food products [25]. It displays an adaptation to the milk environment that is characterized by genome reduction, gene decay and loss of function, which is reflected by the high abundance of pseudogenes in all sequenced S. thermophilus genomes [26,27]. Genome reduction through loss or inactivation of virulence factors and long history of use contributed to the recognition of S. thermophilus by QPS, despite its close genetic relationship to the SBSEC [25-29]. Interestingly, Streptococcus macedonicus ACA-DC 198 (designated S. gallolyticus subsp. macedonicus in this study according to [3]), a Greek cheese isolate, displayed comparable genome decay to S. thermophilus and could indicate parallel evolutionary adaptation to the dairy environment in other members of the SBSEC and important contributions of certain members of the SBSEC to dairy fermentations in Europe [30].
The predominance and probably exclusive habitat of the African Sii variants in dairy fermentations suggests adaptation to the dairy environment similar to S. thermophilus[2,7]. This predominance seems directly related to the presence of a gal-lac operon in the African variant of Sii[7], a feature that is absent in other members of the SBSEC. Furthermore, African strains display a lactose fermentation pattern paralleling that of S. thermophilus[7]. The high prevalence of bacteriocin producers among African Sii isolates likely contributes to the predominance of Sii in African dairy fermentations [2].
In this work, we present the complete genome sequence of Sii CJ18 isolated as representative predominant strain from spontaneously fermented camel milk suusac from Kenya at over 108 CFU mL-1. CJ18 does not produce bacteriocin-like inhibitory substances [2]. It was selected for genome sequencing due to genetic and metabolic evidence of a lactose fermentation pattern similar to S. thermophilus after studying of 3 different African Sii isolates [7]. A genomic comparison of strain CJ18 to other pathogenic and non-pathogenic streptococci was performed in order to identify dairy adaptations and potential virulence factors in CJ18. Our study provides new insight into streptococcal evolution in the previously untouched ecosystem of dairy fermentations in Africa and provides new insight on safety and occurrence of horizontal gene transfer (HGT) of streptococci in food fermentations.
Results
General genome properties
The genome of Sii CJ18 consists of a 1,988,420-bp circular molecule encoding 2050 genes of which 1867 encode for proteins [GenBank:CP003295, GenBank:CP003296] (Table 1) [31]. Comparison of genes with their homologues in other streptococcal genomes, resulted in detection of 97 (4.9%) genes that carry a deletion, insertion or premature stop, and that were therefore assigned as pseudogenes. Additionally, 19,829 bp of plasmid related DNA, designated pSICJ18-1, providing 35 coding DNA sequences (CDS) with only limited similarity to SBSEC sequences were detected. The nucleotide sequence (96-100% identity) and G + C mol%-content of 30 out of 35 CDS suggest a lactococcal origin [Additional file 1].
Table 1.
General features of the Sii CJ18 genome and other sequenced genomes of streptococci
| |
S. infantarius subsp. infantarius |
S. gallolyticus subsp. gallolyticus |
S. gallolyticus subsp. macedonicus |
S. gallolyticus subsp. pasteurianus ATCC 43144 | S. agalactiae 2603 V/R | S. pyogenes M1 GAS | S. pneumoniae D39 |
S. thermophilus |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CJ18 | ATCC BAA-102T | ATCC 43143 | ATCC BAA-2069 | UCN34 | ACA-DC 198 | LMD-9 | CNRZ 1066 | LMG 18311 | |||||
| length (bp) |
1,988,420 + 19,829 pSICJ18-1 |
1,938,634 |
2,362,241 |
2,356,444 |
2,350,911 |
2,130,034 + 12,728 pSMA198 |
2,100,077 |
2,160,267 |
1,852,441 |
2,046,115 |
1,856,368 |
1,796,226 |
1,796,846 |
| G + C (mol%) |
37.6 |
37.6 |
37.5 |
37.6 |
37.6 |
37.6 |
37.4 |
35.6 |
38.5 |
39.7 |
39.1 |
39.1 |
39.1 |
| genes |
2050 + 35 pSICJ18-1 |
1988 |
2371 |
2410 + 21 pSGG1 |
2349 |
2280 + 17 pSMA198 |
2102 |
2276 |
1810 |
2069 |
2002 + 4 Plsm1 + 2 Plsm 2 |
2000 |
1973 |
| pseudogenes/truncated proteins (%)b) |
97 (4.9%) |
n/a a) |
49 (2.1%) |
0 |
37 (1.6%) |
215 (9.8%) |
157 (7.9%) |
0 |
35 (2.0%) |
82 (4.1%) |
206 (10.8%) |
~19% |
~19% |
| protein (non tRNA/rRNA) |
1867 |
|
2246 |
2309 |
2223 |
1977 |
1869 |
2124 |
1696 |
1914 |
1709 |
1915 |
1888 |
| tRNA genes |
68 |
46c) |
60 |
80 |
71 |
70 |
61 |
80 |
60 |
58 |
67 |
67 |
67 |
| rRNA genes |
18 |
8c) |
15 |
21 |
18 |
17 |
15 |
21 |
18 |
12 |
19 |
18 |
18 |
| source | fermented camel milk suusac[31] | baby feces (HMP) | human clinical specimen, blood [32] | human clinical specimen, blood [33] | human clinical specimen, blood [13] | Greek Kasseri cheese [30] | human clinical specimen, blood [32] | human clinical specimen [34] | human clinical specimen [35] | human clinical specimen [36] | yogurt [27] | yogurt [26] | yogurt [26] |
a) n/a: Not available; b) Calculated according to: #pseudogenes/(#pseudogenes + total proteins)*100; c) The genome of ATCC BAA-102T was only aligned to that of CJ18 but not completely assembled. tRNA and rRNA genes are possibly underestimated in the type strain as genome gaps were not closed. HMP: Human Microbiome Project http://www.hmpdacc.org.
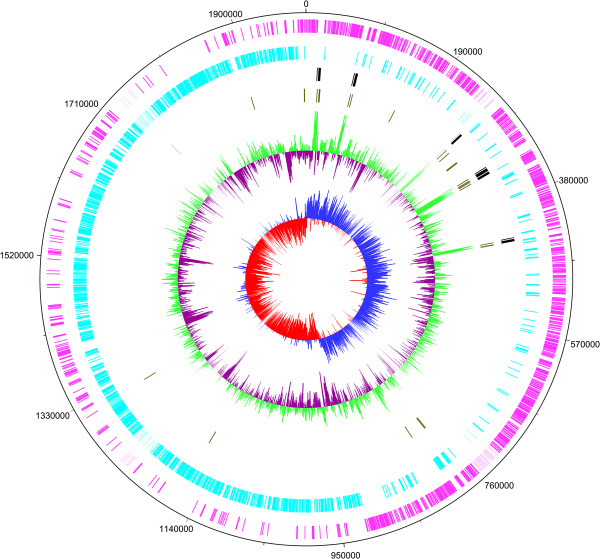
The origin of the genome of CJ18 was determined upstream of the dnaA gene and corresponds to the switch in GC-skew (Figure 1). However, a shift towards the 5 o’clock position was detected for the terminus position, as is reflected by a switch in the GC-skew and in the CDS-density on the forward and reverse strand (Figure 1), a feature also observed in S. gallolyticus subsp. gallolyticus ATCC 43143 and S. gallolyticus subsp. pasteurianus ATCC 43144 [32].
Figure 1.
Circular genome of Sii CJ18. The inner most circle shows the GC-skew of higher (blue) and lower (red) than average followed by the GC-content with higher (green) and lower (dark purple) than average. The third (olive green) and fourth circle (black) display tRNA and rRNA, respectively. The two outermost circles indicate the position of coding sequences on the forward (pink) or reverse (ice blue) strand.
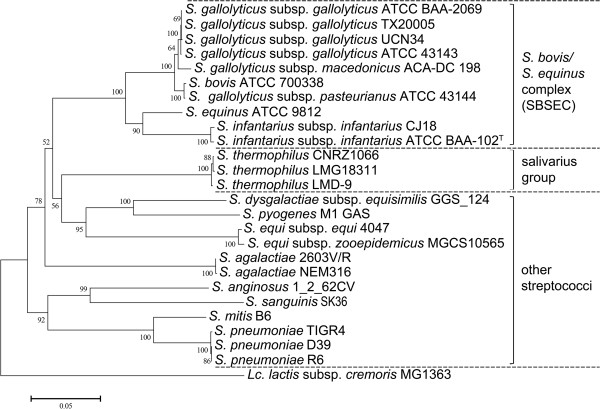
The complete genome sequence was used to confirm the taxonomy of CJ18 through alignment and subsequent phylogenetic analysis using 16S rRNA and eight typical streptococcal genes (groEL, gyrB, recA, recN, rpoB, secA, secY and sodA). All genes clearly positioned CJ18 within the SBSEC on the same branch as its closest relative Sii ATCC-BAA-102T (Figure 2, tree only shown for groEL). The highest bootstrap percentages were obtained for trees based on groEL, recN and secY sequences (data not shown).
Figure 2.
Rooted phylogenetic tree calculated for groEL sequences of Sii CJ18 and related streptococci. Rooted phylogenetic tree was calculated for the groEL genes of Sii CJ18, related SBSEC members and other streptococci. CJ18 was clearly positioned on the same branch as Sii ATCC BAA-102T within the SBSEC. The same phylogenetic position of CJ18 was obtained for the 16S rRNA gene and gyrB, recA, recN, rpoB, secA, secY and sodA with groEL, secY and recN yielding highest bootstrap percentages (data not shown). The evolutionary distances indicated by the horizontal bar below the figure are in the units of the number of base substitutions per site.
Comparison of CJ18 to ATCC BAA-102T and other SBSEC strains
The draft genome sequence of the Sii ATCC BAA-102T type strain was used for a comparison to the African isolate CJ18. An in silico hybridization revealed that the organization of loci was highly conserved between CJ18 and ATCC BAA-102T (Figure 3) and to the closely related species S. gallolyticus subsp. macedonicus and S. gallolyticus subsp. gallolyticus, albeit at a lesser degree [Additional file 2]. The genome of CJ18 is 37 kb larger than that of ATCC BAA-102T and harbors a number of variable regions and insertions compared to other streptococci, designated R1-R15 (Figure 4 and [Additional file 1]). The major variable regions comprise phage-related proteins (R4, 34.2 kb), proteins with high sequence identity to S. thermophilus (R6, 25.6 kb) and a cluster of metabolic and hypothetical proteins specific for CJ18 (R9, 26.1 kb). Interestingly, R14 comprises many hypothetical proteins shared to the largest extent with the Greek cheese isolate S. gallolyticus subsp. macedonicus ACA-DC 198 (R14, 52.5 kb) and second to ATCC BAA-102T. This suggests a closer relationship among these SBSEC strains compared to the other strains used in genome analysis and might possibly even be related to the dairy origin. Remarkably, variable regions often possess a distinct base-deviation index in CJ18, indicating recent evolutionary origin due to little advanced amelioration (Figure 4).
Figure 3.
Synteny plot of genomes Sii CJ18 (x) vs. Sii ATCC BAA-102T (y). Both genomes of the Sii strains display a high degree of conservation indicated by the alignment near the diagonal line. Major insertion sites can be identified as R4 (34.2 kb) consisting largely of phage-related genes; R6 (25.6 kb) encompassing a 13.2-kb S. thermophilus-gene cluster comprising the additional gal-lac operon; and R9 (26.1 kb) containing among others an HTH-type transcriptional regulator Rgg, primosomal protein N’ (replication factor Y) – superfamily II helicase, an FtsK/SpoIIIE family protein and a conjugal transfer protein. The major gap α (34.6 kb) in ATCC BAA-102T corresponds to a phage region.
Figure 4.
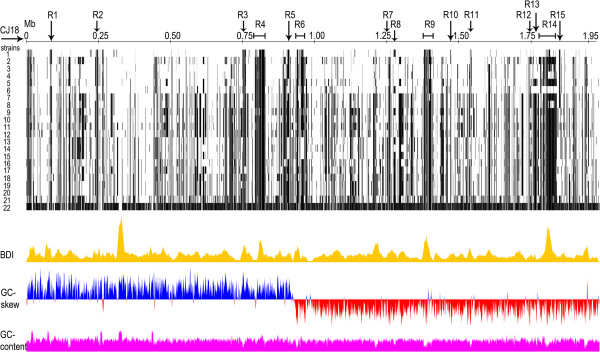
Barcode plot of whole genome comparison of Sii CJ18 with genomes of related species. Whole genomes of Streptococcus and Lactococcus strains were compared for the absence (black bar) and presence (white space) of certain genes related to those of CJ18. Several relevant regions (R1-15) were detected in CJ18 containing the following proteins: (R1) phage-related, (R2) cell-/environment signalling, (R3) Eps/Cps synthesis, (R4) phage-related, (R5) restriction endonuclease and methylase, (R6) S. thermophilus-related e.g. LacS/LacZ, (R7) metabolism, (R8) CRISPR-associated, (R9) hypothetical proteins unique for CJ18, (R10) S. infantarius-species-specific, (R11) surface antigen, (R12) putative bacteriocin locus inactive in CJ18, (R13) adhesion-related proteins, (R14) hypothetical proteins shared between S. infantarius and dairy S. gallolyticus subsp. macedonicus and (R15) 2nd oligopeptide transport operon. Genome characteristic base-deviation index (BDI), GC-skew and GC-content of CJ18 are depicted below the comparison barcode chart. The strains used in this comparison are: 1.) Sii ATCC BAA-102T, 2.) S. equinus ATCC 9812, 3.) S. gallolyticus subsp. gallolyticus ATCC BAA-2069, 4.) S. gallolyticus subsp. gallolyticus UCN34, 5.) S. bovis ATCC 700338, 6.) S. gallolyticus subsp. macedonicus ACA-DC 198, 7.) S. agalactiae 2603 V/R, 8.) S. agalactiae NEM316, 9.) S. equi subsp. equi 4047, 10.) S. dysgalactiae subsp. equisimilis GGS_124, 11.) S. pyogenes M1 GAS, 12.) S. equi subsp. zooepidemicus MGCS10565, 13.) S. thermophilus LMG18311, 14.) S. thermophilus LMD-9, 15.) S. thermophilus CNRZ1066, 16.) S. sanguinis SK36, 17.) S anginosus 1_2_62CV, 18.) S. mitis B6, 19.) S. pneumoniae D39, 20.) S. pneumoniae R6, 21.) Lc. lactis subsp. cremoris MG1363 and 22.) artificial antibiotic resistance genome [37].
For 179 CDSs in CJ18, no homologous CDS were detected in ATCC BAA-102T. However, homologous CDS were detected in other streptococci for 103 of them, whereas for the other 76 CDS no significant hits were found in related strains [Additional file 1] (Table 2). The reverse comparison revealed 310 CDS from ATCC BAA-102T without orthologous CDS in CJ18, 97 of which encoded for hypothetical proteins [Additional file 1]. This comparison of the African dairy isolate CJ18 to the type strain reveals a high similarity in gene content and organisation. However, there are some remarkable differences in gene content suggesting a distinct evolution of the two strains.
Table 2.
GenBank accession numbers and reference sequence numbers of strains used in this study
| Species | Strain | Source | Genbank accession or reference sequence number | Reference |
|---|---|---|---|---|
| artificial antibiotic resistance genome |
various |
gene sequences of published antibiotic resistance genes |
none |
[37] |
|
Lactococcus lactis subsp. cremoris |
MG1363 |
international prototype for LAB genetics; plasmid-free descendant of NCDO712, a cheese starter |
[GenBank:NC_009004] |
[38] |
|
S. agalactiae |
2603 V/R |
human clinical specimen |
[GenBank:NC_004116] |
[34] |
|
S. agalactiae |
NEM316 |
human clinical specimen |
[GenBank:NC_004368] |
[39] |
|
S. anginosus |
1_2_62CV |
human clinical specimen |
[GenBank:NZ_ADME00000000] |
HMP a) |
|
S. bovis |
ATCC 700338 |
human clinical specimen, synovial fluid from knee |
[GenBank:NZ_AEEL00000000] |
HMP a) |
|
S. dysgalactiae subsp. equisimilis |
GGS_124 |
human clinical specimen |
[GenBank:AP010935] |
[40] |
|
S. equi subsp. equi |
4047 |
horse clinical specimen |
[GenBank:FM204883] |
[41] |
|
S. equi subsp. zooepidemicus |
MGCS10565 |
human clinical specimen |
[GenBank:CP001129] |
[42] |
|
S. equinus |
ATCC 9812 |
human clinical specimen, gut |
[GenBank: AEVB00000000] |
HMPa) |
|
S. gallolyticus subsp. gallolyticus |
ATCC 43143 |
human clinical specimen, blood |
[GenBank:AP012053] |
[32] |
|
S. gallolyticus subsp. gallolyticus |
ATCC BAA-2069 |
human clinical specimen, blood |
[GenBank:FR824043] |
[33] |
|
S. gallolyticus subsp. gallolyticus |
UCN34 |
human clinical specimen, blood |
[GenBank:FN597254] |
[13] |
|
S. gallolyticus subsp. gallolyticus |
TX20005 |
human clinical specimen, heart |
[GenBank:NZ_AEEM00000000] |
HMP a) |
|
S. gallolyticus subsp. macedonicus (=S. macedonicus) |
ACA-DC 198 |
Greek kasseri cheese, dairy isolate |
[GenBank:HE613569] (genome) and [GenBank:HE613570] (plasmid pSMA198) |
[30,43,44] |
|
S. gallolyticus subsp. pasteurianus |
ATCC 43144 |
human clinical specimen, blood |
[GenBank:AP012054] |
[32] |
|
S. infantarius subsp. infantarius |
ATCC BAA-102T (isogenetic strain of CCUG 43820T) |
human infant, feces |
[GenBank: ABJK00000000] |
HMP a) |
|
S. infantarius subsp. infantarius |
CJ18 |
fermented camel milk suusac |
[GenBank:CP003295] (genome) and [GenBank:CP003296] (plasmid pSICJ18-1) |
this study and [2,7,31] |
|
S. infantarius subsp. infantarius |
LP90 |
dairy origin |
[GenBank:HM008642] |
none |
|
S. mitis |
B6 |
hospital isolate Germany |
[GenBank:NC_013853] |
[45] |
|
S. pneumoniae |
D39 (=NCTC 7466) |
virulent human clinical isolate |
[GenBank:NC_008533] |
[36] |
|
S. pneumoniae |
R6 (=ATCC BAA-255) |
unencapsulated, parent strain R36A derived from D39 |
[GenBank:NC_003098] |
[46] |
|
S. pneumoniae |
TIGR4 |
human clinical isolate |
[GenBank:NC_003028] |
[47] |
|
S. pyogenes |
M1 GAS (=SF370) |
human clinical isolate |
[GenBank:NC_002737] |
[35] |
|
S. salivarius |
ATCC 25975 |
human saliva |
[GenBank:AF389474] |
[48] |
|
S. sanguinis |
SK36 |
human dental plaque |
[GenBank:NC_009009] |
[49] |
|
S. thermophilus |
CNRZ1066 |
yogurt |
[GenBank:NC_006449] |
[26] |
|
S. thermophilus |
LMG18311 |
yogurt |
[GenBank:NC_006448] |
[26] |
| S. thermophilus | LMD-9 (=ATCC BAA-491) | yogurt | [GenBank:NC_008532] | [27] |
a)HMP Human microbiome project http://www.hmpdacc.org.
Carbohydrate metabolism
Carbohydrate transport in bacteria is frequently mediated via phosphotransferase systems (PTSs). PTS encoding operons were detected in both Sii strains for the uptake of β-glucosides, lactose, fructose/mannose, fructose, sucrose, maltose/glucose and cellobiose. Such a wide variety of transport systems is often observed in GI-tract associated microbes [50]. Remarkably, the lactose PTS gene locus in CJ18 (Sinf_0190-0195) is interrupted by three transposases, two truncating the β-glucoside Bgl operon antiterminator upstream of the PTS genes and one within the 6-phospho-β-galactosidase downstream of the PTS genes, suggesting that the lactose PTS in CJ18 is not involved anymore in lactose utilization.
Genes involved in galactose utilization in CJ18 are organized in the operon galRKTE2 operon (Sinf_0205-0208). However, compared to ATCC BAA-102T, CJ18 harbors an additional gal-lac operon comprising genes galT(truncated)/galE1M/lacSZ (Sinf_0939-Sinf_0935) with high sequence identity (>91%) to S. thermophilus[7] and localized in region R6 [Additional file 3]. Also genes in the proximity of this gal-lac operon display high sequence identity to S. thermophilus, comprising among others the putative virulence gene encoding exfoliative toxin B (Sinf_0933), an acyl-CoA dehydrogenase (Sinf_0932) and a macrophage infectivity potentiator (Sinf_0931) [Additional file 1][Additional file 3]. Although the high sequence conservation indicates an S. thermophilus origin, the sequential order of genes is only conserved in the gal-lac operon. Mainly non-conserved DNA sequences were localized downstream of the gal-lac operon and the truncated galT.
Surprisingly, a second lacS (Sinf_1514) was detected in both Sii strains not adjacent to either the gal or gal-lac operon. This second LacS displays 98.9% amino acid sequence identity between the two S. infantarius strains and lower identity (60%) to the S. thermophilus-like LacS (Sinf_0936). The physiological role of this second LacS is unknown.
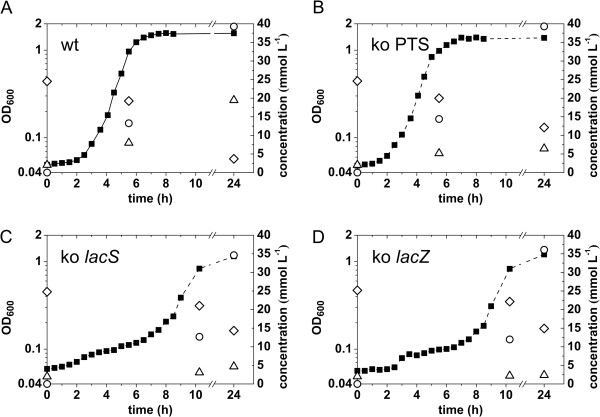
To elucidate the role of two lactose transport systems in lactose metabolism of CJ18, knock-out (KO) strains were constructed in the lactose translocater lacS (Sinf_0936), the β-galactosidase lacZ (Sinf_0935) and the permease unit of the lactose PTS encoding gene lacIIC (Sinf_0192) using a single-cross-over strategy (Table 3). Phenotypes of KO strains were confirmed on BHI/X-Gal/IPTG agar media yielding blue colonies for CJ18WT (wild type), CJ18ΔlacIIC, CJ18ΔlacS and white colonies for CJ18ΔlacZ. This indicates no polar effects of lacS disruption on the expression of the lacZ gene downstream of lacS [Additional file 4]. The wild type CJ18 and its mutant derivatives CJ18ΔlacIIC, CJ18ΔlacS and CJ18ΔlacZ grew similarly in control medium containing glucose as sole carbon source [Additional file 5]. When grown with lactose as sole carbon source, CJ18ΔlacIIC displayed a similar growth pattern as the wild type CJ18 (Figure 5), indicating that lactose uptake in CJ18 is not mediated by the lactose PTS. Strains disrupted in genes of the gal-lac operon, CJ18ΔlacS and CJ18ΔlacZ had clearly an impaired growth rate on lactose (Figure 5). The growth characteristics of the mutant strains CJ18ΔlacS and CJ18ΔlacZ on lactose show that lactose is utilized in CJ18 via uptake by LacS and subsequently cleaved by LacZ with a similar mechanism to the lactose metabolism of S. thermophilus.
Table 3.
Strains and plasmids used in this study
| Material | Relevant featuresa | Source |
|---|---|---|
|
Strains |
|
|
|
Streptococcus infantarius subsp. infantarius | ||
| CJ18 |
Wild type strain, suusac isolate |
[2,7,31] |
| CJ18/pVE6007 |
CJ18 derivative carrying pVE6007, CmR |
this study |
| CJ18ΔlacIIC |
lacIIC::pLFB1005, lacIIC gene disruption derivative of CJ18, EmR |
this study |
| CJ18ΔlacZ |
lacZ::pLFB1006, lacZ gene disruption derivative of CJ18, EmR |
this study |
| CJ18ΔlacS |
lacS::pLFB1007, lacS gene disruption derivative of CJ18, EmR |
this study |
|
Lactococcus lactis |
|
|
| LL302 |
RepA+ derivative of MG1363, host for pORI28 |
[51] |
|
Plasmids |
|
|
| pORI28 |
EmR, Ori+, RepA-, pWV01 derivative, vector for chromosomal insertions in Gram-positive bacteria |
[52] |
| pVE6007 |
CmR, thermosensitive derivative of pWV01, carrier plasmid for pORI28 |
[53] |
| pLFB1005 |
EmR, pORI28 derivative containing a 939-bp internal fragment of lacIIC. |
this study |
| pLFB1006 |
EmR, pORI28 derivative containing an 1177-bp internal fragment of lacZ. |
this study |
| pLFB1007 | EmR, pORI28 derivative containing a 900-bp internal fragment of lacS. | this study |
aCmR Chloramphenicol resistant; EmR Erythromycin resistant.
Figure 5.
Growth kinetics of wild type and knock-out (KO) strains of Sii CJ18 in lactose medium. Growth kinetics of CJ18 wild type (A), CJ18ΔlacIIC (B), CJ18ΔlacS (C) and CJ18ΔlacZ (D) were compared in Elliker-based lactose medium for optical density (OD600 ■) and for metabolites lactose (◊),lactate (○) and galactose (ᐃ) in cell-free supernatant. Representative curves of two independent repetitions per strain are shown.
Additional features related to dairy environment
Oligopeptide transporters are important during growth in milk for the uptake of peptides and amino acids [54,55]. Similar to ATCC BAA-102T, CJ18 possesses an OppABCDF peptide transport system (Sinf_0305-0309) but the genome of CJ18 encodes two additional OppA (Sinf_1225 and Sinf_1226) and, remarkably, a second OppABCDF encoding operon (Sinf_1825-1821, region R15, Figure 4) with high sequence identity to Streptococcus equi, Streptococcus pyogenes or Streptococcus gordonii [Additional file 1]. Single amino acid transport systems are conserved in both strains and in contrast to S. thermophilus strains, no reduction in amino acid biosynthesis pathways was observed for CJ18. Both S. infantarius strains encode apparent complete pathways, such as histidine and glutamate biosynthesis or arginine catabolism (CJ18).
Capsular polysaccharides (CPS) and exopolysaccharides (EPS) are involved in the adhesion properties of bacteria through biofilm formation and serve as a defense mechanism against immune responses [56,57]. Furthermore, EPS may contribute to the texture of many dairy products. CJ18 and ATCC BAA-102T both possess a conserved 5-kb operon for EPS biosynthesis. The genetic organization downstream of this cluster differs between the two S. infantarius strains. CJ18 harbors a number of additional EPS and CPS biosynthesis genes (R3, Figure 4) that share highest protein sequence identities with proteins of species outside of the SBSEC. Remarkably, the same region in CJ18 contains wefC encoding a receptor polysaccharide phosphotransferase, also termed stealth protein. This gene is absent in ATCC BAA-102T and displays high sequence homology to CpsJ of S. thermophilus (99%). Based on in silico analysis it was hypothesized to be involved in protection from the host immune system [58]. The presence of a high variety of EPS genes could be caused by selection during suusac manufacturing, but could also imply an additional virulence risk if a strain displays further virulence factors for e.g. invasion, infection or toxin production.
Adhesion and other virulence factors
Adhesion of bacteria to surfaces is influenced by many factors such as EPS or CPS production as mentioned above, but also certain specific proteins. A fibronectin binding protein Fpb involved with adhesion to fibronectin and fibrinogen is present in both ATCC BAA-102T and CJ18. Streptococcus bovis group surface proteins (Sbs) are also involved in adhesion and found in both CJ18 (7 genes) and in ATCC BAA-102T (8 genes). Five of these Sbs are organized in a 13.7-kb region (R13, Figure 4) in CJ18 comprising a truncated Sbs 13 (collagen binding protein, Sinf_1737), an LPXTG-specific A/C-type sortase (Sinf_1742), Sbs14 (autotransporter adhesion/cell wall anchored protein, Sinf_1743) and Sbs15 (ribonuclease G and E/peptidoglycan linked protein, Sinf_1744). This region upstream of Sbs 13 is conserved in CJ18 and ATCC BAA-102T, the dairy isolate S. gallolyticus subsp. macedonicus ACA-DC 198 and other S. gallolyticus strains. The presence of Sbs4 and Sbs9 suggests that certain adhesion factors are shared among SBSEC as commensal inhabitants of gastrointestinal tracts and detected also in the dairy strain S. thermophilus LMG18311. These factors might only contribute to virulence if further factors for invasion or toxin production are present as well. Other adhesion factors like S. bovis adhesion proteins (Acb) or others from non-SBSEC origin, such as FimA and FimB, are not present in both Sii strains.
A hemolysin III protein highly identical to that of the S. gallolyticus group including S. gallolyticus subsp. macedonicus ACA-DC 198 (91%) as well as that of S. thermophilus LMD-9 (80%) is encoded in both Sii strains. No defibrinated sheep blood hemolysing activity was detected for both strains. A direct implication of virulence from the presence of a hemolysin gene except streptolysin O is not yet established for streptococci [59].
Typical virulence factors of non-SBSEC-members S. pyogenes, S. agalactiae and S. pneumoniae had been used for the safety evaluation of S. thermophilus[26]. Some of these virulence factors were previously found in S. gallolyticus UCN34 such as ssaB/scaA/psaA (locus tag Gallo_2047), pilB (Gallo_0087), gtfbC (Gallo_1055), atlA (Gallo_1368) [32] and used to screen strains in this study. ssaB/scaA/psaA was not detected in Sii strains whereas atlA displayed a lower protein sequence identity in CJ18 (49%) compared to the cheese isolate S. gallolyticus subsp. macedonicus ACA-DC 198 (91%). Pro-inflammatory proteins [23] were detected in both Sii strains but also in S. thermophilus since they encode basic metabolic functions. Finally, comparison with an in silico genome containing antimicrobial resistance and virulence factor genes [37,60] did not result in significant hits with any typical or concerning streptococcal virulence factors for both CJ18 and ATCC BAA-102T.
Natural competence
Several regions potentially involved in natural competence were detected in both S. infantarius strains. These include a competence operon (comGA/GB/GC/GD/GE/GF/GG), separate competence genes and a CoiA encoding gene involved in DNA uptake. Furthermore, a CJ18-unique restriction endonuclease and methylase were detected in region R5 (Figure 4). In addition, both strains contain recombination proteins like RecA, the Rossman fold nucleotide-binding protein Smf/DprA and the single-strand DNA binding protein SsbB [61,62]. The organization and mechanism of the competence-related genes (comX/sigX and comS promoters) seems to be conserved in both ATCC BAA-102T and CJ18 as well as most other streptococci [63]. However, CJ18 harbors an additional conjugal transfer protein (Sinf_1366 region R9, Figure 3) with high protein sequence identity (82%) to S. thermophilus variant, suggesting a potentially increased capability for DNA uptake compared to ATCC BAA-102T.
This is further supported by the apparent reduced activity of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPRs) and CRISPR associated genes (cas) forming the CRISPR/Cas system for defense against foreign DNA [64]. Both CJ18 and ATCC BAA-102T harbor single copies of csn2, cas1 and cas2 in region R8 (Figure 4). But remarkably, the CJ18 proteins Csn2, Cas1 and Cas2 had higher identity (88-93%) with the corresponding proteins in S. gallolyticus, S. bovis and S. equinus than with ATCC BAA-102T. A CRISPR array comprises a leader sequence followed by identical repeated DNA sequences intersected by highly variable spacer sequences. CJ18 comprises a CRISPR/Cas section with 9 spacers whereas ATCC BAA-102T harbors 29 spacers. The relative low number of CRISPR spacers predicts a lower CRISPR activity in CJ18 and thus a decreased protection against foreign DNA.
No DNA sequence identity was detected between any of the spacers. This indicates strain dependent Cas/CRISPR activity in S. infantarius also reported for S. thermophilus strains [65].
Other features in the CJ18 genome
Production of bacteriocins is widely distributed among streptococci [66]. S. infantarius CJ18, ATCC BAA-102T and also LP90 (Table 2) possess a highly conserved bacteriocin ABC-transporter accessory protein InfAE-acc, shared also with S. gallolyticus strains (competence-stimulating peptide ABC transporter-permease ComB Sinf_1732) and the bacteriocin ABC-transporter InfAE-ABC (competence-stimulating peptide ABC transporter ATP-binding protein ComA Sinf_1731) located in region R12. Putative bacteriocin encoding genes were detected in ATCC BAA-102T, but none in strain CJ18, which confirms previous findings on its inability to produce bacteriocin-like inhibitory substances [2].
Unique phage-related genes are located in CJ18 in regions R1 and R4 (Figure 4). CJ18 and ATCC BAA102T harbor both four and five phage integrase genes, respectively. However, only one of them (Sinf_0428) has a homologous gene in ATCC BAA-102T (100% nucleotide identity), indicating possible distant relationship between these strains.
Remarkable differences between both S. infantarius strains and their closest related species within the SBSEC S. gallolyticus UCN34 (Figure 2) were a reduction in carbohydrate transport systems, e.g. the absence of trehalose and mannitol transporting and degrading enzymes which play a role in maintenance in the bovine rumen. This indicates a generally lower adaptation of S. infantarius to the bovine rumen as a habitat compared to S. gallolyticus and provides additional evidence to separate both species from each other.
Discussion
Fermented dairy products are important in Africa as source of nutrients and as weaning food. Fermentation is an essential preservation method in the absence of refrigeration [67-69]. Analyses of dairy adaptations and potential virulence factors of bacteria leading spontaneous fermentation processes is therefore important to identify consumers’ health risk potential and unravel novel fermentative lactic acid bacteria strains.
In this study, we report the complete genome sequence of the African dairy isolate Sii CJ18, the first complete assembled genome of a S. infantarius species. Whole genome comparison of Sii CJ18 to Sii ATCC BAA-102T and related streptococci revealed substantial adaptations to the dairy environment in CJ18, paralleling that of S. thermophilus. However, our data indicates that genome decay of Sii CJ18 is in a less advanced state compared to S. thermophilus, since most biosynthesis pathways seem to be intact and the number of pseudogenes (4.9%) is smaller than for S. thermophilus (10-19%). This suggests that establishment of CJ18 in the dairy environment is more recent than S. thermophilus strains or S. gallolyticus subsp. macedonicus ACA-DC 198. Based on genome decay, the most recent common ancestor for S. thermophilus strains was estimated to have lived 3,000-30,000 years ago, which is approximately the duration of human dairy activity [26,70]. Camels, however, were introduced in East Africa only around 2,500 years ago [71-73], and the less advanced state of genome decay in CJ18 may be related to the later start of African camel milk fermentation.
Adaptation to the dairy environment in S. thermophilus consists of enhanced uptake of lactose and peptides and loss of other metabolic pathways. CJ18 displays a similar adaptation in the lactose metabolism through the transporter LacS and β-galactosidase LacZ. Truncation of either LacS or LacZ resulted in significant impaired growth on lactose, confirming the functionality of this acquired lactose utilization path. Neither the second LacS (Sinf_1514), present in both CJ18 and ATCC BAA-102T, nor the lactose PTS could take over lactose transport in the LacS KO strain. The integration of transposases in the corresponding lactose PTS gene cluster seems therefore a result of loss of essentiality after the acquirement of lacS and lacZ. Moreover, a concurrent activity of both transporters potentially leads to misbalance in redox or phosphorylation status of the cell, and hence positive selection on truncation of the lactose PTS gene cluster might have even occurred after acquirement of LacSZ. The release of galactose into the growth medium shows that LacS in CJ18 functions as a highly efficient antiporter and the competitiveness of CJ18 in the dairy environment seems therefore based on the acquired LacSZ. This facilitates efficient transport of lactose and as a consequence an increased lactose consumption and lactate production compared to ATCC BAA-102T (isogenetic strain of CCUG 43820T) [7].
The role of other adaptations to the dairy environment, such as the presence of a second oppABCDF operon and an extended EPS biosynthesis cluster is less clear. Enhanced uptake of casein derived peptides by the second peptide transporter could contribute to increased competiveness in milk. The enlarged cluster of Eps/Cps-related proteins could contribute to survival during the suusac back-slopping process, via improved biofilm formation capabilities. Furthermore, EPS contribute to texture of the fermented dairy product and the selection of strains for these textural properties might have occurred in the past [18,26].
The more recent adaptation to the dairy environment of C18 is reflected by the lower number of pseudogenes and CRISPR spacers in CJ18 compared to S. thermophilus or S. gallolyticus subsp. macedonicus ACA-DC 198. CJ18 harbors nine CRISPR spacers whereas typical widespread dairy starter strains of S. thermophilus such as CNRZ 1066 and LMG 18311 harbor 42 and 39 spacers, respectively [26,65]. Phage infection and phage-related fermentation losses are major problems in dairy technology. The number of CRISPR spacer in a bacterial genome is directly linked to phage contact history and presumptive resistance against phages of that particular strain [74]. The African strain CJ18 was apparently not continuously exposed to phage infections over prolonged periods. This could be a result of the spontaneous nature of the traditional fermentation, which in contrast to industrial starter culture fermentations, does not rely on selected starter strains. The absence of CRISPR spacer identity between CJ18 and ATCC BAA-102T further shows that the African CJ18 is only a distant relative of ATCC BAA-102T as previously observed in microevolution of CRISPR spacers in other genera [75]. Additionally, the presence of 103 CDS in CJ18 shared only with other streptococci but not with ATCC BAA-102T as well as the absence of 310 CDS in CJ18 present in ATCC BAA-102T indicates an ancestral streptococcal origin of these CDSs and again only distant relation between the two Sii strains.
Another interesting feature of CJ18 is its natural competence and DNA uptake capability, paralleling that of other streptococci and lactic acid bacteria (LAB) [27,76]. As a possible result of this, the genome displays traces of HGT events from commensal bacteria encountered in milk such as Lactococcus spp. and S. thermophilus but also pathogens like S. agalactiae. Furthermore, the natural competence could potentially contribute to the uptake of mobile genetic elements and to spread of antibiotic resistance genes [2]. Therefore the apparent intact competence machinery is probably of high importance for persistence of the strain in the African dairy environment.
CJ18 harbors none of the concerning typical streptococcal virulence factors [60] and less SBSEC-related virulence factors compared to e.g. S. gallolyticus and S. bovis. Moreover, most of these potential virulence factors are related to adhesion and not directly to infection, cytotoxicity or toxin production and are therefore of less concern. Many factors found in CJ18 are also present in the proclaimed safe strain S. gallolyticus subsp. macedonicus ACA-DC 198, a species without QPS-approval [25,77]. Some potential virulence factors or artifacts thereof were even found in S. thermophilus. Consequently, relying on genomic information alone, ingestion and digestion of large amounts of Sii via suusac does not seem to be a direct health risk for adults. However, the SBSEC-associated health risks for immune-deprived people, a major concern in Africa, and for children are less understood as epidemiological data on these diseases are not available. Furthermore, the uncertain association of Sii with human diseases necessitates further elucidation of presumptive Sii-specific virulence factors or the absence thereof in Sii.
Conclusions
We assembled and analyzed the first complete genome sequence of the species S. infantarius. The African dairy strain Sii CJ18 revealed many genetic adaptations to the dairy environment through acquired carbohydrate utilization pathways resulting in a lactose metabolism paralleling that of S. thermophilus. Potential mutations and insertions resulting in pseudogenes or truncated gene clusters indicate further evolution paralleling S. thermophilus. However, gene decay is not as advanced as in the dairy isolates S. thermophilus or S. gallolyticus subsp. macedonicus ACA-DC 198 and the establishment in the dairy environment is therefore likely from a younger evolutionary period.
The species S. infantarius harbors less virulence factors compared to the S. gallolyticus group. However, specific virulence factors for S. infantarius are not yet identified and epidemiological studies are necessary to prove the innocuity of African dairy Sii strains and milks predominantly fermented with these strains. This could prove traditional dairy fermentation in Africa as ideal process to enhance food safety and shelf life as well as the later application of Sii in an enhanced traditional fermentation technology paralleling the Western dairy industry, but specific for Africa. Conclusively, this study provides insight into the evolution of a novel dairy species and dairy environment in parallel to the Western counterpart.
Methods
Bacterial strains and culture conditions
Strains and plasmids used in this study are listed in Table 3. Lactococcus lactis LL302 was used as intermediate cloning host and cultured without agitation at 30°C in M17 (Biolife, Milan, Italy) [78], supplemented with 0.5% glucose (G-M17). Sii strains were grown overnight in G-M17 at 37°C for production of pre-cultures, or anaerobically on G-M17 plates at 37°C.
For growth profiling on specific carbohydrates, a pre-culture of Sii in G-M17 was used to inoculate (1% v/v) Elliker-based single carbohydrate medium [79,80], containing either glucose (1%) or lactose (1%). Growth profiling was performed in 125-mL butyl-rubber stoppered serum flasks [7] at 37°C for the determination of growth curves.
When appropriate, chloramphenicol and erythromycin were added to the media at a final concentration of 8 μg mL-1 and 10 μg mL-1, respectively. BHI agar media (Biolife) supplemented with 80 mg mL-1 5-bromo-4-chlor-3-indolyl-b-D-galactopyranoside (X-Gal, AppliChem, Darmstadt, Germany) and 0.5 mM isopropyl-b-D-thiogalactopyranosid (IPTG, AppliChem) was used to confirm phenotypes of KO strains. AnaeroGen packs (Oxoid, Pratteln, Switzerland) were used as oxygen scavengers for agar plate incubation in anaerobic jars. Stock cultures of all strains were stored at −80°C in 30% glycerol (v/v). All chemicals and enzymes used in this study were obtained from Sigma-Aldrich (Buchs, Switzerland), unless stated otherwise.
Genbank and reference sequence accession numbers
The genome sequence and plasmid pSICJ18-1 of Sii CJ18 is available in the nucleotide database GenBank under the accession numbers [GenBank: CP003295, GenBank: CP003296] [31]. A summary of GenBank accession and reference sequence numbers of strains used in this study for bioinformatic analyses are provided in Table 2.
Electroporation of Sii CJ18 and Lactococcus lactis LL302
Lc. lactis LL302 and Sii strains were transformed by electroporation using a procedure developed for Lc. lactis[81]. Positive transformants were selected on G-M17 agar media supplemented with chloramphenicol (8 μg mL-1) or erythromycin (10 μg mL-1) as required after aerobic incubation at 30°C for 1–2 days.
DNA manipulations
Molecular cloning and DNA manipulations were essentially performed as described by Sambrook et al. [82]. Plasmid DNA isolation from Lc. lactis LL302 was performed using an alkali cell lysis method after lysozyme treatment with subsequent purification [83] using a Midiprep Kit (Qiagen, Basel, Switzerland). Restriction enzymes and Phusion-polymerase were obtained from New England Biolabs (Frankfurt am Main, Germany) and T4-ligase from Invitrogen (Basel, Switzerland). Primers were purchased from Microsynth (Balgach, Switzerland).
Construction of mutant strains
For inactivation of the lactose PTS, the permease encoding lacIIBC gene (Sinf_0192) was disrupted using a single-cross-over strategy. A 959-bp internal fragment of lacIIC was amplified using a PCR master mix (Thermo Scientific, St. Leon-Rot, Germany), chromosomal DNA of CJ18 as template and the primers lacIIC_for and lacIIC_rev (Table 4). The obtained product was purified using a GFX purification column (GE Healthcare, Glattbrugg, Switzerland) and digested with BamHI and EcoRI (restriction sites introduced in primers). The restricted fragment (939 bp) was cloned into a BamHI/EcoRI digested pORI28 resulting in pLFB1005, a lacIIBC disruption vector. Similarly, a 900-bp internal fragment of the lactose transporter gene lacS was amplified using primers lacS_for and lacS_rev. The product was purified, restricted with BamHI and EcoRI, and cloned into a BamHI/EcoRI digested pORI28, resulting in pLFB1007, a lacS interrupting vector.
Table 4.
Oligonucleotides used to amplify internal fragments of target genes to construct knock-out strains
| Name | Sequence (5’ to 3’)a | Name | Sequence (5’ to 3’) |
|---|---|---|---|
| lacS_for |
GATCGGATCCGATCCAAAGCAAAATAGTCA |
lacS_con_for |
TCCTATGCAGCGGGTGCTT |
| lacS_rev |
GATCGAATTCTGCAGTCAAGATAATTGGA |
lacS_con_rev |
GAGATAATCATAAGGATAACAA |
| lacZ_for |
GATCCTGCAGGCGTTAATACAGTTGACGCTCAC |
lacZ_con_for |
TTACTTAAACGATCCAAAGA |
| lacZ_rev |
GATCGGATCCTTTGCCATGTACCGTGTGTT |
lacZ_con_rev |
CATGTTATTGGCACGATCCA |
| lacIIC_for |
GATCGGATCCAATATTTGCGAGCGATTCGT |
lacIIC_con_for |
GGAAACCATTCTTTGAGAG |
| lacIIC_rev |
GATCGAATTCTACAATTGGAGCACCGAACA |
lacIIC_con_rev |
ATTTGAAGATCCACACGTT |
| pORI_for | TTG ATA ATG AAC TGT GCT GA | pORI _rev | ACG AAT CGC CAA CGT TTT CG |
a) Endonuclease restriction sites introduced in primers are underlined.
For disruption of lacZ, an 1177-bp internal fragment was amplified using primers lacZ_for and lacZ_rev. The product was digested with BamHI and PstI and cloned into a similar digested pORI28, resulting in pLFB1006, a disruption vector for lacZ.
The obtained plasmids were first transformed into Lc. lactis LL302 for multiplication. After extraction, they were transformed into Sii CJ18 harboring the thermosensitive plasmid pVE6007 (CmR) as carrier plasmid for pORI28 derivatives (EmR, Table 3). Transformants were isolated on G-M17 supplemented with 10 μg ml-1 erythromycin at 30°C. Growth of transformants at 37°C results in loss of pVE6007 and pORI28-derivatives cannot replicate anymore in the cells, forcing the plasmids to integrate into the chromosome. Therefore, colonies were picked, the presence of the correct plasmids confirmed by PCR and subsequently grown at 37°C in G-M17 supplemented with erythromycin for 24 h. Primary integrants were then isolated on G-M17 supplemented with erythromycin. To check for the loss of pVE6007, colonies were picked and transferred to G-M17 plates with 10 μg mL-1 chloramphenicol and grown overnight at 30°C. Colonies displaying an erythromycin resistant and chloramphenicol sensitive phenotype were checked for correct integration by PCR, using primers annealing outside of the region of integration in the chromosome (control primers in Table 4) and primers annealing in pORI28 (pORI28_for and pORI28_rev). Integrants showing the correct phenotype and positive PCR analyses were streaked on G-M17 with erythromycin and a single colony isolate was checked again by PCR. Phenotypes of KO strains were confirmed using BHI/X-Gal/IPTG agar media.
Metabolite analysis by HPLC
Carbohydrate metabolites lactose, glucose, galactose, lactate and acetate were analyzed from bacterial culture supernatants on a Merck Hitachi HPLC system (Merck Hitachi, Darmstadt, Germany) as previously described [7].
Genome annotation
DNA isolation, sequencing and assembly of the genome of CJ18 was previously described [31]. Annotation of the assembled Sii CJ18 and metabolic reconstruction was performed on the RAST server [84]. The primary gene annotation by RAST was verified by comparing each RAST-predicted gene to the annotated genes of the species listed in Table 1. The genes were categorized into four groups: correct, possible frameshift, possible wrong start/stop assignment and non-conserved hypothetical. Each gene predicted by RAST plus 60-bp flanking regions were translated in silico and the three possible reading frames were compared to all annotated genes within genomes of related species (Table 1) using the Smith-Waterman algorithm [85] on the basis of the BLOSUM62 substitution matrix. The score of the best match was compared to the self-alignment score of the original gene. If the highest score/self-alignment score-ratio was above 0.6, the gene was categorized as correct. If one of the two alternative reading frames had a score ratio above 0.75, the gene was assigned as having a possible frameshift. If the original gene was aligned to its best match with the number of either starting or ending gaps of more than 20-bp, it was categorized as possible wrong start/stop assignment. Genes with highest score/self-alignment score-ratios below 0.35, or a Needleman-Wunsch-Alignment to its best match with a negative score, were assigned as non-conserved hypothetical. The prediction of the oriC region upstream of dnaA was performed using Ori-finder [86].
Phylogenetic analyses
DNA sequences were retrieved from GenBank or sequenced in this study (Table 2). The following genes were used: groEL, gyrB, recA, recN, rpoB, secA, secY, sodA and 16S rRNA encoding genes.
Sequences were aligned in MEGA4.0 [87] using the ClustalW algorithm and then trimmed to equal lengths. Construction of phylogenetic trees was performed in MEGA4.0 using the Neighbor-Joining method and a bootstrap test with 1000 repetitions followed by the computation of evolutionary distances using the Maximum Composite Likelihood method [87-90]. The resulting trees were rooted using Lactococcus lactis subsp. cremoris MG1363 as outgroup.
Genome comparison – synteny plots
The raw scores for the local alignment of all putative proteins of Sii CJ18 versus all proteins of the strains of interest (Table 2) were calculated using the Smith-Waterman algorithm [85] on the basis of the BLOSUM62 substitution matrix [91]. The score ratio is calculated by dividing the raw score by the score of the protein of interest aligned to itself. A threshold of 0.4 was used to distinguish between similar and non-similar proteins [92]. A synteny plot was created by plotting the genomic location of all proteins of Sii CJ18 on the X-axis and the genomic location of all similar proteins of the strain of interest on the Y-axis.
The available contigs of Sii ATCC BAA-102T were putatively assembled using Projector 2 [93]. The contigs of Sii ATCC BAA-102T were re-annotated through the RAST pipeline to facilitate highest comparability with the genome of CJ18 annotated also via the RAST pipeline [84].
Construction of genome comparison graphs
The Base Deviation Index (BDI) is calculated as the deviation of the base composition in a sliding 10-kb window to the average base composition over the entire genome using the X2 statistics [94]. The GC skew is calculated as with G and C being the number of guanin and cytosin in a sliding 1-kb window. The GC content is calculated as the percentage of guanin and cytosin in a sliding 1-kb window. Circular genome graphs were created using DNA Plotter [95].
Search for bacteriocins
The genomic sequence of Sii CJ18 was translated in silico in all three possible reading frames. All peptides available in the BAGEL2-Bacteriocin-Database [96] were searched in the translated sequences using the Smith-Waterman algorithm [85] on the basis of the BLOSUM62 substitution matrix [91]. High scoring matches were further evaluated by hand.
CRISPR/Cas analysis
CRISPRs were detected in the genomes of Sii CJ18 and ATCC BAA-102T using CRISPRfinder and CRISPRdb [97,98]. Spacer sequences were aligned in BioEdit [99] through ClustalW after which DNA sequence identities were calculated. Amino acid sequences of CRISPR-associated (cas) proteins were analyzed analogous.
Abbreviations
CDS: Coding DNA sequence; CPS: Capsular polysaccharides; CRISPR: Clustered regularly interspaced short palindromic repeats; EPS: Exopolysaccharides; KO: Knock-out; LAB: Lactic acid bacteria; PTS: Phosphotransferase system; QPS: Qualified presumption of safety; SBSEC: Streptococcus bovis/Streptococcus equinus complex; Sii: Streptococcus infantarius subsp. infantarius.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CJ performed genome assembly and analysis; RF performed bioinformatic analysis; MH performed construction of knock-out strains and experiments; CL and LM initiated and supervised the project; MJAS designed and supervised experiments and CJ, MJAS, LM and CL wrote/revised the paper. All authors have read and approved the final manuscript.
Supplementary Material
Complete table of CDS in Sii genome CJ18 in comparison with reference strain genomes. Description of data: This table contains all CDS of the African Sii genome CJ18 (excluding the plasmid) in comparison with reference strain genomes used in this study. A reverse comparison of CDS present in Sii ATCC BAA-102T versus CJ18 is included. The CDS of the plasmid pSICJ18-1 are included in a separate tab with their highest NCBI Blast database gene match.
Synteni plot of genome Sii CJ18 (x) vs. (A) S. gallolyticus subsp. macedonicus ACA-DC 198 (y), (B) S. gallolyticus subsp. gallolyticus UCN34 (y) and (C) S. gallolyticus subsp. gallolyticus ATCC BAA-2069 (y). Description of data: Similar to the genome of Sii ATCC BAA-102T, Sii CJ18 and S. gallolyticus strains ACA-DC 198, ATCC BAA-2069 and UCN34 display a high degree of conservation indicated by the alignment near the diagonal line. The same major insertion sites as in ATCC BAA-102T can be identified as R4 (34.2 kb) consisting largely of phage-related genes; R6 (25.6 kb) encompassing a 13.2-kb S. thermophilus-gene cluster comprising the additional gal-lac operon; and R9 (26.1 kb) containing among others an HTH-type transcriptional regulator rgg, primosomal protein N’ (replication factor Y) – superfamily II helicase and an FtsK/SpoIIIE family protein. The dairy isolate S. gallolyticus subsp. macedonicus ACA-DC 198 features an additional unique region R12 comprising bacteriocin-related structures of macedocin, salavaricin, lantibiotic modifying enzymes and transporters.
Unique 13.2-kb gene locus with high DNA sequence identity to S. thermophilus in Sii genome CJ18. Description of data: The African Sii CJ18 harbors an approximately 13.2-kb insert of DNA with high sequence identity to S. thermophilus LMD-9 (white arrows) within a 25.6-kb insert (R6, Sinf_0915-Sinf_0939). The 18.4-kb gap between Sinf_0910 to Sinf_0928 was largely occupied with hypothetical proteins of unknown origin, few transporters and phage-related genes. Genes are not drawn to scale. Gene numbering corresponds to CDS region Sinf_0910-Sinf_0938. Black arrows indicate S. infantarius identity; grey other streptococci and white S. thermophilus identity.
Phenotypes of CJ18WT and mutant KO derivatives on BHI/X-Gal/IPTG agar media. Description of data: Confirmation of phenotypes of wild type, reference and KO strains on BHI/X-Gal/IPTG agar media yielding blue colonies for CJ18WT, CJ18ΔlacIIC, CJ18ΔlacS and white colonies for CJ18ΔlacZ and CCUG 43820T.
Growth kinetics of wild type and knock-out strains of Sii CJ18 in glucose medium. Description of data: Growth kinetics of CJ18 wild type (A), CJ18ΔlacIIC (B), CJ18ΔlacS (C) and CJ18ΔlacZ (D) were compared in Elliker-based glucose medium for optical density (OD600 ■) and for metabolites glucose (∇),lactate (○) and galactose (ᐃ) in cell-free supernatant. Representative curves of two independent repetitions per strain are shown.
Contributor Information
Christoph Jans, Email: christoph.jans@hest.ethz.ch.
Rainer Follador, Email: r.follador@gmail.com.
Mira Hochstrasser, Email: mira.hochstrasser@student.ethz.ch.
Christophe Lacroix, Email: christophe.lacroix@hest.ethz.ch.
Leo Meile, Email: leo.meile@hest.ethz.ch.
Marc J A Stevens, Email: marc.stevens@hest.ethz.ch.
Acknowledgements
This study was funded by the UBS Optimus Foundation, Switzerland and the North South Centre at ETH Zurich, Switzerland.
The authors would like to thank Jan Kok, Department of Molecular Genetics, University of Groningen, for the gift of pORI28 and Lactococcus lactis LL302; and Emmanuelle Maguin, INRA Research Centre Jouy-en-Josas, for the gift of pVE6007.
References
- Herrera P, Min Kwon Y, Ricke SC. Ecology and pathogenicity of gastrointestinal Streptococcus bovis. Anaerobe. 2009;15:44–54. doi: 10.1016/j.anaerobe.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Jans C, Bugnard J, Njage PMK, Lacroix C, Meile L. Lactic acid bacteria diversity of African raw and fermented camel milk products reveals a highly competitive, potentially health-threatening predominant microflora. LWT-Food Sci Technol. 2012;47:371–379. [Google Scholar]
- Schlegel L, Grimont F, Ageron E, Grimont PAD, Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. pasteurianus subsp. nov. Int J Syst Evol Microbiol. 2003;53:631–645. doi: 10.1099/ijs.0.02361-0. [DOI] [PubMed] [Google Scholar]
- Schlegel L, Grimont F, Collins MD, Régnault B, Grimont PAD, Bouvet A. Streptococcus infantarius sp. nov., Streptococcus infantarius subsp. infantarius subsp. nov. and Streptococcus infantarius subsp. coli subsp. nov., isolated from humans and food. Int J Syst Evol Microbiol. 2000;50:1425–1434. doi: 10.1099/00207713-50-4-1425. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Lacroix C, Bonfoh B, Sissoko-Thiam A, Hugenschmidt S, Romanens E, Baumgartner S, Traoré I, Yaffee M, Jans C, Meile L. Analysis of lactic acid bacteria communities and their seasonal variations in a spontaneously fermented dairy product (Malian fènè) by applying a cultivation/genotype-based binary model. Int Dairy J. 2013;29:28–35. [Google Scholar]
- Abdelgadir W, Nielsen DS, Hamad S, Jakobsen M. A traditional Sudanese fermented camel’s milk product, Gariss, as a habitat of Streptococcus infantarius subsp. infantarius. Int J Food Microbiol. 2008;127:215–219. doi: 10.1016/j.ijfoodmicro.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Jans C, Gerber A, Bugnard J, Njage PMK, Lacroix C, Meile L. Novel Streptococcus infantarius subsp. infantarius variants harboring lactose metabolism genes homologous to Streptococcus thermophilus. Food Microbiol. 2012;31:33–42. doi: 10.1016/j.fm.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Díaz-Ruiz G, Guyot JP, Ruiz-Teran F, Morlon-Guyot J, Wacher C. Microbial and physiological characterization of weakly amylolytic but fast-growing lactic acid bacteria: a functional role in supporting microbial diversity in pozol, a Mexican fermented maize beverage. Appl Environ Microbiol. 2003;69:4367–4374. doi: 10.1128/AEM.69.8.4367-4374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyart C, Quesne G, Trieu-Cuot P. Taxonomic dissection of the Streptococcus bovis group by analysis of manganese-dependent superoxide dismutase gene (sodA) sequences: reclassification of ‘Streptococcus infantarius subsp. coli’ as Streptococcus lutetiensis sp. nov. and of Streptococcus bovis biotype II.2 as Streptococcus pasteurianus sp. nov. Int J Syst Evol Microbiol. 2002;52:1247–1255. doi: 10.1099/00207713-52-4-1247. [DOI] [PubMed] [Google Scholar]
- Beck M, Frodl R, Funke G. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J Clin Microbiol. 2008;46:2966–2972. doi: 10.1128/JCM.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Recco RA, Catalano MT, Edberg SC, Casey JI, Steigbigel NH. Association of Streptococcus bovis with carcinoma of colon. New Engl J Med. 1977;297:800–802. doi: 10.1056/NEJM197710132971503. [DOI] [PubMed] [Google Scholar]
- Abdulamir AS, Hafidh RR, Abu Bakar F. Molecular detection, quantification, and isolation of Streptococcus gallolyticus bacteria colonizing colorectal tumors: inflammation-driven potential of carcinogenesis via IL-1, COX-2, and IL-8. Mol Cancer. 2010;9:249. doi: 10.1186/1476-4598-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusniok C, Couvé E, Da Cunha V, El Gana R, Zidane N, Bouchier C, Poyart C, Leclercq R, Trieu-Cuot P, Glaser P. Genome sequence of Streptococcus gallolyticus: insights into its adaptation to the bovine rumen and its ability to cause endocarditis. J Bacteriol. 2010;192:2266–2276. doi: 10.1128/JB.01659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää J, Nallapareddy SR, Qin X, Singh KV, Muzny DM, Kovar CL, Nazareth LV, Gibbs RA, Ferraro MJ, Steckelberg JM, Weinstock GM, Murray BE. A collagen-binding adhesin, Acb, and ten other putative MSCRAMM and pilus family proteins of Streptococcus gallolyticus subsp. gallolyticus (Streptococcus bovis group, biotype I) J Bacteriol. 2009;191:6643–6653. doi: 10.1128/JB.00909-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää J, Nallapareddy SR, Singh KV, Ferraro MJ, Murray BE. Adherence characteristics of endocarditis-derived Streptococcus gallolyticus ssp. gallolyticus (Streptococcus bovis biotype I) isolates to host extracellular matrix proteins. FEMS Microbiol Lett. 2008;289:104–109. doi: 10.1111/j.1574-6968.2008.01378.x. [DOI] [PubMed] [Google Scholar]
- Vollmer T, Hinse D, Kleesiek K, Dreier J. Interactions between endocarditis-derived Streptococcus gallolyticus subsp gallolyticus isolates and human endothelial cells. BMC Microbiol. 2010;10:78. doi: 10.1186/1471-2180-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinse D, Vollmer T, Kleesiek K, Dreier J. Characterisation of Streptococcus gallolyticus subsp gallolyticus virulence factors. Int J Med Microbiol. 2008;298:64. [Google Scholar]
- Monchois V, Willemot RM, Monsan P. Glucansucrases: mechanism of action and structure-function relationships. FEMS Microbiol Rev. 1999;23:131–151. doi: 10.1111/j.1574-6976.1999.tb00394.x. [DOI] [PubMed] [Google Scholar]
- Shun CT, Lu SY, Yeh CY, Chiang CP, Chia JS, Chen JY. Glucosyltransferases of viridans streptococci are modulins of interleukin-6 induction in infective endocarditis. Infect Immun. 2005;73:3261–3270. doi: 10.1128/IAI.73.6.3261-3270.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boleij A, Schaeps RMJ, de Kleijn S, Hermans PW, Glaser P, Pancholi V, Swinkels DW, Tjalsma H. Surface-exposed histone-like protein A modulates adherence of Streptococcus gallolyticus to colon adenocarcinoma cells. Infect Immun. 2009;77:5519–5527. doi: 10.1128/IAI.00384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. Virulence or niche factors: what’s in a name? J Bacteriol. 2012;194:5725–5727. doi: 10.1128/JB.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredoira J, Alonso MP, Coira A, Varela J. Association between Streptococcus infantarius (formerly S. bovis II/I) bacteremia and noncolonic cancer. J Clin Microbiol. 2008;46:1570. doi: 10.1128/JCM.00129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biarc J, Nguyen IS, Pini A, Gossé F, Richert S, Thiersé D, Van Dorsselaer A, Leize-Wagner E, Raul F, Klein JP, Schöller-Guinard M. Carcinogenic properties of proteins with pro-inflammatory activity from Streptococcus infantarius (formerly S. bovis) Carcinogenesis. 2004;25:1477–1484. doi: 10.1093/carcin/bgh091. [DOI] [PubMed] [Google Scholar]
- Boleij A, Muytjens CMJ, Bukhari SI, Cayet N, Glaser P, Hermans PWM, Swinkels DW, Bolhuis A, Tjalsma H. Novel clues on the specific association of Streptococcus gallolyticus subsp gallolyticus with colorectal cancer. J Infect Dis. 2011;203:1101–1109. doi: 10.1093/infdis/jiq169. [DOI] [PubMed] [Google Scholar]
- Leuschner RGK, Robinson TP, Hugas M, Cocconcelli PS, Richard-Forget F, Klein G, Licht TR, Nguyen-The C, Querol A, Richardson M, Suarez JE, Thrane U, Vlak JM, von Wright A. Qualified presumption of safety (QPS): a generic risk assessment approach for biological agents notified to the european food safety authority (EFSA) Trends Food Sci Technol. 2010;21:425–435. [Google Scholar]
- Bolotin A, Quinquis B, Renault P, Sorokin A, Ehrlich SD, Kulakauskas S, Lapidus A, Goltsman E, Mazur M, Pusch GD, Fonstein M, Overbeek R, Kyprides N, Purnelle B, Prozzi D, Ngui K, Masuy D, Hancy F, Burteau S, Boutry M, Delcour J, Goffeau A, Hols P. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat Biotechnol. 2004;22:1554–1558. doi: 10.1038/nbt1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova K. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A. 2006;103:15611–15616. doi: 10.1073/pnas.0607117103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hols P, Hancy F, Fontaine L, Grossiord B, Prozzi D, Leblond-Bourget N, Decaris B, Bolotin A, Delorme C, Ehrlich SD, Guédon E, Monnet W, Renault P, Kleerebezem M. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol Rev. 2005;29:435–463. doi: 10.1016/j.femsre.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kilpper-Bälz R, Fischer G, Schleifer KH. Nucleic acid hybridization of group N and group D streptococci. Curr Microbiol. 1982;7:245–250. [Google Scholar]
- Papadimitriou K, Ferreira S, Papandreou NC, Mavrogonatou E, Supply P, Pot B, Tsakalidou E. Complete genome sequence of the dairy isolate Streptococcus macedonicus ACA-DC 198. J Bacteriol. 2012;194:1838. doi: 10.1128/JB.06804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans C, Follador R, Lacroix C, Meile L, Stevens MJA. Complete genome sequence of the African dairy isolate Streptococcus infantarius subsp. infantarius strain CJ18. J Bacteriol. 2012;194:2105–2106. doi: 10.1128/JB.00160-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin IH, Liu T-T, Teng Y-T, Wu H-L, Liu Y-M, Wu K-M, Chang C-H, Hsu M-T. Sequencing and comparative genome analysis of two pathogenic Streptococcus gallolyticus subspecies: genome plasticity, adaptation and virulence. PLoS One. 2011;6:e20519. doi: 10.1371/journal.pone.0020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinse D, Vollmer T, Rückert C, Blom J, Kalinowski J, Knabbe C, Dreier J. Complete genome and comparative analysis of Streptococcus gallolyticus subsp gallolyticus, an emerging pathogen of infective endocarditis. BMC Genomics. 2011;12:400. doi: 10.1186/1471-2164-12-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A. 2002;99:12391–12396. doi: 10.1073/pnas.182380799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian YD, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan XL, Clifton SW, Roe BA, McLaughlin R. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanie JA, Ng W-LN, Kazmierczak KM, Andrzejewski TM, Davidsen TM, Wayne KJ, Tettelin H, Glass JI, Winkler ME. Genome sequence of Avery’s virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol. 2007;189:38–51. doi: 10.1128/JB.01148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennedsen M, Stuer-Lauridsen B, Danielsen M, Johansen E. Screening for antimicrobial resistance genes and virulence factors via genome sequencing. Appl Environ Microbiol. 2011;77:2785–2787. doi: 10.1128/AEM.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann U, O’Connell-Motherway M, Zomer A, Buist G, Shearman C, Canchaya C, Ventura M, Goesmann A, Gasson MJ, Kuipers OP, van Sinderen D, Kok J. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J Bacteriol. 2007;189:3256–3270. doi: 10.1128/JB.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkiewicz I, Green NM, Guo N, Bongiovanni AM, Witkin SS, Musser JM. Transcriptome adaptation of group B Streptococcus to growth in human amniotic fluid. PLoS One. 2009;4:e6114. doi: 10.1371/journal.pone.0006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Okumura K, Murayama SY, Yagi J, Ubukata K, Kirikae T, Miyoshi-Akiyama T. Complete genome sequencing and analysis of a Lancefield group G Streptococcus dysgalactiae subsp. equisimilis strain causing streptococcal toxic shock syndrome (STSS) BMC Genomics. 2011;12:17. doi: 10.1186/1471-2164-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden MTG. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis. PLoS One. 2009;4:e6072. doi: 10.1371/journal.pone.0006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres SB, Sesso R, Pinto SWL, Hoe NP, Porcella SF, DeLeo FR, Musser JM. Genome sequence of a Lancefield group C Streptococcus zooepidemicus strain causing epidemic nephritis: new information about an old disease. PLoS One. 2008;3:e3026. doi: 10.1371/journal.pone.0003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgalaki MD, Van den Berghe E, Kritikos D, Devreese B, Van Beeumen J, Kalantzopoulos G, De Vuyst L, Tsakalidou E. Macedocin, a food grade lantibiotic produced by Streptococcus macedonicus ACA-DC 198. Appl Environ Microbiol. 2002;68:5891–5903. doi: 10.1128/AEM.68.12.5891-5903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakalidou E, Zoidou E, Pot B, Wassill L, Ludwig W, Devriese LA, Kalantzopoulos G, Schleifer KH, Kersters K. Identification of streptococci from Greek Kasseri cheese and description of Streptococcus macedonicus sp. nov. Int J Syst Bacteriol. 1998;48:519–527. doi: 10.1099/00207713-48-2-519. [DOI] [PubMed] [Google Scholar]
- Denapaite D, Brückner R, Nuhn M, Reichmann P, Henrich B, Maurer P, Schähle Y, Selbmann P, Zimmermann W, Wambutt R, Hakenbeck R. The genome of Streptococcus mitis B6 - what is a commensal? PLoS One. 2010;5:e9426. doi: 10.1371/journal.pone.0009426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins J. Genome of the bacterium Streptococcus pneumoniae strain R6. J Bacteriol. 2001;183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- Vaillancourt K, Moineau S, Frenette M, Lessard C, Vadeboncoeur C. Galactose and lactose genes from the galactose-positive bacterium Streptococcus salivarius and the phylogenetically related galactose-negative bacterium Streptococcus thermophilus: organization, sequence, transcription, and activity of the gal gene products. J Bacteriol. 2002;184:785–793. doi: 10.1128/JB.184.3.785-793.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Alves JM, Kitten T, Brown A, Chen ZM, Ozaki LS, Manque P, Ge XC, Serrano MG, Puiu D, Hendricks S, Wang YP, Chaplin MD, Akan D, Paik S, Peterson DL, Macrina FL, Buck GA. Genome of the opportunistic pathogen Streptococcus sanguinis. J Bacteriol. 2007;189:3166–3175. doi: 10.1128/JB.01808-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CCGM, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K, Bolhuis A, Venema G, Kok J. Construction of a food-grade multiple-copy integration system for Lactococcus lactis. Appl Microbiol Biotechnol. 1998;49:417–423. doi: 10.1007/s002530051192. [DOI] [PubMed] [Google Scholar]
- Leenhouts K, Venema G. In: Plasmids: a practical approach. 2. Hardy KG, editor. Oxford, UK: IRL Press, Inc; 1993. Lactococcal plasmid vectors; pp. 65–94. [Google Scholar]
- Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for Gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet V. Bacterial oligopeptide-binding proteins. Cell Mol Life Sci. 2003;60:2100–2114. doi: 10.1007/s00018-003-3054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garault P, Le Bars D, Besset C, Monnet V. Three oligopeptide-binding proteins are involved in the oligopeptide transport of Streptococcus thermophilus. J Biol Chem. 2002;277:32–39. doi: 10.1074/jbc.M107002200. [DOI] [PubMed] [Google Scholar]
- Smith HE, Damman M, van der Velde J, Wagenaar F, Wisselink HJ, Stockhofe-Zurwieden N, Smits MA. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperisen P, Schmid CD, Bucher P, Zilian O. Stealth proteins: in silico identification of a novel protein family rendering bacterial pathogens invisible to host immune defense. PLoS Comp Biol. 2005;1:492–499. doi: 10.1371/journal.pcbi.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P, Cheng Q. In: The Prokaryotes, an evolving electronic resource for the microbiological community. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editor. New York, NY, USA: Springer Verlag; 2006. Medically important beta-hemolytic streptococci; pp. 108–148. [Google Scholar]
- Yang J, Chen LH, Sun LL, Yu J, Jin Q. VFDB 2008 release: an enhanced web-based resource for comparative pathogenomics. Nucleic Acids Res. 2008;36:D539–D542. doi: 10.1093/nar/gkm951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton B, Dubnau D. Membrane-associated DNA transport machines. Cold Spring Harb Perspect Biol. 2010;2:a000406. doi: 10.1101/cshperspect.a000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverys JP, Martin B, Polard P. The genetic transformation machinery: composition, localization, and mechanism. FEMS Microbiol Rev. 2009;33:643–656. doi: 10.1111/j.1574-6976.2009.00164.x. [DOI] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Attar S, Westra ER, van der Oost J, Brouns SJJ. Clustered regularly interspaced short palindromic repeats (CRISPRs): the hallmark of an ingenious antiviral defense mechanism in prokaryotes. Biol Chem. 2011;392:277–289. doi: 10.1515/BC.2011.042. [DOI] [PubMed] [Google Scholar]
- Horvath P, Romero DA, Coute-Monvoisin AC, Richards M, Deveau H, Moineau S, Boyaval P, Fremaux C, Barrangou R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J Bacteriol. 2008;190:1401–1412. doi: 10.1128/JB.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes IF, Diep DB, Holo H. Bacteriocin diversity in Streptococcus and Enterococcus. J Bacteriol. 2007;189:1189–1198. doi: 10.1128/JB.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel W. Use of starter cultures in fermentation on a household scale. Food Control. 1997;8:241–258. [Google Scholar]
- Motarjemi Y, Käferstein F, Moy G, Quevedo F. Contaminated weaning food: a major risk factor for diarrhea and associated malnutrition. Bull WHO. 1993;71:79–92. [PMC free article] [PubMed] [Google Scholar]
- Oyewole OB. Lactic fermented foods in Africa and their benefits. Food Control. 1997;8:289–297. [Google Scholar]
- Fox PF. Cheese: chemistry, physics and microbiology. London, UK: Chapman & Hall; 1993. [Google Scholar]
- Epstein H. The origin of domestic animals of Africa. Volume I and II. New York, NY, USA: Africana Publ. Corp; 1971. [Google Scholar]
- Bulliet RW. The camel and the wheel. Cambridge, MA, USA: Harvard University Press; 1975. [Google Scholar]
- Mikesell MW. Notes on the dispersal of the dromedary. Southwest J Anthrop. 1955;11:231–245. [Google Scholar]
- Mills S, Griffin C, Coffey A, Meijer WC, Hafkamp B, Ross RP. CRISPR analysis of bacteriophage-insensitive mutants (BIMs) of industrial Streptococcus thermophilus- implications for starter design. J Appl Microbiol. 2010;108:945–955. doi: 10.1111/j.1365-2672.2009.04486.x. [DOI] [PubMed] [Google Scholar]
- Cui YJ, Li YJ, Gorgé O, Platonov ME, Yan YF, Guo ZB, Pourcel C, Dentovskaya SV, Balakhonov SV, Wang XY, Song YJ, Anisimov AP, Vergnaud G, Yang RF. Insight into microevolution of Yersinia pestis by clustered regularly interspaced short palindromic repeats. PLoS One. 2008;3:e2652. doi: 10.1371/journal.pone.0002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C, Poyart C, Ehrlich SD, Renault P. Extent of horizontal gene transfer in evolution of streptococci of the salivarius group. J Bacteriol. 2007;189:1330–1341. doi: 10.1128/JB.01058-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragkoudakis PA, Papadelli M, Georgalaki M, Panayotopoulou EG, Martinez-Gonzalez B, Mentis AF, Petraki K, Sgouras DN, Tsakalidou E. In vitro and in vivo safety evaluation of the bacteriocin producer Streptococcus macedonicus ACA-DC 198. Int J Food Microbiol. 2009;133:141–147. doi: 10.1016/j.ijfoodmicro.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Terzaghi BE, Sandine WE. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliker PR, Anderson AW, Hannesson G. An agar culture medium for lactic acid streptococci and lactobacilli. J Dairy Sci. 1956;39:1611–1612. [Google Scholar]
- Lee R, Molsknes T, Sandine WE, Elliker PR. Carbohydrate metabolism in lactic streptococci: fate of galactose supplied in free or disaccharide form. Appl Microbiol. 1973;26:951–958. doi: 10.1128/am.26.6.951-958.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holo H, Nes IF. In: Methods in Molecular Biology; Electroporation protocols for microorganisms. Volume 47. Nickoloff JA, editor. 1995. Transformation of Lactococcus by electroporation; pp. 195–199. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russel DW. Molecular Cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Birnboim HC, Doly J. Rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol. 1981;147:195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Gao F, Zhang CT. Ori-Finder: a web-based system for finding oriCs in unannotated bacterial genomes. BMC Bioinformatics. 2008;9:79. doi: 10.1186/1471-2105-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci U S A. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, Myers GSA, Ravel J. Visualization of comparative genomic analyses by BLAST score ratio. BMC Bioinformatics. 2005;6:2. doi: 10.1186/1471-2105-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hijum S, Zomer AL, Kuipers OP, Kok J. Projector 2: contig mapping for efficient gap-closure of prokaryotic genome sequence assemblies. Nucleic Acids Res. 2005;33:W560–W566. doi: 10.1093/nar/gki356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar D, Bringel F, Schuren FH, de Vos WM, Siezen RJ, Kleerebezem M. Exploring Lactobacillus plantarum genome diversity by using microarrays. J Bacteriol. 2005;187:6119–6127. doi: 10.1128/JB.187.17.6119-6127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver T, Thomson N, Bleasby A, Berriman M, Parkhill J. DNAPlotter: circular and linear interactive genome visualization. Bioinformatics. 2009;25:119–120. doi: 10.1093/bioinformatics/btn578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong A, van Heel AJ, Kok J, Kuipers OP. BAGEL2: mining for bacteriocins in genomic data. Nucleic Acids Res. 2010;38:W647–W651. doi: 10.1093/nar/gkq365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa I, Vergnaud G, Pourcel C. CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 2007;35:W52–W57. doi: 10.1093/nar/gkm360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissa I, Vergnaud G, Pourcel C. The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats. BMC Bioinformatics. 2007;8:172. doi: 10.1186/1471-2105-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete table of CDS in Sii genome CJ18 in comparison with reference strain genomes. Description of data: This table contains all CDS of the African Sii genome CJ18 (excluding the plasmid) in comparison with reference strain genomes used in this study. A reverse comparison of CDS present in Sii ATCC BAA-102T versus CJ18 is included. The CDS of the plasmid pSICJ18-1 are included in a separate tab with their highest NCBI Blast database gene match.
Synteni plot of genome Sii CJ18 (x) vs. (A) S. gallolyticus subsp. macedonicus ACA-DC 198 (y), (B) S. gallolyticus subsp. gallolyticus UCN34 (y) and (C) S. gallolyticus subsp. gallolyticus ATCC BAA-2069 (y). Description of data: Similar to the genome of Sii ATCC BAA-102T, Sii CJ18 and S. gallolyticus strains ACA-DC 198, ATCC BAA-2069 and UCN34 display a high degree of conservation indicated by the alignment near the diagonal line. The same major insertion sites as in ATCC BAA-102T can be identified as R4 (34.2 kb) consisting largely of phage-related genes; R6 (25.6 kb) encompassing a 13.2-kb S. thermophilus-gene cluster comprising the additional gal-lac operon; and R9 (26.1 kb) containing among others an HTH-type transcriptional regulator rgg, primosomal protein N’ (replication factor Y) – superfamily II helicase and an FtsK/SpoIIIE family protein. The dairy isolate S. gallolyticus subsp. macedonicus ACA-DC 198 features an additional unique region R12 comprising bacteriocin-related structures of macedocin, salavaricin, lantibiotic modifying enzymes and transporters.
Unique 13.2-kb gene locus with high DNA sequence identity to S. thermophilus in Sii genome CJ18. Description of data: The African Sii CJ18 harbors an approximately 13.2-kb insert of DNA with high sequence identity to S. thermophilus LMD-9 (white arrows) within a 25.6-kb insert (R6, Sinf_0915-Sinf_0939). The 18.4-kb gap between Sinf_0910 to Sinf_0928 was largely occupied with hypothetical proteins of unknown origin, few transporters and phage-related genes. Genes are not drawn to scale. Gene numbering corresponds to CDS region Sinf_0910-Sinf_0938. Black arrows indicate S. infantarius identity; grey other streptococci and white S. thermophilus identity.
Phenotypes of CJ18WT and mutant KO derivatives on BHI/X-Gal/IPTG agar media. Description of data: Confirmation of phenotypes of wild type, reference and KO strains on BHI/X-Gal/IPTG agar media yielding blue colonies for CJ18WT, CJ18ΔlacIIC, CJ18ΔlacS and white colonies for CJ18ΔlacZ and CCUG 43820T.
Growth kinetics of wild type and knock-out strains of Sii CJ18 in glucose medium. Description of data: Growth kinetics of CJ18 wild type (A), CJ18ΔlacIIC (B), CJ18ΔlacS (C) and CJ18ΔlacZ (D) were compared in Elliker-based glucose medium for optical density (OD600 ■) and for metabolites glucose (∇),lactate (○) and galactose (ᐃ) in cell-free supernatant. Representative curves of two independent repetitions per strain are shown.