Abstract
Promastigotes of Leishmania (Viannia) panamensis were successfully transfected with p6.5-egfp to express green fluorescent protein. The transfectants remained infective to macrophages, providing an in vitro model for screening antileishmanial drugs. This was demonstrated by flow cytometry of macrophage-associated GFP after exposure of infected cultures to known anti-leishmanial drugs, i. e. amphotericin B and glucantime®. Fluorescence of GFP diminished progressively from infected cells with increasing drug concentrations used in both cases. The availability of this fluorescent assay for infection of macrophages by L. (V.) panamensis facilitates drug discovery program for the Viannia species, which differ significantly from those of the Leishmania subgenus.
Index descriptor: L. (V.) panamensis, L. (L.) amazonensis, L. (L.) major, L. (L.) donovani, Viannia subgenus, Leishmania subgenus, leishmaniasis, green fluorescent protein, antileishmanial drug screening
Introduction
Leishmania spp. are responsible for leishmaniasis - a major parasitic disease of more than 12 million people in 88 countries. There are two million new cases annually, i. e. 1.5 million cutaneous cases and 500,000 visceral cases, respectively (WHO, 2004). Colombia is one of the major endemic places where 99% of the cases are cutaneous leishmaniasis and 70% are caused by Leishmania (V.) panamensis. Chemotherapy of this disease has relied mainly on pentavalent antimonials, but also on amphotericin B and pentamidine as the second line alternative drugs. Development of leishmanial resistance to antimonials (Rojas et al., 2006) necessitates prolonged treatments of patients with increasing dosages, resulting in severe side effects including death (Cesur et al., 2002; Oliveira et al., 2005). Recent evaluation of miltefosine yielded promising results (Soto and Soto, 2006), but the development of resistance to this drug in laboratory studies predicts its emergence in the clinics. There is thus always an urgent need to find new antileishmanial drugs.
Candidate compounds are usually screened against the amastigote as the clinically relevant stage by quantitative microscopy and/or by other assays for cell viability, e. g. propidium iodide permeability (Kamau et al., 2001) or radioactive biosynthetic labeling (Goto et al., 1995). Automation of such assays has been facilitated by transfection of Leishmania to express reporter genes encoding, for example, green fluorescent protein (GFP), luciferase and β-galactosidase that can be used for rapid and high-throughput drug screening. GFP of the jellyfish, Aequorea victoria, is often the marker of choice because it is non-toxic and auto-fluorescent for easy imaging by fluorescent microscopy and ready quantification by flow cytometry or fluorimetry. GFP also compares favorably for the simplicity of its use against other markers, i. e. luciferase, β-lactamase and β-galactosidase. Recently, GFP expressing transfectants have been developed from many species in the Leishmania subgenus, e. g. L. (L.) infantum (Kamau et al., 2001), L. (L.) donovani (Ha et al., 1996; Singh and Dube., 2004; Dube et al., 2005) and L. (L.) amazonensis (Chan et al., 2003; Okuno et al., 2003; Dutta et al., 2005). This is also true for other markers, e. g. luciferase in L. (L.) donovani (Roy et al., 2000; Gupta el al., 2005; Ashutosh et al., 2005), L. (L.) infantum (Sereno et al., 2001), L. (L.) major (Roy et al., 2000) and L. (L.) amazonensis (Lang et al., 2005), and β-lactamase and β-galactosidase in L.(L.) amazonensis (Buckner and Wilson, 2005; Okuno et al., 2003). In contrast, similar transfectants have not been made available for those in the Viannia subgenus, except two luciferase-transfected L. (V.) panamensis for drug studies (Romero et al., 2005; Henao et al., 2004).
Leishmania and Viannia are thought to have diverged 40 to 80 million years ago (McMahon-Pratt et al., 1992; McMahon-Pratt and Alexander, 2004). Leishmania panamensis of the Viannia subgenus differs significantly from its counterpart in the Leishmania subgenus, i. e. L. (L.) major in infectivity to laboratory animals (Goto et al., 1995; Guevara-Mendoza et al., 1997). Different species within the Viannia subgenus also vary among themselves in pathogenicity (Colmenares et al., 2002; McMahon-Pratt and Alexander, 2004), producing a spectrum of simple cutaneous, diffused cutaneous and mucocutaneous leishmaniasis. Intra-species heterogeneity is further noted in the Viannia subgenus (Cupolillo et al., 1997; Muskus et al., 1997; Pacheco et al., 2000; Saravia et al., 2002), including strain-specific drug sensitivity (Croft and Brun, 2003). Thus, additional models are needed for drug-screening to develop Viannia-specific chemotherapy.
In this study we report the development of GFP-transfectants from promastigotes of L. (V.) panamensis. These transfectants retain their infectivity to macrophage cell lines and continue to express GFP intracellularly in vitro. We demonstrated the utility of this infection model for potential high throughput screening of antileishmanial drugs in vitro by flow cytometry.
Materials and methods
Parasites and transfection
Promastigotes of L. (V.) panamensis (MHOM/87/CO/UA140), a Colombian isolate, was cultured at 25°C in Schneider’s medium (Gibco) with 10% HIFBS in 25 cm2 tissue culture flasks (Cellstar). For transfection, these cells grown to late-log phase were washed by centrifugation at 3,500 g at 4°C for 10 min once with PBS and twice in chilled electroporation buffer (25 mM HEPES, 137 mM NaCl, 5 mM KCl, 0.7 mM NaH2PO4 and 6 mM glucose). The parasite suspension (0.4 ml at 50 × 106 cells/ml) was mixed with ~30 μg of p6.5-egfp (Dutta et al., 2005) in a 2 mm-gapped cuvette (BioRad) for electroporation in a Gene Pulser (BioRad) at 0.45 kV and 500 μF (Chen et al., 2000). After ~24 hrs of incubation for recovery in drug-free medium with 20% HIFBS, the transfectants were selected for resistance to tunicamycin at 1.25, 2.5 and 5 μg/ml. Cells were microscopically monitored daily for viability and growth (Nikon Labophot-2).
Transfectants obtained were cloned by limiting dilution in 96-well tissue culture plates. Fluorescent clones emerged were picked and further selected with increasing concentrations of tunicamycin from 5 to 25 μg/ml. Transfectants grown at increasing selective pressures were harvest and fixed with 0.5% paraformaldehyde in PBS for flow cytometry (Beckman–coulter Epics XL) using an argon laser at 488 nm for excitation and 525 nm for emission. GFP-expressing promastigotes were analyzed flow cytometrically in 10,000 gated events and the numeric data were processed by using WinMDI software.
Infection of macrophages with GFP transfectants and determination of their median infective dose (IC50)
Monocyte-derived macrophages of the human cell line U937 were used to determine this after infection with GFP-transfectants (Sundstrom and Nilsson, 1976). The non-infected monocytes were cultured in RPMI 1640 medium (Sigma) plus 10% HIFBS at 37°C in 5% CO2 and passaged every 2 days. These monocytes were treated at 3 × 105 cell/ml per well with 1 μM of phorbol myristate acetate (PMA) (Sigma) for 48 hr at 37°C in 24-well culture plates (Fisher). The macrophages so obtained were infected for two hrs at 34 °C with the GFP-transfectants at different parasite-to-host ratios. The cultures were washed three times with pre-warmed fresh medium to remove un-ingested parasites and further incubated for 24 hrs at 34°C. Cells were then removed from individual wells by vigorous aspiration with cold medium for reaction at 4 °C for 30 min with PE conjugated anti-CD33 (Pharmingen) (5 μl per sample) specific to the monocytic/myeloid lineage (Simmons and Seed, 1988). PE-conjugated isotype control was included to estimate the non-specific binding of the primary antibody to CD33. After washing, individual cell samples were subjected to two-color flow cytometric analysis, i. e. emission at 488 nm and excitation at 530 nm for GFP and 575 nm for PE. Aliquots of these samples at different parasite-to-macrophage ratios were simultaneously collected for microscopic evaluation of the percentage of infection after fixation in methanol and staining with 10% Giemsa (Merck). Median infective dose or IC50 was calculated from the data obtained by both methods using the probit method (Finney, 1971). Macrophages of the J774 line were also infected with the GFP-transfectants under conditions as described (Dutta et al., 2005).
Utility of the GFP tansfectant-infected cultures for screening antileishmanial drugs by fluorescent assay
Two known antileishmanial drugs were included for comparison, i. e. meglumine antimoniate at 0.3125, 1.25, 5 and 20 μg/ml (Glucantime®, Aventis Pharma Ltd, Brasil) and amphotericin B at 0.1563, 0.625, 2.5 and 10 μg/ml (Bristol-Myers Squibb). Controls were diluents alone at the highest concentration used for each compound. The macrophages cultured in 24-well plates as described were infected for 2 hrs at 34 °C with the stationary phase GFP transfectants at the IC50 determined, i. e. a promastigote-to-macrophage ratio of 35:1. Cultures were washed to remove un-ingested promastigotes as before and then treated for 96 hrs at 34°C with the drugs at different concentrations as indicated. The drug effects were analyzed by flow cytometry of cell associated GFP and by microscopy as described. The EC50 was calculated from the fluorescence intensities of CD33 and GFP positive infected macrophages by using a logarithmic regression with the probit method (Finney, 1971).
Results
Expression of GFP in transfected L. (V.) panamensis
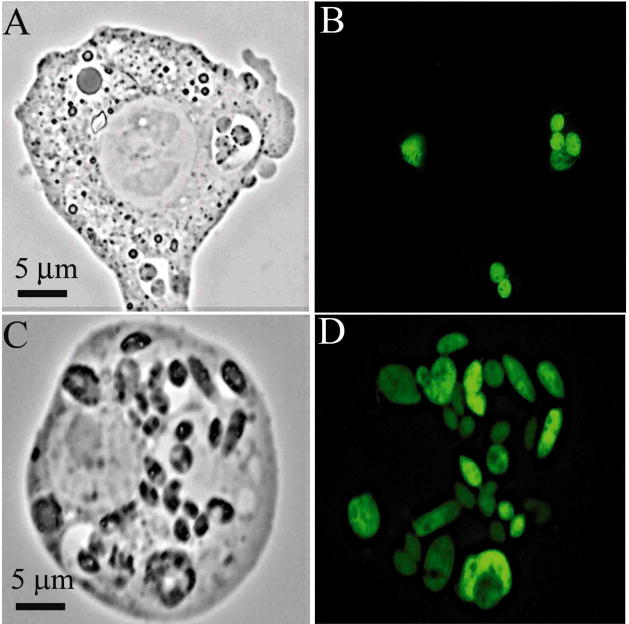
Fluorescent transfectants emerged after electroporation of cells under the initial selective pressures of ≤5 ug tunicamycin/ml, reaching a level of 50–60% of the total population by day 25. The proportion of GFP fluorescent cells increased significantly to 96% when clones were obtained and further selected for resistance to higher concentrations of tunicamycin from 10 to 25 μg/ml. The GFP fluorescence of individual cells appeared largely cytosolic and varied in intensity (Fig. 1A–D). Transfectants grown continuously with increasing selective pressures from 0 to 25 μg of tunicamycin/ml showed an increase in the fluorescent cells from 0 to 96% of the population (Fig. 2).
Figure 1.
Fluorescent promastigotes of L. (V.) panamensis (UA-140) transfected with the p6.5-egfp constructs. (A and C), phase contrast; (B and D), fluorescent microscopy.
Figure 2.
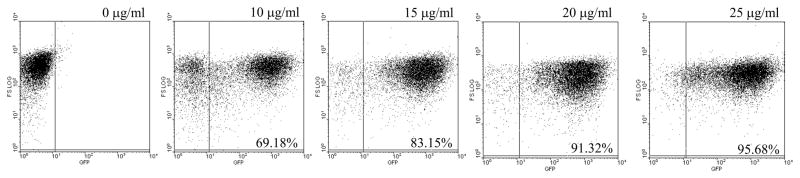
Fluorescent cell population of GFP-expressing L. (V.) panamensis promastigotes increased with increasing selective pressure from 10–25 μg/ml of tunicamycin. Parasites used were grown for one cycle in drug-free medium before flow cytometry.
Assay for the activities of known antileishmanial drugs based on Leishmania GFP fluorescence intensities in infected macrophages
The transfectants retained their infectivity to the macrophages and therein differentiated into amastigotes, which remained fluorescent for the duration of the experiments, as seen by both fluorescent microscopy (Fig. 3) and flow cytometry (Fig. 4). GFP fluorescence was associated strictly with intracellular Leishmania in the parasitophorous vacuoles (Fig. 3A–D). Flow cytometry of the macrophages infected for 4 days showed that the percentage of GFP+ parasites increased in the CD33+ cells proportionally with increasing promastigote-to-macrophage ratios, indicative of increasing infection, as expected (Fig. 4A). The presence of CD33-negative cells (Fig. 4A, Lower left quadrant) suggests that a low percentage of PMA-treated U937 cells did not react with the anti-CD33-PE used. Estimation of the infection rate by the GFP fluorescence of the CD33+ cells is comparable to that obtained by microscopic enumeration of intracellular Leishmania in the transfectant-infected U937 cells, showing an increase with increasing promastigote-to-macrophage ratios from 19% to 74% by microscopy (data not shown) and from 17% to 58%, by flow citometry. The median infective dose of the GFP transfectants is estimated to fall within the range of their number used at a parasite-to-macrophage ratio between 25:1 and 35:1.
Figure 3.
GFP fluorescence of intracellular L. (V.) panamensis after infection of macrophages. (A and C), Phase contrast microscopy; (B and D), fluorescent microscopy. Macrophages of the J774 line were infected with L. (V.) panamensis transfectants as promastigotes at a parasite-to-macrophage ratio of 10:1 under the same conditions as described (Dutta et al., 2005). Culture medium was renewed daily for four days when samples were taken for imaging. U937 cells infected with these transfectants gave similar results.
Figure 4.
(A) Median infective dose (IC50) of GFP-expressing L.(V.) panamensis in U937 cells. The IC50 was calculated from CD33 and GFP double-fluorescent cells in samples at increasing promastigote-to-macrophage ratios from 0:1 to 40:1 as indicated. [1] Isotype control: cells stained with a PE-conjugated mouse IgG1; [2] Non-infected cells stained with PE conjugated anti-CD33; [3–6] Infected cells using different parasite-to-macrophage ratio (5:1, 10:1, 20:1 and 40:1, respectively). (B) Determination of EC50 of two antileishmanial drugs for GFP-expressing L. (V.) panamensis in U937 cells. The EC50 was calculated from double emitting fluorescence cells for GFP and CD33 from a Dose-Response curve for meglumine antimoniate (upper graph) and amphotericin B (lower graph) by probit transformation. Curves show activities of antimoniate and amphotericin B at each concentration against intracellular amastigotes, as indicated by decreases in the percentage of fluorescence that is proportional to the number of parasites within cells when they are in presence of the corresponding drug concentration. (C) Infection rate of L. (V.) panamensis infected U-937 cells after treatment with increasing drug concentrations. Dot plots show leishmanicidal activities of meglumine antimoniate and amphotericin B each at the highest and the lowest concentrations against intracellular amastigotes, as indicated by decreases in the percentage of infection. [1] L. (V.) panamensis infected U-937 cells; [2–3] Infected cells after treatment with meglumine antimoniate at 0.3125 and 20 μg/ml, respectively, and [4–5] Infected cells treated with amphotericin B at 0.156 and 10 μg/ml, respectively.
Numerical values are specific cell population in % ± S.E. Left upper quadrant: CD33+, GFP−(uninfected U937 cells); right upper quadrant: CD33+, GFP+ (infected U937 cells); right lower quadrant: CD33−, GFP+ (extracellular parasites). Data presented were representatives of four independent experiments each performed in triplicate.
Macrophages infected with the median infective dose of the GFP-transfectants were subsequently treated with meglumine antimoniate and amphotericin B for ~4 days at increasing drug concentrations mentioned. The EC50s’ of both antileishmanial drugs were determined by flow cytometry of GFP fluorescence levels in the CD33+ cells. The fluorescence intensities of GFP in arbitrary fluorescent unit (FU) decreased in these CD33+ populations gradually from 95.5 FU of the infected and untreated control to 48.17 and 41.03 FU, in those exposed to the highest concentrations of antimoniate and amphotericin B, respectively (data not shown). When these FU were converted to percentage of viable intracellular parasites, the viability decreased from 100% to 52% with antimoniate and from 100% to 0.8% with amphotericin B, in the infected cells exposed to the highest concentrations of these antileishmanial drugs (Fig. 4B). Namely, the CD33+GFP+ double fluorescent cells or infected macrophages decreased from 36.77% of the untreated control proportionally with increasing drug concentrations to 13.31% (Fig. 4C 1–3) and 7.61% (Fig. 4C 1, 4–5) for antimoniate and amphotericin B, respectively. The GFP+ CD33− populations were present in all cases, apparently representing amastigotes, which were inadvertently released from infected cells during their removal from the culture flasks by vigorous aspiration or as result of the infection itself. The proportion of CD33+ cells was reduced slightly with increasing concentration of both drugs, indicating that they are marginally toxic to U937 cells under the experimental conditions. However, the drug concentrations used were always below the LC50. The EC50s’ of the antileishmanial compounds were determined to be 20 μg/ml and 0.078 μg/ml for meglumine antimoniate and Amphotericin B, respectively. These values are comparable, although not identical, to those previously determined for wildtype cells by the conventional methods, e. g. an EC50 value of 0.132 ± 0.09 μg/ml for amphotericin B (Unpublished).
Discussion
Genetic engineering of Leishmania to express fluorescent markers, such as GFP, facilitates drug screening automation. We produced such transfectants for L. (V.) panamensis with the p6.5-egfp constructs, which have been used previously only for species of the Leishmania subgenus (Chan et al., 2003; Dutta et al., 2005). Clonal variation in GFP expression was evident among the emerging transfectants in the initial selection. Stable clones were subsequently selected with increasing drug concentrations, giving intensive GFP fluorescence in almost 100% of the population (Figs 1 and 2). The vector p6.5 contains DNA sequences from a member of the Leishmania subgenus, L. (L.) amazonensis, including nagt as the selective marker for tunicamycin-resistance (Liu and Chang, 1992) and the precise deletion of a constitutively expressed gene (p36) 2.3 kb downstream of nagt as the expression site (Liu and Chang, 1994). Previously, p6.5 has been shown to express foreign genes with high yield in L. (L.) amazonensis (Sah et al., 2002) and other members of the Leishmania subgenus (Dutta et al, 2008). The work presented here represents first report for the utility of this Leishmania subgenus-derived vector for transfection of a member in the Viannia subgenus. Cross-subgenus functionality of the selective marker and the expression site in p6.5 is thus demonstrated. Whether this may contribute to the initial heterogeneity of GFP expression seen, is unknown. Inherent clonal variations of L. (V.) panamensis in gene expression and low selective pressures used initially are alternative possibilities, pending further investigation. The egfp-transfectants of L. (V.) panamensis retain not only its infectivity in vitro to human monocyte-derived macrophages of the cell line U937 and J774 macrophages of murine origin but also the ability to differentiate from promastigotes into amastigotes in these macrophages. More importantly, the intracellular parasites contain sufficient GFP fluorescence readily detectable by fluorescent microscopy (Fig. 3) and by flow cytometry (Figs. 4). There is a good correlation between parasite burdens and fluorescence quantitatively determined by microscopy and by flow cytometry. The latter method is less laborious, more accurate and higher in capacity to evaluate a large number of cells than possible by microscopy. This may be further simplified by fluorimetry of cell suspensions for quantitative measurement of GFP fluorescence.
Here, we also demonstrate the utility of L. (V.) panamensis transfected with GFP for evaluating their response to known antileishmanial compounds in the in vitro infection model described. The GFP-expression by the transfectants allows us to evaluate their infection of macrophages by measuring fluorescence, as shown previously for other intracellular pathogens, e. g. Mycobacterium tuberculosis (Collins et al., 1998), Toxoplasma gondii (Gubbels et al., 2004) and Leishmania subgenus species (Kamau et al., 2001; Singh and Dube, 2004; Dube et al., 2005). In our case, the stability of GFP fluorescence in the amastigotes of L. (V.) panamensis in infected cells makes it possible to show a dose-dependent antileishmanial activities of both glucantime® and amphotericin B. Their differential antileishmanial activities in favor of the latter drug are in agreement with previous reports (Escobar et al., 2001; Sereno et al., 2000).
In summary, data presented show that L. (V.) panamensis can be transfected to produce GFP in p6.5 expression vector, which has been shown to express foreign proteins efficiently in members of the Leishmania subgenus. This is the first report for such transfection of L. (V.) panamensis and its utility to evaluate antileishmanial compounds. The availability of these transfectants will facilitate the screening of drugs specific for the Viannia group and also the study of host-parasite interactions.
Acknowledgments
This work was financed by the Colombian funding agency, Colciencias (contract numbers 110 and 257, project codes identification 11150412982 and 11150416514, respectively) and USA NIH AI-20486 to KPC. We thank Miguel Toro for assistance with the figure preparation.
Abbreviations
- GFP
Green fluorescent protein
- HIFBS
heat-inactivated fetal bovine serum
- PMA
phorbol myristate acetate
- PE
phycoerythrin
- nagt
N-acetylglucosamine-1-phosphate transferase gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashutosh Gupta S, Ramesh Sundar S, Goyal N. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrobial Agents and Chemotherapy. 2005;49:3776–3783. doi: 10.1128/AAC.49.9.3776-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner FS, Wilson AJ. Colorimetric assay for screening compounds against Leishmania amastigotes grown in macrophages. American Journal of Tropical Medicine and Hygiene. 2005;72:600–605. [PubMed] [Google Scholar]
- Chan MM, Bulinski JC, Chang KP, Fong D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitology Research. 2003;89:266–271. doi: 10.1007/s00436-002-0706-4. [DOI] [PubMed] [Google Scholar]
- Chen DQ, Kolli BK, Yadava N, Lu HG, Gilman-Sachs A, Peterson DA, Chang KP. Episomal expression of specific sense and antisense mRNAs in Leishmania amazonensis: modulation of gp63 level in promastigotes and their infection of macrophages in vitro. Infection and Immunity. 2000;68:80–86. doi: 10.1128/iai.68.1.80-86.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesur S, Bahar K, Erekul S. Death from cumulative sodium stibogluconate toxicity on Kala-Azar. Clinical Microbiololy and Infection. 2002;8:606. doi: 10.1046/j.1469-0691.2002.00456.x. [DOI] [PubMed] [Google Scholar]
- Collins LA, Torrero MN, Franzblau SG. Green fluorescent protein reporter microplate assay for high-throughput screening of compounds against Mycobacterium tuberculosis. Antimicrobial Agents and Chemotherapy. 1998;4:344–347. doi: 10.1128/aac.42.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares M, Kar S, Goldsmith-Pestana K, McMahon-Pratt D. Mechanisms of pathogenesis: differences amongst Leishmania species. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2002;96:S3–S7. doi: 10.1016/s0035-9203(02)90044-1. [DOI] [PubMed] [Google Scholar]
- Croft SL, Brun R. In vitro and in vivo models for the identification and evaluation of drugs active against Trypanosoma and Leishmania. In: Fairlamb AH, Ridley R, Vial H, editors. Drugs against parasitic diseases: Rmethodologies and issues [Online] World Health Organization; Geneva, Switzerland: 2003. pp. 165–175. [Google Scholar]
- Cupolillo E, Grimaldi G, Jr, Momen H. Genetic diversity among Leishmania (Viannia) parasites. Annals of Tropical Medicine and Parasitology. 1997;91:617–626. doi: 10.1080/00034989760716. [DOI] [PubMed] [Google Scholar]
- Dube A, Singh N, Sundar S, Singh N. Refractoriness to the treatment of sodium stibogluconate in Indian kala-azar field isolates persists in in vitro and in vivo experimental models. Parasitology Research. 2005;96:216–223. doi: 10.1007/s00436-005-1339-1. [DOI] [PubMed] [Google Scholar]
- Dutta S, Ray D, Kolli BK, Chang KP. Photodynamic sensitization of Leishmania amazonensis in both extracellular and intracellular stages with aluminum phthalocyanine chloride for photolysis in vitro. Antimicrobial Agents and Chemotherapy. 2005;49:4474–4484. doi: 10.1128/AAC.49.11.4474-4484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Furuyama K, Sassa S, Chang KP. Leishmania spp.: Delta-aminolevulinate-inducible porphyria by genetic complementation of incomplete heme biosynthesis. Experimental Parasitology. 2008;118:629–636. doi: 10.1016/j.exppara.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar P, Yardley V, Croft SL. Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (Pentostam) against Leishmania donovani in immunodeficient scid mice. Antimicrobial Agents and Chemotherapy. 2001;45:1872–1875. doi: 10.1128/AAC.45.6.1872-1875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney JD. Statisical logic in the monitoring of reactions to therapeutic drugs. Methods of Information in Medicine. 1971;10:237–245. [PubMed] [Google Scholar]
- Fumarola L, Spinelli R, Brandonisio O. In vitro assays for evaluation of drug activity against Leishmania spp. Research in Microbiology. 2004;155:224–230. doi: 10.1016/j.resmic.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Goto H, Rojas JI, Sporrong L, De Carreira P, Sanchez C, Orn A. Leishmania (Viannia) panamensis-induced cutaneous leishmaniasis in susceptible and resistant mouse strains. Revista do Instituto de Medicina Tropical de São Paulo. 1995;37:475–481. doi: 10.1590/s0036-46651995000600001. [DOI] [PubMed] [Google Scholar]
- Gubbels MJ, Striepen B. Studying the cell biology of apicomplexan parasites using fluorescent proteins. Microscopy and Microanalysis. 2004;10:568–579. doi: 10.1017/S1431927604040899. [DOI] [PubMed] [Google Scholar]
- Guevara-Mendoza O, Une C, Franceschi Carreira P, Orn A. Experimental infection of Balb/c mice with Leishmania panamensis and Leishmania mexicana: induction of early IFN-gamma but not IL-4 is associated with the development of cutaneous lesions. Scandinavian Journal of Immunology. 1997;46:35–40. doi: 10.1046/j.1365-3083.1997.d01-96.x. [DOI] [PubMed] [Google Scholar]
- Ha DS, Schwarz JK, Turco SJ, Beverley SM. Use of the green fluorescent protein as a marker in transfected Leishmania. Molecular and Biochemical Parasitology. 1996;77:57–64. doi: 10.1016/0166-6851(96)02580-7. [DOI] [PubMed] [Google Scholar]
- Henao HH, Osorio Y, Saravia NG, Gomez A, Travi B. Efficacy and toxicity of pentavalent antimonials (Glucantime and Pentostam) in an American cutaneous leishmaniasis animal model: luminometry application. Biomedica. 2004;24:393–402. [PubMed] [Google Scholar]
- Kamau SW, Grimm F, Hehl AB. Expression of green fluorescent protein as a marker for effects of antileishmanial compounds in vitro. Antimicrobial Agents and Chemotherapy. 2001;45:3654–3656. doi: 10.1128/AAC.45.12.3654-3656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Goyard S, Lebastard M, Milon G. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cellular Microbiology. 2005;7:383–392. doi: 10.1111/j.1462-5822.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Chang KP. The 63-kilobase circular amplicon of tunicamycin-resistant Leishmania amazonensis contains a functional N-acetylglucosamine-1-phosphate transferase gene that can be used as a dominant selectable marker in transfection. Molecular and Cellular Biology. 1992;12:4112–4122. doi: 10.1128/mcb.12.9.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chang KP. Identification by extrachromosomal amplification and overexpression of a zeta-crystallin/NADPH-oxidoreductase homologue constitutively expressed in Leishmania spp. Molecular and Biochemical Parasitology. 1994;66:201–210. doi: 10.1016/0166-6851(94)90147-3. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Alexander J. Does the L. major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunological Review. 2004;201:206–224. doi: 10.1111/j.0105-2896.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- McMahon-Pratt D, Traub-Cseko Y, Lohman KL, Rogers DD, Beverly SM. Loss of the GP46/M-2 surface membrane glycoprotein gene family in the Leishmania braziliensis complex. Molecular and Biochemical Parasitology. 1992;50:151–160. doi: 10.1016/0166-6851(92)90252-f. [DOI] [PubMed] [Google Scholar]
- Misslitz A, Mottram JC, Overath P, Aebischerm T. Targeted integration into a rRNA locus results in uniform and high level expression of transgenes in Leishmania amastigotes. Molecular and Biochemical Parasitology. 2000;107:251–261. doi: 10.1016/s0166-6851(00)00195-x. [DOI] [PubMed] [Google Scholar]
- Muskus C, Segura I, Oddone R, Turco SJ, Leiby DA, Toro L, Robledo S, Saravia NG. Carbohydrate and LPG expression in Leishmania Viannia subgenus. Journal of Parasitology. 1997;83:671–678. [PubMed] [Google Scholar]
- Okuno T, Goto Y, Matsumoto Y, Otsuka H, Masumoto Y. Applications of recombinant Leishmania amazonensis expressing egfp or the beta-galactosidase gene for drug screening and histopathological analysis. Experimental Animal. 2003;52:109–118. doi: 10.1538/expanim.52.109. [DOI] [PubMed] [Google Scholar]
- Oliveira MC, Amorim RF, de Freitas RA, de Costa AL. A fatal case of mucocutaneous leishmaniasis after pentavalent antimonial use. Revista da Sociedade Brasileira de Medicina Tropical. 2005;38:258–260. doi: 10.1590/s0037-86822005000300011. [DOI] [PubMed] [Google Scholar]
- Pacheco RS, Fernandes O, Salinas G, Segura I, Momen H, Degrave W, Saravia NG, Campbell DA. Intraspecies-specific heterogeneity in the mini-exon gene localization of Leishmania (Viannia) panamensis and Leishmania (Viannia) guyanensis from Colombia. Journal of Parasitology. 2000;86:1250–1253. doi: 10.1645/0022-3395(2000)086[1250:IHITME]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. Journal of Infectious Diseases. 2006;193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- Romero IC, Saravia NG, Walker J. Selective action of fluoroquinolones against intracellular amastigotes of Leishmania (Viannia) panamensis in vitro. Journal of Parasitology. 2005;91:1474–1479. doi: 10.1645/GE-3489.1. [DOI] [PubMed] [Google Scholar]
- Roy G, Dumas C, Sereno D, Wu Y, Singh AK, Tremblay MJ, Ouellette M, Olivier M, Papadopoulou B. Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Molecular and Biochemical Parasitology. 2000;110:195–206. doi: 10.1016/s0166-6851(00)00270-x. [DOI] [PubMed] [Google Scholar]
- Sah JF, Ito H, Kolli BK, Peterson DA, Sassa S, Chang KP. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. Journal of Biological Chemistry. 2002;277:14902–14909. doi: 10.1074/jbc.M200107200. [DOI] [PubMed] [Google Scholar]
- Saravia NG, Weigle K, Navas C, Segura I, Valderrama L, Valencia AZ, Escorcia B, McMahon-Pratt D. Heterogeneity, geographic distribution, and pathogenicity of serodemes of Leishmania Viannia in Colombia. American Journal of Tropical Medicine and Hygiene. 2002;66:738–744. doi: 10.4269/ajtmh.2002.66.738. [DOI] [PubMed] [Google Scholar]
- Sereno D, Cordeiro da Silva A, Mathieu-Daude F, Ouaissi A. Advances and perspectives in Leishmania cell based drug-screening procedures. Parasitology International. 2007;56:3–7. doi: 10.1016/j.parint.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Sereno D, Holzmuller P, Lemesre JL. Efficacy of second line drugs on antimonyl-resistant amastigotes of Leishmania infantum. Acta Tropica. 2000;74:25–31. doi: 10.1016/s0001-706x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Sereno D, Roy G, Lemesre JL, Papadopoulou B, Ouellette M. DNA transformation of Leishmania infantum axenic amastigotes and their use in drug screening. Antimicrobial Agents and Chemotherapy. 2001;45:1168–1173. doi: 10.1128/AAC.45.4.1168-1173.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Seed B. Isolation of a cDNA encoding CD33, a differentiation antigen of myeloid progenitor cells. Journal of Immunology. 1988;141:2797–2800. [PubMed] [Google Scholar]
- Singh N, Dube A. Short report: fluorescent Leishmania: application to anti-leishmanial drug testing. American Journal of Tropical Medicine and Hygiene. 2004;71:400–402. [PubMed] [Google Scholar]
- Soto J, Soto P. Miltefosine: oral treatment of leishmaniasis. Expert Review of Anti-infective Therapy. 2006;4:177–185. doi: 10.1586/14787210.4.2.177. [DOI] [PubMed] [Google Scholar]
- Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) International Journal of Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Tropical disease research: progress Leishmaniasis. 2003–2004 [Online.] http://www.who.int/tdr/publications/Publications/pdf/pr17/leishmaniasis.pdf.