Abstract
Background
Random periareolar fine needle aspiration (RPFNA) is a research technique developed to assess short-term breast cancer risk in women at increased risk of breast cancer. Although there is increasing acceptance of RPFNA, neither the reproducibility nor the inter–institutional compatibility of RPFNA has been established. To address these key limitations, the Cancer and Leukemia Group B (CALGB) Prevention Group tested the reproducibility of RPFNA in a multi-institutional cross-sectional study.
Methods
Sixty-three high-risk women from five CALGB institutions (Duke, Ohio State, Roswell Park, Dana Farber, and Vermont) underwent RPFNA from July 1, 2007 to June 30, 2008. Duplicate bilateral RPFNA was performed on each woman by a single investigator on a single day. Masood Cytology Index score was assessed by a single blinded cytopathologist.
Results
There was a high degree of statistical agreement in the Masood Cytology Index scores of duplicate RPFNA samples from the same breast, with a Spearman correlation coefficient of 0.8312 (P < 0.0001). Importantly, although there was agreement in duplicate samples from the same breast, there was lack of agreement between duplicate samples from the opposite breast.
Conclusions
This multi-institutional study shows that RPFNA is a highly reproducible measure of breast cytology in a cooperative group cross-sectional trial. RPFNA did not show a high degree of agreement between breasts, suggesting that breast cancer risk and progression may occur at different rates in individual breasts from a single woman. These studies provide proof-of-principle for future RPFNA-based cooperative group prevention studies.
Introduction
There is evidence that breast cancer incidence may be significantly reduced in high-risk cohorts by treatment with tamoxifen, prophylactic mastectomy, or prophylactic surgery (1–4). However, these risk-reduction strategies carry potential side effects, and prophylactic surgery is expensive. There remains a need for biomarkers to accurately predict short-term risk and identify women most likely to benefit from prevention. Biomarkers that vary with risk and response to prevention interventions are referred to as “surrogate end point biomarkers” (5). As outlined by Fabian et al., surrogate end point biomarkers should be (a) biologically and statistically significantly associated with cancer development, (b) present in a reasonable proportion of at-risk individuals, (c) obtainable by minimally invasive procedures, and (c) reversible with prevention interventions that have been validated to decrease cancer incidence (6). Many modalities have been suggested as potential surrogate end point biomarkers for breast cancer, including mammographic density, serum biomarkers, and breast tissue biomarkers (7–10). Currently, there is no consensus as to the optimal surrogate end point biomarker.
Breast tissue biomarkers offer the advantage of directly testing for pre – cancerous changes in the breast. Atypia and lobular carcinoma in situ are associated with increased breast cancer risk (11). Moreover, breast cancer incidence in women with atypical hyperplasia or lobular carcinoma in situ is substantially reduced after treatment with tamoxifen (1, 2). However, the optimal method to repeatedly sample breast tissue remains controversial. Repeated random core needle biopsies for risk surveillance and/or for measurement of response to a prevention intervention are problematic because, unless the biopsy specimens are obtained from mammographically dense areas, the biopsy is likely to contain few terminal ductal-lobule units (12). Nipple aspirates have shown some promise, but approximately 40% of nipple aspirates are acellular (13).
Random periareolar fine needle aspiration (RPFNA) is a research technique that was developed to assess short-term breast cancer risk in women at high risk of breast cancer (6, 14). RPFNA has the advantage of being inexpensive and may be performed repeatedly, and the majority of samples are cellular in high-risk premenopausal and perimenopausal women (15, 16). In a single institution study, cytologic atypia in RPFNA predicted a 5.6-fold independent increase in breast cancer risk in high-risk women (6). This observation supports the use of cytologic atypia in RPFNA as a marker for high-risk benign lesions in high-risk women.
Although RPFNA has gained acceptance (6, 14, 17–24), several issues limit its use: (a) the reproducibility of RPFNA has not been determined, and (b) RPFNA has the potential to be operator-dependent. To address these potential limitations, the Cancer and Leukemia Group B (CALGB) Prevention Group tested the reproducibility of RPFNA in a multi-institutional cross-sectional study of high-risk women.
The purpose of our study was to determine the agreement between two Masood Cytology Index scores and two cell count measurements from RPFNA samples of individual women. From July 1, 2007 to June 30, 2008, we prospectively tested the reproducibility of duplicate RPFNA samples from 63 high-risk women. RPFNA was performed on the same breast, on the same day, by the same investigator, using separate needles for sequential aspirations.
Materials and Methods
CALGB
Our study was approved by the CALGB Prevention Committee and received pilot funding from CALGB. There was central committee review of this study. All CALGB institutions participating in the Prevention Committee were given the opportunity to participate. The five institutions listed in this study elected to participate. All participating sites are CALGB members and the site principal investigators are members of the CALGB Prevention Committee.
Human Subjects
The study was approved by the Human Subjects Committee and the Institutional Review Boards at the Duke University, Ohio State University (OSU), Roswell Park Cancer Institute (RPCI), Dana Farber Cancer Institute (DFCI), and University of Vermont (UV) in accordance with assurances approved by the Department of Health and Human Services.
Inclusion/Exclusion
Women were required to (a) have at least one of the following risk factors for breast cancer: 5-year Gail risk ≥1.7%,7 a prior excisional biopsy exhibiting atypical hyperplasia or lobular carcinoma in situ, or a known BRCA1/2 mutation (25); (b) be 35 to 55 y old; and (c) have a mammogram read as extreme breast density or heterogeneously dense. Women were excluded for prior mastectomy, breast radiation, or invasive breast cancer.
Recruitment/Demographic Information
Women were recruited from the high-risk breast clinic at each recruiting site. Women were recruited in person and in the order of presentation to the clinic. No patients were excluded; five women declined participation. Demographic data were collected from study participants by patient interview. These data are listed in Table 1. Information obtained about family history included history of breast, ovarian, and other (colon, prostate, pancreatic, brain, lung, esophageal, head and neck, leukemia/lymphoma) cancers in parents, siblings, grandparents, paternal/maternal aunts/uncles, and paternal/maternal cousins.
Table 1.
Demographic and risk data for study participants
| Characteristics | (N = 63) |
|---|---|
| Recruitment | |
| Duke University | 34 |
| Ohio State University (OSU) | 21 |
| Roswell Park Cancer Institute (RPCI) | 6 |
| Dana Farber Cancer Institute (DFCI) | 1 |
| University of Vermont (UV) | 1 |
| Mean age in y (range) | 45.3 (36–55) |
| Menopausal status | |
| Postmenopausal | 8 (13%) |
| Pre/Perimenopausal | 55 (87%) |
| Race | |
| Caucasian | 59 (94%) |
| African American | 2 (3%) |
| Hispanic | 2 (3%) |
| Body mass index in kg/m2 (range) | 25.3 (17.9–36.3) |
| Average 5-y Gail model risk score (range) | 3.2 (0.6–12.9) |
| Known or suspected BRCA1/2 mutation carrier (total) | 11 (17%) |
RPFNA Training
All investigators were trained to do RPFNA according to the published methods of Carol Fabian (6, 14, 16–24) and were individually trained by Carol Fabian at the University of Kansas. One investigator at each recruiting site performed the RPFNA. The respective professions of the investigators who performed the RPFNA at the recruiting sites were as follows: Duke, physician assistant; OSU, surgeon; RPCI, surgeon; DFCI, physician assistant; and UV, pathologist. Prior to initiating the trial, investigators from OSU, UV, and DFCI traveled to Duke University and individually reviewed their RPFNA technique with investigators at Duke. All sites used the same sequence of aspiration, needle size, aspiration fluid, and RPFNA specimen preservative.
RPFNA Aspiration
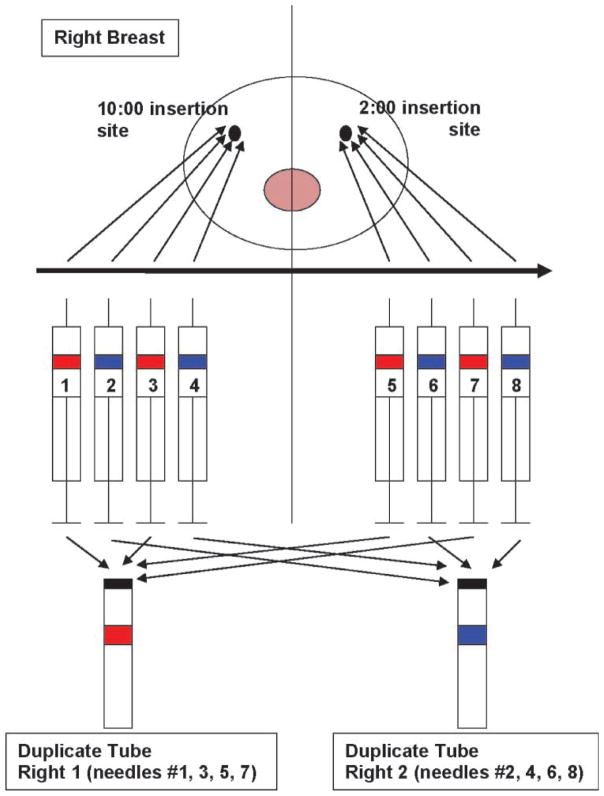
Both breasts were aspirated in all women. The RPFNA aspiration sequence for the right breast is depicted in Fig. 1. The same sequence of aspiration was used for the left breast. Two areas in each breast (10:00 and 2:00 positions) were infiltrated with lidocaine. Breast tissue was aspirated using breast density as a guide. For each breast, eight 10-mL syringes with 21-gauge (1.5 inch) needles were primed with 0.2 mL sterile media. Eight needles were used per breast; multiple passes were made with each needle. Half of the aspirations were made through the 2:00 skin site (four needles) and half through the 10:00 skin site (four needles). The breast was aspirated in the order outlined in Fig. 1. Duplicate samples were sequentially pooled in the appropriate collection tube containing modified CytoLyt Solution (Cytyc). Cytology slides were prepared as previously published (18–24).
Figure 1.
Aspiration plan for the right breast. Multiple passes were made through the breast tissue with each needle, with the goal of covering as much of the dense breast tissue as possible. A total of eight needles were used per breast. The needles were used in sequential order (1, 2, 3, etc). Half of the aspirations were made through the 2:00 skin site (four needles) and half through the 10:00 skin site (four needles). The syringes and tubes were color-coded before the aspiration started to ensure that the correct needle went in the correct tube.
Masood Cytology and Cell Count
Masood Cytology Index score and epithelial cell count for duplicate RPFNA samples were assigned by a blinded, single dedicated cytopathologist (C.M.Z.; refs. 6, 14, 16–24). A minimum of one epithelial cell cluster with >10 epithelial cells was required; the most abnormal cell cluster was scored (26, 27). RPFNA cytology was given a semiquantitative Masood Cytology Index score (26, 27). Cells were given a score of 1 to 4 points for each of the six morphological characteristics: cell arrangement, pleomorphism, number of myoepithelial cells, anisonucleosis, nucleoli, and chromatin clumping. The sum of these points computed the Masood score: ≤10, nonproliferative (normal); 11–13, hyperplasia; 14–17, atypia; and >17, suspicious cytology (26, 27). The numbers of epithelial cells were quantified and classified as <10 cells (insufficient), 10–100 cells, 100–500 cells, 500–1,000 cells, 1,000–5,000 cells, and >5,000 cells.
Statistical Analysis
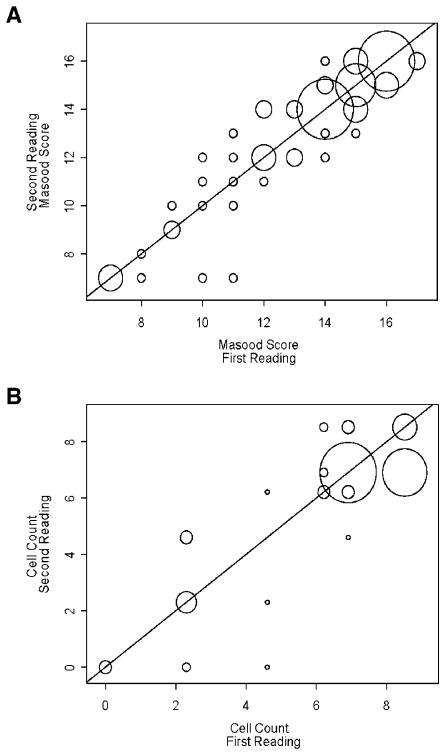
Duplicate RPFNA samples were obtained from 63 high-risk women. The units of observation were individual breast and right versus left breast for an individual woman. Measurements taken within one breast were considered to be independent from the measures taken in the other breast. Data are displayed by plotting the first Masood score against the second (Fig. 2A) and the first epithelial cell count against the second (Fig. 2B), using a line of identity to focus on the area of the plot that indicates perfect agreement. The size of each circle in Fig. 2 corresponds to the number of observations with each pair of readings.
Figure 2.
Plot of duplicate Masood scores and epithelial cell counts from the same breast. Samples were taken from both breasts of 63 women. The unit of observation was the individual breast within each woman. Measurements taken within one breast were considered to be independent from the measures taken in the other breast. Data are displayed by plotting the (A) first Masood score against the second and (B) first epithelial cell count against the second. The line of identity indicates perfect agreement between the two Masood scores or between the two epithelial cell counts.
Concordance between the two duplicate readings from one individual was tested within the left or right breast (Table 2) or between the left and right breasts (Table 3). Agreement between the two readings was initially validated by multiple measures, including Pearson, Spearman, and intraclass correlation coefficients. However, due to the skewed nature of the data, we reported the Spearman and/or intraclass correlation coefficients (Tables 2 and 3). Agreement between the two RPFNA Masood scores and epithelial cell counts within the same breast and between each breast were considered, both by study site and overall. Log transformation was used in calculating all statistics for the RPFNA cell count.
Table 2.
Correlation between two RPFNA measurements of Masood score and cell count of the same breast
| Breast | Variables | Correlation coefficients
|
|
|---|---|---|---|
| Spearman* | Intraclass (95% CI) | ||
| Right | Masood score | 0.9063 | 0.919 (0.870–0.950) |
| Cell count | 0.7488 | 0.860 (0.779–0.913) | |
| Left | Masood score | 0.7428 | 0.721 (0.579–0.820) |
| Cell count | 0.6852 | 0.621 (0.445–0.751) | |
| Overall | Masood score | 0.8312 | ND |
| Cell count | 0.7260 | ND | |
NOTE: 95% CI, 95% confidence interval; ND, not determined.
P < 0.0001.
Table 3.
Lack of agreement between RPFNA measurements of the opposite breast
| Study site | Variables | Spearman correlation coefficient | P |
|---|---|---|---|
| Duke | Masood score | 0.2260 | 0.1987 |
| Cell count | 0.1703 | 0.3356 | |
| OSU | Masood score | −0.0326 | 0.8916 |
| Cell count | 0.0662 | 0.7816 | |
| RPCI | Masood score | −0.9411 | 0.0051 |
| Cell count | −0.0290 | 0.9565 | |
| Overall | Masood score | 0.2215 | 0.0890 |
| Cell count | 0.3240 | 0.0115 |
ANOVA was used to determine if three continuous variables (age, 5-year Gail risk score, and body mass index) differed by site (Table 4). Some of the discrete variables considered for this analysis were menopausal status (pre, peri, and post), BRCA1 or 2 mutation (present or not present), and number of first-degree family members (dichotomized to >2 members or ≤2 members) diagnosed with breast and/or ovarian cancer. Fisher’s exact test was used to determine whether these variables differed significantly by site (Table 5).
Table 4.
Distribution of age, Gail risk score, and body mass index for each of the three highest accruing study sites
| Variable | Study site | Median | Minimum | Maximum | P* |
|---|---|---|---|---|---|
| Age, y | Duke (n = 34) | 44.50 | 36.00 | 55.00 | |
| OSU (n = 21) | 47.00 | 38.00 | 53.00 | ||
| RPCI (n = 6) | 43.50 | 38.00 | 48.00 | ||
| Overall (n = 61) | 45.00 | 36.00 | 55.00 | 0.0258 | |
| Gail score | Duke (n = 34) | 2.15 | 0.90 | 12.90 | |
| OSU (n = 21) | 2.70 | 0.60 | 10.40 | ||
| RPCI (n = 6) | 2.30 | 0.80 | 3.40 | ||
| Overall (n = 61) | 2.30 | 0.60 | 12.90 | <0.0001 | |
| Body mass index, kg/m2 | Duke (n = 34) | 22.70 | 17.90 | 31.50 | |
| OSU (n = 21) | 28.69 | 20.96 | 36.03 | ||
| RPCI (n = 6) | 29.25 | 22.60 | 36.30 | ||
| Overall (n = 61) | 23.90 | 17.90 | 36.30 | 0.0054 |
ANOVA, P < 0.05 is considered statistically significant.
Table 5.
Distribution of menopausal status, BRCA1/2 mutation, and number of first-degree family members with breast and/or ovarian cancer for each of the three highest accruing sites
| Variable | Study site | Frequency (%) | P* |
|---|---|---|---|
| Menopausal status | 0.2917 | ||
| Pre | Duke | 21 (62) | |
| OSU | 15 (71) | ||
| RPCI | 6 (100) | ||
| Overall | 42 (69) | ||
| Peri | Duke | 6 (18) | |
| OSU | 5 (24) | ||
| RPCI | 0 (0) | ||
| Overall | 11 (18) | ||
| Post | Duke | 7 (21) | |
| OSU | 1 (5) | ||
| RPCI | 0 (0) | ||
| Overall | 8 (13) | ||
| BRCA1/2 mutation | 0.1751 | ||
| No | Duke | 25 (74) | |
| OSU | 19 (90) | ||
| RPCI | 6 (100) | ||
| Overall | 50 (82) | ||
| Yes | Duke | 9 (26) | |
| OSU | 2 (10) | ||
| RPCI | 0 (0) | ||
| Overall | 11 (18) | ||
| First-degree family members with breast/ovarian cancer | 0.0077 | ||
| ≤2 members | Duke | 31 (91) | |
| OSU | 12 (57) | ||
| RPCI | 5 (83) | ||
| Overall | 48 (79) | ||
| >2 members | Duke | 3 (9) | |
| OSU | 9 (43) | ||
| RPCI | 1 (17) | ||
| Overall | 13 (21) |
Fisher’s exact test.
Results
Statistical Agreement of Duplicate RPFNA from the Same Breast
Duplicate RPFNA samples were obtained from both breasts of all 63 high-risk women. There was a high level of agreement for Masood Cytology Index score and cell count measured in duplicate RPFNA samples obtained from the same breast (Fig. 2 and Table 2). In particular, the overall Spearman correlation coefficients for Masood score and cell count were 0.8312 (P < 0.0001) and 0.7260 (P < 0.0001), respectively (Table 2). The reproducibility of duplicate RPFNA samples from the same breast was not affected by age (Masood, P = 0.2498; cell count, P = 0.2725), body mass index (Masood, P = 0.1154; cell count, P = 0.8808), 5-year Gail risk score (Masood, P = 0.9385; cell count, P = 0.3976), menopausal status (Masood, P = 0.3603; cell count, P = 0.6086), BRCA1/2 mutation (Masood, P = 0.2151; cell count, P = 0.5781), or the number of first-degree family members with breast and/or ovarian cancer (Masood, P = 0.4022; cell count, P = 0.6823).
Lack of Agreement of RPFNA from the Opposite Breasts
While the duplicate samples within the same breast had a high level of agreement, duplicate samples from opposite breasts were not similar. Agreement between Masood Cytology Index scores and epithelial cell counts was tested by Spearman correlation coefficients. Neither the Masood Cytology Index score nor the cell count produced statistically significant agreement between the right and left breasts from the same individual (Table 3).
Differences in Cohort Composition and Institutional RPFNA Measurements
Duke, OSU, and RPCI contributed the majority of women for this study. All three cohorts differed significantly in their demographic composition (Tables 4 and 5). ANOVA showed that all three continuous demographic variables (age, P = 0.0258; 5-year Gail risk score, P < 0.0001; body mass index, P = 0.0054) differed by site (Table 4). Fisher’s exact test showed that the two dichotomous variables, BRCA1/2 mutation status (P = 0.1751) and menopausal status (P = 0.2917), did not differ significantly by site (Table 5). However, the number of first-degree family members (dichotomized to >2 or ≤2 members) differed among study sites (P = 0.0077; Table 5).
Given the differences in institutional cohort composition, we tested whether there was an institutional effect on RPFNA Masood Cytology Index score and/or epithelial cell count. Specifically, we tested whether overall readings differed among institutional sites (i.e., did one site have higher or lower readings than the other sites?). To carry out this analysis, we used a repeated measure ANOVA to account for the expected correlation found in readings taken from the same woman. We observed that there was a significant difference between Masood Cytology Index score (P = 0.0002) and epithelial cell count (P = 0.0194) obtained from the three highest accruing sites. Based on this result, we examined further the actual differences between pairs of study sites. The least squares mean for each study site (Table 6) indicates that Duke and OSU have significantly higher Masood Cytology Index scores and cell counts than RPCI. However, Duke and OSU do not significantly differ from one another (Table 6).
Table 6.
Pair-wise comparisons of Masood Cytology Index score and epithelial cell count among study sites
| Variable | Study site | Least squares mean | Pairwise comparison | P |
|---|---|---|---|---|
| Masood score | Duke | 13.32 | Duke vs. OSU | 0.4113 |
| OSU | 13.69 | OSU vs. RPCI | <0.0001 | |
| RPCI | 10.38 | RPCI vs. Duke | 0.0002 | |
| Cell count | Duke | 5.90 | Duke vs. OSU | 0.5523 |
| OSU | 6.80 | OSU vs. RPCI | 0.0058 | |
| RPCI | 2.80 | RPCI vs. Duke | 0.0113 |
Discussion
The study described herein shows for the first time that RPFNA is reproducible in a cooperative group setting. Our findings show that RPFNA provides reproducible sampling of mammary epithelial cells from high-risk women and support the future use of RPFNA in multi-institutional trials.
Significantly, although RPFNA was highly reproducible within the same breast, we did not observe agreement between Masood Cytology Index score and cell count measurements of the right and left breasts from the same woman. This observation suggests that breast cancer risk and progression in the individual breasts of a single woman occur at different rates.
We observed institutional differences among women selected for RPFNA in our three highest accruing sites. Masood Cytology Index score and epithelial cell count varied among institutional sites, likely reflecting differences in cohort composition. Despite these institutional differences, it is even more remarkable that we observed reproducible RPFNA measurements from the same breast.
There are several limitations to our present study. First, our multi-institutional cohort is small. Second, we assessed intra-operator variability of RPFNA, but not inter-operator variability. The concordance between RPFNA measurements suggests that single operators in multiple institutions can produce similar results, but does not address whether different operators would have similar findings in the same woman.
Despite the limitations of our study, these data provide important validation of the reproducibility of RPFNA in a multi-institutional cross-sectional study that included cohorts that varied in demographic composition. Important future directions will include a larger RPFNA cohort study and testing for the reproducibility of RPFNA samples with atypia before and after administration of chemoprevention agents.
Currently, we lack agents to prevent estrogen receptor – negative breast cancer. A wealth of targeted inhibitors are undergoing clinical testing for the treatment of estrogen receptor – negative breast cancer. However, in order to effectively test targeted agents for prevention, we first need to develop biomarkers to identify women who have the highest likelihood of response, and then develop strategies to test for response and resistance. Our study provides evidence that RPFNA shows promise to reproducibly test mammary epitehelial cells during adminstration of targeted agents or prevention agents.
Presently, we have few therapeutic options for women who fail to respond to tamoxifen. Low-toxicity targeted agents hold promise for the prevention of estrogen receptor – negative breast cancer. However, emerging evidence suggests that some targeted agents, due to their specificity, may have unanticipated mechanisms of resistance (28). Recently, it was shown that women receiving lapatinib for invasive breast cancer developed resistance via paradoxical activation of quiescent signaling pathways. Suppression of ErbB2-signaling by lapatinib in estrogen receptor – negative breast cancer resulted in activation of FOXO3a and caveolin-1, leading to activation of estrogen receptor signaling (29). This result was unexpected and highlights the importance of directly testing for protein signaling in mammary cytology, rather than relying on indirect markers of response, during administration of targeted agents.
Acknowledgments
Grant support: Primary Support was provided by the Cancer and Leukemia Group B (CALGB) pilot grant and parent grant CA33601 as well as NIH/NCI grants R01CA88799, R01CA98441, and R01CA114068 (V.L. Seewaldt).
Footnotes
Breast Cancer Risk Assessment Tool is available at http://www.cancer.gov/bcrisktool/.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371– 88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727– 41. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann LC, Schaid DJ, Woods JE, et al. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340:77– 84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 4.Rebbeck TR, Levin AM, Eisen A, et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475– 9. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 5.Kelloff GJ, Boone CW, Crowell JA, et al. Risk biomarkers and current strategies in chemoprevention. J Cell Biochem Suppl. 1996;25:1– 14. [PubMed] [Google Scholar]
- 6.Fabian CJ, Kimler BF, Zalles CM, et al. Short-term breast cancer prediction by random periareolar fine-needle aspiration cytology and the Gail risk model. J Natl Cancer Inst. 2000;92:1217– 27. doi: 10.1093/jnci/92.15.1217. [DOI] [PubMed] [Google Scholar]
- 7.Byrne C, Schairer C, Wolfe J, et al. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87:1622– 9. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 8.Jernstrom HC, Olsson H, Borg A. Reduced testosterone, 17 β-oestradiol and sexual hormone binding globulin, and increased insulin-like growth factor-1 concentrations, in healthy nulligravid women aged 19 – 25 years who were first and/or second degree relatives to breast cancer patients. Eur J Cancer Prev. 1997;6:330– 40. doi: 10.1097/00008469-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393– 6. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 10.Allred DC, Hilsenbeck SG. Biomarkers in benign breast tissue disease: risk factors for breast cancer [editorial] J Natl Cancer Inst. 1998;90:1247– 8. doi: 10.1093/jnci/90.17.1247. [DOI] [PubMed] [Google Scholar]
- 11.Marshall LM, Hunter DJ, Connolly JL, et al. Risk of breast cancer associated with atypical hyperplasia of lobular and ductal types. Cancer Epidemiol Biomarkers Prev. 1997;6:297– 301. [PubMed] [Google Scholar]
- 12.Mansoor S, Ip C, Stomper C. Yield of terminal ductal lobule units (TLDU) in normal breast stereotactic core biopsy specimens: implications of biomarker studies. Breast J. 2000;6:220– 4. doi: 10.1046/j.1524-4741.2000.99043.x. [DOI] [PubMed] [Google Scholar]
- 13.Sauter ER, Ross E, Daly M, et al. Nipple aspirate fluid: a promising non-invasive method to identify cellular markers of breast cancer risk. Br J Cancer. 1997;76:494– 501. doi: 10.1038/bjc.1997.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian CJ, Kimler BF, Brady DA, et al. A phase II breast cancer chemoprevention trial of oral alpha-difluoromethylornithine: breast tissue, imaging, and serum and urine biomarkers. Clin Cancer Res. 2002;8:3105– 17. [PubMed] [Google Scholar]
- 15.Khan SA, Masood S, Miller L, Numann PJ. Random fine needle aspiration of the breast of women at increased breast cancer risk and standard risk controls. Breast J. 1998;4:420– 5. [Google Scholar]
- 16.Fabian CJ, Kamel S, Zalles C, Kimler BF. Identification of a chemoprevention cohort from a population of women at high risk for breast cancer. J Cell Biochem Suppl. 1996;25:112– 22. [PubMed] [Google Scholar]
- 17.Khan QJ, Kimler BF, O’Dea AP, Zalles CM, Sharma P, Fabian CJ. Mammographic density does not correlate with Ki-67 expression or cytomorphology in benign breast cells obtained by random periareolar fine needle aspiration from women at high risk for breast cancer. Breast Cancer Res. 2007;9:R35. doi: 10.1186/bcr1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bean GR, Scott V, Yee L, et al. Retinoic acid receptor-β2 promoter methylation in random periareolar fine needle aspiration. Cancer Epidemiol Biomarkers Prev. 2005;14:790– 8. doi: 10.1158/1055-9965.EPI-04-0580. [DOI] [PubMed] [Google Scholar]
- 19.Bean GR, Kimler BF, Seewaldt VL. Long-term raloxifene in a woman at high risk for breast cancer. N Engl J Med. 2006;355:1620– 2. doi: 10.1056/NEJMc061954. [DOI] [PubMed] [Google Scholar]
- 20.Bean GR, Ibarra Drendall C, Goldenberg VK, et al. Hypermethylation of the breast cancer- associated gene 1 promoter does not predict cytologic atypia or correlate with surrogate end points of breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16:50– 6. doi: 10.1158/1055-9965.EPI-06-0598. [DOI] [PubMed] [Google Scholar]
- 21.Goldenberg V, Seewaldt VL, Scott V, et al. Atypia in random periareolar fine needle aspiration affects the decision of high-risk women to take tamoxifen for breast cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2007;16:1032– 43. doi: 10.1158/1055-9965.EPI-06-0910. [DOI] [PubMed] [Google Scholar]
- 22.Seewaldt VL, Goldenberg V, Jones L, et al. Overweight and obese perimenopausal and postmenopausal women exhibit increased abnormal mammary epithelial cytology. Cancer Epidemiol Biomarkers Prev. 2007;16:613– 6. doi: 10.1158/1055-9965.EPI-06-0878. [DOI] [PubMed] [Google Scholar]
- 23.Bean GR, Bryson AD, Pilie PG, et al. Morphologically normal-appearing mammary epithelial cells obtained from high-risk women exhibit methylation silencing of INK4a/ARF. Clin Cancer Res. 2007;13:6834– 41. doi: 10.1158/1078-0432.CCR-07-0407. [DOI] [PubMed] [Google Scholar]
- 24.Baker JC, Ostrander JH, Lem S, et al. ESR1 promoter hypermethylation does not predict atypia in random periareolar fine needle aspiration nor persistent atypia after 12 months tamoxifen chemoprevention. Cancer Epidemiol Biomarkers Prev. 2008;17:1884– 90. doi: 10.1158/1055-9965.EPI-07-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer- susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62:145– 58. doi: 10.1086/301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zalles CM, Kimler BF, Kamel S, et al. Cytology patterns in random aspirates from women at high and low risk for breast cancer. Breast J. 1995;1:343– 9. [Google Scholar]
- 27.Masood S, Frykberg ER, McLellan GL, Scalapino MC, Mitchum DG, Bullard JB. Prospective evaluation of radiologically directed fine-needle aspiration biopsy of nonpalpable breast lesions. Cancer. 1990;66:1480– 7. doi: 10.1002/1097-0142(19901001)66:7<1480::aid-cncr2820660708>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 28.Xia W, Bacus S, Hedge P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795– 800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen FL, Xia W, Spector NL. Acquired resistance to small molecule ErbB2 tyrosine kinase inhibitors. Clin Cancer Res. 2008;14:6730– 4. doi: 10.1158/1078-0432.CCR-08-0581. [DOI] [PubMed] [Google Scholar]