Abstract
Introduction
Steroid receptor coactivator-3 (SRC-3), also called amplified-in-breast cancer-1 (AIB1), is an oncogenic coactivator in endocrine and non-endocrine cancers. Functional studies demonstrate SRC-3 promotes numerous aspects of cancer, through its capacity as a coactivator for nuclear hormone receptors and other transcription factors, and via its ability to control multiple growth pathways simultaneously. Targeting SRC-3 with specific inhibitors therefore holds future promise for clinical cancer therapy.
Areas covered
We discuss critical advances in understanding SRC-3 as a cancer mediator and prospective drug target. We review SRC-3 structure and function and its role in distinct aspects of cancer. In addition, we discuss SRC-3 regulation and degradation. Finally, we comment on a recently discovered SRC-3 small molecular inhibitor.
Expert opinion
Most targeted chemotherapeutic drugs block only a single cellular pathway. In response, cancers frequently acquire resistance by up-regulating alternative pathways. SRC-3 coordinates multiple signaling networks, suggesting SRC-3 inhibition offers a promising therapeutic strategy. Development of an effective SRC-3 inhibitor faces critical challenges. Better understanding of SRC-3 function and interacting partners, in both the nucleus and cytosol, is required for optimized inhibitor development. Ultimately, blockade of SRC-3 oncogenic function may inhibit multiple cancer-related signaling pathways.
Keywords: AIB1, cancer, gene expression, molecular target, nuclear receptor coactivator, SRC-3
1. Introduction
Nuclear hormone receptors (NRs) bind DNA at specific sites to regulate gene expression and impact many physiological processes. Upon binding to its ligand, a NR commonly undergoes a conformational change and forms a dimer. The liganded NR complex then translocates into nucleus, recognizes specific regulatory DNA sequences, and binds response elements upstream of target genes to activate gene transcription. By themselves, NRs cannot initiate optimal transcriptional activation. It is through interaction with coactivator proteins that hormone-activated NRs can direct the assembly and stabilization of a preinitiation complex that ultimately conducts the transcription of the target genes1. The p160 steroid receptor coactivator (SRC) family, consisting of three members (SRC-12/NCOA1, SRC-2/TIF23/GRIP14/NCOA2, and SRC-3/p/CIP5, RAC36, AIB17, ACTR8, TRAM19 and NCOA3), is a key coactivator group for serving as a bridge between the hormone-activated NRs, other co-regulators, and the basal transcriptional machinery. These SRC proteins can also act as coactivators for non-NR transcription factors to regulate target gene transcription and impact multiple growth factor pathways. In general, SRC proteins sit at the nexus of multiple cancer signaling pathways that impact cancer initiation, growth, migration, invasion, metastasis and chemotherapeutic resistance. In this review, we will focus on SRC-3 and discuss the role of SRC-3 in these distinct aspects of carcinogenesis. We will then evaluate the advantages and feasibility of designing SRC-3 inhibitors as therapeutic agents for cancer.
2. Steroid receptor coactivator-3 (SRC-3)
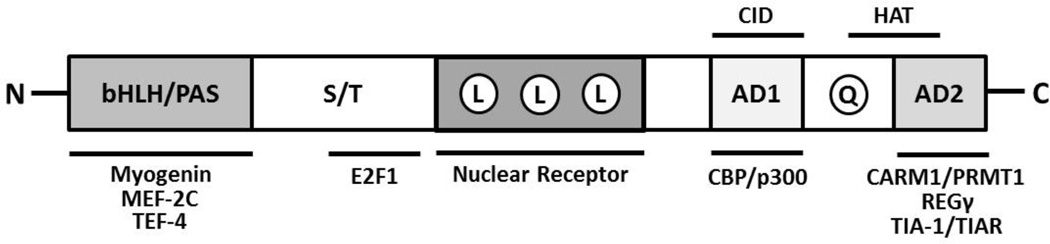
SRC-3 was initially found to be amplified and overexpressed in human breast cancer cell lines and a subset of breast tumors10. AIB1 (SRC-3) was identified during a search on the long arm of chromosome 20 for genes with overexpression and increased copy number in breast cancers10. Serving as an adapter to recruit chromatin remodeling proteins and other transcriptional enzymes, SRC-3 is known to mediate transcriptional activities of NRs such as ER (estrogen receptor) and PR (progesterone receptor). SRC-3 was found to promote hormone-dependent growth of human MCF-7 breast cancer cells by coactivating ERα and PRβ11. SRC-3 also potentiates the transcriptional activities of other TFs (transcription factors) such as E2F-1, PEA3, AP-1 and NF-κB (nuclear factor-kappa B)12–15. SRC-3 shares a common structure with the other members of the p160 family, containing domains with well-studied functional relevance. The structural and functional domains of SRC-3 are summarized in Figure 1. The N-terminal region is the most conserved among p160 family members and consists of bHLH (basic helix-loop-helix) region and a PAS (Per/ARNT/Sim) motif16, 17. This region is necessary for several protein-protein interactions, including association with TFs such as myogenin and MEF2C18–20. In addition, the bHLH-PAS region contains multiple nuclear localization signals21. A region between the bHLH-PAS domain has been linked to the turnover and degradation of SRC-322. The central region of the SRC proteins contains three LXXLL (L, Leucine; X, any amino acid) motifs which form amphipathic α-helices and are essential for direct interactions with NRs in a ligand-dependent manner23–26. Finally, the C-terminal region contains two transcriptional activation domains (AD1 and AD2). The AD1 domain directly binds to CBP (CREB-binding protein) and p300 proteins27. The recruitment of CBP/p300 to the chromatin by SRC proteins results in histone acetylation necessary for SRC-mediated transcriptional activation. The AD2 domain interacts with histone methyltransferases such as CARM1 (co-activator-associated arginine methyltransferase 1) and PRMT1 (protein arginine N-methyltransferase 1) to promote histone methylation and subsequently facilitate chromatin remodeling28, 29. Interestingly, the C termini of SRC-1 and SRC-3 also contain HAT (histone acetyltransferase) activity30. These structural elements allow SRC proteins to provide a platform through which transcription factors can interact with additional coregulators that promote chromatin remodeling and assembly of general transcription machinery. In sum, their structure underlies the ability of SRC proteins to coordinate signals from a myriad of cellular signaling pathways in regulating gene expression output.
Figure 1. Structural and Functional Domains of SRC-3 protein.
The letters within the bar figure indicates functional domains. bHLH, basic helix-loop-helix domain; PAS, Per/ARNT/Sim homologous domain; S/T, serine/threonine-rich region; L, LXLL α -helix motif; AD1, Activation Domain 1; Q, glutamine-rich domain; AD2, Activation Domain 2. AD1 contains CID (CBP/p300 interacting domain) and AD2 contains HAT (histone acetyltransferase domains). The proteins listed below the bar figure are known to interact with SRC-3. This list is incomplete.
SRC proteins exist at limited concentrations in normal physiology. They are considered “master regulators” of differential gene expression and accomplish this through combinatorial codes of post-translational modifications (PTMs). Known SRC-3 PTMs, the responsible modifying proteins, and the modified sites are listed in Table 1. Extracellular stimuli such as hormones, growth factors and cytokines activate signaling pathways that may post-translationally modify SRCs through phosphorylation, ubiquitylation, sumoylation etc. The combinatorial codes of PTMs determine protein stability, interaction specificity and transcriptional activity of SRCs. Deregulation of these PTMs has a significant impact on cellular physiology and results in human diseases such as cancer. Early observation that SRC-3 localization and transcriptional activity could be regulated by IKKβ (IκB kinase β) phosphorylation provided the initial clue that SRC-3 is subject to PTM31. SRC-3 contains at least eight specific phosphorylation sites32. Seven Serine/Threonine (Thr24, Ser 505, Ser 543, Ser 601, Ser 857, Ser 860 and Ser 867) phosphorylation sites and one Tyr (Tyr 1357) phosphorylation site have been demonstrated to be functionally important33, 34. These sites are phosphorylated by a number of different kinases including MAPK, IKK, GSK3α, GSK3β, and CK1d. SRC-3 is also a target of ABL tyrosine kinase which can be activated by estrogen and growth factors. Phosphorylation of Tyr1357 on SRC-3 by ABL tyrosine kinase increases binding of SRC-3 to p300 and transcription factors, thus mediating ER, PR and NF-κB-dependent transcription activities34. Furthermore, Tyr1357 phosphorylation has been shown to increase in ERBB2-induced breast tumors in mice, suggesting an oncogenic function of SRC-3 with Tyr 1357 phosphorylation. In conclusion, phosphorylation can convert inactive SRC-3 into a potent transcriptional coactivator, resulting in differential gene expression with relevance to the development of cancer.
Table 1.
SRC-3 is subject to multiple post-translational modifications
| PTM | SRC-3 Modifier | Effect on SRC-3 | Modified Residue |
Ref |
|---|---|---|---|---|
| Acetylation | CBP/p300 | Inactivation | K626, K629, K630 | 89 |
| Dephosphorylation | PP2A | Inactivation | S505, S543 | 22 |
| PP1 | Stabilization | S101, S102 | 22 | |
| Methylation | CARM1 | Inactivation, then Degradation | R1171 | 90 |
| Phosphorylation | JNK | Activation | T24, S505, S543, S860, S867 | 32 |
| ERK | Activation | S505, S543 | 32 | |
| p38MAPK | Activation | T24, S505, S543, S860, S867 | 32 | |
| PKA | Activation | S857 | 32 | |
| c-Abl | Activation | Y1357 | 34 | |
| IKK | Activation | S857 | 32 | |
| GSK3β | Activation, then Degradation | S505 | 77 | |
| aPKC | Stabilization | S/T sites in a.a. 1031–1130 region | 91 | |
| Ubiquitinylation | E6-AP | Degradation | Unknown | 78 |
| SCFFbw7α | Degradation | K723, K786 | 77 | |
| CUL-3 and RBX1 | Degradation | Unknown | 80 | |
| CHIP | Degradation | Unknown | 81 | |
| SPOP | Degradation | N-terminal | 83 | |
| SUMOylation | SUMO-1 | Inactivation | K731 | 92 |
Notes for Table 1: Created by proteins from multiple intracellular signaling pathways, these modifications constitute a combinatorial code through which SRC-3 can integrate pathway-specific information to coordinate cellular outcomes. CBP, CREB-binding protein; PP2A, protein phosphatase 2A; PP1, protein phosphatase 1; CARM1, coactivator-associated arginine methyltransferase 1; JNK, c-Jun N-terminal kinase; ERK, extracellular-signal-regulated kinase; p38MAPK, p38 mitogen-activated protein kinases; PKA, protein kinase A; c-Abl, Abelson tyrosine kinase; GSK3β, glycogen synthase kinase 3; IKK, IκB kinase; aPKC, atypical protein kinase C; E6-AP, E6-associated protein; SCF, SKP1–cullin-1–F-box; Fbw7a, F-box and WD repeat domain-containing 7; CUL-3, Cullin-3; RBX1, RING box protein 1; CHIP, carboxyl terminus of Hsc70-interacting protein; SPOP, speckle-type POZ protein.
3. SRC-3 impacts multiple axes in cancer
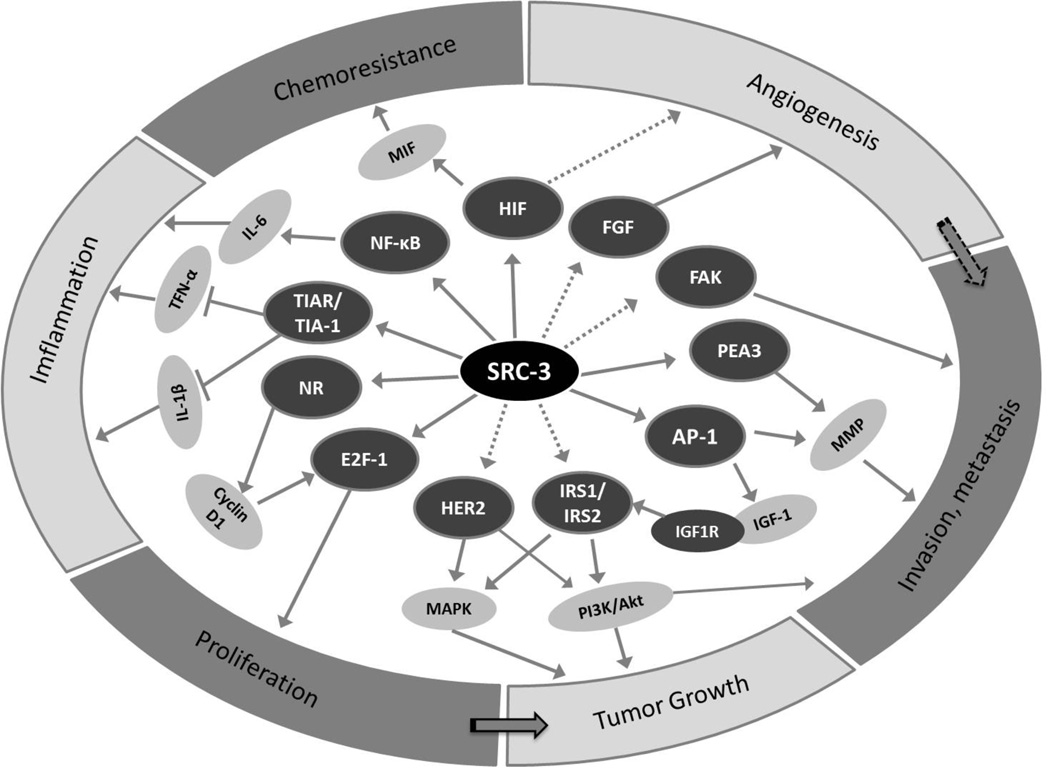
The role of SRC-3 as a master regulator in cellular growth and development places it at the nexus of several intracellular signaling pathways critical for cancer (Figure 2). As SRC-3 amplification and overexpression have been correlated in multiple clinical studies with tumor aggressiveness or poor patient outcome (Table 2), it has become imperative to perform detailed mechanistic studies on the role of SRC-3 in tumorigenesis and cancer metastasis.
Figure 2. SRC-3 integrates multiple signaling pathways.
Depending on the cellular context, SRC-3 can coactivate different nuclear receptors and transcription factors to promote multiple hallmarks of cancer. SRC-3 classically facilitates the transcriptional activities of nuclear receptors to promote cell growth. It also coactivates E2F-1 to promote cell division. To impact on tumor expansion, SRC-3 serves as a coactivator for AP1 to upregulate IGF-Akt pathway and may be involved in HER2 pathway. SRC-3 also acts as a pro-metastatic factor by coactivating PEA3 and AP1 to upregulate MMP production, a requisite process in extracellular matrix breakdown that accompanies invasive tumor behavior. A spliced SRC-3 isoform located in cytoplasm can also contribute to metastasis by serving as an adaptor for FAK-Src signal pathway. SRC-3 has been implied to bind NFκB and upregulate MIF through the HIF transcription factor. This, in turn, contributes to chemoresistance, as well as angiogenesis through the FGF signaling pathway. NR, nuclear receptor such as ER, PR or AR. The solid arrow indicates direct interaction or known pathways while the dotted arrow indicates indirect interaction.
Table 2.
SRC-3 overexpression and amplification are associated with aggressive tumor phenotype or poor prognosis in human carcinomas of endocrine and non-endocrine organs.
| Cancer type | Change | Detection | Frequency | Clinical Associations | Ref |
|---|---|---|---|---|---|
| Endocrine Carcinomas | |||||
| Breast | Gene amplification | FISH | 9.50% | Undetermined | 10 |
| mRNA Overexpression | ISH | 64% | Positive correlation between expression and tumor size | 10 | |
| Protein Overexpression | WB | 25% | Positive correlation between expression and tamoxifen resistance | 62 | |
| Protein Overexpression | WB | 25% | Highly correlated with proliferation and expression of ERBB2 and PR | 62 | |
| Protein Overexpression | IHC | 73.8% | Positive correlation between expression and MMP2, MMP9, and PEA3 protein levels | 13 | |
| Prostate | mRNA Overexpression | ISH | ND | Positive correlation between expression and tumor grade | 93 |
| Protein Overexpression | IHC | ND | Positive correlation between expression and tumor grade | 93, 94 | |
| Endometrial | mRNA Overexpression | qPCR | 17% | Undetermined | 95 |
| Protein Overexpression | IHC | 97% | Associated with poor prognosis | 96 | |
| Ovarian | Gene Amplification | FISH | 25% | Associated with DNA amplification at 20q12-q13 | 97 |
| Protein Overexpression | IHC | 64% | expression of SRC-3 positively correlates with invasiveness of tumor | 44 | |
| Non-Endocrine Carcinomas | |||||
| Esophageal squamous cell carcinoma | Gene Amplification | FISH | 4.3–4.9% | Associated with chromosome 20q amplification and increased copy number | 98, 99 |
| Protein Overexpression | IHC | 46% | Associated with large tumor size and Ki67 proliferation staining | 99 | |
| Gastric | Gene Amplification | FISH | 7% | Amplification positively correlated with metastasis | 100 |
| mRNA Overexpression | Northern, qPCR | 40% | 100 | ||
| Colorectal | Gene Amplification | FISH | 10% | Nuclear expression positively correlated with metastasis | 101 |
| mRNA Overexpression | IHC | 35% | 101 | ||
| Pancreatic | Gene Amplification | FISH | 37% | Undetermined | 102 |
| mRNA Overexpression | ISH | >65% | 102 | ||
| Protein Overexpression | IHC | >65% | 102 | ||
| Hepatocellular | Gene Amplification | FISH | 25% | Undetermined | 103 |
| Protein Overexpression | WB | 68% | Undetermined | 104 | |
| Urothelial | Gene Amplification | FISH | 7% | Undetermined | 105 |
| Protein Overexpression | IHC | 33% | Overexpression positively correlated with Ki67 proliferation staining | 105 | |
| Nasopharyngeal | Gene Amplification | FISH | 7% | Overexpression positively associated with large tumor and Ki67 index | 106 |
| Protein Overexpression | IHC | 51% | Undetermined | 106 |
Notes for Table 2: FISH, fluorescence in situ hybridization; ISH, in situ hybridization; WB, western blot; IHC, immunohistochemistry; qPCR, quantitative PCR.
3.1 SRC-3 in cancer initiation and tumorigenesis
The proliferative role of SRC-3 in primary tumor formation has been extensively studied. Many mouse models have been employed to investigate the function of SRC-3 in cancer, particularly breast cancer. In transgenic mouse models where overexpression of SRC-3 in mammary epithelial cells was driven by MMTV (mouse mammary tumor virus) promoter, mammary hyperplasia and spontaneous development of mammary tumors were observed, directly supporting the role of SRC-3 in breast cancer initiation35. Hyperactive IGF-1 signaling was found in these MMTV-SRC-3 transgenic mice35. Consistent with results in this model, when SRC-3 knockout mice were crossed with MMTV-v-ras transgenic mice, mammary tumor incidence and growth rate were reduced dramatically in SRC-3−/−;MMTV-v-ras bigenic virgin mice and inhibited completely in ovariectomized SRC-3−/−;MMTV-v-ras bigenic mice36. Further molecular analysis indicated SRC-3 deficiency did not alter estrogen and progesterone-responsive gene levels, but decreased IRS-1 (insulin receptor substrate-1) and IRS-2, resulting in impaired IGF-1 signaling pathway36. Along the same lines, ablation of SRC-3 in mice treated with chemical carcinogen DMBA (7,12-dimethylbenz[α]anthracene) protected against mammary gland tumorigenesis37. Another mouse model was used to assess the impact of SRC-3 ablation on MMTV-Erbb2 (erythroblastosis oncogene B 2)-induced mammary tumors. Knockout of SRC-3 in MMTV-Erbb2 mice completely suppressed tumorigenesis and reduced levels of phosphorylated ERBB2, cyclin D1 and cyclin E38. All these findings suggest that SRC-3 plays an important role in tumor initiation and growth and that targeting SRC-3 can effectively suppress tumor initiation and growth.
In addition to the full-length SRC-3, a splice variant of SRC-3 (SRC-3delta4) which lacks the N-terminal bHLH-PAS domain has been identified and found to be an even more potent coactivator for ER and PR than the full-length SRC-339. Transgenic mice overexpressing SRC3delta4 develop mammary gland hyperplasia, increased cyclin D1 expression, and increased IGF-1 receptor level40. Furthermore, combined overexpression of SRC3delta4 and ERα in mouse mammary gland resulted in increased incidence of hyperplasia and adenocarcinoma with increased stromal collagen deposition41. SRC-3delta4 also plays an important role in cancer metastasis and its function will be discussed in detail in a later section.
Besides breast cancer, SRC-3 was also found to be important for tumorigenesis in the prostate. SRC-3 knockout mice were crossed with TRAMP (transgenic adenocarcinoma mouse prostate mice), a mouse prostate cancer model. Total ablation of SRC-3 in TRAMP mice arrested prostate tumor growth at well-differentiated stages. Even though initiation of prostate tumors in this mouse model was not delayed, the progression of prostate tumorigenesis substantially declined42.
3.2 SRC-3 in cell motility, invasion, and metastasis
In order for metastasis to manifest, cancer cells need to gain motility and invasive potential that will allow them to escape the primary tumor site, invade surrounding stroma and enter the blood stream. The first evidence that SRC-3 plays a role in cell migration and invasion comes from the studies of fruit fly ovary43. In the absence of Taiman, the Drosophila homolog of SRC-3, ecdysone receptor-dependent border cell motility and invasiveness were markedly suppressed and there was an abnormal cellular build-up of E-cadherin, β-catenin, and focal adhesion complexes43. Subsequently, in human ovarian cancer cells, SRC-3 was demonstrated to be important for cellular spreading migration on the substratum44. The most relevant in vivo study of SRC-3 function in metastasis originates from MMTV-polyoma middle T antigen (PyMT) transgenic mouse model. Absence of SRC-3 in PyMT transgenic breast cancer mouse model significantly suppressed mammary tumor metastasis to the lung13. Molecular studies showed that SRC-3 could impact the expression levels of MMPs (matrix metalloproteinases) that allow tumor cells to break down the extracellular matrix and invade into stromal compartment. In both human (MDA-MB-231) and PyMT tumor cells in culture, SRC-3 regulates MMP2 and MMP9 by directly binding and potentiating activity of the PEA3 transcription factor13. SRC-3 also serves as a coactivator for AP-1 (activator protein 1) to drive expression of MMP7 and MMP10 in MDA-MC-231 human breast cancer cells45. Furthermore, in prostate cancer, SRC-3 simultaneously coactivates AP-1 and PEA3 to upregulate expression of MMP2 and MMP1315. By serving as a coactivator for a number of TFs responsible for the expression of MMP family members, nuclear SRC-3 promotes invasion of cancer cells into the surrounding stromal compartment. Recently, in a lung cancer cell line, ERK3 was shown to phosphorylate SRC-3 at S857, a modification essential for the binding of SRC-3 with PEA3 and promotion of MMP gene expression46. In sum, SRC-3 clearly promotes cancer invasion by coactivating non-nuclear receptor TFs to regulate MMP gene expression.
SRC-3delta4 also has been implicated in promotion of metastasis. SRC-3delta4 is mainly sequestered in the cytosol and acts as a signal adaptor for EGFR (epidermal growth factor receptor) and FAK (focal adhesion kinase-1) at the plasma membrane47. EGF is a critical mediator for cancer cell migration and metastasis48. Extracellular EGF binds EGFR on the cell membrane and activates a number of intracellular protein kinases including PAK1 (p21-activated kinase 1)49, FAK50 and c-Src51 in a cascade signaling fashion. SRC-3delta4 serves as a bridge between EGFR and FAK to allow optimal activation of EGF-FAK-cSrc signal transduction47. Activation of this signal pathway promotes the movement and invasion of cancer cells.
3.3 SRC-3 in inflammation and angiogenesis
Persistent inflammation is a characteristic of the tumor microenvironment and is recognized as a hallmark of cancer. Inflammation can promote proliferation and survival of cancer cells, facilitate angiogenesis and metastasis, destabilize adaptive immunity, and reduce response to hormone therapy and chemotherapy52. As in other inflammatory contexts, accumulating evidence shows NF-κB is a key mediator of tumor inflammation. SRC-3 has been shown to interact with and coactivate NF-κB in HeLa cancer cells14. In response to TNF-α(tumor necrosis factorα), SRC-3 is phosphorylated by IKK in cytosol of HeLa cells31. SRC-3 translocates along with NF-κB into the nucleus where, aided by SRC-3, NF-κB can bind promoters of target genes and promote the initiation of inflammatory responses. One important NF-κB target gene is IL-6 (interleukin-6), a pro-inflammatory cytokine that plays an important role in tumor metastasis and inflammation53. IL-6 is elevated in prostate cancer tissues and acts as an autocrine growth factor in prostate cancer54, 55. This suggests SRC-3 can serve as a coactivator for NF-κB to promote inflammation in cancer. Demonstrating the context specificity of inflammatory responses, SRC-3 knockout mice are actually more susceptible to acute inflammatory responses than controls56. SRC-3 knockout macrophages are more sensitive to LPS (lipopolysaccharide)-induced endotoxic shock and they produce more proinflammatory cytokines. In these cells, SRC-3 was demonstrated to bind translational repressors TIA-1 (T cell intracellular antigen-1) and TIAR (TIA1-related protein) to inhibit translation of TNF-α, IL-6, and IL-156. In addition, SRC-3 is required for clearing bacteria, and represses inflammatory response in E. Coli-induced septic peritonitis57. These findings suggest that the role of SRC-3 in NF-κB-mediated cytokine expression may be specific to cell types.
Angiogenesis is another important process in cancer progression because the growth of a tumor relies on a sufficient blood supply. Many studies have been focused on investigating the function of SRC-3 in growth factor signaling, with the main focus on cell-autonomous regulation of proliferation and invasive capacity. However, less is known about the SRC-3 function in stroma. A recent study elucidates the role of SRC-3 in angiogenesis and wound healing58. SRC-3 was shown to promote proliferation and motility of endothelial cells, such that neoangiogenesis was dependent on the presence of SRC-358. The study also demonstrated that both alleles of SRC-3 were required for proper wound healing in vivo and that SRC-3 may cross-talk with FGF (fibroblast growth factor) signaling to regulate wound healing process58.
The tumor microenvironment plays a critical role in cancer progression. By immunohistochemistry, SRC-3 protein expression is found in stromal compartment. However, the in vivo function of SRC-3 in tumor microenvironment has not been clearly defined due to lack of appropriate models. Future investigation of SRC-3 in tumor microenvironment can be aided by the generation of mice with floxed SRC-3 alleles59, so that SRC-3 may be deleted in specific cell types with relevance to these novel functions.
3.4 SRC-3 in endocrine therapy-resistant cancer and chemoresistant cancer
SRC-3 has been implicated in the development of resistance to chemotherapeutic agents. Tamoxifen is an antagonist that competes with estrogen for binding to ER, resulting in the inhibition of ER-mediated transcription and thus estrogen dependent cancer growth. Tamoxifen has been the standard endocrine therapy for women with ER-positive breast cancer. However, only 50% of ER-positive breast cancer patients respond to tamoxifen therapy60. Other patients treated with tamoxifen for long periods tend to acquire resistance to the therapy. Resistance to endocrine therapy often has been associated with activation of growth factor signaling pathways such as EGFR pathway61. There is a positive correlation between SRC-3 protein expression and the levels of HER family proteins in the breast cancer patients with recurrence after tamoxifen treatment62. Recently, it was demonstrated PAX2 (paired box gene 2) competes with SRC-3 for binding and regulation of HER2 transcription. High SRC-3 expression was associated with high recurrence rate in patients with ER-positive tumors and treated with tamoxifen63. Another class of endocrine therapeutic agent, aromatase inhibitors, acts by blocking conversion of testosterone and androstenedione into estrogen. Aromatase inhibitors are used to treat postmenopausal women with ERα-positive breast cancer. However, breast tumors with high HER2 and SRC-3 expression may also develop resistance to aromatase inhibitors, as its family member SRC-1 does64.
Bortezomib (PS-314 or Velcade) is a proteasome inhibitor that has anti-cancer activity in various cancer cell lines including prostate cancer cell and prostate cancer xenograft models. In a neoadjuvant clinical trial of bortezomib in men with prostate cancer at high risk of recurrence, unexpected increase in proliferation in treated tissues and cultured cells was found65. In these treated tissues and cell lines, SRC-3 level and phosphorylated Akt level were found to be increased. Knockdown of SRC-3 decreased the level of the phosphorylated Akt. These data suggest that SRC-3 may contribute to chemo-resistant prostate cancer65.
A recent paper identified MIF (macrophage migration inhibitory factor) as a new target gene of SRC-3 and demonstrated MIF is a suppressor of autophagic cell death66. Upregulation of MIF expression by SRC-3 in cancer cells can contribute to chemoresistance. Inhibition of MIF expression can sensitive cancer cells to anti-cancer drugs such as doxorubicin and etoposide66.
4. SRC-3 in development, metabolism and other physiological process
Genetically engineered mouse models have been employed to study the physiological relevance of steroid receptor coregulators. Much of our understanding about SRC-3 function in vivo stems from characterization of SRC-3 knockout mice. Targeted deletion of SRC-3 in mice has revealed its critical role for normal somatic growth, mammary gland development and female reproduction67, 68. Circulating IGF-1(insulin-like growth factor-1) level was found to be significantly reduced in SRC-3 knockout mice69. All three members of SRC family play a critical role in metabolic regulation. In particular, loss of SRC-3 impairs white adipogenic differentiation through decreased PPARγ2 (peroxisome proliferator-activated receptor-γ activity)70. SRC-3 knockout mice are resistant to high-fat diet-induced obesity and have improved insulin sensitivity71. The phenotype was partly due to the regulation of PGC-1 (peroxisome proliferator-activated receptor-γ coactivator-1) acetylation by SRC-371. Furthermore, a knock-in mouse model with mutations at four conserved phosphorylation sites displayed increased body weight and adiposity, and reduced peripheral insulin sensitivity. These mice were also more susceptible to carcinogen-induced liver tumorigenesis. These results support the idea that PTMs are important for the normal function of SRC-3 and that changes in PTMs are sufficient to alter glucose homeostasis and cancer susceptibility72. Because all cancer cells rely on changes in metabolism to support growth and survive, targeting metabolism for anti-cancer therapy has been a recent focus in cancer research. Owning to its close relevance to metabolism, the exploration on the function of SRC-3 in the regulation cancer metabolism might provide some insights into successful cancer therapy.
5. Regulation of SRC-3 mRNA/protein levels
SRC-3 expression and protein amount can be regulated at three different levels: Transcription, translation and protein degradation. SRC-3 is a coactivator for E2F-112, 73 and SRC-3 gene contains E2F-1 binding sites at its promoter region suggesting SRC-3 can self-regulate and form a positive feedback loop for its own expression12. Another binding site on the SRC-3 promoter region is for the SP1 (Specificity Protein 1) transcription factor. Interestingly, E2F-1-dependent transcription of SRC-3 did not require E2F-1 binding to its binding site but rather the binding of SP1 to the SP1 binding site in a proximal SRC-3 promoter region. At translational level, SRC-3 can be regulated by miRNAs (microRNAs). Endogenous miRNAs bind on site-specific sequences within the 3-untranslated regions and inhibit the translation. miRNA Mir-17-5p was found to specifically inhibit the translation of SRC-3 mRNA. In breast cancer cell lines with high levels of SRC-3 protein, Mir-17-5p was found to be at low levels 74.
Specific post translational modifications such as phosphorylation and methylation serve as a code that mediates SRC-3 interaction, function and degradation75. SRC-3 protein turnover is mediated by proteasomal degradation pathways76 and the NLS (nuclear localization signal) within the bHLH domain of SRC-3 is critical for this proteasome-dependent turnover 21. In the ubiquitin-dependent proteasome degradation, ubiquitin molecules are linked to the target proteins by E3 ligases. The ubiquitinylated proteins are then degraded by the 26S proteasome in an ATP –dependent manner. Both SCFFbw7α and E6-AP are examples of E3 ligase that can interact with SRC-3, targeting SRC-3 for degradation77, 78. REGγ, 20S proteasome regulator, can also interact with SRC-3 and mediates its turnover in an ubiquitin-independent manner79. Recently, the components of E3 ligase, CUL1 and RBX1, were shown to be involved in SRC-3 ubiquitinylation and degradation, in response to retinoid acid treatment80. CHIP (carboxyl terminus of Hsc70-interacting protein) is a U-box-type ubiquitin ligase that induces ubiquitinylation and degradation of its substrates. SRC-3 was also found to be a target of CHIP and knockdown of SRC-3 reduces Smad and Twist expression81. In human hepatocellular carcinoma, Hepatitis B virus X protein (HBx) stabilizes SRC-3 so SRC-3 cannot be targeted for degradation by E3 ligase82. Recently, speckle-type POZ protein (SPOP), a cullin 3 (CUL3)-based ubiquitin ligase, was found to promote SRC-3 ubiquitinylation and degradation83. Interestingly, loss-of-function mutations of SPOP were identified in 6–13% of human prostate cancers that do not contain PTEN mutation or TMPRSS2:ERG fusion rearrangement84. In addition, a recent study of exome sequencing in 112 human prostate tumor and normal tissue pairs identified SPOP as one of the most frequently mutated gene in 13% of prostate tumors and all the SPOP mutation affected in the structure that involves in substrate-binding function85. Thus, it would be interesting to find out whether SPOP mutation is associated with SRC-3 protein elevation and human prostate carcinogenesis.
6. Development of an SRC-3 inhibitor
A specific SRC-3 inhibitor has not yet been generated. This is largely due to an incomplete understanding of the protein’s structure and lack of crystallography data. By employing high throughput screening assays, the O’Malley lab recently identified gossypol as a small molecule inhibitor of SRC-1 and SRC-386. Gossypol, a compound derived from the cotton plant, has been shown to partially inhibit SRC-3 function in cell culture, stemming the growth of cancerous but not non-cancerous cells. Gossypol selectively reduces SRC-1 and SRC-3 protein levels in cancer cell lines including breast, prostate, lung, and liver cells. In fact, gossypol could reduce cell viability while also sensitizing lung and breast cancer cell lines to other chemotherapeutic agents such as MEK (MAPK kinase) inhibitor and EGFR inhibitors. Identified as an inhibitor for Bcl-2, gossypol was already demonstrated to be a proapoptotic agent for cancer cells and is currently being evaluated as a therapeutic agent for prostate cancer and lung cancer in clinical trials87, 88. O’Malley’s group identified that gossypol could directly bind to SRC-3 and reduce its protein levels without affecting its mRNA level. They also demonstrated gossypol could reduce mRNA levels of Bcl2. To further explain, genomic study of SRC-3 cistrome in MCF-7 identified several SRC-3 binding sites within Bcl-2 and Bcl-X genes. Knockdown of SRC-3 in MCF-7 cells decreased both Bcl-2 mRNA and protein levels. These findings further support that the anti-cancer mechanism of gossypol is through downregulation of SRC-3 protein levels, impairing anti-apoptotic pathways in cancer cells.
In pre-clinical and clinical studies, gossypol has had mixed results as a chemotherapeutic agent in the treatment of serious malignancies like small cell lung and adrenal cancers. Although gossypol has its own weaknesses including in vivo toxicity to serve as an effective therapeutic cancer drug in human87, 88, the identification of gossypol to be a small molecule inhibitor of SRC-1 and SRC-3 is a proof-of-principle study that oncogenic coactivators can be directly targeted for inhibiting cancer growth. Taken together, gossypol studies suggest direct inhibition of SRC-3 by small molecular inhibitors is possible and has specific impact on cancerous cells. Nonetheless, the failure to achieve near complete SRC-3 inhibition, even in culture, indicates we do not yet understand the full potential for SRC-3 inhibition in modulating disease. Given promising results of SRC-3 knockout in animal models, it will be critical to search for improved inhibitors whose efficacy against real tumors can be evaluated.
7. Expert opinion
Most chemotherapeutic drugs have been designed to target one particular growth factor pathway. For examples, tamoxifen is an antagonist for ERα in breast cancer while Herceptin and Lapatinib target to inhibit ERBB2. Even though these drugs are effective initially, cancer cells eventually upregulate different growth factor pathways to acquire resistance. Therefore, it is important to rationally design a drug that targets multiple pathways simultaneously. SRC-3 is a potential therapeutic target that impacts multiple growth pathways. SRC-3, like its family members, has been shown to play important roles in many aspects of cancer. SRC-3 is an oncogene in that its overexpression is associated with cancer initiation, progression, invasion, metastasis, and chemoresistance. Detailed knowledge of transcription regulation mediated by SRC-3 has been acquired through many in vitro studies, while the functional relevance of SRC-3 in multiple cancer pathways has been illustrated in vivo. It is well established that SRC-3 functions as a coactivator for NR and promotes NR-dependent cell proliferation. SRC-3 can also affect NR-independent cancer motility and invasion by serving as a coregulator for other TFs, such as E2F-1, AP1 and PEA3. Functions of SRC-3 outside its capacity as a transcription regulator have also been demonstrated. SRC-3 was identified as a translational corepressor for TIA-1/TIAR, which inhibits the production of pro-inflammatory cytokines, while SRC-3delta 4 interacts with EGFR and FAK1 to regulate cell invasion and migration. Together, these findings suggest SRC-3 functions to promote many aspects of carcinogenesis and impact multiple cancer pathways (Figure 2). Furthermore, SRC-3 knockout mice have elucidated the physiological relevance of SRC-3 in multiple cancers and served as models for preclinical trials of SRC-3 inhibition. Genetic ablation of SRC-3 in both breast and prostate cancer mouse models inhibits tumorigenesis and blocks metastasis. Given the fact that some SRC-3 knockout mice survived through embryonic and neonatal stages and all survived beyond these early stages have a nearly normal life span, specific inhibition of SRC-3 function may be an ideal approach to control cancer growth without severe side effect.
Ideal therapeutic agents should selectively kill tumor cells while sparing surrounding normal cells. SRC-3 is present at limiting concentration in normal cells and overexpressed in cancer cells. Overexpression of SRC-3 in cancer cells provides a growth advantage, such that cancer cells become “addicted” to SRC-3. Therefore, an SRC-3 inhibitor can theoretically target cancer cells to a greater degree than normal cells. Inhibition of SRC-3 may upregulate cytokine production in some cells of the innate immune, rendering increased risk of cytokine storm56. While altered immunity is a potentially serious side effect, the normal viability and health of SRC-3 knockout mice suggests SRC-3 inhibition may be relatively safe.
Many important questions remain to be addressed. First, SRC-3 functions outside transcriptional regulation appear multiple, but remain poorly understood. Second, many in vivo studies of SRC-3 have been carried out in SRC-3 total knockout mice. However, the roles of SRC-3 in specific cell types such as epithelial vs. stromal cells remain to be investigated. It is important to utilize conditional knockout mice to study cell-specific functions of SRC-3 in carcinogenesis. Temporal deletion of overexpressed SRC-3 in mouse models may also provide insights as the stages of cancer progression for which SRC-3 is most relevant. In addition, clearly mapping genes regulated by SRC-3 is important. Even though a small molecule inhibitor of SRC-3 has been identified, better and improved inhibitors for SRC-3 are still necessary. To develop such inhibitors, a crystal structure of SRC-3 protein may be advantageous (although analysis of large proteins like SRC-3 in this manner is extremely challenging). Other detailed research on the regulation of SRC-3 function and degradation may also provide insights for rational design of SRC-3 inhibitors.
The ultimate goal of future research is to block SRC-3 oncogenic function and inhibit multiple cancer-related signaling pathways. However, better knowledge of structure, interaction partners and the manner in which these interaction partners change during cancer progression will be important areas of research.
Article Hightlights Box.
SRC-3 has been linked to various aspects of carcinogenesis ranging from cancer initiation, progression, cell motility and invasion, inflammation, angiogenesis and resistance to chemotherapy.
SRC-3 can undergo post-translational modification (PTM) and PTM is responsible for its diverse functions.
SRC-3 promotes cancer by activating nuclear receptors (NRs) such as ER and AR and facilitating transcription of multiple transcription factors. Thus SRC-3 sits at the nexus of multiple cellular pathways to promote cancer.
SRC-3delta4, a splicing isoform of SRC-3, has been identified to play an important role in cell invasion and metastasis.
SRC-3 can be regulated at the mRNA level by transcriptional regulation and microRNA and at protein levels by protein degradation pathways. Knowledge of SRC-3 regulation provides insights into designing small molecules for inhibiting SRC-3 function.
Gossypol was recently identified to be an SRC-3 inhibitor that can degrade SRC-3 at protein level.
Acknowledgements
We apologize to those investigators whose studies were relevant to SRC-3 but could not be cited in this mini-review article because of our limited space or knowledge. This study is partially supported by the grant RP121051-P4 funded by Cancer Prevention and Research Institute of Texas and the grants R01CA112403 and R01DK058242 funded by National Institutes of Health. Ms. Tien is a recipient of the prostate cancer predoctoral training award (W81XWH11-1-0194) funded by US Department of Defense.
References
- 1.McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinology. 2002 Jul;143(7):2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- 2.Onate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995 Nov 24;270(5240):1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 3.Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996 Jul 15;15(14):3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 4.Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997 May;17(5):2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, et al. The transcriptional coactivator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997 Jun 12;387(6634):677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Gomes PJ, Chen JD. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan XY, Xu J, Anzick SL, Zhang H, Trent JM, Meltzer PS. Hybrid selection of transcribed sequences from microdissected DNA: isolation of genes within amplified region at 20q11-q13.2 in breast cancer. Cancer Res. 1996 Aug 1;56(15):3446–3450. [PubMed] [Google Scholar]
- 8.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997 Aug 8;90(3):569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita A, Cardona GR, Koibuchi N, Suen CS, Chin WW. TRAM-1, A novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivator-1. J Biol Chem. 1997 Oct 31;272(44):27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 10.Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, et al. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997 Aug 15;277(5328):965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 11.List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem. 2001 Jun 29;276(26):23763–23768. doi: 10.1074/jbc.M102397200. [DOI] [PubMed] [Google Scholar]
- 12.Mussi P, Yu C, O'Malley BW, Xu J. Stimulation of steroid receptor coactivator-3 (SRC-3) gene overexpression by a positive regulatory loop of E2F1 and SRC-3. Mol Endocrinol. 2006 Dec;20(12):3105–3119. doi: 10.1210/me.2005-0522. [DOI] [PubMed] [Google Scholar]
- 13.Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008 Oct;28(19):5937–5950. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werbajh S, Nojek I, Lanz R, Costas MA. RAC-3 is a NF-kappa B coactivator. FEBS Lett. 2000 Nov 24;485(2–3):195–199. doi: 10.1016/s0014-5793(00)02223-7. [DOI] [PubMed] [Google Scholar]
- 15.Yan J, Erdem H, Li R, Cai Y, Ayala G, Ittmann M, et al. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008 Jul 1;68(13):5460–5468. doi: 10.1158/0008-5472.CAN-08-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YH, Kim JH, Stallcup MR. GAC63, a GRIP1-dependent nuclear receptor coactivator. Mol Cell Biol. 2005 Jul;25(14):5965–5972. doi: 10.1128/MCB.25.14.5965-5972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Li H, Stallcup MR. CoCoA, a nuclear receptor coactivator which acts through an N-terminal activation domain of p160 coactivators. Mol Cell. 2003 Dec;12(6):1537–1549. doi: 10.1016/s1097-2765(03)00450-7. [DOI] [PubMed] [Google Scholar]
- 18.Lee YH, Campbell HD, Stallcup MR. Developmentally essential protein flightless I is a nuclear receptor coactivator with actin binding activity. Mol Cell Biol. 2004 Mar;24(5):2103–2117. doi: 10.1128/MCB.24.5.2103-2117.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belandia B, Parker MG. Functional interaction between the p160 coactivator proteins and the transcriptional enhancer factor family of transcription factors. J Biol Chem. 2000 Oct 6;275(40):30801–30805. doi: 10.1074/jbc.C000484200. [DOI] [PubMed] [Google Scholar]
- 20.Chen SL, Dowhan DH, Hosking BM, Muscat GE. The steroid receptor coactivator, GRIP-1, is necessary for MEF-2C-dependent gene expression and skeletal muscle differentiation. Genes Dev. 2000 May 15;14(10):1209–1228. [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Wu RC, Amazit L, Tsai SY, Tsai MJ, O'Malley BW. Specific amino acid residues in the basic helix-loop-helix domain of SRC-3 are essential for its nuclear localization and proteasome-dependent turnover. Mol Cell Biol. 2007 Feb;27(4):1296–1308. doi: 10.1128/MCB.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Liang YY, Feng XH, Tsai SY, Tsai MJ, O'Malley BW. Essential phosphatases and a phosphodegron are critical for regulation of SRC-3/AIB1 coactivator function and turnover. Mol Cell. 2008 Sep 26;31(6):835–849. doi: 10.1016/j.molcel.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savkur RS, Burris TP. The coactivator LXXLL nuclear receptor recognition motif. J Pept Res. 2004 Mar;63(3):207–212. doi: 10.1111/j.1399-3011.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 24.Darimont BD, Wagner RL, Apriletti JW, Stallcup MR, Kushner PJ, Baxter JD, et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998 Nov 1;12(21):3343–3356. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997 Jun 12;387(6634):733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 26.Voegel JJ, Heine MJ, Tini M, Vivat V, Chambon P, Gronemeyer H. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 1998 Jan 15;17(2):507–519. doi: 10.1093/emboj/17.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996 Nov 29;87(5):953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 28.Teyssier C, Chen D, Stallcup MR. Requirement for multiple domains of the protein arginine methyltransferase CARM1 in its transcriptional coactivator function. J Biol Chem. 2002 Nov 29;277(48):46066–46072. doi: 10.1074/jbc.M207623200. [DOI] [PubMed] [Google Scholar]
- 29.Koh SS, Chen D, Lee YH, Stallcup MR. Synergistic enhancement of nuclear receptor function by p160 coactivators and two coactivators with protein methyltransferase activities. J Biol Chem. 2001 Jan 12;276(2):1089–1098. doi: 10.1074/jbc.M004228200. [DOI] [PubMed] [Google Scholar]
- 30.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997 Sep 11;389(6647):194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 31.Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol. 2002 May;22(10):3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, et al. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell. 2004 Sep 24;15(6):937–949. doi: 10.1016/j.molcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Giamas G, Castellano L, Feng Q, Knippschild U, Jacob J, Thomas RS, et al. CK1delta modulates the transcriptional activity of ERalpha via AIB1 in an estrogen-dependent manner and regulates ERalpha-AIB1 interactions. Nucleic Acids Res. 2009 May;37(9):3110–3123. doi: 10.1093/nar/gkp136. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Oh AS, Lahusen JT, Chien CD, Fereshteh MP, Zhang X, Dakshanamurthy S, et al. Tyrosine phosphorylation of the nuclear receptor coactivator AIB1/SRC-3 is enhanced by Abl kinase and is required for its activity in cancer cells. Mol Cell Biol. 2008 Nov;28(21):6580–6593. doi: 10.1128/MCB.00118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, et al. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell. 2004 Sep;6(3):263–274. doi: 10.1016/j.ccr.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 36. Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004 Mar 1;64(5):1875–1885. doi: 10.1158/0008-5472.can-03-3745.. This was the first paper to report that SRC-3 deficiency inhibited tumor initiation, progression, and metastasis in an animal model and effect of SRC-3 was through downregulation of IGF pathway.
- 37.Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005 Sep 1;65(17):7993–8002. doi: 10.1158/0008-5472.CAN-05-1179. [DOI] [PubMed] [Google Scholar]
- 38. Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A, et al. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008 May 15;68(10):3697–3706. doi: 10.1158/0008-5472.CAN-07-6702.. This study demonstrates ablation of SRC-3 arrests prostate cancer progression at early stage in an animal model
- 39.Reiter R, Wellstein A, Riegel AT. An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J Biol Chem. 2001 Oct 26;276(43):39736–39741. doi: 10.1074/jbc.M104744200. [DOI] [PubMed] [Google Scholar]
- 40.Tilli MT, Reiter R, Oh AS, Henke RT, McDonnell K, Gallicano GI, et al. Overexpression of an N-terminally truncated isoform of the nuclear receptor coactivator amplified in breast cancer 1 leads to altered proliferation of mammary epithelial cells in transgenic mice. Mol Endocrinol. 2005 Mar;19(3):644–656. doi: 10.1210/me.2004-0106. [DOI] [PubMed] [Google Scholar]
- 41.Nakles RE, Shiffert MT, Diaz-Cruz ES, Cabrera MC, Alotaiby M, Miermont AM, et al. Altered AIB1 or AIB1Delta3 expression impacts ERalpha effects on mammary gland stromal and epithelial content. Mol Endocrinol. 2011 Apr;25(4):549–563. doi: 10.1210/me.2010-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung AC, Zhou S, Liao L, Tien JC, Greenberg NM, Xu J. Genetic ablation of the amplified-in-breast cancer 1 inhibits spontaneous prostate cancer progression in mice. Cancer Res. 2007 Jun 15;67(12):5965–5975. doi: 10.1158/0008-5472.CAN-06-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000 Dec 22;103(7):1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida H, Liu J, Samuel S, Cheng W, Rosen D, Naora H. Steroid receptor coactivator-3, a homolog of Taiman that controls cell migration in the Drosophila ovary, regulates migration of human ovarian cancer cells. Mol Cell Endocrinol. 2005 Dec 21;245(1–2):77–85. doi: 10.1016/j.mce.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Li LB, Louie MC, Chen HW, Zou JX. Proto-oncogene ACTR/AIB1 promotes cancer cell invasion by up-regulating specific matrix metalloproteinase expression. Cancer Lett. 2008 Mar 8;261(1):64–73. doi: 10.1016/j.canlet.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Long W, Foulds CE, Qin J, Liu J, Ding C, Lonard DM, et al. ERK3 signals through SRC-3 coactivator to promote human lung cancer cell invasion. J Clin Invest. 2012 Apr 16; doi: 10.1172/JCI61492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, et al. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell. 2010 Feb 12;37(3):321–332. doi: 10.1016/j.molcel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, et al. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- 49.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 50.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000 May;2(5):249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 51.Goi T, Shipitsin M, Lu Z, Foster DA, Klinz SG, Feig LA. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J. 2000 Feb 15;19(4):623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb) 2011;21(3):264–275. doi: 10.11613/bm.2011.036. [DOI] [PubMed] [Google Scholar]
- 53.Pages F, Vives V, Sautes-Fridman C, Fossiez F, Berger A, Cugnenc PH, et al. Control of tumor development by intratumoral cytokines. Immunol Lett. 1999 May 3;68(1):135–139. doi: 10.1016/s0165-2478(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 54.Giri D, Ozen M, Ittmann M. Interleukin-6 is an autocrine growth factor in human prostate cancer. Am J Pathol. 2001 Dec;159(6):2159–2165. doi: 10.1016/S0002-9440(10)63067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001 Dec;58(6):1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 56.Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell. 2007 Mar 9;25(5):765–778. doi: 10.1016/j.molcel.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Q, Chen T, Xu Y, Zhu J, Jiang Y, Zhao Y, et al. Steroid receptor coactivator 3 is required for clearing bacteria and repressing inflammatory response in Escherichia coli-induced septic peritonitis. J Immunol. 2010 Nov 1;185(9):5444–5452. doi: 10.4049/jimmunol.0903802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Otaiby M, Tassi E, Schmidt MO, Chien CD, Baker T, Salas AG, et al. Role of the Nuclear Receptor Coactivator AIB1/SRC-3 in Angiogenesis and Wound Healing. Am J Pathol. 2012 Apr;180(4):1474–1484. doi: 10.1016/j.ajpath.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, Liao L, Zhou S, Xu J. Generation and validation of a mouse line with a floxed SRC-3/AIB1 allele for conditional knockout. Int J Biol Sci. 2008;4(4):202–207. doi: 10.7150/ijbs.4.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 61.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005 Jan 15;11(2 Pt 2):865s–870s. [PubMed] [Google Scholar]
- 62.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, et al. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003 Mar 5;95(5):353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 63.Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, et al. Regulation of ERBB2 by oestrogen receptor-PAX2 determines response to tamoxifen. Nature. 2008 Dec 4;456(7222):663–666. doi: 10.1038/nature07483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McBryan J, Theissen SM, Byrne C, Hughes E, Cocchiglia S, Sande S, et al. Metastatic progression with resistance to aromatase inhibitors is driven by the steroid receptor coactivator SRC-1. Cancer Res. 2012 Jan 15;72(2):548–559. doi: 10.1158/0008-5472.CAN-11-2073. [DOI] [PubMed] [Google Scholar]
- 65.Ayala G, Yan J, Li R, Ding Y, Thompson TC, Mims MP, et al. Bortezomib-mediated inhibition of steroid receptor coactivator-3 degradation leads to activated Akt. Clin Cancer Res. 2008 Nov 15;14(22):7511–7518. doi: 10.1158/1078-0432.CCR-08-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu MY, Fu J, Xu J, O'Malley BW, Wu RC. Steroid receptor coactivator 3 regulates autophagy in breast cancer cells through macrophage migration inhibitory factor. Cell Res. 2012 Mar 20; doi: 10.1038/cr.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6379–6384. doi: 10.1073/pnas.120166297.. This study was the first report of SRC-3 knockout mice and their phenotype.
- 68.Wang Z, Rose DW, Hermanson O, Liu F, Herman T, Wu W, et al. Regulation of somatic growth by the p160 coactivator p/CIP. Proc Natl Acad Sci U S A. 2000 Dec 5;97(25):13549–13554. doi: 10.1073/pnas.260463097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao L, Chen X, Wang S, Parlow AF, Xu J. Steroid receptor coactivator 3 maintains circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. Mol Cell Biol. 2008 Apr;28(7):2460–2469. doi: 10.1128/MCB.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Louet JF, Coste A, Amazit L, Tannour-Louet M, Wu RC, Tsai SY, et al. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc Natl Acad Sci U S A. 2006 Nov 21;103(47):17868–17873. doi: 10.1073/pnas.0608711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coste A, Louet JF, Lagouge M, Lerin C, Antal MC, Meziane H, et al. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1{alpha} Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17187–17192. doi: 10.1073/pnas.0808207105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.York B, Yu C, Sagen JV, Liu Z, Nikolai BC, Wu RC, et al. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci U S A. 2010 Jun 15;107(24):11122–11127. doi: 10.1073/pnas.1005262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Louie MC, Zou JX, Rabinovich A, Chen HW. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol. 2004 Jun;24(12):5157–5171. doi: 10.1128/MCB.24.12.5157-5171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006 Nov;26(21):8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.York B, O'Malley BW. Steroid receptor coactivator (SRC) family: masters of systems biology. J Biol Chem. 2010 Dec 10;285(50):38743–38750. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lonard DM, O'Malley BW. SRC-3 transcription-coupled activation, degradation, and the ubiquitin clock: is there enough coactivator to go around in cells? Sci Signal. 2008;1(13):pe16. doi: 10.1126/stke.113pe16. [DOI] [PubMed] [Google Scholar]
- 77.Wu RC, Feng Q, Lonard DM, O'Malley BW. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell. 2007 Jun 15;129(6):1125–1140. doi: 10.1016/j.cell.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 78.Mani A, Oh AS, Bowden ET, Lahusen T, Lorick KL, Weissman AM, et al. E6AP mediates regulated proteasomal degradation of the nuclear receptor coactivator amplified in breast cancer 1 in immortalized cells. Cancer Res. 2006 Sep 1;66(17):8680–8686. doi: 10.1158/0008-5472.CAN-06-0557. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell. 2006 Jan 27;124(2):381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 80.Ferry C, Gaouar S, Fischer B, Boeglin M, Paul N, Samarut E, et al. Cullin 3 mediates SRC-3 ubiquitination and degradation to control the retinoic acid response. Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20603–20608. doi: 10.1073/pnas.1102572108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kajiro M, Hirota R, Nakajima Y, Kawanowa K, So-ma K, Ito I, et al. The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat Cell Biol. 2009 Mar;11(3):312–319. doi: 10.1038/ncb1839. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Tong Z, Li T, Chen Q, Zhuo L, Li W, et al. Hepatitis B virus X protein stabilizes AIB1 protein and cooperates with it to promote human hepatocellular carcinoma cell invasiveness. Hepatology. 2012 Apr 2; doi: 10.1002/hep.25751. [DOI] [PubMed] [Google Scholar]
- 83.Li C, Ao J, Fu J, Lee DF, Xu J, Lonard D, et al. Tumor-suppressor role for the SPOP ubiquitin ligase in signal-dependent proteolysis of the oncogenic co-activator SRC-3/AIB1. Oncogene. 2011 Oct 20;30(42):4350–4364. doi: 10.1038/onc.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barbieri CE, Demichelis F, Rubin MA. Molecular genetics of prostate cancer: emerging appreciation of genetic complexity. Histopathology. 2012 Jan;60(1):187–198. doi: 10.1111/j.1365-2559.2011.04041.x. [DOI] [PubMed] [Google Scholar]
- 85.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44(6):685–689. doi: 10.1038/ng.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Y, Lonard DM, Yu Y, Chow DC, Palzkill TG, O'Malley BW. Small molecule inhibition of the steroid receptor coactivators, SRC-3 and SRC-1. Mol Endocrinol. 2011 Dec;25(12):2041–2053. doi: 10.1210/me.2011-1222.. This study reported gossypol as a small molecule inhibitor for SRC-3 and SRC-1.
- 87.Baggstrom MQ, Qi Y, Koczywas M, Argiris A, Johnson EA, Millward MJ, et al. A phase II study of AT-101 (Gossypol) in chemotherapy-sensitive recurrent extensive-stage small cell lung cancer. J Thorac Oncol. 2011 Oct;6(10):1757–1760. doi: 10.1097/JTO.0b013e31822e2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu G, Kelly WK, Wilding G, Leopold L, Brill K, Somer B. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin Cancer Res. 2009 May 1;15(9):3172–3176. doi: 10.1158/1078-0432.CCR-08-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999 Sep 3;98(5):675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 90.Feng Q, Yi P, Wong J, O'Malley BW. Signaling within a coactivator complex: methylation of SRC-3/AIB1 is a molecular switch for complex disassembly. Mol Cell Biol. 2006 Nov;26(21):7846–7857. doi: 10.1128/MCB.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yi P, Feng Q, Amazit L, Lonard DM, Tsai SY, Tsai MJ, et al. Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol Cell. 2008 Feb 29;29(4):465–476. doi: 10.1016/j.molcel.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kotaja N, Karvonen U, Janne OA, Palvimo JJ. The nuclear receptor interaction domain of GRIP1 is modulated by covalent attachment of SUMO-1. J Biol Chem. 2002 Aug 16;277(33):30283–30288. doi: 10.1074/jbc.M204768200. [DOI] [PubMed] [Google Scholar]
- 93.Gnanapragasam VJ, Armitage TG. Laparoscopic pyeloplasty, initial experience in the management of UPJO. Ann R Coll Surg Engl. 2001 Sep;83(5):347–352. [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou HJ, Yan J, Luo W, Ayala G, Lin SH, Erdem H, et al. SRC-3 is required for prostate cancer cell proliferation and survival. Cancer Res. 2005 Sep 1;65(17):7976–7983. doi: 10.1158/0008-5472.CAN-04-4076. [DOI] [PubMed] [Google Scholar]
- 95.Glaeser M, Floetotto T, Hanstein B, Beckmann MW, Niederacher D. Gene amplification and expression of the steroid receptor coactivator SRC3 (AIB1) in sporadic breast and endometrial carcinomas. Horm Metab Res. 2001 Mar;33(3):121–126. doi: 10.1055/s-2001-14938. [DOI] [PubMed] [Google Scholar]
- 96.Balmer NN, Richer JK, Spoelstra NS, Torkko KC, Lyle PL, Singh M. Steroid receptor coactivator AIB1 in endometrial carcinoma, hyperplasia and normal endometrium: Correlation with clinicopathologic parameters and biomarkers. Mod Pathol. 2006 Dec;19(12):1593–1605. doi: 10.1038/modpathol.3800696. [DOI] [PubMed] [Google Scholar]
- 97.Tanner MM, Grenman S, Koul A, Johannsson O, Meltzer P, Pejovic T, et al. Frequent amplification of chromosomal region 20q12-q13 in ovarian cancer. Clin Cancer Res. 2000 May;6(5):1833–1839. [PubMed] [Google Scholar]
- 98.Fujita Y, Sakakura C, Shimomura K, Nakanishi M, Yasuoka R, Aragane H, et al. Chromosome arm 20q gains and other genomic alterations in esophageal squamous cell carcinoma, as analyzed by comparative genomic hybridization and fluorescence in situ hybridization. Hepatogastroenterology. 2003 Nov-Dec;50(54):1857–1863. [PubMed] [Google Scholar]
- 99.Xu FP, Xie D, Wen JM, Wu HX, Liu YD, Bi J, et al. SRC-3/AIB1 protein and gene amplification levels in human esophageal squamous cell carcinomas. Cancer Lett. 2007 Jan 8;245(1–2):69–74. doi: 10.1016/j.canlet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 100.Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K, et al. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int J Cancer. 2000 May 20;89(3):217–223. [PubMed] [Google Scholar]
- 101.Xie D, Sham JS, Zeng WF, Lin HL, Bi J, Che LH, et al. Correlation of AIB1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum Pathol. 2005 Jul;36(7):777–783. doi: 10.1016/j.humpath.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 102.Henke RT, Haddad BR, Kim SE, Rone JD, Mani A, Jessup JM, et al. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin Cancer Res. 2004 Sep 15;10(18 Pt 1):6134–6142. doi: 10.1158/1078-0432.CCR-04-0561. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer. 2002 Dec 1;95(11):2346–2352. doi: 10.1002/cncr.10963. [DOI] [PubMed] [Google Scholar]
- 104.Xu Y, Chen Q, Li W, Su X, Chen T, Liu Y, et al. Overexpression of transcriptional coactivator AIB1 promotes hepatocellular carcinoma progression by enhancing cell proliferation and invasiveness. Oncogene. 2010 Jun 10;29(23):3386–3397. doi: 10.1038/onc.2010.90. [DOI] [PubMed] [Google Scholar]
- 105.Luo JH, Xie D, Liu MZ, Chen W, Liu YD, Wu GQ, et al. Protein expression and amplification of AIB1 in human urothelial carcinoma of the bladder and overexpression of AIB1 is a new independent prognostic marker of patient survival. Int J Cancer. 2008 Jun 1;122(11):2554–2561. doi: 10.1002/ijc.23399. [DOI] [PubMed] [Google Scholar]
- 106.Liu MZ, Xie D, Mai SJ, Tong ZT, Shao JY, Fu YS, et al. Overexpression of AIB1 in nasopharyngeal carcinomas correlates closely with advanced tumor stage. Am J Clin Pathol. 2008 May;129(5):728–734. doi: 10.1309/QMDTL82JKEX6E7H2. [DOI] [PubMed] [Google Scholar]