Abstract
Malaria caused by the protozoan parasite Plasmodium falciparum is characterized by long-term, persistent infections that can last for many months. The ability of this parasite to avoid clearance by the human immune system is dependent on its capacity to continuously alter the surface exposed antigenic proteins that that are vulnerable to antibody recognition and attack, a process called antigenic variation. Significant work in recent years has contributed to our understanding of the mechanisms underlying this process, including the genes encoding the antigenic proteins and the DNA sequence elements that control their expression. In addition, the epigenetic “marks” that are associated with activation and silencing of individual genes have been extensively characterized. These studies have led to a model that includes multiple layers of regulation that ultimately lead to the tight coordination of expression of the genes responsible for antigenic variation by malaria parasites. Here we review some more recent data that adds additional complexity to our understanding of these regulatory layers.
Keywords: malaria, gene regulation, chromatin, epigenetic memory, nuclear organization, transcription
INTRODUCTION
Plasmodium falciparum, the causative agent of the most severe form of human malaria, is one of many protozoan parasites that have evolved the ability to maintain long-term, persistent infections of their mammalian hosts. These infections are often characterized by waves of parasitemia that recur over several months of infection, with each wave representing the rise and fall of distinct populations of parasites expressing a particular set of immune antigens(Miller et al. 1994). This is a hallmark of a process called antigenic variation, in which parasites limit the antigens they display to the host immune system to a small number of proteins, then alter these antigenic molecules overtime, thus staying one step ahead of the antibody based immune response of the infected individual(Deitsch et al. 1997). In the case of malaria parasites, which infect red blood cells (RBCs) during the asexual stage of their life cycle, antigenic variation is centered on proteins displayed on the RBC surface(Kyes et al. 2001).
Upon infection of circulating RBCs in its human host, P. falciparum makes a number of alterations to the RBC membrane to enable its survival and proliferation. These include changes in the deformability of the cells(Cooke et al. 2001; Mills et al. 2007; Glenister et al. 2008; Hodder et al. 2008), the induction of new channels for the import and export of nutrients into and out of the cell(Staines et al. 2007), and perhaps most importantly for pathogenesis, the placement of adhesive proteins on the infected cell surface, thus enabling the cell to adhere to the endothelium of the post-capillary venules and avoid circulation through the spleen where infected cells would be destroyed(Baruch 1999). The cytoadhesion of infected RBCs is thought to lead to many of the severe manifestations of the disease, including cerebral malaria and pregnancy associated malaria, in which infected cells tightly adhere within the brain or placenta, respectively(Miller et al. 1994). The many modifications to the RBC membrane leave parasite encoded proteins exposed to the extracellular environment where they are detected by the host immune system, and consequently an antibody response is produced against these proteins. This leads to the requirement for the parasite to continuously vary these proteins in order to avoid destruction and extend the length of the infection. Antigenic variation in P. falciparum malaria is therefore intimately tied to the pathogenesis of the disease, and the primary antigenic molecules are also the most prominent virulence factors of the parasites.
The completion and annotation of the P. falciparum genome, as well as the addition of large amounts of genomic sequence data from numerous field isolates, enabled investigators to search the genome for large multi-copy gene families that might be involved in antigenic variation(Gardner et al. 2002). Four prominent families were identified; var, rifin, stevor and Pfmc-2TM(Smith et al. 1995; Baruch et al. 1995; Su et al. 1995; Kyes et al. 1999; Cheng et al. 1998; Sam-Yellowe et al. 2004). The best characterized of these is var, which encodes PfEMP1, the surface exposed adhesion protein responsible for sequestration of infected RBCs in the deep tissues. The other three families all encode proteins of similar structure but unknown function. Interestingly, when parasites are cultured in vitro, expression of the proteins encoded by these gene families appears not to be required for viability(Lavazec et al. 2007). Nonetheless, parasites taken directly from patients robustly express members of these families(Blythe et al. 2008), suggesting that they are likely involved in direct interactions with the host, or with the parasite’s ability to avoid the immune response, thus explaining why they are not required when the parasites are reared in the absence of a host.
The four gene families mentioned above are found clustered with one another within specific regions of the genome that appear to be dedicated to these hyper-variable genes(Gardner et al. 2002). The subtelomeric regions of most chromosomes, immediately adjacent to the telomeric repeats, contain such clusters of genes, with var genes typically found at the most telomeric extreme, followed by members of the other three families. While they are generally found in close proximity to one another, expression of individual genes within the different families does not appear to be coordinated(Sharp et al. 2006; Lavazec et al. 2007). The process of antigenic variation results from switches in expression between members of a specific gene family, thus altering the form of the surface exposed protein and allowing parasites to avoid antibodies produced against the protein encoded by previously expressed genes. In the case of var genes, expression appears to be strictly mutually exclusive, with one and only one var gene actively transcribed at a time(Chen et al. 1998; Scherf et al. 1998; Voss et al. 2006; Dzikowski et al. 2006). This requires that every var gene in the genome be recognized as part of a single family, and that a mechanism be in place to limit expression to a single copy. In addition, a switch in expression must be coordinated so that activation of one gene coincides with simultaneous silencing of the previously active copy. It is not yet clear if the rifin, stevor and Pfmc-2TM families follow a similarly strict regulatory paradigm. Also inherent to this process is the requirement for cellular memory, meaning that once a particular gene is activated, it tends to remain active through many cell cycles and switches in expression only occur at a relatively low rate. This ensures that the parasite’s repertoire of antigenic types is not exhausted too quickly and results in the waves of parasitemia observed in infected individuals.
The molecular mechanisms that control changes in gene expression and antigenic variation are far from being fully understood. Nonetheless substantial progress has been made in recent years in determining many of the aspects underlying this intricate process. The most detailed work has focused on the var gene family, and several recent reviews have summarized much of this work(Kyes et al. 2007; Coleman and Duraisingh 2008; Scherf et al. 2008; Lopez-Rubio et al. 2007b). Here we describe more recent data that adds to an emerging model that includes multiple “layers” of regulation that control expression of these large gene families.
LAYER ONE: GENE STRUCTURE AND PUTATIVE REGULATORY ELEMENTS
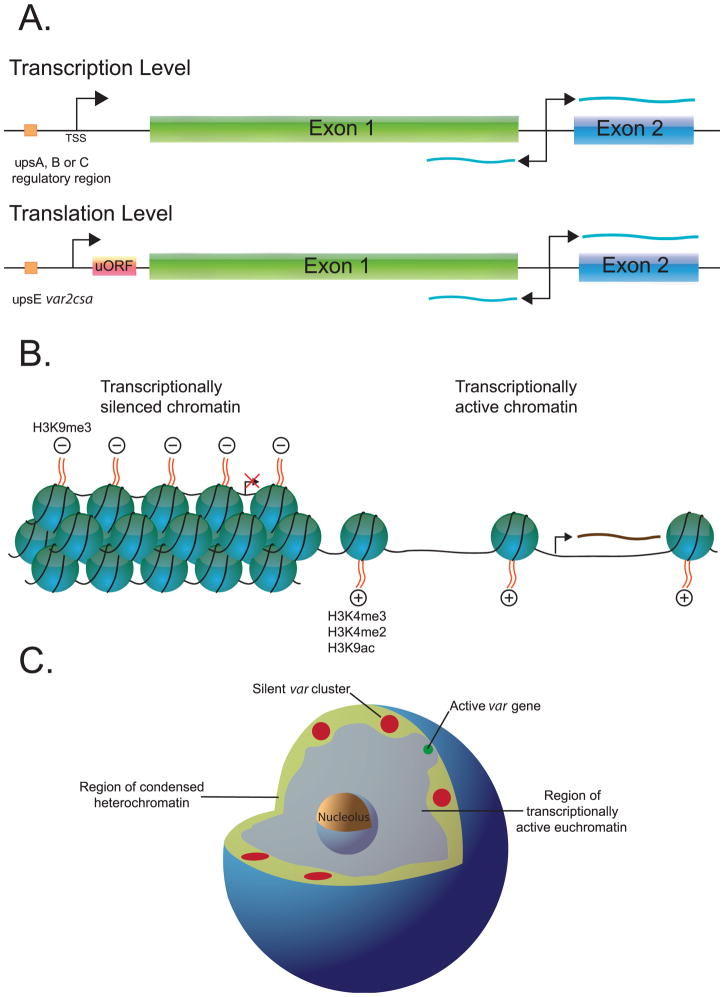
The ~60 var genes found in the genome of P. falciparum all maintain the same basic structure consisting of a large first exon encoding the hyper-variable, extracellular portion of the protein, an ~800 bp intron, and a conserved second exon that encodes the portion of the protein anchored inside the infected RBC membrane (Figure 1A). Each gene also includes two transcriptionally active promoters, one upstream of the first exon and giving rise to the mRNA, and the second within the intron that leads to expression of noncoding RNAs of unknown function(Su et al. 1995; Calderwood et al. 2003). Only the first promoter is subject to mutually exclusive expression and therefore while only one upstream promoter is active at a time (and thus only a single mRNA is made), the intron promoter appears to be active in most or perhaps all var genes(Epp et al. 2008a). Analyses of the DNA sequence of the upstream regulatory regions have shown that they can be classified into three main types, called upsA, B and C(Kraemer and Smith 2003; Lavstsen et al. 2003), and regulatory elements have been mapped within these regions by both deletion analysis and electro-mobility shift assays(EMSA)(Voss et al. 2000; Voss et al. 2003). A member of the ApiAP2, a newly identified class of transcription factors containing AP2 DNA binding domains has been identified as a protein that binds to the upstream regulatory regions of upsB var genes(Llinas et al. 2008), although its exact role has not been defined. There is also evidence that the promoter found within var introns functions as a regulatory element, both as a silencer and in the recognition of var genes by the mechanism that controls mutually exclusive expression(Deitsch et al. 2001; Frank et al. 2006; Dzikowski et al. 2007). A model is therefore emerging that the first layer of var gene regulation is controlled by these elements residing in the regions immediately surrounding each gene.
Figure 1.
Three layers of var gene regulation. A. The conserved structure of var genes and their putative regulatory elements are shown. The transcription start site (TSS) of the mRNA and the “sterile” transcripts are shown with bent arrows. The sterile transcripts are derived from both DNA strands and are shown as green wavy lines. The orange box upstream of the TSS represents regulatory elements mapped by deletion analysis or EMSA. The uORF found in the UpsE promoter of var2csa (red box) functions as a translational repressor. B. Modifications to the structure of chromatin surrounding var genes are shown. Condensed, silent chromatin (left) is characterized by tightly packed nucleosomes carrying the H3K9me3 modification while the nucleosomes found in loose, transcriptionally active chromatin surrounding “on” var genes (right) are marked by H3K4me3, H3K4me2 and H3K9ac histone modifications. C. The hypothetical structure of nuclei from P. falciparum. Regions containing condensed heterochromatin (green) are found at the nuclear periphery while euchromatin is found in the internal regions (grey). Clusters of silent var genes (red) are thought to be tethered to the nuclear membrane. The active var locus (green) relocates to a specific region of euchromatin near the nuclear membrane which may represent a specific var gene expression site.
There are two exceptions to the basic model outlined above. The first is a conserved var pseudogene referred to as var1csa. The upstream regulatory region of this gene is unique (referred to as upsD)(Kraemer and Smith 2003; Lavstsen et al. 2003), and the intron of this gene appears to have a substantial deletion in the region containing the promoter activity. In all parasite isolates examined to date, the gene either is truncated or has a frame shift mutation in exon 1, thus leading to its designation as a pseudogene in the annotated genome of the 3D7 isolate. Interestingly, this gene appears defective in both silencing and in recognition by the mechanism that control mutually exclusive expression(Kyes et al. 2003; Winter et al. 2003); it is constitutively expressed regardless of expression of other members of the var gene family. This provides support for the hypothesis that the promoter activity found in var introns is necessary for both silencing and mutually exclusive expression. The second exception is the so called “type 3” or “var-like” genes(Trimnell et al. 2006). These genes are found in one to three copies in the genomes of most parasite isolates and, unlike other var genes, are conserved in sequence. The intron found in these genes does not resemble those found in the rest of the gene family and does not contain promoter activity(Epp et al. 2008b), nonetheless these genes do get silenced. Whether or not they are recognized by the mutually exclusive expression pathway has not yet been determined, and their role in parasite biology remains an open question.
In addition to the regulation of var gene expression at the level of transcription outlined above, it has recently been shown that the expression of at least one var gene is also regulated at the level of translation. A particular var gene, called var2csa, is found within the genomes of most or all wildtype parasites(Trimnell et al. 2006) and is thought to encode a form of PfEMP1 that specifically binds to the placental receptor chondroitin sulfate A (CSA)(Salanti et al. 2003; Viebig et al. 2005). Antibodies against this protein are only detectable in women who have suffered a bout of malaria during pregnancy(Salanti et al. 2004), suggesting that this gene is not expressed during infection in men, children or non-pregnant women. In addition, in vitro parasites can be isolated that actively transcribe this gene but in which no detectable protein is produced(Mok et al. 2008), suggesting that translation of the mRNA can somehow be repressed. Examination of the upstream untranslated region of the var2csa transcript identified a unique short open reading frame (uORF) that encodes a polypeptide of 120 amino acids(Lavstsen et al. 2003). Using transgenic parasites, it was demonstrated that translation of the uORF suppresses translation of the PfEMP1 encoding portion of the transcript, thus effectively silencing the gene(Amulic et al. 2009). Using selection pressure, it was possible to isolate parasites that had switched to translating the downstream, PfEMP1 encoding ORF, therefore demonstrating that the suppression of translation was reversible(Amulic et al. 2009). This led to the hypothesis that translation of var2csa mRNA is repressed when parasites infect individuals without a placenta, and that this repression can be lifted when a placenta is available for sequestration. Whether reversal of translational repression is triggered by a signal in pregnant women or is simply stochastic is not known.
LAYER TWO: EPIGENETIC MEMORY
The term epigenetic memory refers to the concept that once the transcriptional status of an individual var gene has been established, this state (either active or silent) tends to be maintained through multiple cell cycles and only rarely does a switch event occur(Horrocks et al. 2004; Frank et al. 2007). Thus the transcriptional state of each gene is “remembered” from one cell cycle to the next. It is important for var gene expression switches to be relatively rare in order to prevent premature expenditure of the parasite’s antigenic repertoire, and it is this inherent rate of switching that gives rise to the waves of parasitemia observed in experimental infections with P. falciparum. This memory is encoded into the genome through a series of “marks” placed at each gene that are faithfully replicated along with the genome during S-phase of each cycle(Duraisingh et al. 2005; Freitas-Junior et al. 2005; Chookajorn et al. 2007; Lopez-Rubio et al. 2007a). Significant effort in recent years has been expended to decipher these marks and to determine how they influence var gene expression.
Mutually exclusive expression and var gene expression switching are thought to be controlled epigenetically, referring to the fact that activation and silencing of each individual gene is not accompanied by changes in either DNA sequence or the presence or absence of specific transcription factors. Rather the transcriptional state of a gene is associated with a specific chromatin structure found surrounding the promoter region, and an extensive amount of work has been completed to define the constellation of histone modifications that are associated with either active or silent genes (Figure 1B). The particular modifications identified are similar to those observed at either active or silent genes in most other eukaryotes studied, for example acetylated histone H2 and H3 and methylated H3K27 are found at active genes, while tri-methyl H3K9 is found at silent loci(Duraisingh et al. 2005; Freitas-Junior et al. 2005; Chookajorn et al. 2007; Lopez-Rubio et al. 2007a). Interestingly, Lopez Rubio et al. (2007a) demonstrated that di- and trimethylated H3K4 are enriched in an “on” var gene even during the phase of the cell cycle when the gene is no longer transcriptionally active, indicating that the gene is “poised” and ready to be activated again in the next cell cycle. This suggests that methylated H3K4 plays a signficant role in “bookmarking” the active gene, an important aspect of epigenetic memory. In addition, parasites in which the histone deacetylase SIR2 has been knocked out show leaky silencing of a portion of the var gene family, implicating this histone modifying protein in the maintenance of the silent state(Duraisingh et al. 2005).
In many other models systems, the conventional histone modifications that are associated with the regulation of transcription are augmented by the incorporation of noncoding RNAs (ncRNAs) into the chromatin structure(Andersen and Panning 2003). Examples include the ncRNAs associated with dosage compensation in both mammals (Xist, T-six)(Chang et al. 2006) and Drosophila (Rox1 and 2)(Meller et al. 1997; Meller et al. 2000) as well as the small double-stranded RNAs generated by the RNAi pathway. There is evidence that the genome of P. falciparum encodes numerous ncRNAs(Mourier et al. 2008), and small noncoding transcripts have been found incorporated into the chromatin structure found at centromeres(Li et al. 2008). Additionally, numerous proteins containing RNA binding domains are encoded in the genome(Aravind et al. 2003), suggesting that RNA might play a significant role in regulating gene expression in Plasmodium. In the case of var genes, ncRNAs (originally called “sterile” RNAs) were found to be transcribed from the intron promoter during the late stage of the cell cycle(Kyes et al. 2003; Calderwood et al. 2003), when the genome is being replicated and chromatin assembly is occurring. A more recent paper has shown that these ncRNAs are capped but not polyadenylated, are retained within the nucleus, and are associated with chromatin, thereby suggesting that they might play a role in chromatin assembly and transcriptional regulation of the var gene family(Epp et al. 2008a).
While modifications to chromatin structure clearly play an important role in determining which var gene is active in any given parasite, another interesting question is what is required to maintain the transcription state through multiple cell generations so that the chromatin structure is faithfully replicated along with the genome. Recently it was shown that transcription itself is required for an “on” var gene to continue to be active for multiple cell cycles(Dzikowski and Deitsch 2008). Using a genetic technique referred to as promoter titration(Iyer et al. 2007), it was possible to prevent an “on” var gene from being actively transcribed. This resulted in the gene reverted to the silent state, and essentially erased the epigenetic marks from this gene. This observation led to a model in which transcription, and specifically the movement of the RNA polymerase II complex along the length of the gene, results in the recruitment of histone modifiers to the active locus, leading to the propagation of the chromatin modifications indicative of an active gene. This results in a reinforced feedback loop through the coupling of RNA polymerase to chromatin modifiers and thus leads to the “on” gene remaining “on”. This type of system for perpetuating a particular chromatin state has been observed in other organisms(Hampsey and Reinberg 2003; Eissenberg and Shilatifard 2006), and is thought to require the direct recruitment of histone modifying enzymes by the RNA polymerase II complex. How switching occurs in such a model however remains to be determined.
LAYER THREE: SUBNUCLEAR ORGANIZATION
The nuclear microenvironment and the organization of the genome into different subnuclear compartments appear to play a role as another epigenetic level of transcriptional regulation in eukaryotic organisms. Silent genes tend to localize within regions of the nucleus that contain primarily heterochromatin whereas active transcription generally takes place in euchromatic regions in which chromatin is loose and open for transcription. The role of nuclear structure in antigenic variation was first demonstrated for African trypanosomes where the active vsg gene was shown to localize within a specific subnuclear expression site(Navarro and Gull 2001). There is now evidence from several laboratories indicating that nuclear architecture is also important for gene regulation in P. falciparum and represents another layer of control of var gene expression (Figure 1C). Studies investigating the ultrastructure of the P. falciparum nucleus have demonstrated that it is composed of two distinct compartments; the nuclear periphery consisting mainly of electron dense heterochromatin and the internal part of the nucleus contain primarily loose euchromatin(Ralph et al. 2005). Interestingly however, the periphery of each parasite nucleus contains at least one distinct region that is clear of heterochromatin, indicating that the region next to the nuclear membrane may contain both transcriptionally active and inactive zones. These observations have lead to the suggestion that this region represents an expression site where var genes localize when they are transcriptionally active.
Fluorescent In Situ Hybridization (FISH) has been used by several groups to visualize where in the nucleus various genes or chromosomal regions are localized at any given time. Experiments using var specific probes showed that both subtelomeric and central var genes localize mostly at the nuclear periphery regardless of their activation state(Ralph et al. 2005). In addition, var genes appear to move upon changes in their transcriptional activity. By selecting parasites for the ability to bind to CSA, it is possible to create parasite lines that exclusively express var2csa(Salanti et al. 2003), thus enabling the visualization of a single transcriptionally active var gene by FISH. Using this technique, Ralph et al demonstrated that when var2csa is silent, it highly co-localizes with telomeric clusters (84% of cells visualized) whereas upon activation it moves to another location of the nuclear periphery apart from the telomeric clusters, resulting in a decrease in their co-localization rate to only 26%. Similar results were subsequently observed by Mok and colleagues(Mok et al. 2008). Based on these observations, a model was proposed in which the telomeric clusters are located within the heterochromatic region of the nuclear periphery and upon activation, var genes exit this region and move to the euchromatic portion of the periphery where the chromatin is open for transcription(Ralph et al. 2005). However, contrary data was obtained using transgenic lines in which var promoters were integrated at a centrally located var locus. When selected for activation using a drug selectable marker, the transgenes tended to co-localize with the telomeric clusters independently of their transcriptional status(Voss et al. 2006). In addition, transcriptionally active var promoters found on episomes were reported to co-localize with the telomeric clusters significantly more frequently upon activation, thus suggesting that active var genes are not necessarily separated from silent genes. Nonetheless, both sets of studies are consistent with a specific region within the largely heterochromatic nuclear periphery that is conducive to var gene transcription, and that when activated, var genes must relocate to this expression site. This led to the proposal that mutually exclusive expression could simply be a result of this site being only capable of accommodating a single var gene at a time.
More recent data using a different experimental approach suggests that a var expression site does indeed exist within the P. falciparum nucleus, however it is not limited in its capacity to only a single var promoter at a time and therefore does not control mutually exclusive expression of var genes(Dzikowski et al. 2007). These experiments took advantage of the ability to render a var promoter constitutively active and not counted by the mechanism the controls mutually exclusive expression (by separating it from the regulatory element in the intron), thus enabling the creation of parasites in which at least two var promoters are active simultaneously. Using DNA FISH with gene specific probes targeting either a constitutively active episomal var promoter or a properly regulated transcriptionally active chromosomal var gene, it was possible to demonstrate that two simultaneously active var genes virtually always co-localized within the nucleus (~95% of cells). However, when a silent chromosomal var gene was visualized, it did not co-localized with the active episomal var promoter (~5% of cells). A subsequent study using multicopy concatameric episomes containing constitutively active var promoters indicated that individual cells are capable of expressing up to 20 var promoters at the same time(Dzikowski and Deitsch 2008). These results provided a strong evidence for the existence of a var-specific subnuclear expression site, however, this site can accommodate more than one active gene at a time indicating that mutually exclusive expression of var genes is regulated at a different level than simply nuclear architecture.
CONCLUSIONS
The complex molecular interplay between the regulatory elements found at each var gene, ncRNAs, RNA polymerase II, chromatin modifying enzymes and subnuclear organization leads to the tightly coordinated activation and silencing of individual genes within this large hyper-variable family. As the details of each layer of regulation continue to be elucidated, a better understanding of how all of the pieces fit together will ultimately provide researchers with a basic understanding of how malaria parasites manage their genomes. What is learned from studying this process in malaria parasites is likely to provide insights into how other eukaryotic pathogens regulate similar processes, as well as how eukaryotes in general use epigenetic mechanisms to control gene expression. Therefore the study of var gene regulation should be viewed as more than simply an investigation into how parasites survive within their hosts, but also as a complex problem that involves regulation at the levels of transcription, translation and genome organization. Thus, while hardly a model system, lessons learned from the study of P. falciparum should be valuable to all researchers interested in complex genetic pathways.
Acknowledgments
The authors thank Ira Pasternak for his contributions to the graphic design of Figure 1. The Department of Microbiology and Immunology at Weill Medical College of Cornell University acknowledges the support of the William Randolph Hearst Foundation. KWD is a Stavros S. Niarchos Scholar and is supported by National Institutes of Health grant AI 52390. RD is a Golda Meir Scholar and supported by the Marie Curie International Reintegration Grant (IRG) [203675] and the German Israeli Foundation [2163-1725.11/2006]. KWD and RD are supported by a grant from the United States-Israel Binational Science Foundation.
References
- Amulic B, Salanti A, Lavstsen T, Nielsen MA, Deitsch KW. An upstream open reading frame controls translation of var2csa, a gene implicated in placental malaria. PLoS Pathog. 2009;5:e1000256. doi: 10.1371/journal.ppat.1000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen AA, Panning B. Epigenetic gene regulation by noncoding RNAs. Current Opinion in Cell Biology. 2003;15:281–289. doi: 10.1016/s0955-0674(03)00041-3. [DOI] [PubMed] [Google Scholar]
- Aravind L, Iyer LM, Wellems TE, Miller LH. Plasmodium biology: Genomic gleanings. Cell. 2003;115:771–785. doi: 10.1016/s0092-8674(03)01023-7. [DOI] [PubMed] [Google Scholar]
- Baruch DI. Adhesive receptors on malaria-parasitized red cells. Baillieres Best Pract Res Clin Haematol. 1999;12:747–761. doi: 10.1053/beha.1999.0051. [DOI] [PubMed] [Google Scholar]
- Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, Feldman M, Taraschi TF, Howard RJ. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Blythe JE, Yam XY, Kuss C, Bozdech Z, Holder AA, Marsh K, Langhorne J, Preiser PR. Plasmodium falciparum STEVOR proteins are highly expressed in patient isolates and located in the surface membranes of infected red blood cells and the apical tips of merozoites. Infect Immun. 2008;76:3329–3336. doi: 10.1128/IAI.01460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood MS, Gannoun-Zaki L, Wellems TE, Deitsch KW. Plasmodium falciparum var genes are regulated by two regions with separate promoters, one upstream of the coding region and a second within the intron. Journal of Biological Chemistry. 2003;278:34125–34132. doi: 10.1074/jbc.M213065200. [DOI] [PubMed] [Google Scholar]
- Chang SC, Tucker T, Thorogood NP, Brown CJ. Mechanisms of X-chromosome inactivation. Front Biosci. 2006;11:852–866. doi: 10.2741/1842. [DOI] [PubMed] [Google Scholar]
- Chen Q, Fernandez V, Sundstrom A, Schlichtherle M, Datta S, Hagblom P, Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum. Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Cloonan N, Fischer K, Thompson J, Waine G, Lanzer M, Saul A. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- Chookajorn T, Dzikowski R, Frank M, Li F, Jiwani AZ, Hartl DL, Deitsch KW. Epigenetic memory at malaria virulence genes. Proc Natl Acad Sci U S A. 2007;104:899–902. doi: 10.1073/pnas.0609084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman BI, Duraisingh MT. Transcriptional control and gene silencing in Plasmodium falciparum. Cell Microbiol. 2008;10:1935–1946. doi: 10.1111/j.1462-5822.2008.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol. 2001;50:1–86. doi: 10.1016/S0065-308X(01)50029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitsch KW, Calderwood MS, Wellems TE. Malaria. Cooperative silencing elements in var genes. Nature. 2001;412:875–876. doi: 10.1038/35091146. [DOI] [PubMed] [Google Scholar]
- Deitsch KW, Moxon ER, Wellems TE. Shared themes of antigenic variation and virulence in bacterial, protozoal, and fungal infections. Microbiol Mol Biol Rev. 1997;61:281–293. doi: 10.1128/mmbr.61.3.281-293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh MT, Voss TS, Marty AJ, Duffy MF, Good RT, Thompson JK, Freitas-Junior LH, Scherf A, Crabb BS, Cowman AF. Heterochromatin silencing and locus repositioning linked to regulation of virulence genes in Plasmodium falciparum. Cell. 2005;121:13–24. doi: 10.1016/j.cell.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Dzikowski R, Deitsch KW. Active transcription is required for maintenance of epigenetic memory in the malaria parasite Plasmodium falciparum. J Mol Biol. 2008;382:288–297. doi: 10.1016/j.jmb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikowski R, Frank M, Deitsch K. Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikowski R, Li F, Amulic B, Eisberg A, Frank M, Patel S, Wellems TE, Deitsch KW. Mechanisms underlying mutually exclusive expression of virulence genes by malaria parasites. EMBO Rep. 2007;8:959–965. doi: 10.1038/sj.embor.7401063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, Shilatifard A. Leaving a mark: the many footprints of the elongating RNA polymerase II. Curr Opin Genet Dev. 2006;16:184–190. doi: 10.1016/j.gde.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Epp C, Li F, Howitt CA, Chookajorn T, Deitsch KW. Chromatin associated sense and antisense noncoding RNAs are transcribed from the var gene family of virulence genes of the malaria parasite Plasmodium falciparum. RNA. 2008a;15:116–127. doi: 10.1261/rna.1080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp C, Raskolnikov D, Deitsch KW. A regulatable transgene expression system for cultured Plasmodium falciparum parasites. Malar J. 2008b;7:86. doi: 10.1186/1475-2875-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Dzikowski R, Amulic B, Deitsch K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol Microbiol. 2007;64:1486–1498. doi: 10.1111/j.1365-2958.2007.05736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M, Dzikowski R, Constantini D, Amulic B, Burdougo E, Deitsch K. Strict pairing of var promoters and introns is required for var gene silencing in the malaria parasite Plasmodium falciparum. J Biol Chem. 2006;281:9942–9952. doi: 10.1074/jbc.M513067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Junior LH, Hernandez-Rivas R, Ralph SA, Montiel-Condado D, Ruvalcaba-Salazar OK, Rojas-Meza AP, Mancio-Silva L, Leal-Silvestre RJ, Gontijo AM, Shorte S, Scherf A. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, Carlton JM, Pain A, Nelson KE, Bowman S, Paulsen IT, James K, Eisen JA, Rutherford K, Salzberg SL, Craig A, Kyes S, Chan MS, Nene V, Shallom SJ, Suh B, Peterson J, Angiuoli S, Pertea M, Allen J, Selengut J, Haft D, Mather MW, Vaidya AB, Martin DM, Fairlamb AH, Fraunholz MJ, Roos DS, Ralph SA, McFadden GI, Cummings LM, Subramanian GM, Mungall C, Venter JC, Carucci DJ, Hoffman SL, Newbold C, Davis RW, Fraser CM, Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenister FK, Fernandez KM, Kats LM, Hanssen E, Mohandas N, Coppel RL, Cooke BM. Functional alteration of red blood cells by a Mega-Dalton protein of Plasmodium falciparum. Blood. 2008 doi: 10.1182/blood-2008-05-157735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey M, Reinberg D. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell. 2003;113:429–432. doi: 10.1016/s0092-8674(03)00360-x. [DOI] [PubMed] [Google Scholar]
- Hodder AN, Maier AG, Rug M, Brown M, Hommel M, Pantic I, Puig-de-Morales-Marinkovic M, Smith B, Triglia T, Beeson J, Cowman AF. Analysis of structure and function of the giant protein Pf332 in Plasmodium falciparum. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks P, Pinches R, Christodoulou Z, Kyes SA, Newbold CI. Variable var transition rates underlie antigenic variation in malaria. Proc Natl Acad Sci U S A. 2004;101:11129–11134. doi: 10.1073/pnas.0402347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer JK, Amaladoss A, Genesan S, Preiser PR. Variable expression of the 235 kDa rhoptry protein of Plasmodium yoelii mediate host cell adaptation and immune evasion. Mol Microbiol. 2007;65:333–346. doi: 10.1111/j.1365-2958.2007.05786.x. [DOI] [PubMed] [Google Scholar]
- Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Molecular Microbiology. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annu Rev Microbiol. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- Kyes SA, Christodoulou Z, Raza A, Horrocks P, Pinches R, Rowe JA, Newbold CI. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Molecular Microbiology. 2003;48:1339–1348. doi: 10.1046/j.1365-2958.2003.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes SA, Kraemer SM, Smith JD. Antigenic variation in Plasmodium falciparum: gene organization and regulation of the var multigene family. Eukaryot Cell. 2007;6:1511–1520. doi: 10.1128/EC.00173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes SA, Rowe JA, Kriek N, Newbold CI. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavazec C, Sanyal S, Templeton TJ. Expression switching in the stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Mol Microbiol. 2007;64:1621–1634. doi: 10.1111/j.1365-2958.2007.05767.x. [DOI] [PubMed] [Google Scholar]
- Lavstsen T, Salanti A, Jensen ATR, Arnot DE, Theander TG. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malaria Journal. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Sonbuchner L, Kyes SA, Epp C, Deitsch KW. Nuclear non-coding RNAs are transcribed from the centromeres of Plasmodium falciparum and are associated with centromeric chromatin. J Biol Chem. 2008;283:5692–5698. doi: 10.1074/jbc.M707344200. [DOI] [PubMed] [Google Scholar]
- Llinas M, Deitsch KW, Voss TS. Plasmodium gene regulation: far more to factor in. Trends Parasitol. 2008;24:551–556. doi: 10.1016/j.pt.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubio JJ, Gontijo AM, Nunes MC, Issar N, Hernandez RR, Scherf A. 5′ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol. 2007a;66:1296–1305. doi: 10.1111/j.1365-2958.2007.06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubio JJ, Riviere L, Scherf A. Shared epigenetic mechanisms control virulence factors in protozoan parasites. Curr Opin Microbiol. 2007b;10:560–568. doi: 10.1016/j.mib.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Meller VH, Gordadze PR, Park Y, Chu X, Stuckenholz C, Kelley RL, Kuroda MI. Ordered assembly of roX RNAs into MSL complexes on the dosage-compensated X chromosome in Drosophila. Current Biology. 2000;10:136–143. doi: 10.1016/s0960-9822(00)00311-0. [DOI] [PubMed] [Google Scholar]
- Meller VH, Wu KH, Roman G, Kuroda MI, Davis RL. roX1 RNA paints the X chromosome of male Drosophila and is regulated by the dosage compensation system. Cell. 1997;88:445–457. doi: 10.1016/s0092-8674(00)81885-1. [DOI] [PubMed] [Google Scholar]
- Miller LH, Good MF, Milon G. Malaria Pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- Mills JP, Diez-Silva M, Quinn DJ, Dao M, Lang MJ, Tan KS, Lim CT, Milon G, David PH, Mercereau-Puijalon O, Bonnefoy S, Suresh S. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc Natl Acad Sci U S A. 2007;104:9213–9217. doi: 10.1073/pnas.0703433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok BW, Ribacke U, Rasti N, Kironde F, Chen Q, Nilsson P, Wahlgren M. Default Pathway of var2csa switching and translational repression in Plasmodium falciparum. PLoS ONE. 2008;3:e1982. doi: 10.1371/journal.pone.0001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourier T, Carret C, Kyes S, Christodoulou Z, Gardner PP, Jeffares DC, Pinches R, Barrell B, Berriman M, Griffiths-Jones S, Ivens A, Newbold C, Pain A. Genome-wide discovery and verification of novel structured RNAs in Plasmodium falciparum. Genome Res. 2008;18:281–292. doi: 10.1101/gr.6836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414:759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- Ralph SA, Scheidig-Benatar C, Scherf A. Antigenic variation in Plasmodium falciparum is associated with movement of var loci between subnuclear locations. Proc Natl Acad Sci U S A. 2005;102:5414–5419. doi: 10.1073/pnas.0408883102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti A, Dahlback M, Turner L, Nielsen MA, Barfod L, Magistrado P, Jensen AT, Lavstsen T, Ofori MF, Marsh K, Hviid L, Theander TG. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanti A, Staalsoe T, Lavstsen T, Jensen ATR, Sowa MPK, Arnot DE, Hviid L, Theander TG. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Molecular Microbiology. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- Sam-Yellowe TY, Florens L, Johnson JR, Wang T, Drazba JA, Le Roch KG, Zhou Y, Batalov S, Carucci DJ, Winzeler EA, Yates JR., III A Plasmodium gene family encoding Maurer’s cleft membrane proteins: structural properties and expression profiling. Genome Res. 2004;14:1052–1059. doi: 10.1101/gr.2126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Hernandez-Rivas R, Buffet P, Bottius E, Benatar C, Pouvelle B, Gysin J, Lanzer M. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum. EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf A, Lopez-Rubio JJ, Riviere L. Antigenic variation in Plasmodium falciparum. Annu Rev Microbiol. 2008;62:445–470. doi: 10.1146/annurev.micro.61.080706.093134. [DOI] [PubMed] [Google Scholar]
- Sharp S, Lavstsen T, Fivelman QL, Saeed M, McRobert L, Templeton TJ, Jensen ATR, Baker DA, Theander TG, Sutherland CJ. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryotic Cell. 2006;5:1206–1214. doi: 10.1128/EC.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, Peterson DS, Pinches R, Newbold CI, Miller LH. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, Ginsburg H, Hill DA, Huber SM, Kirk K, Lang F, Lisk G, Oteng E, Pillai AD, Rayavara K, Rouhani S, Saliba KJ, Shen C, Solomon T, Thomas SL, Verloo P, Desai SA. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Heatwole VM, Wertheimer SP, Guinet F, Herrfeldt JV, Peterson DS, Ravetch JV, Wellems TE. A large and diverse gene family (var) encodes 200–350 kD proteins implicated in the antigenic variation and cytoadherence of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Trimnell AR, Kraemer SM, Mukherjee S, Phippard DJ, Janes JH, Flamoe E, Su XZ, Awadalla P, Smith JD. Global genetic diversity and evolution of var genes associated with placental and severe childhood malaria. Mol Biochem Parasitol. 2006;148:169–180. doi: 10.1016/j.molbiopara.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Viebig NK, Gamain B, Scheidig C, Lepolard C, Przyborski J, Lanzer M, Gysin J, Scherf A. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005;6:775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss TS, Healer J, Marty AJ, Duffy MF, Thompson JK, Beeson JG, Reeder JC, Crabb BS, Cowman AF. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- Voss TS, Kaestli M, Vogel D, Bopp S, Beck HP. Identification of nuclear proteins that interact differentially with Plasmodium falciparum var gene promoters. Molecular Microbiology. 2003;48:1593–1607. doi: 10.1046/j.1365-2958.2003.03528.x. [DOI] [PubMed] [Google Scholar]
- Voss TS, Thompson JK, Waterkeyn J, Felger I, Weiss N, Cowman AF, Beck HP. Genomic distribution and functional characterisation of two distinct and conserved Plasmodium falciparum var gene 5′ flanking sequences. Mol Biochem Parasitol. 2000;107:103–115. doi: 10.1016/s0166-6851(00)00176-6. [DOI] [PubMed] [Google Scholar]
- Winter G, Chen QJ, Flick K, Kremsner P, Fernandez V, Wahlgren M. The 3D7var5.2 (var(COMMON)) type var gene family is commonly expressed in non-placental Plasmodium falciparum malaria. Molecular and Biochemical Parasitology. 2003;127:179–191. doi: 10.1016/s0166-6851(03)00004-5. [DOI] [PubMed] [Google Scholar]