Abstract
Naltrexone has high potential for use as a relapse prevention pharmacotherapy for opiate dependence; however suffers from notoriously poor adherence when prescribed for oral self-administration. This study evaluated whether entry to a therapeutic workplace could be used to reinforce adherence with oral naltrexone. Opiate-dependent and cocaine-using injection drug users were detoxified, inducted onto oral naltrexone, and randomly assigned to a Contingency (n=35) or Prescription (n=32) group for a 26-week period. Contingency participants were required to ingest naltrexone under staff observation to gain access to the therapeutic workplace. Prescription participants received a take-home supply of naltrexone and could access the workplace independent of naltrexone ingestion. Primary outcome measures were percent of urine samples positive for naltrexone at 30-day assessments and negative for opiates and cocaine at 30-day assessments. Contingency participants provided significantly more urine samples that were positive for naltrexone compared to Prescription participants (72% vs. 21%, P<.01), however no effect of experimental group was observed on percent opiate-negative (71% vs. 60%, P=.19.) or cocaine-negative (56% vs. 53%, P=.82) samples in the Contingency and Prescription groups, respectively. Opiate-positive samples were significantly more likely to occur in conjunction with cocaine (P<.001), and when not protected by naltrexone (P<.02), independent of experimental group. Overall, these results show that contingent access to a therapeutic workplace significantly promoted adherence to oral naltrexone, and that the majority of opiate use occurred in conjunction with cocaine use, suggesting that untreated cocaine use may limit the effectiveness of oral naltrexone in promoting opiate abstinence.
Keywords: naltrexone, contingency management, therapeutic workplace, incentive, injection drug use
Introduction
Naltrexone is an opioid antagonist that blocks the reinforcing, subjective and physiological effects of opiates (Martin, Jasinski, & Mansky, 1973; Mello, Mendelson, Kuehnle, & Sellers, 1981; Schuh, Walsh, & Stitzer, 1999; Walsh, Sullivan, Preston, Garner, & Bigelow, 1996). Naltrexone has no abuse liability or diversion potential and can be prescribed directly by a physician from a primary care setting. Although these characteristics position naltrexone as an ideal relapse prevention pharmacotherapy, non-adherence with oral naltrexone has severely limited its utility in the treatment of opiate dependence (Adi et al., 2007; Kirchmayer et al., 2002; Minozzi et al., 2011; San, Pomarol, Peri, Olle, & Cami, 1991; Sullivan et al., 2007). The most recent meta-analysis of oral naltrexone reported no statistical benefit of naltrexone over placebo or psychotherapy in preventing relapse (Minozzi et al., 2011); yet when the analysis was restricted to studies that ensured patient adherence, naltrexone was significantly associated with drug abstinence, suggesting its clinical limitations may be due to poor adherence.
Extended release formulations of naltrexone have been developed to reduce the frequency of dosing and improve adherence (Comer, Sullivan, & Hulse, 2007; Krupitsky et al., 2011), and one of those formulations (Vivitrol®) was recently approved by the U.S. Food and Drug Administration (FDA) for treatment of opiate dependence (U.S. Food and Drug Administration, 2010). A randomized controlled trial that evaluated the FDA-approved formulation was conducted in Russia in a group of opiate dependent adults who had a significant other who could participate in treatment and who were not dependent on other substances (Krupitsky et al., 2011). Despite this select population, only 53% of participants adhered to the FDA-approved extended release formulation of naltrexone for 24 weeks. In a recent study in the United States with a less selective population, only 26% of participants in a usual care control condition continued to take monthly injections of the FDA-approved extended release formulation of naltrexone for 24 weeks (Defulio et al., 2012).
Several interventions have been developed to promote better adherence with oral naltrexone. Behavioral interventions that provide monetary reinforcement for naltrexone ingestion or integrate naltrexone adherence into supportive psychosocial treatments have shown particularly promising results and have produced the most robust rates of adherence to date (Carroll, Sinha, Nich, Babuscio, & Rounsaville, 2002; Carroll et al., 2001; Grabowski et al., 1979; Nunes, Rothenberg, Sullivan, Carpenter, & Kleber, 2006; Preston et al., 1999; Rawson, Glazer, Callahan, & Liberman, 1979; Rothenberg et al., 2002). However, these interventions have provided only short-term reinforcement for naltrexone adherence. Methods for promoting long-term adherence to naltrexone are likely necessary to address the chronic nature of opiate addiction (McLellan, Lewis, O’Brien, & Kleber, 2000).
Two recent clinical trials were conducted to determine whether a novel employment-based reinforcement intervention could increase adherence to extended release naltrexone (Defulio et al., 2012; Everly et al., 2011). Workplaces may be ideal contexts in which to arrange contingencies for naltrexone adherence for at least two reasons. First, workplaces contain strong reinforcers (e.g., paychecks, employment benefits) that can be arranged to promote naltrexone adherence. Second, because employment can be maintained over extended periods of time, employment-based reinforcement of naltrexone adherence can be an effective means of promoting long-term naltrexone use and prevent relapse to opiates. In both studies, unemployed, heroin dependent adults were hired into a model employment setting in which they could earn wages for working. After being successfully inducted onto oral naltrexone, participants were prescribed a 6-dose course of extended-release naltrexone injections that were administered every 3 (Everly et al., 2011) or 4 (Defulio et al., 2012) weeks. In both studies participants were assigned to a usual care group that could access the workplace and earn wages independent of their acceptance of extended-release naltrexone, or to a Contingency group that was required to accept extended-release naltrexone injections to access the workplace and earn wages. In both studies, Contingency participants were significantly more likely than usual care participants to take naltrexone injections and to accept all 6 injections. In the study that used the FDA-approved extended release formulation (Vivitrol®), 74% and 26% of participants in the Contingency and usual care groups, respectively, accepted all 6 injections (Defulio et al., 2012).
Despite this success, extended-release naltrexone is substantially more expensive than oral naltrexone and requires medical personnel to administer injections, thus oral naltrexone may still be preferred in many contexts. Oral naltrexone must be taken frequently (i.e., at least three times per week) to produce a continuous opiate blockade, which can be time consuming or hard to manage in therapeutic settings. Employment-based reinforcement holds particular promise for reinforcing adherence with oral naltrexone. First, two studies have demonstrated that employment-based reinforcement successfully reinforces adherence with extended-release naltrexone (DeFulio et al., 2012; Everly et al., 2011). Second, incorporating naltrexone administration into a workplace setting facilitates frequent monitoring of naltrexone ingestion without imposing substantial burden on the patient or the program staff. Given that naltrexone is used to treat both opioid and alcohol dependence, developing an intervention that can help reinforce compliance of this medication within a workplace setting has strong clinical relevance. For these reasons, we assessed the efficacy of an employment-based reinforcement intervention in maintaining adherence to oral naltrexone.
Methods
Study Participants
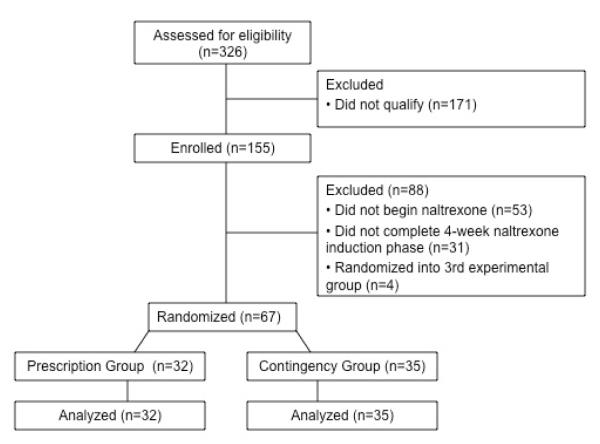
Volunteers were recruited from detoxification programs and through street outreach in Baltimore MD from May 2006 to September 2009. Research assistants assessed volunteers for study eligibility. Volunteers were eligible to participate if they were between the ages of 18-65, were unemployed (i.e., reporting no work in the past 30 days and earning < $200 in taxable income per month), self-reported injection drug use and had visible track marks (assessed via visual inspection), provided a urine sample that tested positive for both opiates and cocaine upon entry into detoxification, met DSM-IV criteria for opiate dependence (assessed using the Composite International Diagnostic Interview; Robins et al., 1989), were medically approved to be maintained on naltrexone by the study physician, and lived within reasonable commuting distance to the research unit (i.e., in Baltimore City and the immediately surrounding area). Cocaine use was originally included as an eligibility criterion to support a third experimental group in which participants would be required to ingest naltrexone and abstain from both opiates and cocaine to maintain maximum pay in the workplace, however this group was ultimately dropped in response to enrollment concerns (see Randomization). Volunteers were excluded if they had active hallucinations, delusions or a thought disorder, were judged to be of imminent threat to harm self or others, were currently incarcerated, in a halfway house or under constant monitoring, were pregnant or breastfeeding, had serum aminotransferase levels over three times normal, required opiates for other medical problems (and thus could not be maintained on naltrexone which would block the effects on any opiate), reported an interest in methadone treatment, had active Tuberculosis or had physical limitations that would have prevented use of a computer keyboard. All participants provided informed written consent to participate and the Johns Hopkins Medicine Institutional Review Board approved the study.
General Therapeutic Workplace Procedures
The study was conducted in a model therapeutic workplace in which employment-based reinforcement contingencies are arranged to promote therapeutic behavior change. All participants were invited to attend the workplace for four hours every weekday to work on training programs that were almost fully automated. Participants earned vouchers that were exchangeable for goods and services in the community. Earnings were based on attendance in the workplace and performance in the training programs. Overall, voucher earnings were arranged such that participants could earn a base pay of $8.00/hour for the hours worked in the workplace plus approximately $2.00/hour for their performance on the training programs, for a total potential wage of $10/hour. Detailed descriptions of the therapeutic workplace, the web-based training programs, the staffing requirements, and the cost of the intervention can be found elsewhere (DeFulio, Donlin, Wong, & Silverman, 2009; Donlin, Knealing, Needham, Wong, & Silverman, 2008; Knealing, Roebuck, Wong, & Silverman, 2008; Silverman et al., 2007). Participants in the Prescription group were eligible to work and earn vouchers independent of naltrexone consumption and/or drug use, however Contingency participants were only permitted to work when naltrexone consumption was objectively confirmed (described in more detail below).
Assessment Procedures
Assessments were conducted at study intake and every 30 days throughout the study. Primary assessment measures included the Addiction Severity Index-Lite (ASI-Lite; McLellan et al., 1985) to evaluate changes in medical, employment, alcohol, drug, social, legal and psychological functioning; the opiate, cocaine, alcohol and nicotine sections of the Composite International Diagnostic Interview (CIDI; (Compton, Cottler, Dorsey, Spitznagel, & Mager, 1996)) to evaluate psychiatric disorders; the Risk Assessment of Behavior (RAB; Navaline et al., 1994) to evaluate HIV-risk behaviors; and the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1996) and Symptom Checklist-90 (SCL-90; Derogatis, 1977) to evaluate psychosocial functioning. Additional measures were collected for exploratory purposes but will not be reported here.
Naltrexone is contraindicated for patients with hepatic damage or reductions in liver functioning, and is rated in the FDA pregnancy category C. Therefore, blood samples were taken at intake, and months 1, 2, 3, and 5 for liver function (aminotransferase) levels, and pregnancy tests were conducted monthly. Naltrexone was discontinued permanently for one Contingency and one Prescription participant, and temporarily for one Contingency and one Prescription participant due to abnormal aminotransferase levels. Naltrexone was discontinued for one Contingency participant due to pregnancy.
Urine samples were collected under same-sex staff observation upon arrival to the workplace every Monday, Wednesday, and Friday and at each 30-day assessment. Urine samples were analyzed immediately onsite for evidence of opiates (morphine, >300ng/ml), and cocaine (benzoylecgonine, >300ng/ml) using an Abbott AxSYM® fluorescent polarization immunoassay system. Samples collected at 30-day assessments were also analyzed for evidence of buprenorphine, methadone, amphetamine, benzodiazepines, and naltrexone. Urine samples were analyzed for naltrexone using an Enzyme Linked Immunosorbent Assay (ELISA) procedure; values <5ng/ml were considered negative for naltrexone (Friends Laboratory, MD). Participants were informed of their urinalysis results but there were no consequences for positive test results.
Participants were instructed to notify study staff of any adverse events they experienced as soon as possible after the occurrence of the event. Any staff member who was notified by a participant of a potential adverse event filed a formal report into an automated system, which immediately distributed the reports to study investigators and key staff. Ongoing or unresolved adverse events were also included in a weekly adverse event report that was distributed to study investigators and key staff. At all 30-day assessments study staff assessed new adverse events and attempted to resolve any ongoing or unresolved adverse events. Participants were referred to medical staff to receive treatments and concomitant medications as needed, and reports were filed with the IRB and funding agency in accordance with the relevant institutional requirements and guidelines.
Oral Naltrexone Treatment
Participants were required to complete opiate detoxification before being invited to attend the therapeutic workplace for induction onto oral naltrexone (Depade®; Mallinckrodt Inc.). All opiate detoxification and naltrexone inductions were overseen by a physician and were guided solely by clinical considerations. All participants were notified of the potential for heightened risk of overdose in relation to naltrexone treatment and after extended periods of opiate abstinence. These notifications were issued by study staff during the study consent process, and at monthly assessments, and were issued by medical staff prior to initiating naltrexone treatment, and whenever a blood draw was conducted. Overdose risk reminders were read aloud and signed by the study participants. Finally, monthly lunch-time overdose prevention seminars were provided and free pizza was provided to encourage seminar attendance.
During the 4-week induction period, participants were required to ingest scheduled doses of oral naltrexone to gain access to the therapeutic workplace. Induction onto oral naltrexone was determined by clinical judgment; participants were generally inducted using a 3-day dose run-up schedule (e.g., 12.5 mg, 25 mg, and 50 mg) in order to reach the full maintenance dose of 100 mg (Monday and Wednesday) and 150 mg (Friday) for the four-week period. Only participants who completed the induction period were considered eligible for randomization; these participants were invited to attend the therapeutic workplace for 26 weeks, and were offered oral naltrexone at no cost for the duration of the study. Participants were also offered access to outpatient drug abuse counseling that was provided independent of the workplace throughout their participation in the study.
Randomization
Prior to group assignment, participants were stratified to an experimental group in a 1:1 ratio according to whether they attended the workplace on >85% of days during the 4-week induction period, provided >1 opiate positive urine sample during the final 2 weeks of the induction period, and provided >75% cocaine-positive urine samples during the 4-week induction period. Eligible participants were assigned to one of two experimental groups (i.e., Prescription or Contingency) using an urn randomization method that ensured all levels of each stratification variable were evenly distributed across the groups (Wei & Lachin, 1988). This research design originally proposed three experimental groups; therefore, four participants were randomized into a third experimental group that required participants to ingest naltrexone under staff observation and provide an opiate and cocaine-negative urine sample to maintain maximum pay in the workplace. Due to logistical difficulties encountered early in the study, it became clear that it would not be possible to randomize a sample size large enough to support a 3-group comparison within the planned study duration. A second power analysis was completed to determine the appropriate sample size for a 2-group comparison (described below) and the third experimental group was eliminated. Data from participants randomized into the third group have not been included in these analyses.
Experimental Groups
All participants were invited to attend the therapeutic workplace for 26 weeks. Prescription group participants were provided a take-home supply of oral naltrexone every 30 days and were allowed to access the workplace independent of naltrexone ingestion. Contingency participants were required to ingest oral naltrexone under staff observation every Monday, Wednesday, and Friday to gain access to the workplace. Contingency participants who missed a naltrexone dose were not permitted to access the workplace until they were able to resume naltrexone. Additionally, missing a scheduled dose resulted in a base pay reset from $8 per hour to $1 per hour. After a reset a participant’s base pay increased by $1 per hour to a maximum of $8 per hour for every day that a participant attended the workplace for at least 5 minutes.
Sample Size
Sample size was determined by a power analysis based on the magnitude of the effect on the percentage of doses taken in a similar study of voucher reinforcement of oral naltrexone (Preston et al., 1999), assuming an alpha of .05 and a power of .80. The resulting sample size was 40 participants per group. The study ended after sponsor funding was exhausted, with 35 and 32 participants randomized into the Contingency and Prescription groups, respectively.
Outcome measures
The primary outcome measures were percentage of urine samples positive for naltrexone, and the percentage of urine samples negative for opiates and/or cocaine, as measured at 30-day assessments. Also analyzed were the results of urinalysis tests analyzed thrice weekly for evidence of opiates and cocaine; the correlation between naltrexone adherence and opiate use; the percentage of days that participants attended the therapeutic workplace; and the relationship between naltrexone adherence, opiate and cocaine urinalysis results and experimental group.
Data Analysis
Participant characteristics at intake were analyzed using Fisher’s Exact tests for dichotomous variables and independent t-tests for continuous variables. Analyses of urine samples were based on monthly and thrice weekly assessments collected after randomization. Naltrexone adherence was defined as having a naltrexone-positive urine sample; missing naltrexone samples were treated as negative for naltrexone. Missing drug use samples were treated positive for opiates and cocaine (e.g., missing = positive). Two alternative methods of handling missing urine samples were also analyzed in which missing samples were not replaced (e.g., missing = missing) or missing samples were interpolated (e.g., missing = interpolated) based on the result of the urine sample collected before and after the missing sample or group of samples. All methods of handling missing samples produced essentially the same results, therefore only missing = negative (naltrexone), missing = positive (opiates and cocaine), and missing = missing are reported here. Longitudinal dichotomous measures were analyzed using generalized estimating questions (GEE;(Zeger, Liang, & Albert, 1988)), and a Spearman’s rank correlation was used to evaluate the association between naltrexone adherence and opiate-negative and cocaine-negative urine samples. Retention in oral naltrexone treatment and the therapeutic workplace were analyzed using a Cox proportional hazard model. A participant was considered to have completed naltrexone treatment if he or she provided a naltrexone positive urine sample during the final study assessment (month 6), and to have completed the study if he or she attended the workplace >1 day during the final two weeks of the intervention. Total vouchers as a function of experimental group was compared using an independent t-test. The overall rate of adverse events was too low to merit quantitative analysis. A complete description of all adverse events that may have been related to the study appears below. Unless otherwise stated (e.g., missing=missing), all analyses were intent-to-treat. Two-tailed tests were used and results were considered statistically significant if P<.05. Statistical analyses were conducted using SAS software version 9.1
Results
Participant Characteristics and Flow Through the Study
Table 1 presents the characteristics of participants assessed at intake. Compared to the Prescription participants during the 30 days prior to intake, Contingency participants spent more money on drugs in the past 30 days than Prescription participants (P<.01). No additional group differences were observed.1 shows the flow of participants.
Table 1.
Participant characteristics at intake.
| Characteristica | Naltrexone Prescription (n=32) |
Naltrexone Contingency (n=35) |
Fisher’s Exact (p) |
t-test (p) |
|---|---|---|---|---|
| Age, mean (SEM), years | 46.82 (1.32) | 43.13 (1.53) | 0.08 | |
| Female, % | 31 | 46 | 0.17 | |
| Black, % | 91 | 80 | 0.89 | |
| Married, % | 56 | 63 | 0.89 | |
| High school diploma or GED, % | 62 | 51 | 0.36 | |
| Opiate dependent, %b | 100 | 100 | 0.99 | |
| Cocaine dependent, %b | 97 | 86 | 0.11 | |
| Alcohol use, mean days (SEM), past 30 days | 7.2 (1.9) | 4.9 (1.4) | 0.09 | |
| Majority unemployed past 3 yrs, % | 63 | 66 | 0.80 | |
| Past 30 days income, mean (SEM), $ | ||||
| Employment | 12 (7) | 0 (0) | 0.09 | |
| Welfare | 157 (29) | 108 (26) | 0.21 | |
| Pension, benefits, Social Security | 208 (114) | 71 (34) | 0.26 | |
| Mate, family, friends | 105 (39) | 110 (57) | 0.94 | |
| Illegal | 650 (261) | 1417 (501) | 0.18 | |
| Total income | 1132 (280) | 1707 (486) | 0.32 | |
| $ spent on drugs, mean (SEM), past 30 days | 956 (177) | 2253(535) | 0.03 | |
| Currently on parole/probation, % | 43 | 46 | 0.99 | |
| Lifetime >1 felony conviction, % | 97 | 88 | 0.20 | |
| HIV positive (%) | 16 | 11 | 0.58 | |
| Injection primary route of heroin administration, % | 91 | 97 | 0.26 | |
| Injection primary route of cocaine administration, % | 59 | 81 | 0.06 | |
| Grade levels, mean (SEM)c | ||||
| Reading | 9 (1) | 9 (1) | 0.79 | |
| Spelling | 8 (1) | 8 (1) | 0.75 | |
| Arithmetic | 6 (1) | 7 (1) | 0.53 |
Note. Results for Fisher’s Exact tests and t-tests are based on two-tailed tests with an alpha of .05.
Unless otherwise noted, characteristics are taken from the Addiction Severity Index – Lite.
Taken from the Composite International Diagnostic Interview.
Taken from the Wide Range Achievement Test.
Naltrexone Adherence and Retention
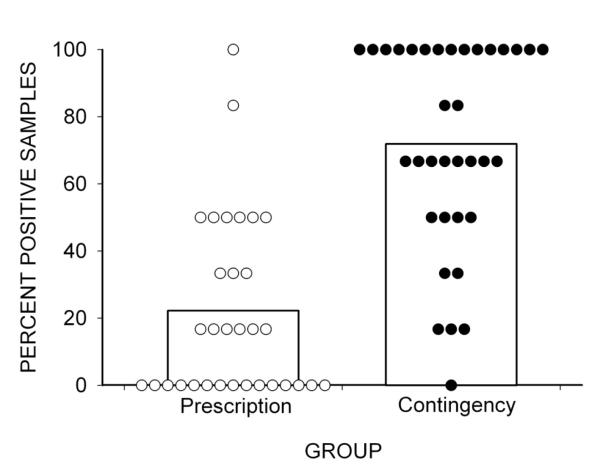
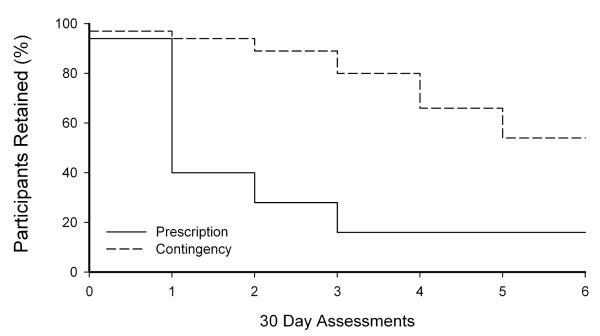
As seen in Figures 2 and 3, Table 2, and supplementary material, analysis of monthly urinalysis samples indicated that Contingency participants provided significantly more naltrexone-positive samples collected at 30-day assessments than Prescription participants (72% and 21%, respectively, P <.01). More Contingency participants also provided 100% naltrexone-positive urine samples (43%) than Prescription participants (3%; χ2 (1)= 14.52, P <.01, CI=2.85-190.0). A survival analysis revealed a statistically different pattern of naltrexone treatment adherence between the two groups [Figure 3; χ2 (1) = 21.29; P <.0001; HR = 0.22; 95% CI = 0.12 - 0.42], and Contingency participants were significantly more likely than Prescription participants to complete naltrexone treatment (54% vs. 16%, (χ2 (1)= 9.81, P <.01, CI=2.04 - 20.52).
Figure 2.
The percentage of urine samples that tested positive for naltrexone in the Prescription and Contingency groups. Bars show group percentages and circles show individual percentages.
Figure 3.
The percentage of participants retained in Naltrexone treatment (calculated by determining the final naltrexone-positive urinalysis sample provided).
Table 2.
Naltrexone, opiate, and cocaine urinalysis results, and workplace attendance for participants in the two study groups.
|
|
|||||
|---|---|---|---|---|---|
| Percentage |
|||||
| Prescription | Contingency | χ2 (1) | OR (95% CI) | P | |
| Monthly Urinalysis | |||||
| Naltrexone | |||||
| Missing negative | 21 | 72 | 27.53 | 8.67 (4.24-17.73) | <0.01 |
| Missing missing | 23 | 83 | 33.11 | 13.58 (6.62-27.89) | <0.01 |
| Opiate-negative | |||||
| Missing positive | 60 | 71 | 1.70 | 0.61 (0.29-1.26) | 0.19 |
| Missing missing | 65 | 81 | 3.43 | 0.46 (0.21-1.04) | 0.06 |
| Cocaine-negative | |||||
| Missing positive | 53 | 56 | 0.05 | 0.91 (0.43-1.95) | 0.82 |
| Missing missing | 58 | 63 | 0.10 | 0.88 (0.40-1.94) | 0.75 |
| Collected Samples | 92 | 87 | 0.98 | 1.77 (0.54-5.82) | 0.32 |
| Thrice Weekly Urinalysis | |||||
| Opiate-negative | |||||
| Missing positive | 60 | 68 | 1.35 | 0.71 (0.40-1.23) | 0.25 |
| Missing missing | 78 | 93 | 8.33 | 0.32 (0.16-0.64) | <0.01 |
| Cocaine-negative | |||||
| Missing positive | 56 | 58 | 0.03 | 0.95 (0.54-1.68) | 0.86 |
| Missing missing | 73 | 79 | 0.53 | 0.79 (0.42-1.49) | 0.47 |
| Collected Samples | 77 | 74 | 0.35 | 0.82 (0.44-1.56) | 0.55 |
| Days in Attendance | 69 | 62 | 1.05 | 1.34 (0.77–2.36) | 0.31 |
Opiate and Cocaine use
As shown in Table 2, collection rates of urine samples were high and similar across both groups. Analysis of monthly urine samples revealed no significant differences between Contingency and Prescription groups in percent of opiate negative or cocaine-negative urine samples, independent of how missing samples were handled. Analysis of thrice-weekly urine samples showed the Contingency and Prescription participants provided similar rates of opiate negative and cocaine negative urine samples when missing samples were considered positive. In contrast, when missing samples were not replaced (missing = missing), Contingency participants provided significantly more (P=.01) opiate negative thrice-weekly urine samples than Prescription participants.
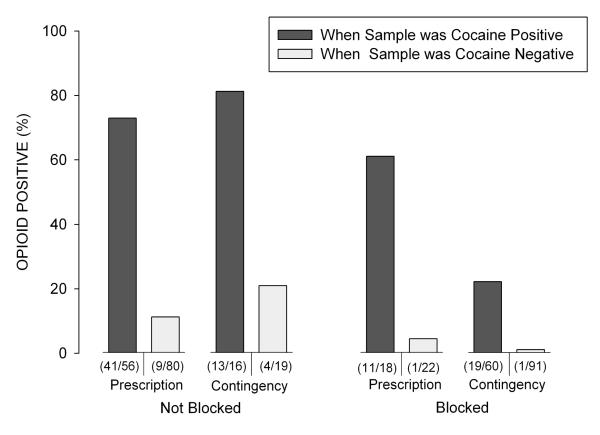
Independent of experimental group, the majority of urine samples collected at the monthly assessments that tested positive for opiates also tested positive for cocaine (84/151, 56%). A high rate of co-occurrence was seen both under conditions of naltrexone-positive (30/84, 36%) and naltrexone-negative (54/84, 64%) samples. GEE analyses (excluding missing samples) were conducted to determine whether opiate urinalysis results were associated with group (Contingency/ Prescription), naltrexone adherence (naltrexone positive urine sample, yes/no), cocaine use (urine sample positive for cocaine, yes/no), or interactions of those variables. As shown in Figure 4 and the supplementary material, these analyses indicated that opiate positive urine samples were significantly associated with cocaine positive urine samples (χ2 (1)= 27.17, P <.01, CI=7.43-50.97), and with naltrexone adherence (χ2 (1)= 5.28, P <.02, CI=1.59-7.71), independent of group assignment (P=0.40). A Spearman’s rank correlation test revealed that opiate negative urine samples collected during monthly assessments were negatively associated with cocaine positive samples (P= .004, rho = −0.35), but no association was observed with a naltrexone-positive urinalysis sample (P = .67, rho = −0.05).
Figure 4.
The percentage of monthly opiate positive urine samples as a function of the three different conditions under which the sample was submitted: whether or not the participant was not blocked (e.g., naltrexone negative urine sample) or blocked (e.g., naltrexone positive urine sample) by naltrexone at the time the sample was collected, study group, and whether the sample was cocaine positive.
Other Drug Use
Non-opiate or cocaine drug use was uncommon. Of the 370 monthly samples collected, 6% tested positive for buprenorphine, 3% tested positive for methadone, and 2% tested positive for benzodiazepines. No sample was positive for more than one of these substances.
Attendance and Voucher Earnings
As seen in Table 2, no significant difference in the mean percent workdays attended was identified between Contingency and Prescription participants (62% vs. 69%, respectively; P = .31). A survival analysis also revealed no between-group differences in pattern of therapeutic workplace retention [χ2 (1) = 0.71; P = 0.40; HR = 0.76; 95% CI = 0.41 – 1.44]. Participants in the Prescription group did attend the workplace for significantly more hours than participants in the Contingency group [M (SEM) = 308 (17.3) and 283 (23.8), respectively; P = .04], however the total voucher earnings for Prescription and Contingency participants was similar [M (SEM) = $4,710 ($268.40) and $4,320 ($355.07) respectively; P = .07].
Adverse Events
Relatively few adverse events that were judged to be possibly, probably, or definitely related to the study medication were recorded. Eight participants (12% of the total sample) experienced an adverse event, and a total of 16 events were recorded. The majority of participants who experienced an adverse event were assigned to the Contingency group (n=6). Events (ordered by frequency) included: sexual dysfunction (n=3), abdominal problems (n=2), headache (n=2), sleep problems (n=2), opioid withdrawal (n=2), nausea (n=1), chills (n=1), rapid heart rate (n=1), shakiness (n=1), and opioid overdose (n=1). Events were mild to moderate, with the exception of the opioid overdose, which resulted in death. The participant who died from an overdose had completed a 28-day inpatient opioid detoxification program prior to enrolling in the study. He had been assigned to the Contingency group and took all but three scheduled doses of naltrexone during the study. He provided opiate-negative urine samples consistently throughout the study. At the end of the study, he was offered and accepted a prescription to continue taking naltrexone after completing the study. The death occurred about one month after he completed the study. The cause of death on the death certificate was listed as narcotic intoxication (methadone and heroin) and cocaine use. It is unknown whether the participant was still taking naltrexone at the time of death. No participant discontinued study participation related to adverse effects of the study medication.
Discussion
Oral naltrexone might be an effective tool to protect against relapse to opiate use, however non-adherence has severely limited its utility in the treatment of opiate dependence (Adi et al., 2007; Kirchmayer et al., 2002; Minozzi et al., 2011; San et al., 1991; Sullivan et al., 2007). The current study showed that employment-based reinforcement of naltrexone adherence promoted high rates of adherence to oral naltrexone over a substantial duration of time. Consistent with other research, participants who were required to ingest oral naltrexone under staff observation to gain access to a workplace where they could work and earn monetary vouchers (i.e., Contingency group) showed greater adherence to oral naltrexone than did participants provided a take-home prescription and permitted to work independent of adherence (i.e., Prescription group), despite the fact that both groups were successfully inducted onto oral naltrexone for a 4 week period. Contingency participants were also significantly more likely than Prescription participants to have 100% of monthly urine samples test positive for naltrexone, representing 7 months of continuous naltrexone maintenance. Overall, these data show that an employment based reinforcement intervention was effective in maintaining adherence to oral naltrexone and closely parallel the results of two previous studies (Defulio et al., 2012; Everly et al., 2011) that used an employment-based reinforcement intervention to promote adherence with extended-release naltrexone in unemployed heroin users.
More than 80 percent of the scheduled monthly urine samples were collected for both experimental groups, and no difference in collection rates were observed between the groups. This high rate of urine sample collection permitted a rigorous evaluation of the effects of the study intervention on naltrexone adherence and concurrent drug use. It is interesting that the significant and substantial difference in adherence to oral naltrexone between the two study groups was not associated with a significant difference in opiate abstinence. Yet, these results are consistent with the results of two previous studies that utilized an employment-based reinforcement intervention to promote adherence with extended-release naltrexone (Defulio et al., 2012; Everly et al., 2011).
The nonsignificant between-group difference in opiate abstinence that occurred despite differences in naltrexone adherence may be due to the fact that Contingency participants continued to use opioids despite being maintained on naltrexone. As seen in the supplementary material, many Contingency participants continued to abuse opioids, often in conjunction with cocaine, despite providing a naltrexone-positive urinalysis sample. The phenomenon of challenging a naltrexone blockade with illicit opiates has been previously reported among patients maintained on both oral and extended release naltrexone (Fishman, 2008; Kunoe et al., 2010; Sullivan et al., 2007). One study reported that 25% of participants receiving oral naltrexone used opiates despite providing biochemical evidence of oral naltrexone adherence (Sullivan et al., 2007). A second study that evaluated patients receiving extended release naltrexone reported that 56% of participants used opioids on one or more occasions, though only 30% reported experiencing a partial or full opioid high (Kunoe et al., 2010). These authors commented that the persistent opiate use despite the naltrexone adherence was difficult to understand because it occurred when naltrexone levels are considered sufficient to antagonize the physiological and reinforcing effects of opiates, and because the majority of participants did not endorse achieving any positive subjective effects. The authors also noted that opiate use occurred most often in conjunction with other types of drug use, which is supported by results from our laboratory (Defulio et al., 2012; Everly et al., 2011). In the current study, a secondary analysis that controlled for study group and naltrexone adherence revealed that urine samples were significantly more likely to be opiate positive when they were also cocaine positive (Figure 4 and supplementary material). This finding is consistent with other studies that have reported an association between cocaine use and likelihood of opiate use following detoxification (Broers, Giner, Dumont, & Mino, 2000; Gossop, Stewart, Browne, & Marsden, 2002). Ultimately, untreated cocaine use may have limited the clinical effectiveness of oral naltrexone maintenance on opiate use and may help explain the nonsignificant effect of naltrexone on opioid-positive urinalyses. These data suggest it may be particularly important for providers to offer treatment for polysubstance abuse concurrent with oral naltrexone treatment to prevent relapse to opiates among polysubstance abusers.
Few adverse events of naltrexone were recorded. Adverse events were more prevalent within the Contingency group, which is likely due to the higher rates of naltrexone adherence observed within those participants. These data also revealed no apparent effect of gender on the frequency of adverse events, particularly nausea. This is in contrast to previous studies that have reported a higher incidence of nausea among women taking naltrexone for alcohol dependence (Pettinati, et al., 2008; O’Malley, Krishnan-Sarin, Farren, & O’Connor, 2000; Suh, Pettinati, Kampman, & O’Brien, 2008). In this study, the only report of nausea was provided by a female participant on her first day of naltrexone dosing. The overdose death that occurred about one month after the participant completed the study may have occurred because the participant had decreased tolerance to opioid drugs as a result of the inpatient opioid detoxification that he completed prior to enrolling in the study and the months of opioid abstinence and naltrexone adherence that he experienced during the study. The overdose occurred despite the fact that we repeatedly provided overdose risk reminders to this and other participants in the study. Opioid overdose is a known risk of extended periods of opioid abstinence and naltrexone treatment.
Some limitations should be noted. First, this study did not enroll the intended sample size and therefore may not have been sufficiently powered to detect between-group differences on urinalysis outcomes. Second, only participants who completed the 4-week induction period were randomized into the study. As noted in Figure 1, 31 participants were excluded for failure to complete the induction period. Since access to the workplace was being used to reinforce naltrexone consumption, it was appropriate to only enroll participants who demonstrated that the workplace was functioning as a reinforcer. However, this may have resulted in a sampling bias whereby only participants willing to adhere with an oral naltrexone regimen continued to attend the workplace and ultimately become enrolled. This may have impacted the final study outcomes. Third, the two experimental groups differed both on whether access to the workplace was contingent on naltrexone ingestion and whether naltrexone ingestion was administered at the workplace and observed. It is possible that naltrexone adherence may have been higher among Prescription participants if naltrexone was administered and ingestion was observed at the workplace. However, the Prescription group was intended to provide a real-world comparison of naltrexone adherence. This is important because few studies have followed the natural history of naltrexone adherence for such an extended period (6 months), and we know of no study that has verified adherence with naltrexone urinalysis testing. Thus, this design provided an opportunity to rigorously evaluate naltrexone adherence as it would occur in real-world settings, to provide a strong control for the Contingency group. Despite these limitations this study still showed a robust effect of the workplace on oral naltrexone adherence and highlighted several avenues for future research in this area.
Figure 1.
Flow of participants through the study.
Substance abuse can be a chronic, relapsing problem that may require extended treatment (McLellan et al., 2000). Opiate dependence has been associated with a variety of adverse societal consequences, including HIV transmission, criminal activity, unemployment and death, and overall costs are estimated to exceed $20 billion annually (National Consensus Development Panel on Effective Medical Treatment of Opiate Addiction, 1998; Mark, Woody, Juday, & Kleber, 2001; Wall et al., 2000). Addressing opiate dependence is particularly important considering the recent escalation in prescription opioid abuse and related societal costs (Birnbaum et al., 2011; Strassels, 2009). Maintenance on the opiate agonists methadone or buprenorphine already address the chronic nature of opiate dependence, and naltrexone may represent an additional long-term treatment option that is particularly important for patients who have been successfully detoxified and are seeking to prevent relapse. Several studies have demonstrated that a workplace environment can function as a reinforcer, and this study extends upon this research by suggesting that employment-based interventions can successfully reinforce medication adherence in a relatively noninvasive manner. Medications like naltrexone could be administered in community workplaces and a staff member can be appointed to monitor medication administration prior to an employee beginning his or her shift. Employees may opt into this type of program to help them remain compliant with medications, and this may be particularly valuable for individuals for whom medication adherence helps them to control conditions that might otherwise interfere with or jeopardize their job performance (e.g., alcohol or drug dependence, certain psychiatric conditions). Though there may be ethical concerns with mandating medication compliance as a condition of employment, this approach may preferable to job termination to all involved if an employer requires that the employee maintains drug abstinence but the employee is not successful in controlling their drug use without the benefit of medication. Ultimately, these results suggest employment-based reinforcement interventions that require participants to ingest naltrexone in order to gain access to a workplace can reinforce naltrexone and enhance adherence over an extended period, and have potential for use with other long-term treatments that suffer from poor adherence, such as anti-psychotic or anti-retroviral medications.
Supplementary Material
Acknowledgements and Disclosures
The project described was supported by Award Numbers R01DA019386 and T32DA07209 from the National Institute On Drug Abuse
This research was conducted while Dr. Will M. Aklin was affiliated with Johns Hopkins University. Dr. Aklin is now at the National Institute on Drug Abuse. The views expressed in this article do not necessarily represent the views of the National Institute on Drug Abuse, the National Institutes of Health, the Department of Health and Human Services, or the United States Government.
In recent years George Bigelow has received research support from Pain Therapeutics, Inc. and Titan Pharmaceuticals and consulting payments from Abbott Laboratories, Acura Pharmaceuticals, GW Pharmaceuticals, Pfizer, Teva Pharmaceuticals, and Transcept Pharmaceuticals. Paul A. Nuzzo has been paid as a statistical consultant / project coordinator for the NIDA Clinical Trials Network (CTN), NIDA Clinical Coordinating Center, and Yaupon Therapeutics, Inc.
The authors would like to acknowledge the hard work of the numerous Center for Learning and Health and the Behavioral Pharmacology Research Unit employees who assisted in the administration of this study, without whom this research would not have been possible.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha
Trial Registration: Clinicaltrials.gov Identifier: NCT 00149669
The remaining authors report no conflicts of interest or financial disclosures.
All authors provided substantive contributions to the manuscript and have read and approved the final manuscript.
References
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, Burls A. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: A systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2007;11(6):iii–iv. 1–85. doi: 10.3310/hta11060. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the united states. Pain Medicine (Malden, Mass.) 2011;12(4):657–667. doi: 10.1111/j.1526-4637.2011.01075.x. doi:10.1111/j.1526-4637.2011.01075.x; 10.1111/j.1526-4637.2011.01075.x. [DOI] [PubMed] [Google Scholar]
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in geneva: Follow-up at 1 and 6 months. Drug and Alcohol Dependence. 2000;58(1-2):85–92. doi: 10.1016/s0376-8716(99)00063-0. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O’Connor PG, Eagan DA, Frankforter TL, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: Efficacy of contingency management and significant other involvement. Archives of General Psychiatry. 2001;58(8):755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Sinha R, Nich C, Babuscio T, Rounsaville BJ. Contingency management to enhance naltrexone treatment of opioid dependence: A randomized clinical trial of reinforcement magnitude. Experimental and Clinical Psychopharmacology. 2002;10(1):54–63. doi: 10.1037//1064-1297.10.1.54. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Hulse GK. Sustained-release naltrexone: Novel treatment for opioid dependence. Expert Opinion on Investigational Drugs. 2007;16(8):1285–1294. doi: 10.1517/13543784.16.8.1285. doi:10.1517/13543784.16.8.1285. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Dorsey KB, Spitznagel EL, Mager DE. Comparing assessments of DSM-IV substance dependence disorders using CIDI-SAM and SCAN. Drug and Alcohol Dependence. 1996;41(3):179–187. doi: 10.1016/0376-8716(96)01249-5. [DOI] [PubMed] [Google Scholar]
- DeFulio A, Donlin WD, Wong CJ, Silverman K. Employment-based abstinence reinforcement as a maintenance intervention for the treatment of cocaine dependence: A randomized controlled trial. Addiction (Abingdon, England) 2009;104(9):1530–1538. doi: 10.1111/j.1360-0443.2009.02657.x. doi:10.1111/j.1360-0443.2009.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defulio A, Everly JJ, Leoutsakos JM, Umbricht A, Fingerhood M, Bigelow GE, Silverman K. Employment-based reinforcement of adherence to an FDA approved extended release formulation of naltrexone in opioid-dependent adults: A randomized controlled trial. Drug and Alcohol Dependence. 2012;120(1-3):48–54. doi: 10.1016/j.drugalcdep.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90: Administration, scoring & procedures manual for the revised version. Clinical Psychometric Research; Baltimore, MD: 1977. [Google Scholar]
- Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. Journal of Applied Behavior Analysis. 2008;41(4):499–516. doi: 10.1901/jaba.2008.41-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effective medical treatment of opiate addiction. national consensus development panel on effective medical treatment of opiate addiction. JAMA : The Journal of the American Medical Association. 1998;280(22):1936–1943. [PubMed] [Google Scholar]
- Everly JJ, Defulio A, Koffarnus MN, Leoutsakos JM, Donlin WD, Aklin WM, Silverman K. Employment-based reinforcement of adherence to depot naltrexone in unemployed opioid-dependent adults: A randomized controlled trial. Addiction (Abingdon, England) 2011;106(7):1309–1318. doi: 10.1111/j.1360-0443.2011.03400.x. doi:10.1111/j.1360-0443.2011.03400.x; 10.1111/j.1360-0443.2011.03400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. Precipitated withdrawal during maintenance opioid blockade with extended release naltrexone. Addiction (Abingdon, England) 2008;103(8):1399–1401. doi: 10.1111/j.1360-0443.2008.02252.x. doi:10.1111/j.1360-0443.2008.02252.x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: Protective effect of coping responses. Addiction (Abingdon, England) 2002;97(10):1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- Grabowski J, O’Brien CP, Greenstein R, Ternes J, Long M, Steinberg-Donato S. Effects of contingent payment on compliance with a naltrexone regimen. The American Journal of Drug and Alcohol Abuse. 1979;6(3):355–365. doi: 10.3109/00952997909001724. [DOI] [PubMed] [Google Scholar]
- Kirchmayer U, Davoli M, Verster AD, Amato L, Ferri A, Perucci CA. A systematic review on the efficacy of naltrexone maintenance treatment in opioid dependence. Addiction (Abingdon, England) 2002;97(10):1241–1249. doi: 10.1046/j.1360-0443.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Knealing TW, Roebuck MC, Wong CJ, Silverman K. Economic cost of the therapeutic workplace intervention added to methadone maintenance. Journal of Substance Abuse Treatment. 2008;34(3):326–332. doi: 10.1016/j.jsat.2007.04.013. doi:10.1016/j.jsat.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: A double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. doi: 10.1016/S0140-6736(11)60358-9. doi:10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- Kunoe N, Lobmaier P, Vederhus JK, Hjerkinn B, Gossop M, Hegstad S, Waal H. Challenges to antagonist blockade during sustained-release naltrexone treatment. Addiction (Abingdon, England) 2010;105(9):1633–1639. doi: 10.1111/j.1360-0443.2010.03031.x. doi:10.1111/j.1360-0443.2010.03031.x. [DOI] [PubMed] [Google Scholar]
- Mark TL, Woody GE, Juday T, Kleber HD. The economic costs of heroin addiction in the united states. Drug and Alcohol Dependence. 2001;61(2):195–206. doi: 10.1016/s0376-8716(00)00162-9. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR, Mansky PA. Naltrexone, an antagonist for the treatment of heroin dependence. effects in man. Archives of General Psychiatry. 1973;28(6):784–791. doi: 10.1001/archpsyc.1973.01750360022003. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. JAMA : The Journal of the American Medical Association. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the addiction severity index. reliability and validity in three centers. The Journal of Nervous and Mental Disease. 1985;173(7):412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. Operant analysis of human heroin self-administration and the effects of naltrexone. The Journal of Pharmacology and Experimental Therapeutics. 1981;216(1):45–54. [PubMed] [Google Scholar]
- Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database of Systematic Reviews (Online) 2011;4:CD001333. doi: 10.1002/14651858.CD001333.pub4. doi:10.1002/14651858.CD001333.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, Woody GE. Preparations for AIDS vaccine trials. an automated version of the risk assessment battery (RAB): Enhancing the assessment of risk behaviors. AIDS Research and Human Retroviruses. 1994;10(Suppl 2):S281–3. [PubMed] [Google Scholar]
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: A ceiling on effectiveness? The American Journal of Drug and Alcohol Abuse. 2006;32(4):503–517. doi: 10.1080/00952990600918973. doi:10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, O’Connor PG. Naltrexone-induced nausea in patients treated for alcohol dependence: Clinical predictors and evidence for opioid-mediated effects. Journal of Clinical Psychopharmacology. 2000;20(1):69–76. doi: 10.1097/00004714-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O’Brien CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. Journal of Substance Abuse Treatment. 2008;34(4):378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug and Alcohol Dependence. 1999;54(2):127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Glazer M, Callahan EJ, Liberman RP. Naltrexone and behavior therapy for heroin addiction. NIDA Research Monograph. 1979;(25):26–43. doi: 10.1037/e497382006-005. (25) [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen H-U, Helzer JE, Babor TF, Burke J, Towle LH. The Composite International Diagnostic Interview: An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry. 1989;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: An integrated treatment for opiate dependence. Journal of Substance Abuse Treatment. 2002;23(4):351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- San L, Pomarol G, Peri JM, Olle JM, Cami J. Follow-up after a six-month maintenance period on naltrexone versus placebo in heroin addicts. British Journal of Addiction. 1991;86(8):983–990. doi: 10.1111/j.1360-0443.1991.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Walsh SL, Stitzer ML. Onset, magnitude and duration of opioid blockade produced by buprenorphine and naltrexone in humans. Psychopharmacology. 1999;145(2):162–174. doi: 10.1007/s002130051045. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, Kolodner K. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. Journal of Applied Behavior Analysis. 2007;40(3):387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassels SA. Economic burden of prescription opioid misuse and abuse. Journal of Managed Care Pharmacy : JMCP. 2009;15(7):556–562. doi: 10.18553/jmcp.2009.15.7.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JJ, Pettinati HM, Kampman KM, O’Brien CP. Gender differences in predictors of treatment attrition with high dose naltrexone in cocaine and alcohol dependence. The American Journal on Addictions. 2008;17(6):463–468. doi: 10.1080/10550490802409074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Garawi F, Bisaga A, Comer SD, Carpenter K, Raby WN, Nunes EV. Management of relapse in naltrexone maintenance for heroin dependence. Drug and Alcohol Dependence. 2007;91(2-3):289–292. doi: 10.1016/j.drugalcdep.2007.06.013. doi:10.1016/j.drugalcdep.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration FDA news release: FDA approves injectable drug to treat opioid-dependent patients. 2010.
- Wall R, Rehm J, Fischer B, Brands B, Gliksman L, Stewart J, Blake J. Social costs of untreated opioid dependence. Journal of Urban Health : Bulletin of the New York Academy of Medicine. 2000;77(4):688–722. doi: 10.1007/BF02344032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Sullivan JT, Preston KL, Garner JE, Bigelow GE. Effects of naltrexone on response to intravenous cocaine, hydromorphone and their combination in humans. The Journal of Pharmacology and Experimental Therapeutics. 1996;279(2):524–538. [PubMed] [Google Scholar]
- Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Controlled Clinical Trials. 1988;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.