Abstract
Factors associated with high-dose opioid therapy for non-cancer pain are poorly understood. We document the prevalence of high-dose opioid use, as well as associated demographic, clinical, and health service utilization correlates among low back pain patients. Patients prescribed higher-dose opioids (≥100 mg/day morphine equivalent at last dispensing; n=453) and receiving opioids for 90+ consecutive days were compared to two groups: lower-dose (1–99 mg/day; n=4,815) or no opioid use (n=10,184). Higher-dose opioid use occurred in 2.9% of patients who received any opioids and in 8.6% of patients who received opioids long-term. The median dose in the higher-dose group was 180.0 mg/day. Compared to the no opioid group, higher-dose users reported poorer health. Compared to either comparison group, patients in the higher-dose group had higher rates of mental health and substance use disorders, concurrent sedative-hypnotic use (60.5%; n=274), and health service utilization. After adjusting for select covariates, male gender (Odds ratio (OR) 1.68, 95%CI 1.37,2.06), higher comorbidity, Medicare coverage (OR 1.65, 95% CI 1.22,2.23), any mental health or substance use diagnosis (OR 1.58, 95% CI 1.28,1.95), co-prescriptions of sedative-hypnotics (OR 1.75, 95% CI 1.42,2.16), and more Emergency Department and specialty pain clinic visits were associated with higher likelihood of high-dose prescriptions.
Keywords: Chronic pain, Back pain, Opioids, Epidemiology, Pain/drug therapy
Introduction
Opioid prescribing for the treatment of chronic non-cancer pain (CNCP) in primary care has increased dramatically since the mid 1990s despite ongoing concerns surrounding effectiveness and safety.3,6,19 This increase is mirrored by a parallel increase in the misuse, abuse and overdose of prescription opioids.3 Thus, opioid prescribing requires a balancing act between potential benefits and risks.
Most patients receiving opioid therapy take low to moderate doses.6 However, a substantial number are on higher-dose opioid therapy for CNCP.19,23 Conventional wisdom suggests there is no absolute limit to opioid dose because development of tolerance varies among individuals, and there is no consensus on definitions of lower-dose versus higher-dose therapy. Rather, higher-dose opioid therapy is increasingly defined empirically based on elevated risks associated with certain doses. For example, daily doses of 100 mg or more morphine equivalents may be considered high-dose use because of the substantial risks for side effects, overdose and mortality associated with this dosage or higher.3,6,11,17,26
Evidence for the efficacy of high-dose opioid therapy for CNCP is sparse and mixed.2,7 A three-year registry study found that only 5% of the 233 patients who were on more than 100 mg of oxycodone per day were able to achieve sustained analgesic benefit from opioid therapy.25 In a study of patients with disabling musculoskeletal disorders, Kidner and colleagues19 found that higher doses of opioids (greater than 61mg/day of morphine equivalents) predicted worse outcomes, including program non-completion, lower rates of return to work, and higher healthcare utilization.
Beyond uncertain efficacy, important safety concerns are associated with long-term opioid therapy, including side effects, addiction, misuse, and possible diversion. These harms may be more prominent for patients on high-dose opioid therapy. Prior research suggests that patients on higher-doses of opioids may experience unique side effects, such as endocrinologic abnormalities, arrhythmia (with methadone), and fracture risk.8,9,21,26 Most troubling is the association of higher-doses with increased risk of overdose and death.11,17 Morasco and colleagues23 found that high-dose opioid therapy (180 mg of morphine-equivalent per day or greater) was associated with multiple pain diagnoses and high levels of other medical, mental health and substance use comorbidity in a sample of veterans in the Pacific Northwest.
Identifying the personal, clinical, and health service utilization characteristics of higher-dose opioid users may help to identify patients most likely to progress to higher-dose use and suggest strategies for improving their care. We therefore sought to examine correlates of higher-dose opioid use among patients in a routine primary care setting, taking advantage of the large population and electronic records of an integrated health plan with mainly pre-paid insurance coverage. We focused on patients with back pain because it is common, a leading reason for opioid prescribing, often occurs in the absence of major systemic diseases, and provides a more homogenous sample than considering all pain conditions. Our goals were to: 1) determine the prevalence of higher-dose opioid prescribing, 2) identify the demographic and clinical characteristics of patients receiving higher-dose opioids, and 3) examine health service utilization patterns among higher-dose opioid users. We compared patients receiving more or less than 100 mg of morphine equivalents per day because this dose is associated with substantially increased risk of overdose and death.11 We also compared opioid users with patients who had back pain but were not receiving opioids.
Methods
Our methods and patient population have been described elsewhere.10 This study was conducted in the Kaiser Permanente Northwest (KPNW) region, a large, not-for-profit, integrated health care system. KPNW serves the Portland, Oregon and Vancouver, Washington metropolitan area. KPNW currently has an annual membership of about 470,000 people with demographic characteristics similar to the community it serves, and covers 17% of the metropolitan area.
Dispensed prescriptions are recorded through an automated outpatient pharmacy system. Based on patient surveys, an estimated 90 percent of prescriptions are filled at a program pharmacy.28 While physicians may prescribe any marketed medication, KPNW has a formulary of recommended medications. Data from this system are linked to administrative and research databases with detailed information on the patient, clinician, and medication for each dispensed prescription. KPNW’s data systems are accessible for research purposes. This study was approved by the Institutional Review Boards at the Kaiser Permanente Center for Health Research (KPCHR) and at Oregon Health & Science University (OHSU).
Patient Selection
Participants were adult ambulatory patients aged 18 and older. We made use of electronic medical and pharmacy data in a “virtual data warehouse” at KPCHR. To select patients with back pain, we chose as an index visit the first visit in 2004 with any one of 32 ICD-9 diagnoses associated with low back pain.10 We used electronic pharmacy and medical record data for 6 months before and after the index visit. Because our focus was on patients with musculoskeletal back pain, we excluded patients with cancer, spinal infections, open fractures, or pregnancy.
Defining Opioid Dose Groups
We analyzed electronic pharmacy and medical record data for 6 months before and after the 2004 index visit, including data from 2003 and 2005. Participants were classified into one of three study groups based on their last prescription dispensed: pain patients not prescribed any opioids, those prescribed lower-doses of opioids (defined as 1 – 99 mg morphine equivalent per day), and those prescribed higher-doses of opioids (defined as ≥ 100 mg morphine equivalent per day). Because we were interested in long-term use of opioid medications, for both the lower and higher-dose groups, we selected patients who used opioids for greater than 90 consecutive days. These were patients who met the definition set by Von Korff et al20 as “episodic” or “chronic” opioid use. Patients with short-term use (<90 days) were excluded for several reasons: (1) short-term use is less likely to be higher-dose use, (2) our focus was on long-term use rather than short duration or acute use because these patients are less likely to suffer serious long-term consequences, (3) our goal was to generate data comparable to previous studies that have restricted samples to long-term, higher-dose users, and (4) we strove for straight forward interpretation of our analysis by dosage as we previously described opioid use by duration in a separate report. 10
We considered use of any of the opioids listed by Von Korff et al.20 and classified opioids as long or short acting based on their definitions. To calculate morphine-equivalents, each prescription was given a conversion factor to estimate the same milligram amount per day of morphine, and doses of multiple opioids were summed. Conversion factors were those of Von Korff and colleagues.20
Measures of Psychiatric Diagnoses, Comorbidity and Health Services Utilization
We assessed several patient characteristics, including demographics, medical and psychiatric comorbidity, and health behaviors (e.g., smoking, body mass index). We also measured several aspects of health services utilization, including co-prescription of sedative-hypnotics, emergency department (ED) visits, clinic visits, pain clinic visits, number of opioid providers, and hospital care.
Psychiatric diagnoses were based on medical record search for one year before the index visit for any coded ICD-9 diagnoses for depression (codes 296.2, 296.3, 300.4, 309.0, 309.1, 311); anxiety (codes 300.0 – 300.09); posttraumatic stress disorder (code 309.81); or substance use disorder (codes 303.xx, 304.xx, 305.xx). These diagnoses were based on clinician judgments as detailed in the electronic medical record.
Sedative-hypnotic drugs were those identified in the Medi-Span Generic Product Identifier32 or the American Hospital Formulary Service drug information compendium with Benzodiazepines represented the largest group percentage.1,14
Comorbidity was measured using the RxRisk score. The RxRisk is a pharmacy-based risk assessment model designed to predict future health care costs based on patient age, sex, Medicare or Medicaid coverage, and use of drugs closely linked to specific chronic conditions (e.g., biguanides, insulins, sulfonylureas for diabetes).15,16 It was developed in a managed care system with electronic records very similar to KPNW. A score is calculated from a regression model that weights each diagnosis according to its ability to predict future costs. The RxRisk calculation for adults excludes analgesics, because they are prescribed with too much discretion to be appropriate for a payment adjustment model.16
Statistical Analysis
We used the Wald Chi-square test for trend to compare proportions across ordered categories of opioid dose. For continuous data, which generally had skewed distributions, we used the Kruskall-Wallis nonparametric rank-sum test followed by post-hoc testing using the Wilcoxon. Significance levels for pairwise post-hoc tests were adjusted using a Bonferroni correction. Multivariate analysis used a backward elimination logistic regression to evaluate characteristics associated with prescriptions of higher-dose opioid medications adjusting for age and sex. The dependent variable was prescription for higher-dose (versus lower-dose) of opioids. Because our goal was to examine differences between patients prescribed lower- doses of opioids versus those prescribed higher-dose opioids, this analysis excluded patients who were not prescribed any opioid medications. Independent variables were eligible to be entered into Step 1 if they significantly differed between groups in the bivariate analysis (p < 0.10). Only participants for whom all variables were available were included in the multivariate analysis. All statistical analyses were performed in STATA Version 12 (Stata Corp, College Station, Texas).
Results
Patient Demographics
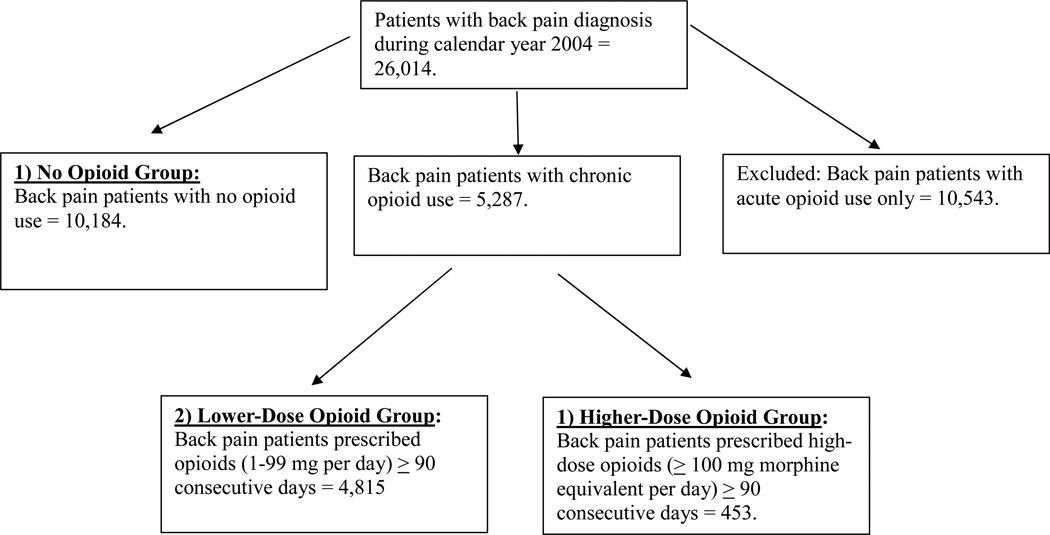
We identified 26,014 patients with a diagnosis of low back pain who met our eligibility criteria (Figure 1). Most patients with back pain (78%) received non-specific diagnoses such as “low back pain,” or “sprains and strains.” Another 12% had herniated discs, sciatica, degenerative discs, or spinal stenosis. The remainder received a variety of diagnoses (e.g. spondylolisthesis, closed vertebral fractures, post-surgical pain).
Figure 1.
Data Flow for Back Pain Patients on Higher Doses of Opioid Medications.
Note. 5,287 patients were initially identified as having a long-term episode of opioid use based on pharmacy record; however, for 19 of these patients dose level was not recorded in the pharmacy record, resulting in a total sample of 5,268 (4,815 + 453) for patients on lower and higher-dose opioids.
Among patients with a back pain diagnosis, 15,830 (61%) received at least one opioid prescription in the year surrounding the index visit, 2.9% of these patients received a higher-dose opioid prescription as their final prescription. Among patients receiving long-term opioids, 8.6% received a higher-dose opioid prescription as their final prescription. Patient demographic characteristics are reported in Table 1. The typical back pain patient was 50.3 years old (SD = 16.6), female (56.5%), and non-Hispanic white (89.3%). Average age did not differ between the lower versus higher-dose opioid users. Non-Hispanic white patients were overrepresented in the higher-dose opioid group compared to Black or Hispanic patients (data missing for race 31.3% and for ethnicity 48.4% of participants). A significantly higher proportion of women were represented in the lower-dose group than in the no-opioid group or higher-dose group. A significantly higher proportion of Medicare patients were in the higher-dose opioid group.
Table 1.
Comparison of Demographic Characteristics by Opioid Dose Group
| No Opioid Group (n=10,184) |
Lower-Dose Opioid Group (n=4,815) |
Higher-Dose Opioid Group (n=453) |
Test (df) | p-value | |
|---|---|---|---|---|---|
| Age, mean (SD)* | 49.1 (16.7)a | 54.7 (15.5)b | 54.7 (15.0)b | χ2 (2, 15452) = 352.8 | < 0.001 |
| Female Gender† % | 54.3a | 63.3b | 55.6ac | χ2 (1) = 72.9 | < 0.001 |
| Race‡ (%) | |||||
| Caucasian | 5,673 (55.7) | 3,614 (75.1) | 362 (79.9) | ||
| Black | 143 (1.4) | 134 (2.8) | 7 (1.6) | ||
| Native American/Alaskan Native | 71 (0.7) | 49 (1.0) | 9 (2.0) | ||
| Asian Pacific Islander | 256 (2.5) | 40 (0.8) | 4 (0.9) | ||
| Other | 270 (2.7) | 99 (2.1) | 7 (1.6) | ||
| Unknown/Declined to answer | 3,771 (37.0) | 879 (18.3) | 64 (14.1) | ||
| Hispanic Ethnicity†,§ | 259 (5.2)a | 93 (3.2)b | 5 (1.8)ab | χ2 (1) = 22.9 | < 0.001 |
| Medicaid†, % | 3 (0.03)a | 3 (0.06)b | 1 (0.22)c | χ2 (0) = 2.7 | 0.14 |
| Medicare†, % | 1,816 (17.8)a | 1,352 (28.l)ab | 154 (34.0)ab | χ2 (1) = 237.4 | <0.001 |
Kruskal-Wallis non-parametric test was used for continuous variables followed by Wilcoxan post-hoc testing. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Wald Chi-Square test for trend was used for categorical variables. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Race data were missing for 8,145 or 31.3% of participants.
Ethnicity data were missing for 12,579 or 48.4% of participants.
Health Behaviors, Psychiatric Characteristics, and Comorbidity
Patients in the higher-dose group had several indications of poorer health than patients in the lower-dose or no opioid groups (Table 2). Health behaviors, including obesity and smoking, were significantly associated with increasing opioid dose in a graded fashion. Among the higher-dose group, 52% had a Body Mass Index (BMI) ≥30 and 57% were recent or current smokers compared to 50% with a BMI ≥30 and 51% smokers in the lower-dose group. Psychiatric diagnoses also increased consistently with opioid dose, with patients in the higher-dose group having higher frequencies than other groups for depression (42%), anxiety (20%), post-traumatic stress disorder (PTSD; 4%), and substance use disorder (SUD; 31%) relative to the lower-dose group which had frequencies for depression (30%), anxiety (11%), PTSD (2%), and SUD (24%). Patients in the higher-dose group and lower-dose group also had significantly higher medical comorbidity scores compared to patients in the no-opioid group.
Table 2.
Health Behaviors, Psychiatric Characteristics, and Comorbidity by Opioid Dose Group
| No- Opioid Group (n=10,184) |
Lower-Dose Opioid Group (n=4,815) |
Higher-Dose Opioid Group (n=453) |
Test (df) | p-value | |
|---|---|---|---|---|---|
|
Health Behaviors | |||||
| BMI ≥ 30*, % | 36.8a | 49.6b | 51.9b | χ2 (1) = 216.8 | <0.001 |
| Smoker*, % | 37.4a | 51.7b | 56.6b | χ2 (1) = 280.6 | <0.001 |
|
Psychiatric Diagnoses | |||||
| Depression Diagnosis*, % | 12.2a | 29.6b | 41.9c | χ2 (1) = 750.0 | < 0.001 |
| Anxiety Diagnosis*, % | 4.41a | 10.6b | 19.7c | χ2 (1) = 293.2 | < 0.001 |
| PTSD Diagnosis*, % | 0.53a | 2.0b | 4.4b | χ2 (1) = 98.3 | < 0.001 |
| Substance Use Disorder diagnosis*, % | 9.29a | 23.9b | 31.1c | χ2 (1) = 601.4 | < 0.001 |
| Any of the 4 mental health diagnoses*, % | 21.5a | 47.0b | 61.8c | χ2 (1) = 1101.4 | < 0.001 |
|
Median Comorbidity score – RxRisk, (IQR)† |
658.1a (520 – 1205) |
895.9b (653– 1432) |
895.9b (653 – 2115) |
χ2 (2) = 190.4 | < 0.001 |
Wald Chi-Square test for trend was used for categorical variables. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Kruskal-Wallis non-parametric test was used for continuous variables followed by Wilcoxon post-hoc testing. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Medications and Health Services Utilization
Patients in the higher-dose group had a median daily opioid dose at last dispensing of 180.0 mg morphine equivalent per day (Interquartile range or IQR = 120 – 257.1; range = 100 – 2160) as noted in Table 3. Patients in the lower-dose group showed a median daily dose of 25.7 (IQR = 13.5 – 41.7) mg morphine equivalent per day (range = 1 - 98.6). Most patients in the higher-dose group were prescribed long-acting opioid medications (88%). Greater sedative-hypnotic use was associated with greater opioid dose in a graded fashion with patients in the higher-dose group showing the highest use (61%).
Table 3.
Medications and Health Services Utilization by Opioid Dose Group
| No- Opioid Group (n=10,184) |
Lower-Dose Opioid Group (n=4,815) |
Higher-Dose Opioid Group (n=453) |
Test (df) | p-value | |
|---|---|---|---|---|---|
| Medications or Type of Health Service Used | |||||
| Median daily dose at last dispensing, morphine equivalent (IQR) † | NA* | 25.7 (13.5 – 41.7) | 180 (120 – 257.1) | χ2 (1, 5268) = 1241.9 | < 0.001 |
| Range | NA* | 1 – 98.6 | 100 – 2160 | χ2 (1, 5268) = 237.4 | <0.001 |
| Long-acting Opioids‡, % | NA* | 34 | 88.4 | χ2 (1, 5268) = 510.6 | <0.001 |
| Median opioid prescribers† | NA* | 3 | 4 | χ2 (1, 5268) = 30.3 | <0.001 |
| Sedative-hypnotic Rx 6- mos. before/after index visit‡, % | 10.0a | 42.0b | 60.5c | χ2 (1, 15452) = 1917.0 | <0.001 |
| ER visit 6 mos. before/after index date‡, % | 16.9a | 38.8b | 49.7c | χ2 (1, 15452) = 895.6 | <0.001 |
| ER visit with back pain diagnosis‡, % of patients with any ER visit | 23.5a | 27.8b | 28.9ab | χ2 (1, 3816) = 9.08 | <0.005 |
| Filled opioid rx after ER visit‡, % of patients with any ER visit | 0.1a | 55.5b | 62.7b | χ2 (1, 15452) = 681.7 | <0.001 |
| Median clinic visits of any type 6-mos before/after index date† | 8a | 17b | 22c | χ2 (2, 15397) = 2715.4 | <0.001 |
| Any pain clinic visit 6 mos. before/after index date‡, % | 0.97a | 10.5b | 22.7c | χ2 (1, 15452) = 787.4 | <0.001 |
| Mean hospitalizations 6 mos. before/after index date†, (SD) | 1.3 (0.73)a | 1.5 (1.1)b | 1.9 (1.3)c | χ2 (2, 1460) = 21.515 | <0.001 |
Not applicable. This category not included in tests of statistical significance.
Kruskal-Wallis non-parametric test was used for continuous variables followed by Wilcoxan post-hoc testing. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Wald Chi-Square test for trend was used for categorical variables. Scores with different superscripts differed significantly (p < 0.05) in post-hoc testing and adjusted with a Bonferroni correction.
Visits to the ER increased steadily with opioid dose, with half the patients in the higher-dose opioid group having an ER visit during the study period; about a third of these were associated with a back pain diagnosis. Patients in the higher-dose group had a higher proportion of filling an opioid prescription within five days following an ER visit (63%) compared to patients in the lower-dose group (56%), though this difference was not statistically significant.
Patients in the higher-dose group also had the greatest number of clinic visits within the study year, with a median number of 22 compared to 8 for those in the no-opioid group, and intermediate for the lower-dose group. Most patients were not concurrently seen in a specialty pain clinic, but relative to those in the lower-dose group (11%), patients in the higher-dose group were twice as likely to attend the pain clinic (23%; p = <.001). Across the dose groups, few hospitalizations occurred; however, patients in the higher-dose group had the highest mean frequency. The number of different prescribers increased with dose, with higher-dose patients having the highest number (Table 3).
Independent Correlates of High Dose Opioid Use
In logistic regressions, after adjusting for age and sex, several characteristics remained independently associated with higher-dose opioid use (Table 4). These included male gender (OR = 1.68, 95% CI = 1.37 – 2.06), higher comorbidity scores (OR = 1.20; 95% CI = 1.06 – 1.37), having Medicare insurance (OR = 1.65, 95% CI = 1.22 – 2.23), any 1 of the 4 mental health diagnoses (OR = 1.58, 95% CI = 1.28 – 1.95), co-prescriptions of sedative-hypnotics (OR = 1.75, 95% CI = 1.42 – 2.16), having an ED visit (OR = 1.29, 95% CI = 1.05 – 1.58), and pain clinic visits (OR = 2.30, 95% CI = 1.80 – 2.94). The discrimination index (C, measured as area under the receiver operating characteristic curve) was 0.67 (95% CI: 0.65 – 0.70) with the final model showing no evidence of a lack of fit (χ2 (8df) = 6.72, p = 0.57; Hosmer – Lemeshow lack of fit test).
Table 4.
Associations with Higher-Dose Opioid Prescribing Compared to Lower-Dose Opioid Prescribing in Logistic Regression Analysis (n = 5,268)
| Adjusted Odds Ratio |
P Value |
95% Confidence Interval |
|
|---|---|---|---|
| Male gender | 1.68 | < 0.001 | 1.37 – 2.06 |
| Age | 0.95 | 0.03 | 0.90 – 1.00 |
| Comorbidity score (RxRisk) | 1.20 | 0.005 | 1.06 – 1.37 |
| Medicare | 1.65 | 0.001 | 1.22 – 2.23 |
| Any 1 of 4 mental health diagnoses | 1.58 | < 0.001 | 1.28 – 1.95 |
| Co-prescription of sedative-hypnotics | 1.75 | < 0.001 | 1.42 – 2.16 |
| ED visit | 1.29 | 0.013 | 1.05 – 1.58 |
| Pain clinic | 2.30 | < 0.001 | 1.80 – 2.94 |
Note. This analysis includes only those patients who had complete data and were currently prescribed at least one opioid medication. Variables that were potentially eligible to be included in the analysis, but ultimately were not included were: BMI ≥ 30, smoker, depression diagnosis, anxiety/PTSD diagnosis, substance use disorder diagnosis, receipt of opioid rx at ED visit, clinic visits, hospitalization, and number of opioid prescribers. Comorbidity score measured in quartiles.
Discussion
High-dose opioid therapy was prescribed to over eight percent of patients with low back pain who received long-term opioids. Patients receiving higher-dose opioid therapy were prescribed a median daily opioid dose of 180 mg per day morphine equivalent at their last dispensing, a dose seven times greater than the average of patients receiving lower-dose opioids. The prevalence of higher-dose use in our study is similar to the prevalence found in a sample of veterans (8.2%),23 suggesting that this pattern of prescribing is not unusual.
High-dose opioid therapy was characterized by certain demographic, clinical, and utilization features. However, the strength of these independent predictors to discriminate individual higher-dose users from lower-dose users was modest, suggesting that predicting individual risk for higher-dose use will require studies with greater individual detail. Being male, white, and having Medicare were significantly associated with higher-dose use. Our finding that Black patients were less likely to be in the higher-dose group is consistent with previous research indicating that Black patients are less likely to receive opioids for pain treatment compared to white patients.23,24 However, whether receiving less opioids for chronic pain treatment reflects better or worse care is unclear.
Our results are consistent with studies suggesting that chronic pain patients with comorbid psychiatric diagnoses are more likely to be prescribed opioids compared to patients without psychiatric diagnoses.4,12,27,31 Moreover, our findings are consistent with reports that chronic pain patients with co-morbid psychiatric diagnoses tend to receive higher-dose opioid prescriptions.4,23,,27 Thus, not only is long-term opioid therapy more common among patients with psychiatric disorders, but they also tend to receive higher doses. Patients with mental health and substance use disorders are routinely excluded from clinical trials of opioid medication efficacy,13,18 yet, these patients clearly suffer from pain indications. Our findings suggest an acute need for inclusion of these patients in clinical trials of pain therapy to help guide prescribing and use of opioids in this population.
Though we cannot identify the reasons for greater use of long-term higher-dose opioids among patients with comorbid psychopathology, there are plausible explanations. We have found that the prevalence of mental health diagnoses increases with increasing duration of opioid use (from acute to chronic), and not just beyond some threshold of duration.10 In sequential surveys, depression and anxiety at the first survey were associated with greater likelihood of opioid initiation and continuation at the second survey 3 years later.31 Kroenke recently demonstrated a bi-directional relationship between depression and persistent pain, suggesting that depression and pain have a potentially causative influence on one another.22 Thus, depression may lead to more opioid use (prevalence and dose), opioid use may cause or exacerbate depression, or both may be true. Our results support the need for providers to carefully screen opioid therapy candidates for mental health and substance use disorders and either treat or refer them for specialty mental health care. The hope is to avoid Sullivan’s concept of “adverse selection:” pairing higher-dose therapy with high-risk patients.29
Most patients prescribed higher-dose opioids received long-acting opioids (88%) rather than short-acting opioids alone, consistent with some expert recommendations. In contrast, the higher rate of concurrent sedative-hypnotic prescriptions associated with higher-dose opioid use (61% vs. 42% in the lower-dose and 10% in the no opioid group) we observed is contrary to most recommendations and presents potential safety risks.11 We previously reported a 44% rate of sedative hypnotic use (mostly benzodiazepines) in long-term opioid users.10 The 61% rate among long-term higher-dose patients may represent an opportunity for improving prescribing, as this is a particularly high-risk group for overdose.
Since these data were collected, Kaiser Permanente Northwest has implemented risk mitigation strategies with the goal of improving the safety of opioid prescribing for pain. For example, clinicians now stratify patients receiving opioids according to their level of risk for misuse and safety depending on their clinical profiles (i.e., current or past history of substance use disorder, opioid dosage, poly-prescriptions, etc.) and tailor follow-up frequency accordingly. Providers could consider other strategies, such as setting and enforcing a maximum recommended daily dosage, requiring urine drug screens, and use of electronic records to identify patients who are receiving opioid medications and risky co-prescriptions.33 Efforts could also be made to reduce dosages for patients already receiving higher-doses; for example, optimizing use of non-opioid medications, and making referrals to specialty mental health, chiropractic and acupuncture treatment.
Patients in the higher-dose group were high utilizers of medical services overall. If patients are on long-term higher-dose opioid therapy, it is reasonable to expect higher utilization attributable to medication refills, in addition to possible mental health visits and management of comorbid conditions. However, the greater emergency department use among higher-dose opioid users suggests that greater utilization is not strictly explained by scheduled visits.
Higher-dose patients had the highest number of different opioid prescribers. This may be an inevitable consequence of long-term higher-dose opioid therapy, requiring providers to be available for medication refills, and increasing the primary care burden. However, it may also suggest continuity of care problems, potential “doctor-shopping,” or more uncontrolled pain.
Strengths of our study include a large study population, use of electronic records, and nearly complete capture of health care utilization. However, there are some important limitations. Though our study population was representative of the racial and ethnic composition of the Portland, Oregon, metropolitan area, our results may not be generalizable to regions with higher concentrations of minority populations. Most of our study population had commercial health insurance. Thus, our findings may not necessarily be generalizable to more socio-economically disadvantaged populations.
While we focused on patients with a known back pain diagnosis, we do not know whether this was the reason for being prescribed higher dose opioid therapy, particularly given high levels of co-morbidity. We also do not know the relative effectiveness of higher-dose treatment, as we did not have measures of pain severity or functional outcomes. We were reliant on clinician diagnoses of mental health disorders rather than standardized measures, limiting comparisons with other studies. Although we found several factors associated with higher-dose opioid prescribing, given our design, we cannot infer causation or the direction of causality.
In conclusion, we found that over eight percent of patients with low back pain were prescribed higher-dose opioid therapy on a long-term basis. Patients on higher-dose opioid therapy were characterized by higher rates of psychiatric comorbidity, co-prescriptions of sedative hypnotics, and higher health service utilization. Further research is needed to ascertain the balance of benefits and harms of long-term higher-dose opioid therapy for chronic non-cancer pain, as well as factors that lead to the progression of higher-dose use.
Acknowledgments
We gratefully acknowledge the statistical consultation received from Robert C. Kobus, MA, and Michael Lasarev, MS, and publication assistance from Ms. LeNeva Spires.
Disclosures:
This study was supported in part by an NRSA T32 Fellowship in Health Services Research from the Agency of Healthcare Research and Quality to Dr. Kobus (5T32HS017582) and a pilot grant from the Oregon Clinical and Translational Research Institute, grant number U11 RR024140 from the National Center for Research Resources, National Institutes of Health; and the National Institutes of Health Roadmap for Medical Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
No conflicts of interest were reported by any of the authors.
Contributor Information
Amy M. Kobus, Email: kobusa@ohsu.edu, Assistant Professor, Departments of Psychiatry & Family Medicine, Oregon Health & Science University, 503-494-0903.
David H. Smith, Email: David.h.smith@kpchr.org, Senior Investigator, Kaiser Permanente Center for Health Research, 503-335-6302.
Benjamin J. Morasco, Email: morascob@ohsu.edu, Assistant Professor, Mental Health and Clinical Neurosciences Division, Oregon Health & Science University, 503-220-8262; Portland VA Medical Center, Portland, OR.
Eric S. Johnson, Email: Eric.S.Johnson@kpchr.org, Investigator, Kaiser Permanente Center for Health Research.
Xiuhai Yang, Email: Xiuhai.Yang@kpchr.org, Senior Research Analyst, Kaiser Permanente Center for Health Research, 503-335-6732.
Amanda F. Petrik, Email: Amanda.F.Petrik@kpchr.org, Research Associate, Kaiser Permanente Center for Health Research, 503-335-2483.
Richard A. Deyo, Email: deyor@ohsu.edu, Professor, Departments of Family Medicine, Internal Medicine, and Public Health and Preventive Medicine, and the Center for Research in Occupational and Environmental Toxicology, OHSU; and Clinical Investigator, Kaiser Permanente Center for Health Research, 503.494.1694.
References
- 1.American Society of Health-System Pharmacists. [Accessed December 20, 2011];AHFS drug information. AHFS Drug Information. Available at: http://www.ahfsdruginformation.com/products_services/di_ahfs.aspx.
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169:64–70. doi: 10.1176/appi.ajp.2011.10101476. [DOI] [PubMed] [Google Scholar]
- 4.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–570. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Accessed December 13, 2011];Unintentional drug poisoning in the United States. Available at: http://www.cdc.gov/HomeandRecreationalSafety/pdf/poison-issue-brief.pdf.
- 6.Chapman CR, Lipschitz DL, Angst MS, Chou R, Denisco RC, Donaldson GW, Fine PG, Foley KM, Gallagher RM, Gilson AM, Haddox JD, Horn SD, Inturrisi CE, Jick SS, Lipman AG, Loeser JD, Noble M, Porter L, Rowbotham MC, Schoelles KM, Turk DC, Volinn E, Von Korff MR, Webster LR, Weisner CM. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010;11:8807–8829. doi: 10.1016/j.jpain.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Ballantyne JC, Fanciullo GJ, Fine PG, Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Daniell HW. Hypogonadism in men consuming sustained-action oral opioids. J Pain. 2002;3:377–384. doi: 10.1054/jpai.2002.126790. [DOI] [PubMed] [Google Scholar]
- 9.Daniell HW. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J Pain. 2008;9:28–36. doi: 10.1016/j.jpain.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Deyo RA, Smith DH, Johnson ES, Donovan M, Tillotson CJ, Yang X, Petrik AF, Dobscha SK. Opioids for back pain patients: primary care prescribing patterns and use of services. J Am Board Fam Med. 2011;24:717–727. doi: 10.3122/jabfm.2011.06.100232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edlund MJ, Sullivan M, Steffick D, Harris KM, Wells KB. Source. Do users of regularly prescribed opioids have higher rates of substance use problems than nonusers? Pain Med. 2007;8:647–656. doi: 10.1111/j.1526-4637.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 14.Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125:172–179. doi: 10.1016/j.pain.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Farley JF, Harley CR, Devine JW. A comparison of comorbidity measurements to predict healthcare expenditures. Am J Manag Care. 2006;12:110–119. [PubMed] [Google Scholar]
- 16.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O’Keeffe Rosetti MC. Source. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 18.Kalso E, Allan L, Dellemijn PL, Faura CC, Ilias WK, Jensen TS, Perrot S, Plaghki LH, Zenz M. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7:381–386. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- 19.Kidner CL, Mayer TG, Gatchel RJ. Higher opioid doses predict poorer functional outcome in patients with chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg Am. 2009;91:919–927. doi: 10.2106/JBJS.H.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korff MV, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–527. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krantz MJ, Lewkowiez L, Hays H, Woodroffe MA, Robertson AD, Mehler PS. Torsade de pointes associated with very-high-dose methadone. Ann Intern Med. 2002;137:501–504. doi: 10.7326/0003-4819-137-6-200209170-00010. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011 Jun 16; doi: 10.1016/j.jpain.2011.03.003. [Epub 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151:625–632. doi: 10.1016/j.pain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299:70–78. doi: 10.1001/jama.2007.64. [DOI] [PubMed] [Google Scholar]
- 25.Portenoy RK, Farrar JT, Backonja MM, Cleeland CS, Yang K, Friedman M, Colucci SV, Richards P. Long-term use of controlled-release oxycodone for noncancer pain: results of a 3-year registry study. Clin J Pain. 2007;23:287–299. doi: 10.1097/AJP.0b013e31802b582f. [DOI] [PubMed] [Google Scholar]
- 26.Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Braden JB, Psaty BM, Von Korff M. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. J Gen Intern Med. 2010;25:310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Source. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;7:940–947. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 28.Selby JV, Smth DH, Johnson ES, Roebel MA, Friedman GD, McFarland BH. Kaiser Permanente Medical Care Program. In: Strom B, editor. Pharmacoepidemiology. West Susgender, UK: John Wiley and Sons; 2005. [Google Scholar]
- 29.Sullivan MD. Who gets high-dose opioid therapy for chronic non-cancer pain? Pain. 2010;151:567–568. doi: 10.1016/j.pain.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan MD, Edlund MJ, Steffick D, Unützer J. Regular use of prescribed opioids: association with common psychiatric disorders. Pain. 2005;119:95–103. doi: 10.1016/j.pain.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan MD, Edlund MJ, Zhang L, Unützer J, Wells KB. Association between mental health disorders, problem drug use, and regular prescription opioid use. Arch Intern Med. 2006;166:2087–2093. doi: 10.1001/archinte.166.19.2087. [DOI] [PubMed] [Google Scholar]
- 32.WoltersKluwer Health, Medi-Span. [Accessed December 20, 2011];Master drug data base v2.5. Available at: http://www.medispan.com/index.aspx.
- 33.Von Korff M. CA8-04: Health Plan Implementation of a Major Risk Mitigation Initiative for Chronic Opioid Therapy. Clin Med Res. 2012;10:177. [Google Scholar]