Abstract
Studies show a protective relationship between physical activity and breast cancer risk across the life course from menarche to postmenopausal years. Mammographic breast density is a known and strong breast cancer risk factor. Whether the association of physical activity with breast cancer risk is mediated through mammographic breast density is poorly understood. This systematic review summarizes published studies that investigated the association between physical activity and mammographic breast density and discusses the methodological issues that need to be addressed. We included in this review studies that were published before October 31, 2011 that were accessible in full-text format and were published in English. We identified 20 studies through the PubMed Central, BioMed Central, Embase, and Scopus and using the search terms “physical activity and breast density” and “exercise and breast density” as well as through manual searches of the bibliographies of the articles identified in electronic searches. We found no evidence of association between physical activity and breast density across the studies by grouping them first by the timing of physical activity assessment (in adolescence, current/recent, past, and lifetime) and then by women’s menopausal status (premenopausal and postmenopausal). Given the strength of the relationship between physical activity and breast cancer and the null findings of this review, it is unlikely that the effect of physical activity is mediated through an effect on breast density.
Keywords: Breast density, Physical activity, Exercise, Breast cancer risk
Introduction
Mammographic breast density is a well-established and very strong predictor of breast cancer risk [1–4]. Appearance of the breast on the mammogram is a reflection of the amount of fat, connective, and epithelial tissue in the breast [3]. Light (non-radiolucent) areas on the mammogram represent the fibrous and glandular tissues (“mammographically dense”) and the dark (radiolucent) areas are primarily fat. Women with 75 % or greater percent density (proportion of the breast that appears dense on the mammogram out of the total breast area) are at four to six times greater risk of breast cancer compared with women with fatty breasts [3, 5–7]. Breast density is not a static characteristic and changes over time [8]. Use of postmenopausal hormones, especially combined estrogen plus progestin therapy, increases breast density as early as after 3–6 months of hormone use [9–11]. However, this increase is reversible [12]. Breast density decreases with age, especially during the menopausal transition and during tamoxifen use [13–15].
In 2007, the World Cancer Research Fund and the American Institute for Cancer Research concluded that high levels of physical activity protect against postmenopausal breast cancer [16]. A recent systematic review of 33 cohort studies and 40 case–control studies reported a lower breast cancer risk in women with highest versus lowest levels of physical activity in 40 % of the studies [17]. Null results were reported in 26 % of the studies, 30 % reported either borderline or not statistically significant breast cancer risk reduction, and 4 % reported a non-statistically significant positive association of physical activity with breast cancer risk [17]. Reduction in breast cancer risk across case–control studies was greater than in cohort studies (30 vs. 20 %) [17]. Physical activity at any age throughout life reduces breast cancer risk, but the strongest reduction was seen for physical activity after age 50 (average risk reduction of 17 %) followed by activity in adolescence (average risk reduction of 16 %), middle adulthood (30s/40s, average risk reduction of 15 %), and early adulthood (20s, average risk reduction of 8 %) [17]. A greater risk reduction was reported for recreational and household activity, followed by walking/cycling and occupational activity as well as for vigorous level and longer duration of physical activity [17]. An inverse association between physical activity and the risk of breast cancer was reported in both pre- and postmenopausal women; the average reduction in breast cancer risk, however, was greater in postmenopausal than in premenopausal women (31 vs. 27 %) [17]. A greater risk reduction was seen in lean women as compared to women with Body Mass Index (BMI) ≥22 kg/m2, in women without a family history of breast cancer as compared to women with a family history, and in parous women as compared to nulliparous women [17].
Several biologic mechanisms have been suggested to explain the inverse association of physical activity with breast cancer risk. Among those mechanisms are decreased body fat, decreased production of steroid hormones and as a result, reduced cumulative exposure to endogenous reproductive hormones, changes in the menstrual cycle frequency and duration, improved insulin sensitivity, reduced adipokine levels, and decreased inflammation [17, 18].
Whether the association of physical activity with breast cancer risk is mediated through mammographic breast density remains unclear. The purpose of this review is to summarize published studies on the association between physical activity and mammographic breast density and to identify the methodological issues that need to be addressed in future studies.
Literature search
Published studies were identified using the PubMed Central (U.S. National Institutes of Health [NIH]), BioMed Central, Embase, and Scopus literature search (through Washington University in St. Louis). We included in this review studies published before October 31, 2011 that were accessible in full-text format and were published in English. Articles were searched using the combination of the terms “physical activity and breast density” and “exercise and breast density.” Bibliographies of the articles identified in the electronic searches were then searched manually for additional relevant references. From this review, we excluded the studies that did not show their findings on the association between physical activity and breast density.
From each identified article, we abstracted the data on study design, sample size, physical activity (timing, type, dose, and intensity), characteristics of the study population (premenopausal, postmenopausal, and race/ethnicity), breast density assessment method and timing of the mammogram, and results. Even though some of the studies that sampled their participants cross-sectionally asked them to recall physical activity earlier in life, those studies are considered as cross-sectional in design for the purpose of this review.
Statistical analysis
We compared the results across the studies by grouping them first by the timing of physical activity assessment (in adolescence, current/recent [within 5 years preceding the mammogram], past [>5 years before the mammogram], and lifetime [from childhood through adulthood]) and then by women’s menopausal status (premenopausal and post-menopausal). In the statistical comparisons, we excluded studies by Lopez et al. [19] (investigation of association between inactivity and breast density), Sala et al. [20] (timing of the physical activity assessment not specified), and Marmara et al. [21] (reported ORs values <0 and 95 % CIs sometimes not centered around the point estimate). These studies, however, were included in the qualitative review. In a secondary analysis, we also excluded two studies in breast cancer survivors.
Variability of the studies with respect to the study populations, type, dose and timing of physical activity, density estimation, and presentation of the results makes a direct comparison across the studies difficult. For these reasons, we were unable to conduct a formal meta-analysis across the studies. Instead, the Mann–Whitney–Wilcoxon rank-sum test was used to combine the results of the studies by the timing of physical activity, overall and by menopausal status [22]. For the studies that did not report the overall findings, but rather presented them stratified by either menopausal status or other characteristics (BMI, age at physical activity, etc.), we extrapolated the overall findings by calculating the average for adjusted mean percent density weighted by the number of participants. Significance of the test was assessed at the alpha = 0.05 significance level. All analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
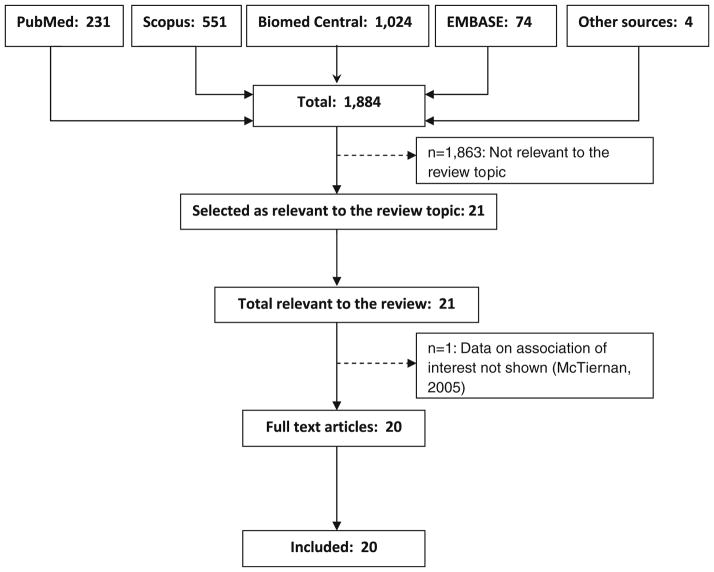
Our search yielded 1,884 manuscripts, from which we identified 20 eligible studies (Fig. 1). Key characteristics of these studies are summarized in Table 1. The earliest study we found was published in 1999. The majority of the published studies originated in the United States (60 %). Most of the studies sampled their participants cross-sectionally (75 %). Three studies were longitudinal and one study utilized a case–control design. All studies, except two whose populations were composed of survivors, investigated the associations in healthy (breast cancer–free) women.
Fig. 1.
Flow diagram of the literature search
Table 1.
Summary characteristics of the studies on association between physical activity/inactivity and mammographic breast density
| Study characteristic | n (%) | Sample size | Total sample range size across all studies |
|---|---|---|---|
| Study population | |||
| Breast cancer survivors | 2 (10) | 474–522 | 996 |
| Healthy women | |||
| Premenopausal/Perimenopausala | 14 (70) | 68–1741 | 5,345 |
| Postmenopausal | 17 (80) | 110–1,520 | 11,324 |
| Race/ethnicity | |||
| Predominantly White | 14 (70) | 320–2,720 | 15,262 |
| Hispanic only | 2 (10) | 95–294 | 389 |
| Asian only | 1 (5) | 201 | 201 |
| Mixed | 3 (15) | 375–772 | 1,869 |
| Study design | |||
| Randomized controlled trial | 1 (5) | 320 | 320 |
| Prospective | 3 (15) | 722–1,666 | 3,160 |
| Case–control | 1 (5) | 400 | 400 |
| Cross-sectional | 15 (75) | 95–2,720 | 13,860 |
| Physical activity/inactivity recalled fromb | |||
| Current or within previous 5 yearsc | 14 (70) | 95–2,720 | 11,052 |
| Past (5+ years before the mammogram) | 4 (20) | 375–1,666 | 3,493 |
| Between age 7 and 20 (adolescent) | 5 (25) | 375–1,893 | 3,825 |
| Lifetime history | 2 (10) | 375–1,900 | 2,275 |
| Breast density assessment approach | |||
| Percent breast density (continuous) | 13 (65) | 95–1,900 | 10,109 |
| Categorical breast density | 7 (35) | 201–2,720 | 7,631 |
Three studies did not report the number of pre- and postmenopausal women in the study population
One study did not specify the timing of physical activity assessment; some studies are included in more than one category
Includes a randomized controlled trial with duration of 1 year
Majority of the studies (65 %) had a large sample size (>500 participants): The mean number ofparticipants was 887 (median 675, range 95–2,720) with the total number of 5,345 pre- or peri-and 11,324 postmenopausal women across all 20 studies. Most of the studies included both pre- and postmenopausal women in their study population (16 or 80 %), two studies had only postmenopausal participants, and one study included only pre-menopausal women. Among studies with mixed population with respect to menopausal status, seven (44 %) reported the results separately for pre- and postmenopausal women.
Most studies (14 or 70 %) assessed the physical activity level at the time of the mammogram or within the 5 years before the mammogram date; four studies assessed physical activity within more than 5 years preceding the mammogram. Five studies investigated the association of physical activity level during adolescence (between 7 and 20 years of age) and adult breast density and two studies investigated the associations of lifetime physical activity with breast density. The majority of the studies (50 %) assessed non-occupational physical activity only, while others (30 %) assessed physical activity across multiple domains (recreational, household/caregiving, and occupational). Nine studies (45 %) used the metabolic equivalent (MET) to assess the intensity of physical activity, by calculating an index or a score incorporated in the analyses.
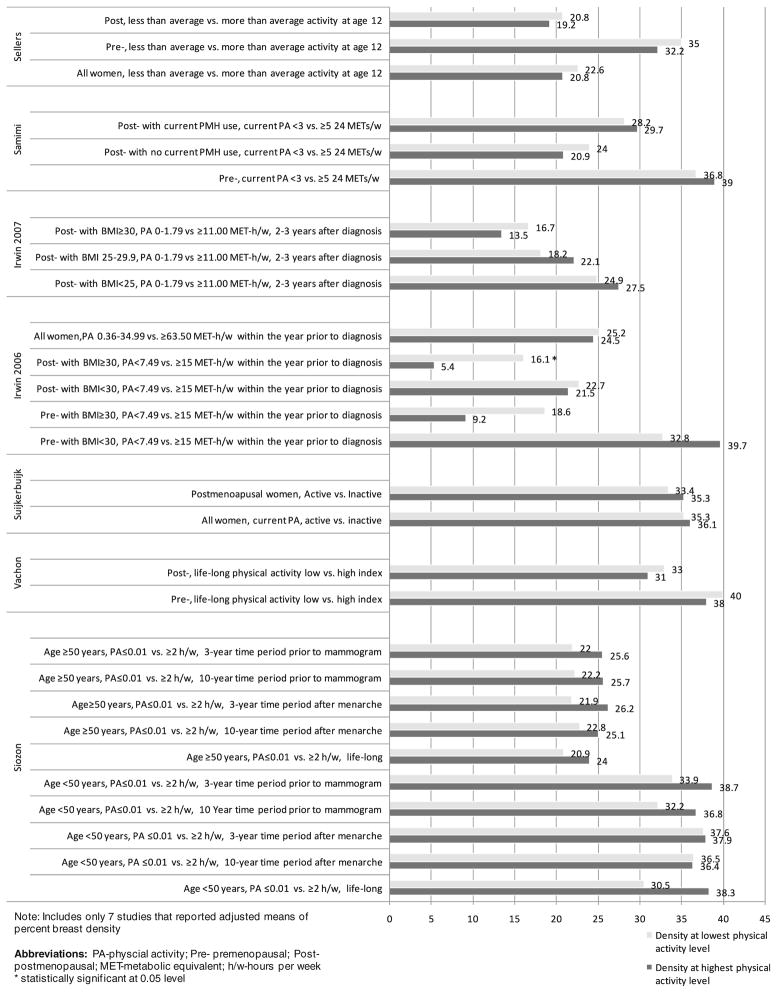
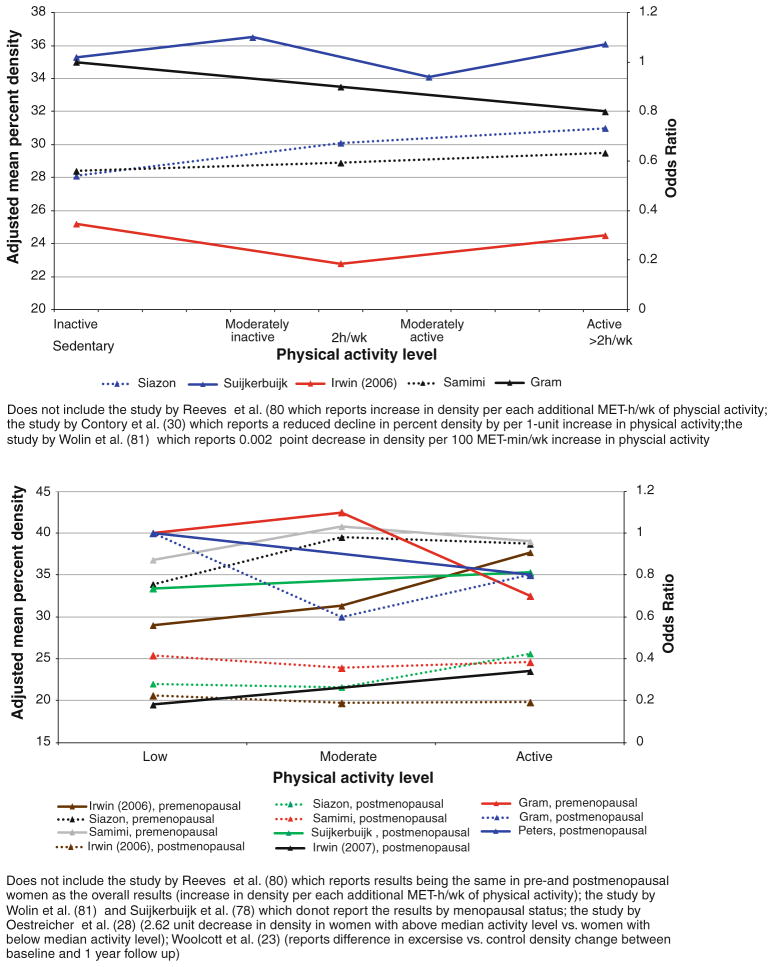
The majority of the studies (14 or 70 %) included predominantly white women. Two studies included only Hispanic women, one study included only Asian women, and two other studies had a mixed population with respect to race/ethnicity. Most of the studies (65 %) quantified percent breast density (proportion of dense breast tissue out of the total breast area) using computerized techniques. Of those, eight studies also measured the absolute dense area. Seven of the studies assessed breast density qualitatively using one of the existing breast density classification systems (Wolfe’s density categories, Tabar’s classification, six-category [Boyd] classification, or BI-RADS breast density classification). The absolute difference between adjusted mean percent breast density at the extremes of physical activity in different subpopulations across seven studies (Fig. 2) varied between 0.1 and 10.7 %. Among the studies that reported their findings as ORs, the change in risk for denser density pattern in women with the highest physical activity level versus the lowest level varied between ≤15 % reduction and ≤17 % increase, depending on the study subpopulation. Among the studies that reported their findings as regression coefficient estimates, beta varied between −2.62 and 3.18.
Fig. 2.
Breast density at the lowest (upper bar) and highest (lower bar) extremes of physical activity
In Table 2, we present for each study the characteristics of the study population as well as details of physical activity assessment and breast density estimation approach (see Table 2). In the following, we summarize findings of the studies on the association between physical activity/inactivity and mammographic breast density by the timing of physical activity and by menopausal status as presented in Supplementary Tables S1–S6.
Table 2.
Summary description of the studies on association between physical activity/inactivity and mammographic breast density
| References | Country | Sample size | N pre-or peri/post- | Physical activity/inactivity assessment method | Timing of physical activity | Breast density assessment |
|---|---|---|---|---|---|---|
| Woolcott et al. [23] | Canada | 320 | 0/320 | Intervention: 45 min, 5/week; control: usual lifestyle | Current/recent (intervention > 1 year) | Percent breast density, total dense area, and total non-dense area |
| Oestreicher et al. [28] | USA | 772 | 772/0 | Self-reported physical activity instrument (Kaiser Physical Activity Survey) | Current/recent | Percent breast density and total dense area |
| Masala et al. [73] | Italy | 1,666 | 610/1,056 | Self-administered questionnaire | Past | Wolfe’s classification |
| Conroy et al. [30] | USA | 722 | 300/422a | Self-administered questionnaire | Current/recent | Percent breast density, total dense area, and total non-dense area |
| Sala et al. [20] | UK | 400 | 68/313b | Self-administered questionnaire | Not specified | Wolfe’s classification |
| Gram et al. [31] | Norway | 2,720 | 1741/979c | Self-administered questionnaire | Current/recent and past | Tabar’s classification |
| Vachon et al. [62] | USA | 1,900 | 380/1,520c | Interviewer-administered phone interview | Lifetime | Percent breast density |
| Lopez et al. [19] | USA | 294 | 105/189 | Interviewer-administered questionnaire | Current/recent | Percent breast density |
| Jeffreys et al [74] | UK | 628 | NR | Self-administered questionnaire | Adolescent | Six-category (Boyd) classification |
| Suijkerbuijk et al. [75] | The Netherlands | 616 | 130/429b | Self-administered questionnaire | Current/recent | Percent breast density and total dense area |
| Irwin et al. [60] | USA | 474 | 151/323 | 29-item, Interviewer- administered questionnaire | Current/recent | Percent breast density, total dense area, and total non-dense area |
| Siozon et al. [76] | USA | 375 | 148/227 | Interviewer-administered questionnaire | Lifetime, adolescent current/recent, past | Percent breast density and total dense area |
| Reeves et al. [77] | USA | 728 | 180/548 | Self-administered questionnaire | Adolescent, current/recent and past | Percent breast density, total dense area, and total non-dense area |
| Sellers et al. [29] | USA | 1,893 | 451/1,442 | Self- administered questionnaire | Adolescent | Percent breast density |
| Wolin et al. [78] | USA | 95 | NR | 27-item, self-administered physical activity instrument | Current/recent | Percent breast density |
| Irwin et al. [79] | USA | 522 | 0/522 | 29-item, Interviewer- administered questionnaire | Current/recent | Percent breast density, total dense area, and total non-dense area |
| Samimi et al. [80] | USA | 1,398 | 369/1,029 | Self-administered questionnaire | Current/recent | Percent breast density |
| Peters et al. [27] | UK | 1,292 | 0/1,292 | Self-administered questionnaire | Current/recent | Six-category (Boyd) classification |
| Marmara et al. [21] | Greece | 724 | 0/724 | 29-item, Interviewer- administered questionnaire | Current/recent | BI-RADS |
| Tseng et al. [81] | USA | 201 | 91/110 | Self- administered questionnaire | Adolescent | BI-RADS categories |
NR Not reported
Based on the data from the visit closest to the index mammogram
Excludes women with missing data
Estimated from reported percents
Physical activity in adolescence and adult breast density
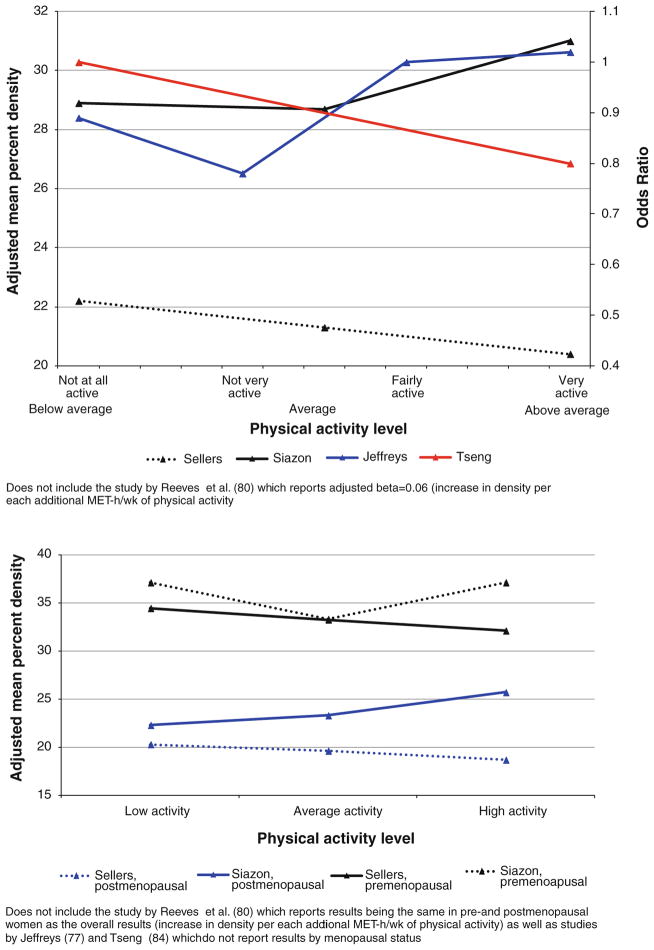
Five studies investigated the association between adult breast density and adolescent physical activity (between age 7 and 20 years) (Fig. 3; Table 2). When we combined data across five studies (Table 3), we observed no statistically significant differences in breast density at the extreme levels of physical activity (p = 0.6). Similarly, no statistically significant differences were observed in either pre- or post-menopausal women across three studies that reported the results separately by menopausal status (p = 1.0 in pre-menopausal women; p = 0.6 in postmenopausal women).
Fig. 3.
Results of the studies on association between adolescent physical activity and adult breast density, overall and by menopausal status
Table 3.
Results of Mann–Whitney tests for combined results across the studies
| Timing of physical activity | All women | Premenopausal women | Postmenopausal women |
|---|---|---|---|
| Adolescent | |||
| Included studies | Reeves, Jeffreys, Sellers, Tseng, Siozona | Reeves,b Siozon, Sellers | Reeves,b Siozon, Sellers |
| p value | 0.6 | 1.0 | 0.6 |
| Early life (5 + years before mammogram) | |||
| Included studies | Reeves,b Masala, Gram, Siozona | Reeves,b Masala, Gram, Siozon | Reeves,b Masala, Gram, Siozon |
| p value | 1.0 | 1.0 | 0.2 |
| Recent | |||
| Included studies | Reeves,b Conroy, Gram, Suijkerbuijk, Irwin, 2006 Wolin, Samimi,a Siozona | Reeves,b Oestreicher, Conroy,b Gram, Irwin, 2006, Siozon, Samimi | Reeves,b Woolcott, Conroy,b Gram, Suijkerbuijk, Irwin, 2006, Siozon, Irwin, 2007,a Samimi, Peters |
| p value | 0.6 (0.1c) | 0.1 (0.3c) | 0.4 (0.4c) |
| Life long | |||
| Included studies | Vachon,d Siozona | Vachon, Siozon | Vachon, Siozon |
| p value | 1.0 | 1.0 | 1.0 |
Note. Does not include studies by Sala et al. [20] (did not report the timing of physical activity), Lopez et al. [19] (investigation of association between inactivity and breast density) and Marmara et al. [21] (reported ORs values<0 and 95 % CIs sometimes not centered around the point estimate)
Weighted averages of adjusted mean densities calculated from estimates reported by menopausal status (Siozon and Samimi) or BMI categories (Irwin et al. [79])
Results were either reported or stated to be similar by the timing of physical activity and/or menopausal status without showing the data
p value for the test excluding studies with breast cancer patients
Even though the overall results cannot be extrapolated, the direction of association is the same in both pre- and postmenopausal women and the overall results are treated as being also in the same direction
Past physical activity (>5 years before the mammogram) and breast density
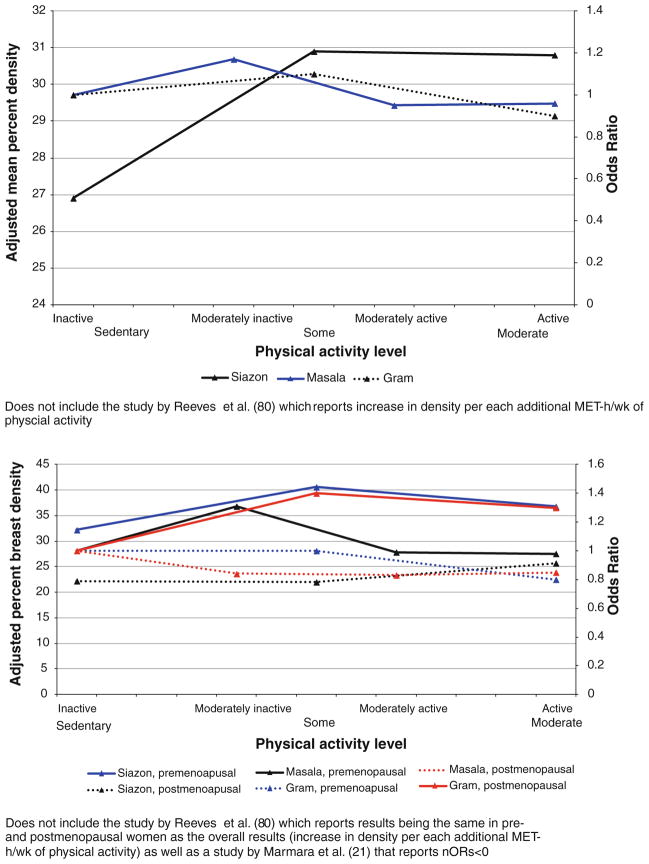
Four studies investigated the association of past physical activity and breast density (Fig. 4; Table 2). We found no statistically significant differences in breast density at the extreme levels of physical activity (p = 1.0) when we combined data across four studies (Table 3). No statistically significant differences were observed in a stratified analysis by menopausal status (p = 1.0 in premenopausal women; p = 0.2 in postmenopausal women).
Fig. 4.
Results of the studies on association between past physical activity (5+ years before mammogram) and breast density, overall and by menopausal status
Recent physical activity (within 5 years before the mammogram) and breast density
Fourteen studies investigated the association of recent physical activity/inactivity (within 5 years before mammogram) with breast density (Fig. 5; Table 2). No statistically significant differences in breast density at the extreme levels of physical activity (p = 0.6) were observed when data across 12 studies (excluding Lopez et al. and Marmara et al.) were combined (Table 3). No statistically significant differences were observed in either pre- or postmenopausal women across the studies (p = 0.1 in premenopausal women; p = 0.4 in postmenopausal women). The results were similar when we excluded two studies with breast cancer survivors from overall (p = 0.1) and stratified analyses (p = 0.3 in premenopausal women; p = 0.4 in postmenopausal women).
Fig. 5.
Results of the studies on association between recent physical activity and breast density, overall and by menopausal status
Only one randomized trial has examined the effect of an exercise intervention on breast density in postmenopausal women. In this trial, which assigned women to either an intervention arm with 1 year aerobic exercise (45 min, 5 days per week) targeting at least 150 min/week of moderate to vigorous physical activity or usual level of physical activity (control arm), found no difference in change of percent density between the arms (intervention minus control: −0.4 %, p = 0.6) [23]. No randomized trials have been conducted in premenopausal women.
Lifetime physical activity and breast density
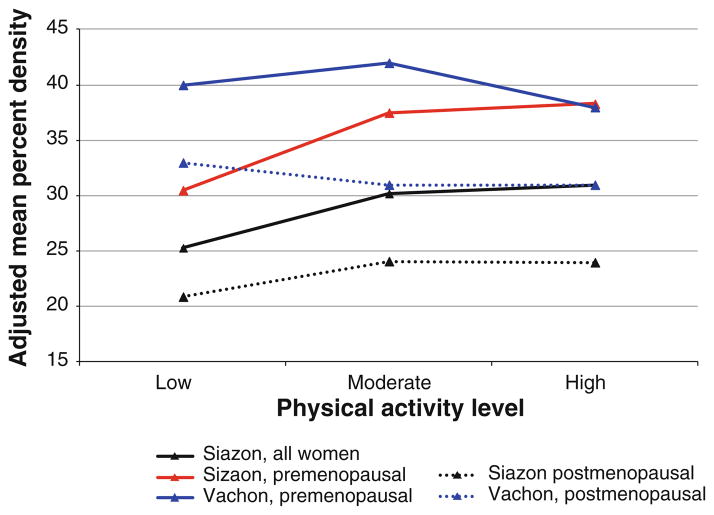
Only two studies examined the association of lifetime physical activity with breast density (Fig. 6; Table 2). When we combined the data (Table 3), we found no statistically significant differences in breast density at the extreme levels of lifetime physical activity either overall (p = 1.0) or when stratified by menopausal status (p = 1.0 in both pre- and postmenopausal women).
Fig. 6.
Results of the studies on association between recent physical activity and breast density, overall and by menopausal status
Discussion
The evidence on association of physical activity and mammographic breast density remains equivocal. We identified 20 published studies that investigated the association between physical activity and breast density. Differences in study designs, including characteristics of the study population, timing of physical activity, and breast density estimation preclude quantitative synthesis of the data via meta-analysis. Similarly, these design differences also precluded drawing conclusions as to whether design features might explain the observed differences in the results. In the following, we discuss some of those methodological issues, including physical assessment methods and associations in pre- and post-menopausal women.
Physical activity assessment
Timing, type, and dose of physical activity
The majority of the studies examined the relationship between recent physical activity and breast density. The biological relevance of physical exercise early versus later in life on breast density remains unclear. Whether physical activity could result in long-term changes in breast tissue and a subsequent reduced risk of high density later in life is unclear. Future studies would benefit from prospective designs with data collection in adolescence and subsequent follow-up of women throughout the adulthood for their breast density assessment.
The majority of the studies assessed non-occupational physical activity only. The focus on recreational/leisure-time physical activity in most studies is unlikely to introduce substantial bias in these studies. Levels of the occupational physical activity in women are generally low and much lower than in men [24]. As a result, restricting assessment of physical activity to non-occupational is considered to cause only very little underestimation of the total activity level in women [25, 26]. Furthermore, this misclassification of overall physical activity, if any, would be non-differential in nature.
Not all of those studies that assessed the intensity of the physical activity commented if the associations for vigorous physical activity were the same as those associations for less intense activity levels. In some studies, the duration of physical activity rather than intensity was used to characterize the physical activity level [27–30]. Previous reports suggest a slightly greater reduction in breast cancer risk in women with a vigorous activity level as compared to women with moderate physical activity (18 vs. 15 %) and much greater reduction with increasing duration of exercise (30 % decrease with ≥6.5 h/week vs. 9 % for 2–3 h/week of activity) [17]. Similarly, two studies on physical activity and breast density observed a pattern suggestive of a dose–response relationship [27, 31].
Objective versus subjective assessment
Detailed validated questionnaires were used by most of the reviewed studies to characterize the physical activity level. These validation studies find a moderate correlation (mean 0.37, range −0.71–0.98) between self-reported and objectively measured physical activity level [32]. Thus, the potential for an exposure misclassification remains, which, depending on characteristics of the exposure and nature of the instrument, might result in either overestimation of the association or null findings. On the other hand, an objective assessment of the physical activity level, for example, with accelerometers could improve the exposure characterization, but such methods are very costly which limits their application in large epidemiologic studies.
Study populations
A vast majority of the studies on the association between physical activity and breast density had racially homogeneous populations and the majority of the studies (14 or 70 %) included predominantly white women. Thus, their findings might be limited to certain racial/ethnical subgroups. In some studies, breast density appeared to be greater in African American women as compared with white women at age 65 and younger, but did not differ by race in women older than 65 years [33]. Inclusion of sufficient number of women from racial minorities would allow to investigate the associations of physical activity and breast density by race/ethnicity.
Two studies assessed the association of physical activity with breast density in breast cancer survivors. In one of those studies, both physical activity and breast density of the contralateral breast were assessed after the breast cancer diagnosis. Even though the risk estimates were adjusted for Tamoxifen use, the time since discontinuation of Tamoxifen use was not taken into account. Whether the effect of Tamoxifen on breast density diminishes with time after cessation of Tamoxifen therapy yet needs to be determined [34–37]; some recent reports, however, suggest that the density might increase after discontinuation of Tamoxifen [37]. Thus, an adjustment for the time since Tamoxifen cessation in addition to the history of Tamoxifen use might be necessary in studies with breast cancer survivors.
All studies with significant time between the exposure and mammograms (≥5 years), with exception of one study [30], did not report on whether there were any women who were premenopausal at the time of the physical activity assessment and became postmenopausal at the time of the mammogram. Breast density declines around menopause because of the breast tissue involution [2, 38] and the portion of that decline that might be attributable to the effect of physical activity is hard to measure. Among studies with a mixed population with respect to the menopausal status, only a few reported the results separately for pre- and postmenopausal women. Some studies suggest that breast density-associated breast cancer risk is different in pre- and postmenopausal women [39]. The underlying biological mechanisms by which physical activity may affect breast density could also be different in pre- and postmenopausal women owing to the differences in the breast tissue proliferation rates [40, 41] and estrogen synthesis before and after menopause [42–44]. Due to these issues, reporting the results separately for pre- and post-menopausal women becomes important.
The studies in premenopausal women did not all assess breast density in the same phase of the menstrual cycle. A few authors have reported changes in breast density during the menstrual cycle [45, 46]. Thus, an adjustment for the phase of the menstrual cycle in premenopausal women or a consistent collection of the mammogram data in one phase is preferable. Finally, some studies in postmenopausal women have not adjusted their risk estimates to the use of hormone replacement therapy, a known and strong risk factor for high breast density [9, 12, 47–59].
Other considerations
Success in revealing true associations depends on variability of the exposure and outcome in the study population. In some studies, the levels of physical activity in their study populations were very low [27, 31]. Similarly, other studies reported having a high proportion of women with low-risk density patterns [21]. The lack of variation in physical activity and in breast density in the study population could attenuate true associations.
All studies adjusted their risk estimates for BMI; some studies also performed analyses stratified by BMI and/or commented on the interaction between physical activity and BMI. In many studies, the observed associations either disappeared or became weaker after the adjustment for BMI. Modification of the association by BMI has also been suggested [60]. Previous studies consistently reported an inverse association of BMI with breast density [9, 47–49, 56–58, 61–71] and physical activity is inversely correlated with BMI (0.31 unit decrease in BMI per one-category increase in the physical activity index, 95 % CI 0.23–0.38) [72]. Because the association of BMI and breast density is well-described and because the potential pathway from physical activity to breast cancer includes changes in body fat, adjustment for BMI is essential in the studies between physical activity and breast density.
Conclusions
We found no evidence of association between physical activity and breast density across the studies included in this review, which might be explained by heterogeneity of the studies. Given the strength of the relationship between physical activity and breast cancer and the null findings of this review, it is unlikely that the effect of physical activity is mediated through an effect on breast density.
Supplementary Material
Acknowledgments
Dr. Graham Colditz is supported by American Cancer Society’s Clinical Research Professorship and U54 CA155496 from the National Cancer Institute’s Centers for Transdisciplinary Research on Energetics and Cancer (TREC). Dr. Kathleen Wolin is supported by grants RO1CA 148791 and U54 CA155496 from the National Cancer Institute and the Barnes Jewish Hospital Foundation. Dr. Yaghjyan is supported by Barnes Jewish Hospital Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-012-2152-z) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no competing interests.
Contributor Information
Lusine Yaghjyan, Email: yaghjyanl@wudosis.wustl.edu, Department of Surgery, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, 660 S. Euclid Avenue, Campus Box 8100, St. Louis 63110, MO, USA.
Graham A. Colditz, Email: colditzg@wudosis.wustl.edu, Department of Surgery, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, 660 S. Euclid Avenue, Campus Box 8100, St. Louis 63110, MO, USA. Institute for Public Health, Washington University in St. Louis, St. Louis, MO, USA. Alvin J Siteman Cancer Center, St. Louis, MO, USA
Kathleen Wolin, Email: wolink@wustl.edu, Department of Surgery, Division of Public Health Sciences, Washington University in St. Louis School of Medicine, 660 S. Euclid Avenue, Campus Box 8100, St. Louis 63110, MO, USA. Institute for Public Health, Washington University in St. Louis, St. Louis, MO, USA. Alvin J Siteman Cancer Center, St. Louis, MO, USA.
References
- 1.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg OM, Martin LJ, Boyd NF. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. 2008;99(9):1369–1374. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 4.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87(9):670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 6.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87(21):1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 7.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 9.Conner P, Svane G, Azavedo E, Soderqvist G, Carlstrom K, Graser T, Walter F, von Schoultz B. Mammographic breast density, hormones, and growth factors during continuous combined hormone therapy. Fertil Steril. 2004;81(6):1617–1623. doi: 10.1016/j.fertnstert.2004.02.096. [DOI] [PubMed] [Google Scholar]
- 10.Marchesoni D, Driul L, Ianni A, Fabiani G, Della Martina M, Zuiani C, Bazzocchi M. Postmenopausal hormone therapy and mammographic breast density. Maturitas. 2006;53(1):59–64. doi: 10.1016/j.maturitas.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Martin LJ, Minkin S, Boyd NF. Hormone therapy, mammographic density, and breast cancer risk. Maturitas. 2009;64(1):20–26. doi: 10.1016/j.maturitas.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285(2):171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 13.Brisson J, Brisson B, Coté G, Maunsell E, Bérubé S, Robert J. Tamoxifen and mammographic breast densities. Cancer Epidemiol Biomark Prev. 2000;9(9):911–915. [PubMed] [Google Scholar]
- 14.Cuzick J, Warwick J, Pinney E, Duffy SW, Cawthorn S, Howell A, Forbes JF, Warren RM. Tamoxifen-induced reduction in mammographic density and breast cancer risk reduction: a nested case–control study. J Natl Cancer Inst. 2011;103(9):744–752. doi: 10.1093/jnci/djr079. [DOI] [PubMed] [Google Scholar]
- 15.Vachon CM, Pankratz VS, Scott CG, Maloney SD, Ghosh K, Brandt KR, Milanese T, Carston MJ, Sellers TA. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(5):921–928. doi: 10.1158/1055-9965.EPI-06-1047. [DOI] [PubMed] [Google Scholar]
- 16.AIRC. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: 2007. [Google Scholar]
- 17.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Lee I-M, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 19.Lopez P, Van Horn L, Colangelo LA, Wolfman JA, Hendrick RE, Gapstur SM. Physical inactivity and percent breast density among Hispanic women. Int J Cancer. 2003;107(6):1012–1016. doi: 10.1002/ijc.11495. [DOI] [PubMed] [Google Scholar]
- 20.Sala E, Warren R, McCann J, Duffy S, Luben R, Day N. High-risk mammographic parenchymal patterns, hormone replacement therapy and other risk factors: a case–control study. Int J Epidemiol. 2000;29(4):629–636. doi: 10.1093/ije/29.4.629. [DOI] [PubMed] [Google Scholar]
- 21.Marmara EA, Papacharalambous XN, Kouloulias VE, Maridaki DM, Baltopoulos JP. Physical activity and mammographic parenchymal patterns among Greek postmenopausal women. Maturitas. 2011;69(1):74–80. doi: 10.1016/j.maturitas.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6(1):5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- 23.Woolcott CG, Courneya KS, Boyd NF, Yaffe MJ, Terry T, McTiernan A, Brant R, Ballard-Barbash R, Irwin ML, Jones CA, Brar S, Campbell KL, McNeely ML, Karvinen KH, Friedenreich CM. Mammographic density change with 1 year of aerobic exercise among postmenopausal women: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):1112–1121. doi: 10.1158/1055-9965.EPI-09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saffer H, Dave DM, Grossman M. Racial, ethnic and gender differences in physical activity. National Bureau of Economic Research, Inc; 2011. p. 17413. NBER Working Papers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthews CE, Hebert JR, Freedson PS, Stanek EJ, III, Merriam PA, Ebbeling CB, Ockene IS. Sources of variance in daily physical activity levels in the seasonal variation of blood cholesterol study. Am J Epidemiol. 2001;153(10):987–995. doi: 10.1093/aje/153.10.987. [DOI] [PubMed] [Google Scholar]
- 26.Greene BL, Haldeman GF, Kaminski A, Neal K, Lim SS, Conn DL. Factors affecting physical activity behavior in urban adults with arthritis who are predominantly African-American and female. Phys Ther. 2006;86(4):510–519. [PubMed] [Google Scholar]
- 27.Peters TM, Ekelund U, Leitzmann M, Easton D, Warren R, Luben R, Bingham S, Khaw KT, Wareham NJ. Physical activity and mammographic breast density in the EPIC-Norfolk cohort study. Am J Epidemiol. 2008;167(5):579–585. doi: 10.1093/aje/kwm350. [DOI] [PubMed] [Google Scholar]
- 28.Oestreicher N, Capra A, Bromberger J, Butler LM, Crandall CJ, Gold EB, Greendale GA, Modugno F, Sternfeld B, Habel LA. Physical activity and mammographic density in a cohort of midlife women. Med Sci Sports Exerc. 2008;40(3):451–456. doi: 10.1249/MSS.0b013e31815f5b47. [DOI] [PubMed] [Google Scholar]
- 29.Sellers TA, Vachon CM, Pankratz VS, Janney CA, Fredericksen Z, Brandt KR, Huang Y, Couch FJ, Kushi LH, Cerhan JR. Association of childhood and adolescent anthropometric factors, physical activity, and diet with adult mammographic breast density. Am J Epidemiol. 2007;166(4):456–464. doi: 10.1093/aje/kwm112. [DOI] [PubMed] [Google Scholar]
- 30.Conroy SM, Butler LM, Harvey D, Gold EB, Sternfeld B, Oestreicher N, Greendale GA, Habel LA. Physical activity and change in mammographic density: the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171(9):960–968. doi: 10.1093/aje/kwq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gram IT, Funkhouser E, Tabar L. Moderate physical activity in relation to mammographic patterns. Cancer Epidemiol Biomarkers Prev. 1999;8(2):117–122. [PubMed] [Google Scholar]
- 32.Prince SA, Adamo KB, Hamel ME, Hardt J, Gorber SC, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Bastawissi AY, White E, Mandelson MT, Taplin S. Variation in mammographic breast density by race. Ann Epidemiol. 2001;11(4):257–263. doi: 10.1016/s1047-2797(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 34.Cuzick J, Warwick J, Pinney E, Warren RML, Duffy SW. Tamoxifen and breast density in women at increased risk of breast cancer. J Natl Cancer Inst. 2004;96(8):621–628. doi: 10.1093/jnci/djh106. [DOI] [PubMed] [Google Scholar]
- 35.Konez O, Goyal M, Reaven RE. Can tamoxifen cause a significant mammographic density change in breast parenchyma? Clin Imaging. 2001;25(5):303–308. doi: 10.1016/s0899-7071(01)00329-1. [DOI] [PubMed] [Google Scholar]
- 36.Ozturk T, Oktay A, Bilgen I, Izmir T. Effect of tamoxifen on breast density in breast cancer patients. Paper presented at the Europena Congress of Radiology; Vienna. 2011. [Google Scholar]
- 37.Gao J, Forbes J, Warren R, Cuzick J, Howell A, D’Este C, Warren-Forward H. Change in mammographic density after cessation of tamoxifen: results from international breast cancer intervention study I (IBIS I). SABCS 2006—San Antonio Breast Cancer Symposium; San Antonio, USA. 2006. [Google Scholar]
- 38.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, Vachon CM. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28(13):2207–2212. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, Miglioretti DL. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal women. Int J Cancer. 2002;98(1):118–127. doi: 10.1002/ijc.10171. [DOI] [PubMed] [Google Scholar]
- 41.Misell L, Hwang E, Au A, Esserman L, Hellerstein M. Development of a novel method for measuring in vivo breast epithelial cell proliferation in humans. Breast Cancer Res Treat. 2005;89(3):257–264. doi: 10.1007/s10549-004-2228-5. [DOI] [PubMed] [Google Scholar]
- 42.Zhu BTCA. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Guillemette C, Belanger A, Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6(6):246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 45.Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2001;10(2):141–142. [PubMed] [Google Scholar]
- 46.White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, Yasui Y, Taplin SH. Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 years. J Natl Cancer Inst. 1998;90(12):906–910. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
- 47.Gapstur SM, Lopez P, Colangelo LA, Wolfman J, Van Horn L, Hendrick RE. Associations of breast cancer risk factors with breast density in Hispanic women. Cancer Epidemiol Bio-markers Prev. 2003;12(10):1074–1080. [PubMed] [Google Scholar]
- 48.Vachon CM, Sellers TA, Vierkant RA, Wu FF, Brandt KR. Case–control study of increased mammographic breast density response to hormone replacement therapy. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1382–1388. [PubMed] [Google Scholar]
- 49.El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States) Cancer Causes Control. 2000;11(10):955–963. doi: 10.1023/a:1026514032085. [DOI] [PubMed] [Google Scholar]
- 50.Harvey J, Scheurer C, Kawakami FT, Quebe-Fehling E, de Palacios PI, Ragavan VV. Hormone replacement therapy and breast density changes. Climacteric. 2005;8(2):185–192. doi: 10.1080/13697130500103458. [DOI] [PubMed] [Google Scholar]
- 51.Persson I, Thurfjell E, Holmberg L. Effect of estrogen and estrogen–progestin replacement regimens on mammographic breast parenchymal density. J Clin Oncol. 1997;15(10):3201–3207. doi: 10.1200/JCO.1997.15.10.3201. [DOI] [PubMed] [Google Scholar]
- 52.Topal NB, Ayhan S, Topal U, Bilgin T. Effects of hormone replacement therapy regimens on mammographic breast density: the role of progestins. J Obstet Gynaecol Res. 2006;32(3):305–308. doi: 10.1111/j.1447-0756.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 53.Aiello EJ, Buist DS, White E. Do breast cancer risk factors modify the association between hormone therapy and mammographic breast density? (United States) Cancer Causes Control. 2006;17(10):1227–1235. doi: 10.1007/s10552-006-0073-z. [DOI] [PubMed] [Google Scholar]
- 54.Pettersen PC, Raundahl J, Loog M, Nielsen M, Tanko LB, Christiansen C. Parallel assessment of the impact of different hormone replacement therapies on breast density by radiologist- and computer-based analyses of mammograms. Climacteric. 2008;11(2):135–143. doi: 10.1080/13697130801930385. [DOI] [PubMed] [Google Scholar]
- 55.Sterns EE, Zee B. Mammographic density changes in perimenopausal and postmenopausal women: is effect of hormone replacement therapy predictable? Breast Cancer Res Treat. 2000;59(2):125–132. doi: 10.1023/a:1006326432340. [DOI] [PubMed] [Google Scholar]
- 56.Erel CT, Esen G, Seyisoglu H, Elter K, Uras C, Ertungealp E, Aksu MF. Mammographic density increase in women receiving different hormone replacement regimens. Maturitas. 2001;40(2):151–157. doi: 10.1016/s0378-5122(01)00236-5. [DOI] [PubMed] [Google Scholar]
- 57.Colacurci N, Fornaro F, De Franciscis P, Palermo M, del Vecchio W. Effects of different types of hormone replacement therapy on mammographic density. Maturitas. 2001;40(2):159–164. doi: 10.1016/s0378-5122(01)00232-8. [DOI] [PubMed] [Google Scholar]
- 58.Titus-Ernstoff L, Tosteson AN, Kasales C, Weiss J, Goodrich M, Hatch EE, Carney PA. Breast cancer risk factors in relation to breast density (United States) Cancer Causes Control. 2006;17(10):1281–1290. doi: 10.1007/s10552-006-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stuedal A. Postmenopausal hormone therapy with estradiol and norethisterone acetate and mammographic density: findings from a cross-sectional study among Norwegian women. Climacteric. 2009;12(3):248–258. doi: 10.1080/13697130802638458. [DOI] [PubMed] [Google Scholar]
- 60.Irwin M, Aiello E, McTiernan A, Baumgartner R, Baumgartner K, Bernstein L, Gilliland F, Ballard-Barbash R. Pre-diagnosis physical activity and mammographic density in breast cancer survivors. Breast Cancer Res Treat. 2006;95(2):171–178. doi: 10.1007/s10549-005-9063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warwick J, Pinney E, Warren RM, Duffy SW, Howell A, Wilson M, Cuzick J. Breast density and breast cancer risk factors in a high-risk population. Breast. 2003;12(1):10–16. doi: 10.1016/s0960-9776(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 62.Vachon CM, Kuni CC, Anderson K, Anderson VE, Sellers TA. Association of mammographically defined percent breast density with epidemiologic risk factors for breast cancer (United States) Cancer Causes Control. 2000;11(7):653–662. doi: 10.1023/a:1008926607428. [DOI] [PubMed] [Google Scholar]
- 63.Vachon CM, Sellers TA, Janney CA, Brandt KR, Carlson EE, Pankratz VS, Wu FF, Therneau TM, Cerhan JR. Alcohol intake in adolescence and mammographic density. Int J Cancer. 2005;117(5):837–841. doi: 10.1002/ijc.21227. [DOI] [PubMed] [Google Scholar]
- 64.Maskarinec G, Takata Y, Pagano I, Lurie G, Wilkens LR, Kolonel LN. Alcohol consumption and mammographic density in a multiethnic population. Int J Cancer. 2006;118(10):2579–2583. doi: 10.1002/ijc.21705. [DOI] [PubMed] [Google Scholar]
- 65.Gram IT, Funkhouser E, Tabar L. Reproductive and menstrual factors in relation to mammographic parenchymal patterns among perimenopausal women. Br J Cancer. 1995;71(3):647–650. doi: 10.1038/bjc.1995.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelemen LE, Pankratz VS, Sellers TA, Brandt KR, Wang A, Janney C, Fredericksen ZS, Cerhan JR, Vachon CM. Age-specific trends in mammographic density: the Minnesota Breast Cancer Family Study. Am J Epidemiol. 2008;167(9):1027–1036. doi: 10.1093/aje/kwn063. [DOI] [PubMed] [Google Scholar]
- 67.Modugno F, Ngo DL, Allen GO, Kuller LH, Ness RB, Vogel VG, Costantino JP, Cauley JA. Breast cancer risk factors and mammographic breast density in women over age 70. Breast Cancer Res Treat. 2006;97(2):157–166. doi: 10.1007/s10549-005-9105-8. [DOI] [PubMed] [Google Scholar]
- 68.Sala E, Warren R, McCann J, Duffy S, Luben R, Day N. High-risk mammographic parenchymal patterns and anthropometric measures: a case–control study. Br J Cancer. 1999;81(7):1257–1261. doi: 10.1038/sj.bjc.6690838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lam PB, Vacek PM, Geller BM, Muss HB. The association of increased weight, body mass index, and tissue density with the risk of breast carcinoma in Vermont. Cancer. 2000;89(2):369–375. doi: 10.1002/1097-0142(20000715)89:2<369::aid-cncr23>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 70.Noh JJ, Maskarinec G, Pagano I, Cheung LW, Stanczyk FZ. Mammographic densities and circulating hormones: a cross-sectional study in premenopausal women. Breast. 2006;15(1):20–28. doi: 10.1016/j.breast.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 71.Johansson H, Gandini S, Bonanni B, Mariette F, Guerrieri-Gonzaga A, Serrano D, Cassano E, Ramazzotto F, Baglietto L, Sandri M, Decensi A. Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res Treat. 2008;108(1):57–67. doi: 10.1007/s10549-007-9577-9. [DOI] [PubMed] [Google Scholar]
- 72.Chaput J-P, Klingenberg L, Rosenkilde M, Gilbert J-A, Tremblay A, Sjordin A. Physical activity plays an important role in body weight regulation. J Obes. 2011;2011:1–11. doi: 10.1155/2011/360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masala G, Assedi M, Ambrogetti D, Sera F, Salvini S, Bendinelli B, Ermini I, Giorgi D, Rosselli del Turco M, Palli D. Physical activity and mammographic breast density in a Mediterranean population: the EPIC Florence longitudinal study. Int J Cancer. 2009;124(7):1654–1661. doi: 10.1002/ijc.24099. [DOI] [PubMed] [Google Scholar]
- 74.Jeffreys M, Warren R, Gunnell D, McCarron P, Smith GD. Life course breast cancer risk factors and adult breast density (United Kingdom) Cancer Causes Control. 2004;15(9):947–955. doi: 10.1007/s10522-004-2473-3. [DOI] [PubMed] [Google Scholar]
- 75.Suijkerbuijk KPM, Van Duijnhoven FJB, Van Gils CH, Van Noord PAH, Peeters PHM, Friedenreich CM, Monninkhof EM. Physical activity in relation to mammographic density in the Dutch prospect-European prospective investigation into cancer and nutrition cohort. Cancer Epidemiol Biomark Prev. 2006;15(3):456–460. doi: 10.1158/1055-9965.EPI-05-0569. [DOI] [PubMed] [Google Scholar]
- 76.Siozon CC, Ma H, Hilsen M, Bernstein L, Ursin G. The association between recreational physical activity and mammographic density. Int J Cancer. 2006;119(7):1695–1701. doi: 10.1002/ijc.22020. [DOI] [PubMed] [Google Scholar]
- 77.Reeves KW, Gierach GL, Modugno F. Recreational physical activity and mammographic breast density characteristics. Cancer Epidemiol Biomarkers Prev. 2007;16(5):934–942. doi: 10.1158/1055-9965.EPI-06-0732. [DOI] [PubMed] [Google Scholar]
- 78.Wolin KY, Colangelo LA, Chiu BC, Ainsworth B, Chatterton R, Gapstur SM. Associations of physical activity, sedentary time, and insulin with percent breast density in Hispanic women. J Womens Health (Larchmt) 2007;16(7):1004–1011. doi: 10.1089/jwh.2006.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Irwin ML, Aiello EJ, McTiernan A, Bernstein L, Gilliland FD, Baumgartner RN, Baumgartner KB, Ballard-Barbash R. Physical activity, body mass index, and mammographic density in postmenopausal breast cancer survivors. J Clin Oncol. 2007;25(9):1061–1066. doi: 10.1200/JCO.2006.07.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Samimi G, Colditz GA, Baer HJ, Tamimi RM. Measures of energy balance and mammographic density in the Nurses’ Health Study. Breast Cancer Res Treat. 2008;109(1):113–122. doi: 10.1007/s10549-007-9631-7. [DOI] [PubMed] [Google Scholar]
- 81.Tseng M, Olufade TO, Evers KA, Byrne C. Adolescent lifestyle factors and adult breast density in U.S. Chinese immigrant women. Nutr Cancer. 2011;63(3):342–349. doi: 10.1080/01635581.2011.535955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.