Abstract
Background
Impaired ability to use contextual information to optimally prepare for tasks contributes to performance deficits in schizophrenia. We used magnetoencephalography (MEG) and an antisaccade task to investigate the neural basis of this deficit.
Methods
In patients with schizophrenia (n=25) and healthy controls (n=18) we examined the difference in preparatory activation to cues indicating an impending antisaccade or prosaccade. We analyzed activation for correct trials only and focused on the network for volitional ocular motor control – frontal eye field (FEF), dorsal anterior cingulate cortex (dACC), and the ventrolateral and dorsolateral prefrontal cortex (VLPFC, DLPFC).
Results
Compared to controls, patients made more antisaccade errors and showed reduced differential preparatory activation in the dACC and increased differential preparatory activation in the VLPFC. In patients only, antisaccade error rates correlated with preparatory activation in the FEF, DLPFC, and VLPFC.
Conclusions
In schizophrenia, reduced differential preparatory activation of the dACC may reflect reduced signaling of the need for control. Greater preparatory activation in the VLPFC and the correlations of error rate with FEF, DLPFC, and VLPFC activation may reflect that patients who are more error-prone require stronger activation in these regions for correct performance. These findings provide the first evidence of abnormal task preparation, distinct from response generation, during volitional saccades in schizophrenia. We conclude that schizophrenia patients are impaired in using task cues to modulate cognitive control and that this contributes to deficits inhibiting prepotent but contextually inappropriate responses and to behavior that is stimulus-bound and error-prone rather than flexibly guided by context.
Keywords: schizophrenia, antisaccade, frontal eye field, anterior cingulate cortex, lateral prefrontal cortex, cognitive control
Introduction
A key feature of schizophrenia is impaired cognitive control, the ability to mobilize cognitive resources to support task goals in the face of response competition (1). This includes difficulty using contextual information to optimally adjust control to prepare for a task, which contributes to rigid and perseverative behavior (2). Here we used a saccadic paradigm to investigate neural responses to contextual cues indicating that the impending task would require a high (antisaccade) vs. low (prosaccade) level of control. Antisaccades require inhibition of the prepotent response of looking towards a visual stimulus (i.e., a prosaccade) and substitution of gaze in the opposite direction (3). Patients with schizophrenia and their first degree relatives consistently show more antisaccade errors than controls (i.e., failures to suppress the prepotent prosaccade, for reviews see 4, 5, 6), but it is unclear whether this reflects a deficit in mobilizing cognitive resources to prepare for the antisaccade, in planning and generating a motor response, or both. The task used in the present study was designed to discriminate between these possibilities by inserting a ‘preparatory interval’ between the task cue and the imperative stimulus. During this interval, because the task is known from the cue but the required response is not (i.e., a saccade to the right or left), activity reflects task preparation rather than motor planning. In the present study we tested the hypothesis that schizophrenia patients fail to optimally modulate control in response to contextual cues, and that this contributes to errors. To this end, we examined preparatory activation in the cognitive control network and its relation with error rate.
Antisaccades are ideal for the study of preparatory activity as their neural underpinnings have been extensively characterized by monkey neurophysiology, human neuroimaging and human lesion studies (7). Single-unit recordings in monkeys (8) and human neuroimaging studies (9) demonstrate that preparatory activity in the frontal eye field (FEF) predicts both the likelihood and the latency of a correct response. FEF neurons show reduced preparatory firing rates in response to antisaccade compared with prosaccade task cues, and greater preparatory suppression predicts longer response latencies and lower error rates. Preparatory activity in the FEF is thought to be modulated by the dorsal anterior cingulate cortex (dACC) and lateral prefrontal cortex (PFC), key regions in the ‘top-down’ control of motor and ocular motor structures (10, 11). The lateral PFC and dACC are structurally (12, 13) and functionally (14–16) connected to the FEF. In human neuroimaging studies, the FEF, dACC, and both the ventrolateral and dorsolateral PFC (VLPFC, DLPFC), show greater activation for antisaccades than for prosaccades (17–19), and lesions of the dACC (20), DLPFC (21), and VLPFC (22) increase antisaccade errors. These findings suggest that the dACC, lateral PFC, and FEF coordinate preparatory activity to establish and maintain the antisaccade task-set.
Prior functional neuroimaging studies of antisaccades in schizophrenia have produced conflicting reports of either decreased (23, 24) or no difference (25–28) in FEF activation compared to controls. Other studies variably report reduced activation of the lateral PFC, dACC, insula, thalamus, and striatum in patients and their healthy relatives (23–27, 29, 30). Discrepant findings may reflect task differences, the inclusion of error trials, and group differences in the timing of the hemodynamic response (28). Two event-related potential studies reported reduced contingent negative variation (CNV) during antisaccades versus prosaccades in schizophrenia, suggesting that patients failed to modulate cognitive control based on task (31, 32). As no prior study distinguished between preparatory activation from that due to planning and generating the motor response, it is unclear which processes contribute to increased antisaccade errors in schizophrenia.
In the present study, we exploited the millisecond temporal resolution of magnetoencephalography (MEG) to restrict our analyses to neural activity during task preparation during correct trials only. We compared groups on the difference in activation for antisaccades, which require a high level of control, compared with prosaccades, which are relatively automatic responses in the volitional ocular motor control network, FEF, dACC, VLPFC and DLPFC. This comparison addressed our primary hypothesis that schizophrenia patients would show reduced modulation of preparatory activation in response to cues indicating that a high vs. low level of control was required. We also tested the hypothesis that preparatory activation in the FEF would predict antisaccade error rate, as it does in monkey neurophysiology studies (8).
Methods
Participants
Twenty-five outpatients with schizophrenia were recruited from an urban mental health center. With the exception of one patient who took fluphenazine, all patients had been maintained on stable doses of atypical antipsychotic medications for at least six weeks. Diagnoses were confirmed with Structured Clinical Interviews for DSM-IV (33). Clinical status was characterized with the Positive and Negative Syndrome Scale (PANSS, 34), the Scale for the Assessment of Negative Symptoms (SANS, 35), and the Brief Psychiatric Rating Scale (BPRS, 36).
Twenty healthy control participants, screened to exclude a personal history of mental illness (SCID-Non-patient edition, 37) or a family history of schizophrenia spectrum disorder, were recruited from the community by poster and website advertisements. Two control participants were excluded, one for excessive blink artifacts, and one for an antisaccade error rate greater than two standard deviations higher than the group mean. All participants were screened to exclude substance abuse or dependence within the preceding six months and any independent conditions that might affect brain function. The final groups of 25 schizophrenia patients and 18 controls did not differ significantly in age, sex, handedness (38, 39), or mean parental education (Table 1). The study was approved by the Partners Human Research Committee and all participants gave written informed consent.
Table 1.
Means, standard deviations and group comparisons of demographic data and rating scale scores. The Phi value is the result of a Fisher’s Exact Test.
| Subject characteristics | Healthy controls (n=18) | Schizophrenia patients (n=25) | t | p |
|---|---|---|---|---|
| Age | 31 ± 10 | 34 ± 13 | .86 | .39 |
| Sex | 10M/8F | 19M/6F | Phi = .21 | .20 |
| Handedness | 81 ± 40 | 76 ± 38 | .39 | .69 |
| Parental Education (years) | 15.5 ± 3.5 | 14.4 ± 2.4 | 1.17 | .25 |
| Age of Onset | 25 ± 6 | |||
| Length of Illness (years) | 12 ± 11 | Level of Severity | ||
| BPRS | 14 ± 8 | Minimal | ||
| PANSS positive | 13 ± 5 | Mild | ||
| PANSS negative | 15 ± 5 | Mild | ||
| SANS | 29 ± 17 | Questionable | ||
Procedures
Please see our prior publication for details of the saccadic paradigm and MEG analysis (40).
Saccadic paradigm
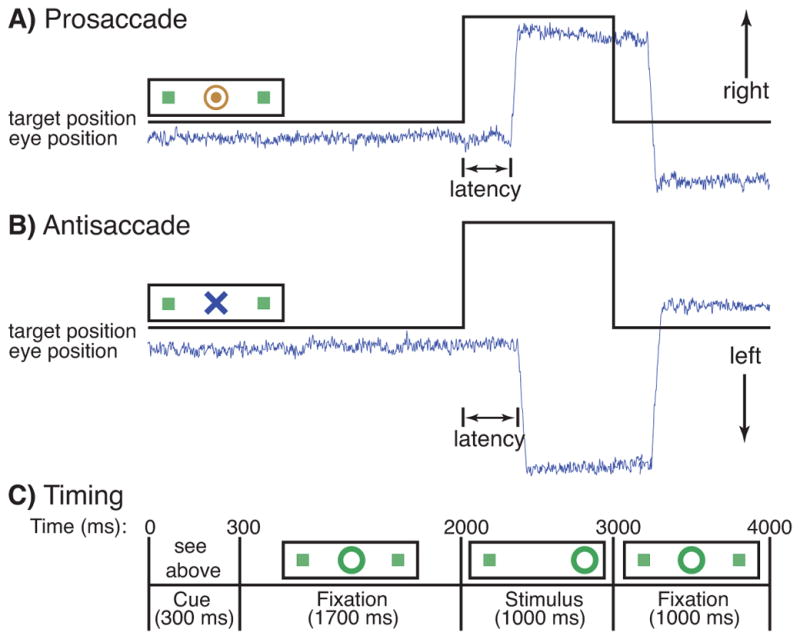
The task consisted of a pseudorandom sequence of prosaccade and antisaccade trials that were balanced for right and left movements. Each saccadic trial lasted 4s and began with an instructional cue, which, at 300ms, was replaced by a central fixation ring. At 2s the fixation ring shifted to either the right or left of center for 1s. This was the stimulus to which participants responded. During the final second of the trial the fixation ring returned to center. Task details are provided in Figure 1. We analyzed the preparatory interval, which was the first 2s of the trial, prior to the appearance of the imperative stimulus. Saccadic trials were intermixed with intervals of fixation lasting 2, 4, or 6s. Fixation intervals provided breaks and their lengths were varied to decrease the predictability of trial onset and thereby enhance attention. Participants performed eight runs of the task, each lasting 5min 22s, for a total time of approximately 1h. The experiment generated a total of 278 prosaccade, 285 antisaccade, and 107 fixation trials. The horizontal and vertical components of eye movements were recorded concurrently with the MEG, using two pairs of bipolar electro-oculogram (EOG) electrodes.
Figure 1.
Saccadic paradigm with idealized eye position traces. Saccadic trials lasted 4000 ms and began with an instructional cue at the center of the screen. For half of the participants, orange concentric rings were the cue for a prosaccade trial a) and a blue X was the cue for an antisaccade trial b). These cues were reversed for the rest of the participants. The cue was flanked horizontally by two small green squares of 0.2° wide that marked the potential locations of targets, 10° left and right of center. These squares remained on the screen for the duration of each run. c) At 300 ms, the instructional cue was replaced by a green fixation ring at the center of the screen, of 0.4° diameter and luminance of 20 cd/m2. At 2000 ms, the ring shifted to one of the two peripheral locations, right or left, with equal probability. This was the stimulus to which the participant responded. The green ring remained at the peripheral location for 1000 ms and then returned to center for the final 1000 ms of the trial. Fixation intervals were simply a continuation of the fixation display that constituted the final second of the previous saccadic trial.
MEG data acquisition
MEG data were acquired inside a magnetically shielded room (IMEDCO, Hagendorf, Switzerland) using a dc-SQUID Neuromag™ VectorView system (Elekta-Neuromag, Helsinki, Finland) comprising 306 sensors arranged in triplets of two orthogonal planar gradiometers and a magnetometer, distributed at 102 locations around the entire scalp. During the MEG recording, the position and orientation of the head with respect to the MEG sensor array were determined with four head position indicator (HPI) coils.
Structural MRI acquisition
Two T1-weighted high-resolution structural images were acquired for spatial normalization and cortical surface reconstruction using a 3.0T Siemens (Erlangen, Germany) Trio whole body high-speed imaging device equipped for echo planar imaging (EPI) and a 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence.
Scoring of eye movement data
EOG data were scored in MATLAB (Mathworks, Natick, MA) using a partially automated program that determined the directional accuracy of each saccade with respect to the required response, and the latency from stimulus onset. Only correct trials were analyzed. A breakdown of trial exclusions for MEG analysis and their rationale is provided in the Supplement. Latency and error rate analyses included all scorable trials, regardless of blinks, losses of fixation, or prior errors. Error rate data were logit-transformed before analysis. We employed ANOVAs with factors for group (SZ, HC) and trial type as a repeated measure, and their interaction.
Offline analysis of MEG data
All channels were processed using the signal-space separation method (SSS, 41). The data of three patients were also processed using spatio-temporal signal-space separation (tSSS, 42) with a correlation of limit value of 0.95 or higher to suppress magnetic artifact due to dental work. For off-line averaging, each participant’s continuous MEG data were low-pass filtered at 40Hz. The waveforms for correct prosaccades and antisaccades were then averaged for each participant. A 200ms interval prior to the appearance of the cue was used as baseline and subtracted from each epoch before the trial was added to the average.
For source estimation, the geometry of each participant’s cortical surface was reconstructed from 3D structural MRI data using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu). To display activity in the sulci, inflated cortical surfaces were employed in visualization. The forward solution was calculated using a single-compartment boundary-element model (43) with the inner skull surface segmented from the MRI data. The head position information from the start of each run was used in the calculation of the forward solution for each run. Activity at each cortical location was estimated every 4ms using the anatomically-constrained linear estimation approach (44–46). In calculating the average dipole waveforms, the orientation of the dipole moment was loosely constrained to the cortical normal direction by setting source variances for the transverse current components to be 0.1 times the variance of the currents normal to the cortical surface (47). The inverse solutions were temporally smoothed by integrating over an interval extending 2 ms in each direction.
Inter-subject registration for group analysis
Each participant’s inflated cortical surface was registered to a template brain by optimally aligning individual sulcal-gyral patterns (48). Individual data were registered to the averaged cortical surface and the results were averaged across participants.
Region of interest (ROI) definition
We defined ROIs for the FEF, VLPFC, DLPFC, and dACC using anatomical labels provided by an automated cortical surface-based parcellation (49). We used sulcal anatomical labels for ROIs on the lateral cortical surface since MEG is best able to detect tangential sources (i.e., those in sulci rather than on gyri on the lateral surface). We used the superior and inferior precentral sulci as the FEF ROI (40, 50), since the FEF is located in and around the superior and inferior portions of the precentral sulcus and gyrus (51–54). The DLPFC and VLPFC ROIs were defined as the superior and inferior frontal sulci, respectively. We defined the dACC ROI by combining the anterior cingulate sulci and gyri and dividing them into dorsal and rostral segments by drawing a line perpendicular to the intercommissural plane at the anterior boundary of the genu of the corpus callosum (55). As we had no a priori basis to expect lateralized effects for these regions, our ROIs included both hemispheres.
Evaluation of preparatory activation
We examined ROI activation in the preparatory (cue-stimulus) interval from 0–2000ms locked to the appearance of the task cue (Figure 1). Activity was averaged across all of the vertices in each ROI at each 4ms epoch for antisaccades and prosaccades in each participant.
We first compared activation for antisaccades vs. prosaccades within each group using pairwise t-tests. We considered a difference to be significant only if five consecutive 4ms epochs met a threshold of p<0.05. This method corrects for multiple comparisons over time and sets the overall alpha to p<0.05 (56).
To compare groups on activation for antisaccades vs. prosaccades and to test the hypothesis that preparatory activation predicts error rate, we performed a mixed model regression. We treated participant as a random effect and regressed activation on the following fixed covariates: logit transformed error rate, the interaction of error rate with group, and a full factorial of time interval (at six 250ms epochs from 500–2000ms), group, and ROI. Using this model, we used ANOVA to assess the effects of: (1) group and the interaction of group by ROI on activation and (2) error rate and the interaction of error rate with group on activation. We then examined each ROI separately to determine the direction and timing of both group differences in activation and the relations of activation with error rate.
To compare the groups on activation in each ROI, we used pairwise t-tests and bootstrapping analyses. Our index of activation was the difference between antisaccade and prosaccade activation normalized by the sum of activation (i.e., (AS−PS)/(AS+PS)) in each participant at each ROI. We normalized the difference score to mitigate against the effects of variation in the amplitude of the MEG signal across participants and groups. The normalized difference scores were compared between groups at each 4ms epoch using pairwise t-tests.
As a second confirmatory analysis of group differences, we employed a bootstrapping procedure (57) to test for sustained differences in activity between groups at each ROI. These analyses used the normalized difference scores for each participant averaged over 250ms windows, between 500ms and 2000ms after cue onset. The bootstrapping procedures are described in the Supplement.
To examine the relations between preparatory activity and antisaccade error rate in each ROI, we regressed activation on error rate, time interval, group, and the group by time interaction. A compound symmetric error model was assumed for the repeated measures of activation at the six time intervals within participant. Hypothesis testing was based on sequential sums of squares.
Results
Behavioral data
Schizophrenia patients made more errors than controls (F(1,41)=4.23, p=.05) and there was a group by task interaction (F(1,41)=4.70, p=.04) reflecting that while patients made almost twice as many antisaccade errors as controls (21±16% vs. 11±11%; t(41)=2.95, p=.004, effect size (ES)=.52), they did not differ in prosaccade errors (5±5 vs. 4±3%; t(41)=0.31, p=.75, ES=.17). Overall, patients responded more slowly than controls on correct trials, but not significantly so (F(1,41)=2.42, p=.13), and this did not differ by task (F(1,41)=0.05, p=.81; antisaccades: 318±72 vs. 288±47 ms, t(41)=1.57, p=.12; ES=.35) prosaccades: 269±72 vs. 241±39 ms, t(41)=1.47, p=.15; ES=.34).
Preparatory MEG activation
The mixed model regression of activation including all ROIs showed a highly significant group by region interaction (Chisquare=44.7(3) p=1.1*10−9) indicating that group differences in activation depend strongly on ROI.
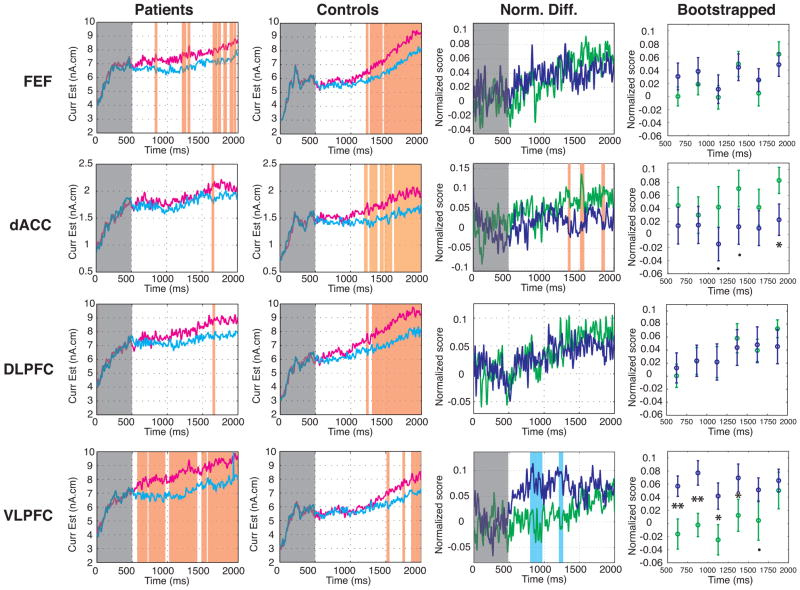
Separate analysis of each ROI revealed that in the FEF, both groups showed greater activation for antisaccades than prosaccades, which first reached significance at 828ms for patients and at 1220ms for controls (Figure 2). Comparisons of the normalized difference scores showed that activation for antisaccades vs. prosaccades did not differ by group.
Figure 2.
Preparatory activation for antisaccades (red) and prosaccades (blue) in the FEF, dACC, DLPFC, and VLPFC. First column: patients; second column: controls. Significant differences between trial types (based on five consecutive 4 ms epochs at p<.05) are denoted by orange vertical stripes. The third column displays normalized difference scores of activation for antisaccades minus prosaccades in patients (purple) and controls (green). Significant differences between groups (based on five consecutive 4 ms epochs at p<.05) are denoted by orange (controls > patients) or blue (patients > controls) vertical stripes. The fourth column displays the means and standard deviations of the bootstrapped normalized difference scores for the patient (yellow) and control (green) groups.. Two asterisks denotes statistical significance at p < 0.01, an asterisk denotes p < 0.05 and a dot denotes p < 0.1.
In both the dACC and DLPFC, controls showed a sustained significant increase in activation for antisaccades vs. prosaccades beginning at 1200ms. Patients, in contrast, showed only one 4ms epoch of significantly greater activation for antisaccades vs. prosaccades in each ROI. In the dACC, compared to controls, patients showed significantly reduced differential preparatory activation for antisaccades vs. prosaccades at several intervals between 1352 and1860ms. The bootstrapping analysis confirmed a significant reduction during the interval immediately prior to stimulus onset, 1750–2000ms. In the DLPFC, group differences in preparatory activation for antisaccades vs. prosaccades did not reach significance.
In the VLPFC both groups showed greater antisaccade than prosaccade activation, which first reached significance at 580ms for patients and at 1520ms for controls. This difference activation was greater for patients than controls during several intervals between 808 and 1260ms, and the bootstrapping analysis confirmed significantly increased activation for patients between 500 and 1500ms.
Regressions with error rate
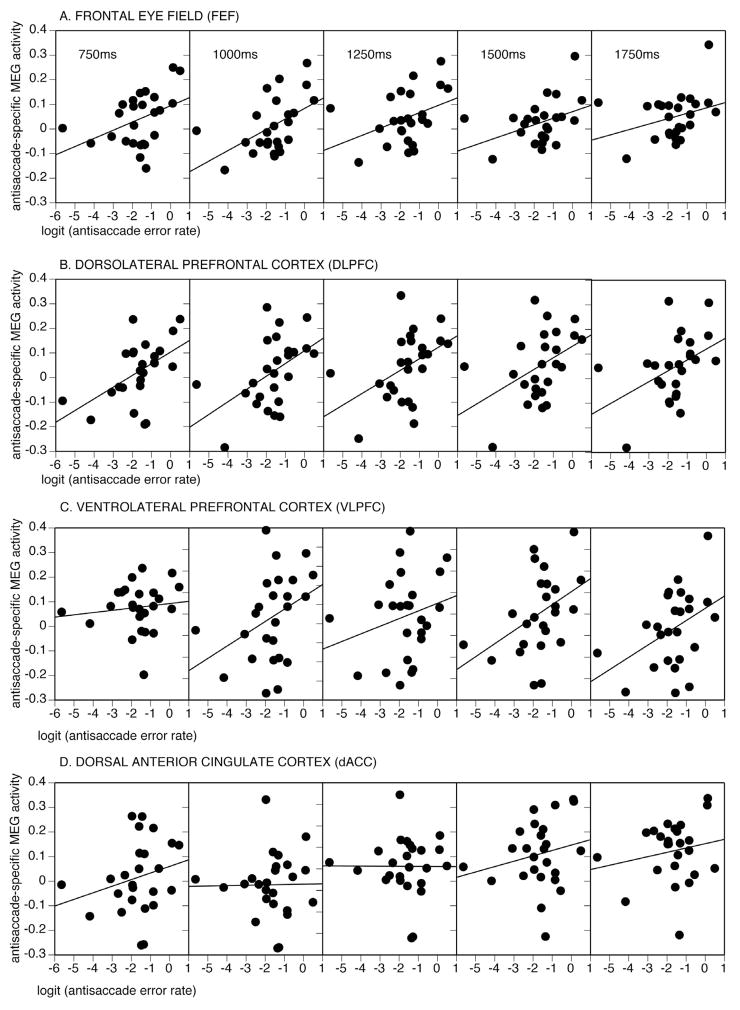
The mixed model regression showed trends for error rate (Chisquare=2.8(1), p=.09) and the interaction of error rate by group to predict activation (Chisquare=3.6(1), p=.056). This suggests that error rate predicts activation and that this effect may differ by group. To determine the timing and direction of these relations in each ROI, we performed separate regressions. In the FEF and DLPFC, activation was not significantly related to errors in patients and controls combined, but there were significant group by time interactions (Table 2). These reflected that in patients only, increased activation in the FEF and DLPFC was associated with higher error rates during multiple 250 ms epochs (Figure 3). In the VLPFC, increased activation predicted a higher error rate at a trend level in the combined group, and did not differ significantly by group. Only in patients, however, was greater VLPFC activation significantly associated with a higher error rate in the intervals immediately prior to stimulus appearance. dACC activation was not significantly related to error rate in either group.
Table 2.
Regressions of preparatory activation for antisaccades vs. prosaccades on logit transformed error rate with t-tests for each group at the six 250ms epochs from 500–2000ms following the cue.
| Region | AS Error Rate | Error × Group | Interval | Controls | Patients | ||||
|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | t | p | t | p | ||
| FEF | 1.57 | .21 | 5.83 | .02* | 500 | −0.14 | .89 | 0.75 | .46 |
| 750 | −1.06 | .30 | 2.16 | .04* | |||||
| 1000 | −1.08 | .29 | 2.79 | .01* | |||||
| 1250 | −1.45 | .17 | 2.01 | .06 | |||||
| 1500 | 0.58 | .57 | 2.06 | .05* | |||||
| 1750 | 0.39 | .70 | 1.57 | .13 | |||||
| DLPFC | 0.16 | .69 | 9.08 | .005* | 500 | −1.22 | .24 | 1.81 | .08 |
| 750 | −1.36 | .19 | 2.88 | .009* | |||||
| 1000 | −1.96 | .07 | 2.66 | .01* | |||||
| 1250 | −2.29 | .04* | 2.40 | .02* | |||||
| 1500 | −0.88 | .39 | 2.36 | .03* | |||||
| 1750 | −0.31 | .76 | 2.30 | .03* | |||||
| VLPFC | 3.10 | .08 | 0.00 | .95 | 500 | 0.83 | .42 | −0.31 | .76 |
| 750 | 0.46 | .65 | 0.61 | .55 | |||||
| 1000 | 0.21 | .83 | 1.9 | .07 | |||||
| 1250 | 0.09 | .93 | 1.2 | .24 | |||||
| 1500 | 0.80 | .44 | 2.28 | .03* | |||||
| 1750 | 0.94 | .36 | 2.23 | .04* | |||||
| dACC | 0.28 | .60 | 2.73 | .11 | 500 | −0.44 | .66 | 1.27 | .21 |
| 750 | −0.91 | .38 | 1.19 | .24 | |||||
| 1000 | −1.18 | .26 | 0.07 | .94 | |||||
| 1250 | −0.96 | .35 | −0.02 | .98 | |||||
| 1500 | 0.01 | .99 | 1.03 | .31 | |||||
| 1750 | 0.02 | .99 | 0.91 | .37 | |||||
Figure 3.
Relations of logit transformed antisaccade error rate with FEF, DLPFC, VLPFC, and dACC activation in patients averaged over 250 ms intervals from 750–2000.
Discussion
The present findings provide the first evidence of abnormal task preparation, distinct from response generation, during volitional saccades in schizophrenia. Patients made more antisaccade errors than controls and, during correct antisaccade vs. prosaccade trials, showed an abnormal pattern of preparatory activation in the network for volitional ocular motor control. Specifically, while healthy controls responded to cues indicating that the impending task would require a high (antisaccade) vs. low (prosaccade) level of control with sustained significant increases in network activation, patients failed to show sustained significant increases in activation in either the dACC or DLPFC, and differed significantly from controls in the dACC. In contrast, in the VLPFC, patients showed an earlier, greater and more sustained increase in preparatory activation than controls. Finally, preparatory activation of the FEF, DLPFC, and VLPFC predicted antisaccade error rate in patients only. We interpret these findings to reflect that schizophrenia patients show aberrant use of task cues to modulate cognitive control and that this contributes to deficient inhibition of prepotent but contextually inappropriate responses.
According to current theory, the dACC, DLPFC, and VLPFC are key components of a network for implementing task control across modalities (58, 59). In the ocular motor system, these regions are thought to exert top-down control on the FEF (10), the key cortical region for generating volitional saccades (60). These regions show greater fMRI activation for antisaccades vs. prosaccades (17–19) and lesions are associated with increased antisaccade errors (20–22). While the relative specialization of each region is a topic of active study, current models propose that in response to contextual cues, the dACC signals the need for adjustments in control and modulates the involvement of the DLPFC, which coordinates processing across the brain to support performance (11, 61). In healthy controls, the remarkably similar timing of increased activation in the dACC, DLPFC, and FEF (Figure 2) is consistent with the theory that the dACC and DLPFC act together to exert control over the FEF in preparation for a challenging task. In the context of these models, reduced preparatory dACC activation in schizophrenia may reflect impaired recognition and signaling of the need for greater control and may lead to reduced DLPFC recruitment (61). Other potentially compatible interpretations of reduced dACC activation include that it reflects impaired motivation, attention, and recognition and preparation for response conflict.
The finding of increased differential preparatory activation of the VLPFC in schizophrenia was unexpected and should therefore be considered preliminary. Accumulating evidence suggests that the VLPFC contributes to task rule representation and inhibitory control (22, 58, 62). One plausible interpretation of the pattern of findings in schizophrenia is that to compensate for reduced top-down control by the dACC, patients increase the engagement of processes mediated by the VLPFC. These processes may include updating and maintaining the task-set and increasing inhibitory control. This increased engagement may be reflected in the markedly earlier and stronger recruitment of VLPFC in patients than controls who, in contrast to patients, showed relatively less VLPFC than DLPFC or dACC activation (Figure 2).
The pattern of findings in patients resonate with a recent meta-analysis of functional neuroimaging studies of executive function in schizophrenia that revealed weaker dACC and DLPFC activation in the context of greater VLPFC activation (63). This suggests that this pattern of regional recruitment is general to tasks requiring cognitive control in schizophrenia, regardless of task and measurement modality. The present study adds to this literature by demonstrating anomalous function of the cognitive control network specifically during task preparation that predicts deficient task performance.
Increased activation in the DLPFC, VLPFC and FEF during correct trials predicted more antisaccade errors in patients. While errors reflect a failure of response inhibition, activation reflects the magnitude of the difference in activation between antisaccades and prosaccades during correct trials. Thus, these relations may reflect that within the schizophrenia group, individuals who are more prone to errors (i.e., have a higher error rate) require stronger top-down control from lateral PFC and stronger inhibition of the FEF to successfully inhibit prepotent responses. This interpretation assumes that saccadic inhibition requires neuronal inhibition in the FEF. Evidence for this comes from studies of monkey FEF showing that antisaccade versus prosaccade cues result in reduced neuronal firing (8) and that infusions of a 3-aminobutyric acid (GABA) agonist interfere with the generation of volitional saccades while a GABA antagonist facilitates them (64, 65). As MEG source signals primarily reflect postsynaptic currents, it is possible that increased MEG activation of the FEF reflects increased inhibitory input. The relations of error rate with lateral PFC activation are consistent with its putative role in modulating FEF activity during antisaccades. Only in the dACC did relations of activation with error rate not reach significance. It is unclear whether the dACC directly modulates FEF activity or whether it does so via the lateral PFC (10). If the latter option, the less direct influence of the dACC on FEF activity may account for its weaker relations with error rate. The lack of any significant relations of activation with error rate in controls may reflect the more restricted range of errors.
The present findings suggest that abnormal preparatory recruitment of the cognitive control network in response to task cues contributes to antisaccade errors in schizophrenia. But abnormal preparation is unlikely to be the only culprit. Other possible contributors include less efficient implementation of inhibition, slower activation of the antisaccade task goal (e.g., 66), and perseveration of prior responses that interferes with performance (28, 62, 66–68). By allowing an examination of temporally-separated epochs of task performance, MEG can delineate spared and impaired processes.
A limitation to the interpretation of the present findings is that we did not investigate whether they reflect a specific deficit in the use of context to prepare, or a more general deficit in the ability to prepare. In addition, we did not directly compare groups on activation for single trial types, leaving open the possibility that an abnormal response to task cues on prosaccade rather than, or in addition to, antisaccade trials accounts for our findings. For example, increased preparatory activation on prepotent prosaccades along with reduced activation on the more effortful antisaccades, consistent with the cortical ‘inefficiency’ hypothesis of schizophrenia (69–71), could account for reduced differential activity. Previous work, however, shows normal fMRI activation for prosaccades, but not antisaccades in patients with schizophrenia (e.g., 25, 28). The present findings of an elevated error rate for antisaccades but not prosaccades, and that antisaccade error rate correlates with preparatory activation in patients suggests that abnormal preparation for antisaccades is an important contributor to the group differences we observed. Finally, the effects of chronic illness and antipsychotic medications may have contributed to our results. Prior work has shown reduced neural and behavioral responses to contextual cues in first-episode antipsychotic-naïve patients using an AX-CPT task, indicating that abnormal context processing is present early in the illness, prior to treatment with medications (2). In the present study, although it is difficult to ascribe the pattern of increased, decreased, and comparable activation in schizophrenia to medication or chronicity, we cannot exclude a contribution from these factors.
In summary, the present findings suggest that patients with schizophrenia are less able to use contextual cues to mobilize cognitive resources in preparation for challenging tasks. This deficit may compromise their ability to rapidly adjust behavior in response to the demands of the moment. These dynamic adjustments are fundamental to adaptive, flexible behavior and impairments may contribute to behavior that is stimulus-bound and error-prone rather than flexibly guided by context.
Supplementary Material
Acknowledgments
The authors wish to thank Jay Edelman for programming the saccadic task and Matthew Cain, Katy Thakkar, Frida Polli, and Szymon Mikulski for their contributions to data collection and analysis.
Footnotes
Drs. Lee, Dyckman, Hämäläinen, Vangel, and Barton and Mr. Freidman reported no biomedical financial interests or potential conflicts of interest.
Financial Disclosures: Dr. Manoach reports having received research funding from Sepracor, Inc and an honorarium from Lilly, Inc. Dr. Goff reports having served as a consultant or advisor to: Xytis, Forest Labs, Pfizer, Indevus Pharmaceuticals, H. Lundbeck, Schering-Plough, Eli Lilly, Takeda, Biovail, Solvay, Hoffman- La Roche, Cypress, and Dianippon Sumitomo. He served on a DSMB for Otsuka and Wyeth. He received research funding from Pfizer, Janssen, Novartis, and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol Psychiatry. 2011;70:64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, Noll DC, et al. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 3.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 4.Levy DL, Mendell NR, LaVancher CA, Brownstein J, Krastoshevsky O, Teraspulsky L, et al. Disinhibition in antisaccade performance in schizophrenia. In: Lenzenweger MF, Dworkin RH, editors. Origins and Development of Schizophrenia. Washington DC: American Psychological Association; 1998. pp. 185–210. [Google Scholar]
- 5.Gooding DC, Basso MA. The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 2008;68:371–390. doi: 10.1016/j.bandc.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDowell JE, Myles-Worsley M, Coon H, Byerley W, Clementz BA. Measuring liability for schizophrenia using optimized antisaccade stimulus parameters. Psychophysiology. 1999;36:138–141. doi: 10.1017/s0048577299980836. [DOI] [PubMed] [Google Scholar]
- 7.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 8.Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connolly JD, Goodale MA, Goltz HC, Munoz DP. fMRI activation in the human frontal eye field is correlated with saccadic reaction time. J Neurophysiol. 2005;94:605–611. doi: 10.1152/jn.00830.2004. [DOI] [PubMed] [Google Scholar]
- 10.Johnston K, Levin HM, Koval MJ, Everling S. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron. 2007;53:453–462. doi: 10.1016/j.neuron.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Matsuzaka Y, Shima K, Tanji J. Cingulate cortical cells projecting to monkey frontal eye field and primary motor cortex. Neuroreport. 2004;15:1559–1563. doi: 10.1097/01.wnr.0000133300.62031.9b. [DOI] [PubMed] [Google Scholar]
- 13.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu P, Buckner RL, Zollei L, Dyckman KA, Manoach DS. Reduced functional connectivity in a right-hemisphere network for volitional ocular motor control in schizophrenia. Brain. 2010;133:625–637. doi: 10.1093/brain/awp317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- 17.McDowell JE, Dyckman KA, Austin BP, Clementz BA. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyckman KA, Camchong J, Clementz BA, McDowell JE. An effect of context on saccade-related behavior and brain activity. Neuroimage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Matsuda T, Matsuura M, Ohkubo T, Ohkubo H, Matsushima E, Inoue K, et al. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Res. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Milea D, Lehericy S, Rivaud-Pechoux S, Duffau H, Lobel E, Capelle L, et al. Antisaccade deficit after anterior cingulate cortex resection. Neuroreport. 2003;14:283–287. doi: 10.1097/00001756-200302100-00026. [DOI] [PubMed] [Google Scholar]
- 21.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Demeret S, Rivaud-Pechoux S. Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain. 2003;126:1460–1473. doi: 10.1093/brain/awg148. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson T, Chamberlain M, Parris B, James M, Gutowski N, Husain M, et al. The role of the ventrolateral frontal cortex in inhibitory oculomotor control. Brain. 2007 doi: 10.1093/brain/awm064. [DOI] [PubMed] [Google Scholar]
- 23.Camchong J, Dyckman KA, Austin BP, Clementz BA, McDowell JE. Common neural circuitry supporting volitional saccades and its disruption in schizophrenia patients and relatives. Biol Psychiatry. 2008;64:1042–1050. doi: 10.1016/j.biopsych.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima Y, Momose T, Sano I, Katayama S, Nakajima T, Niwa S, et al. Cortical control of saccade in normal and schizophrenic subjects: a PET study using a task-evoked rCBF paradigm. Schizophr Res. 1994;12:259–264. doi: 10.1016/0920-9964(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 25.McDowell JE, Brown GG, Paulus M, Martinez A, Stewart SE, Dubowitz DJ, et al. Neural correlates of refixation saccades and antisaccades in normal and schizophrenia subjects. Biol Psychiatry. 2002;51:216–223. doi: 10.1016/s0006-3223(01)01204-5. [DOI] [PubMed] [Google Scholar]
- 26.Raemaekers M, Jansma JM, Cahn W, Van der Geest JN, van der Linden JA, Kahn RS, et al. Neuronal substrate of the saccadic inhibition deficit in schizophrenia investigated with 3-dimensional event-related functional magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:313–320. doi: 10.1001/archpsyc.59.4.313. [DOI] [PubMed] [Google Scholar]
- 27.Tu PC, Yang TH, Kuo WJ, Hsieh JC, Su TP. Neural correlates of antisaccade deficits in schizophrenia, an fMRI study. J Psychiatr Res. 2006;40:606–612. doi: 10.1016/j.jpsychires.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Dyckman KA, Lee AKC, Agam Y, Vangel M, Goff DC, Barton JJS, et al. Abnormally persistent fMRI activation during antisaccades in schizophrenia: a neural correlate of perseveration? Schizophr Res. 2011;132:62–68. doi: 10.1016/j.schres.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crawford TJ, Puri BK, Nijran KS, Jones B, Kennard C, Lewis SW. Abnormal saccadic distractibility in patients with schizophrenia: a 99mTc-HMPAO SPET study. Psychol Med. 1996;26:265–277. doi: 10.1017/s0033291700034668. [DOI] [PubMed] [Google Scholar]
- 30.Raemaekers M, Ramsey NF, Vink M, van den Heuvel MP, Kahn RS. Brain Activation During Antisaccades in Unaffected Relatives of Schizophrenic Patients. Biol Psychiatry. 2005 doi: 10.1016/j.biopsych.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 31.Klein C, Heinks T, Andresen B, Berg P, Moritz S. Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biol Psychiatry. 2000;47:978–990. doi: 10.1016/s0006-3223(00)00234-1. [DOI] [PubMed] [Google Scholar]
- 32.Reuter B, Herzog E, Endrass T, Kathmann N. Brain potentials indicate poor preparation for action in schizophrenia. Psychophysiology. 2006;43:604–611. doi: 10.1111/j.1469-8986.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN) New York: Biometrics Research, New York State Psychiatric Institute; 1997. [Google Scholar]
- 34.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC. Scale for the assessment of negative symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 36.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Reports. 1962;10:799–812. [Google Scholar]
- 37.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Nonpatient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 38.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 39.White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- 40.Lee AKC, Hämäläinen MS, Dyckman KA, Barton JJS, Manoach DS. Saccadic preparation in frontal eye field is modulated by distinct trial history effects as revealed by magnetoencephalography. Cerebral Cortex. 2011;21:245–253. doi: 10.1093/cercor/bhq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taulu S, Simola J, Kajola M. Applications of the signal space separation method. IEEE Trans Signal Processing. 2005;53:3359–3372. [Google Scholar]
- 42.Medvedovsky M, Taulu S, Bikmullina R, Ahonen A, Paetau R. Fine tuning the correlation limit of spatio-temporal signal space separation for magnetoencephalography. J Neurosci Methods. 2009;177:203–211. doi: 10.1016/j.jneumeth.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 43.Hämäläinen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- 44.Hämäläinen MS, Ilmoniemi R. Interpreting measured magnetic fields of the brain: estimates of current distribution. Helsinki: University of Technology, Dept. of Technical Physics Report; 1984. p. TKK-F-A559. [Google Scholar]
- 45.Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cog Neursci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- 46.Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- 47.Lin FH, Belliveau JW, Dale AM, Hämäläinen MS. Distributed current estimates using cortical orientation constraints. Hum Brain Mapp. 2006;27:1–13. doi: 10.1002/hbm.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 50.Moon SY, Barton JJS, Mikulski S, Polli FE, Cain MS, Hämäläinen MS, et al. Where left becomes right: A magnetoencephalographic study of sensorimotor transformation for antisaccades. NeuroImage. 2007;36:1313–1323. doi: 10.1016/j.neuroimage.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paus T. Location and function of the human frontal eye-field: a selective review. Neuropsychologia. 1996;34:475–483. doi: 10.1016/0028-3932(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 52.Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron. 2004;41:795–807. doi: 10.1016/s0896-6273(04)00047-9. [DOI] [PubMed] [Google Scholar]
- 53.Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, et al. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- 54.Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- 55.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 56.Lee AK, Hamalainen MS, Dyckman KA, Barton JJ, Manoach DS. Saccadic preparation in the frontal eye field is modulated by distinct trial history effects as revealed by magnetoencephalography. Cereb Cortex. 2011;21:245–253. doi: 10.1093/cercor/bhq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Efron B, Tibshirani RJ. An introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 58.Braver TS, Barch DM. Extracting core components of cognitive control. Trends Cogn Sci. 2006;10:529–532. doi: 10.1016/j.tics.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierrot-Deseilligny C, Muri RM, Ploner CJ, Gaymard B, Rivaud-Pechoux S. Cortical control of ocular saccades in humans: a model for motricity. Prog Brain Res. 2003;142:3–17. doi: 10.1016/S0079-6123(03)42003-7. [DOI] [PubMed] [Google Scholar]
- 61.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 62.Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. J Neurosci. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dias EC, Segraves MA. Muscimol-induced inactivation of monkey frontal eye field: effects on visually and memory-guided saccades. J Neurophysiol. 1999;81:2191–2214. doi: 10.1152/jn.1999.81.5.2191. [DOI] [PubMed] [Google Scholar]
- 65.Schiller PH, Tehovnik EJ. Cortical inhibitory circuits in eye-movement generation. Eur J Neurosci. 2003;18:3127–3133. doi: 10.1111/j.1460-9568.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- 66.Reuter B, Jager M, Bottlender R, Kathmann N. Impaired action control in schizophrenia: the role of volitional saccade initiation. Neuropsychologia. 2007;45:1840–1848. doi: 10.1016/j.neuropsychologia.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Barton JJ, Cherkasova MV, Lindgren KA, Goff DC, Manoach DS. What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. J Abnorm Psychol. 2005;114:75–84. doi: 10.1037/0021-843X.114.1.75. [DOI] [PubMed] [Google Scholar]
- 68.Barton JJ, Goff DC, Manoach DS. The inter-trial effects of stimulus and saccadic direction on prosaccades and antisaccades, in controls and schizophrenia patients. Exp Brain Res. 2006;174:487–498. doi: 10.1007/s00221-006-0492-9. [DOI] [PubMed] [Google Scholar]
- 69.Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 70.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 71.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.