Abstract
Objective
Infants with Congenital Heart Disease (CHD) often exhibit growth failure. This can affect anthropometric and neurodevelopmental outcomes well into childhood. To determine the resting energy expenditure (REE), body composition, and growth in infants with CHD at 3 months of age, with the secondary aim to identify predictors of REE as compared to healthy infants.
Design and Methods
This descriptive study is a sub-analysis of a prospective study investigating predictors of growth in postoperative infants with CHD compared to healthy infants. Growth measurements, REE, and body composition were obtained in all infants. Analysis included chi-square for association between categorical variables, t-tests, ANOVA and ANCOVA. Outcome measures included the REE as determined by indirect calorimetry, anthropometric z-scores and body composition at 3-months of age.
Setting
Participants were recruited from the Cardiac Intensive Care Unit of a large, urban, pediatric cardiac center and pediatric primary care practices.
Results
The analysis included 93 infants, 44 (47%) with CHD. Of the infants with CHD, 39% had single ventricle (SV) physiology. There was no difference in REE related to cardiac physiology between infants with CHD and healthy infants or between infants with SV and biventricular (BV) physiology. Anthropometric z-scores for weight (−1.1 ± 1.1, p<0.001), length (−0.7 ± 1.1, p<0.05) and head circumference (−0.6 ± 1.2, p<0.001) were lower in infants with CHD at 3-months of age. The % body fat (%FAT) in postoperative infants with SV (24% ± 6, p=0.02) and BV (23% ± 5, p<0.001) physiology were lower than healthy infants (27% ± 5), with no difference in REE.
Conclusion
At 3-months of age, there was no difference in REE between postsurgical infants with CHD and healthy infants. Infants with CHD had lower growth z-scores and %FAT. These data demonstrate decreased %FAT contributed to growth failure in the infants with CHD.
Keywords: congenital heart disease, infant growth, growth failure, resting energy expenditure
Introduction
Growth failure is a well-recognized, common occurrence in infants with Congenital Heart Disease (CHD). Despite surgical intervention in the neonatal period, more than 50% of these infants exhibit inadequate growth,(1, 2) with greater than 30% falling below the third percentile in weight-for-age early in life.(3) Poor somatic growth in infancy has the potential to impact both anthropometric and neurodevelopmental outcomes into childhood and adolescence.(4, 5) The etiology of poor growth in infants following neonatal surgery for CHD is likely multi-factorial and may in part be from inadequate energy intake or an increase in energy expenditure, resulting in an energy imbalance. Strong correlations have been demonstrated between growth failure early in life and long term cognitive deficiencies, including poor arithmetic performance, attention deficit, aggressive behavior and poor social and emotional development.(4, 5) We reported a high rate of growth failure at hospital discharge in infants with both single ventricle (SV) and biventricular (BV) physiology following neonatal surgery.(6, 7) Poor weight gain in the postoperative period prior to hospital discharge was associated with post-operative complications, and timing of initiation of nutrient intake. These findings are similar to other reports that suggest infants with CHD receive inadequate caloric intake to support weight gain and growth that is comparable to healthy children.(8–13)
There have been multiple investigations into the energy needs of infants with CHD in the pre-operative and post-operative period. Findings from these studies are conflicting, some show increased resting energy expenditure (REE) preoperatively,(14–17) while studies conducted postoperatively show either no difference in measured REE from predicted or they demonstrate a decreased REE from predicted or from a control group(16, 18–21). The primary aims of this study were to determine whether there are differences in REE, body composition, and somatic growth at 3-months of age in infants who underwent neonatal surgery for CHD compared to healthy infants, and whether differences were present among infants with CHD classified postoperatively as SV or BV physiology. A secondary aim was to identify predictors of REE in infants with CHD compared to healthy infants.
Design and Methods
This is a sub-analysis from a prospective, cohort study investigating predictors of growth in postoperative infants with CHD conducted at The Children’s Hospital of Philadelphia (CHOP) from March 2003 through May 2007. Study approval was obtained from the CHOP Institutional Review Board. Informed consent was obtained from a parent or guardian of each participant prior to initiation of study protocol.
Sample Population
Study participants were recruited from the Cardiac Intensive Care Unit (CICU) at CHOP. Healthy infants served as the control group and were recruited during the study period from multiple primary care practices associated with CHOP and the community at large. Providers in primary care practices, received an electronic reminder of eligibility criteria, if eligible, the family was queried for study interest. Those families who indicated interest were then contacted by a member of the study team and enrolled if all criteria were met.
Eligibility for all infants included post-menstrual age ≥ 36 weeks and birth weight ≥ 2500 grams. Infants with CHD who underwent cardiac surgery during the neonatal period (first 30 days of life) and had no known multiple congenital, facial, chromosomal or complex gastrointestinal anomalies or congenital and/or acquired neurological insults were eligible. Families of all infants with CHD who met eligibility criteria and had a parent or guardian available in the CICU or by phone were approached daily for study enrollment. Study participation was a one year commitment; families whose primary residence was a long distance from CHOP were offered travel support for study participation. Of the infants with CHD, 29 underwent cardiopulmonary bypass for their surgical procedure, including four of the six infants with a pre-operative diagnosis of Coarctation of the Aorta. Infants were classified postoperatively as SV or BV physiology by the CICU cardiology team.
Race and ethnicity were assigned by the mother’s self-identification. Race designation is African-American, Asian, Caucasian or other. Ethnicity is defined as Latin/Hispanic, Non Latin/Hispanic or other. The category of other, in each instance includes those families who declined to answer the questions of race or ethnicity or they self-described as other. There were no differences found between race and ethnicity in any demographic variables. All study measurements were obtained during the 3-month outpatient visit to the Clinical and Translational Research Center (CTRC), by research personnel according to standard protocol.(22)
Anthropometric Measurements
Birth weight was extracted from the transfer records accompanying the infant to CHOP and by parental report for healthy infants. Weight, length and head circumference were obtained prior to measurement of REE and body composition on all participants. Weight was measured in kilograms (kg) using a scale accurate to 5 grams (Scaletronix, White Plains, NY, USA). Infant recumbent length was assessed using an infant length board (Holtain Limited, Crymuch, UK) accurate to 0.1 cm and head circumference was measured using a non-stretchable measuring tape accurate to 0.1 cm (McCOY Health Science Supply, Maryland Heights, MO, USA). Measurements were obtained in triplicate and the calculated mean used in analysis. All measurements were converted to z-scores using current World Health Organization (WHO) standards.(23)
Resting Energy Expenditure
REE was measured in the CTRC by open-circuit indirect calorimetry using a canopy based computerized metabolic cart (Sensor Medic 2900 Z; Sensor Medics, Yorba Linda, CA, USA) in a thermal-neutral, noise-restricted environment. Measurements were performed during a minimum 30-minute period of infant sleep following an ad libitum feeding of breast milk or the infant’s usual formula given within one hour of the start of REE measurement. Infants who were enteral tube fed did not have feeds infusing during REE measurement. All infants were receiving enteral nutrition. In infants, sleeping energy expenditure is used as a proxy for REE due to the practical considerations of measuring energy expenditure in this age group.(24) The metabolic cart measures infant respiratory gas exchange of oxygen consumption (VO2) and carbon dioxide production (VCO2) in 1-minute intervals. The initial period of infant adjustment and any period of significant movement that altered REE were extracted prior to analysis. Studies with less than 15 minutes of usable data were also eliminated from analysis. The remaining data points were averaged and the modified Weir equation(25) was used to calculate the REE. The results of the measured REE are expressed as kcal/day, and as a percent of the predicted values using Schofield weight-height (%Sch)(26) and WHO (%WHO)(27) prediction equations to estimate energy needs. The Schofield equation adjusts for age, gender, weight and length, while the WHO equation adjusts for age, gender and weight.
Body composition
Body composition was measured using the Total Body Electrical Conductivity (TOBEC) instrument (TOBEC; model HP- Pediatric, 2 EM-SCAN, Springfield, IL).(28) TOBEC is based on a two-compartment model consisting of fat mass (FM) and fat free mass (FFM). Infants were swaddled in a blanket to restrict movement with extremities extended and held parallel to the trunk of the body. The swaddled infant was then placed supine on the TOBEC sled. A minimum of five measurements were performed and the mean FFM in kg, FM in kg and % body fat (%FAT) are reported.
Nutrition Intake
By study protocol, families were instructed to collect infant nutrition intake for three days either immediately preceding or immediately following the 3-month visit to the CTRC. A feeding diary with written instructions along with a pan scale to weight the infant if breast-fed, or a food scale, to weigh the container before and after each feeding from which the infant was fed were distributed. Families reported numerous challenges surrounding the collection and documentation of nutrient intake data, including an inability to weigh and record each feeding; therefore nutrition data was not included in the final analysis.
Statistical Analysis
Statistical analysis was performed using SAS V9.2 (SAS Institute, Cary, NC). Infants with CHD were studied based on their post-operative physiology classification. Initial analysis was as a combined group of infants with CHD and subsequent analyses were by post-operative physiology classification to identify if there were differences between the groups. In addition, the SV and BV physiology groups were separately compared to the group of healthy infants. Distribution plots were used to assess normality of all variables, which appeared to be normal and did not require transformation. Chi-square was used to test the association between the categorical variables. Descriptive statistics of the means, standard deviations, and the minimum and maximum values for the continuous variables with computation of frequencies and percentages for categorical variables were calculated. Statistical significance was determined at the p<0.05 level. Two sided t-tests were used to compare mean differences in variables between the combined CHD physiology group and healthy infants and separately for the SV and BV physiology groups and healthy infants. In addition, the mean difference between the SV and BV physiology groups were compared using ANOVA. Linear relationships between all continuous variables were examined using Pearson’s correlation coefficient. Additionally, Pearson’s correlations were used to explore linear relationships between the continuous variables and REE, and to determine the independent variables to be included in a model to predict REE. Due to high correlation among many of the independent variables, the number of covariates in the regression model was restricted to minimize multicollinearity. ANCOVA models were constructed to examine the differences in REE for each CHD physiology group and the healthy infants while controlling for particular continuous covariates. The least squares means and the difference of the means were used to evaluate differences among the healthy infants and the CHD physiology groups. The variance of inflation factor (VIF), a measure of the degree of multicollinearity present in the model was used to assess collinearity among the independent variables.(29) All models had a VIF <10, indicating there was minimal collinearity in the models constructed.
Results
The study included 93 infants (44 with CHD, 49 healthy infants) with data suitable for analysis for both the REE and TOBEC measurements. Of the 44 infants with CHD, 17 (39%) had SV physiology and 27 (61%) had BV physiology. The distribution of cardiac primary diagnoses is presented in Table 1. Characteristics of the study sample are presented in Table 2. Infants with CHD were more likely to be Caucasian (p=0.02). All other characteristics were similar between the healthy infants, the combined CHD group and between the SV and BV physiology groups. Mean age was similar between the groups at the 3-month visit (Table 3). The WHO z-score means (Table 3, pFigure 1) for weight, length and head circumference for age were all significantly lower in the combined CHD group when compared to healthy infants. The SV and BV physiology groups were also lower when compared separately to healthy infants, with the exception of length-for-age z-score for the BV physiology group (=0.06). The SV and BV physiology groups differed only in head circumference z-score; infants with SV physiology had smaller head size (p=0.03).
Table 1.
Congenital Heart Disease pre-operative diagnoses and post-operative physiology classification of study sample.
| Pre-Operative Diagnosis | Post-Operative Physiology Classification | |
|---|---|---|
|
| ||
| Single Ventricle n = 17 (%) | Biventricular n = 27 (%) | |
| Hypoplastic Left Heart Syndrome | 10(59) | |
| Double Outlet Right Ventricle | 2(12) | |
| Double Inlet Left Ventriclea | 3(18) | |
| Tricuspid Atresia | 1(6) | |
| Valvular Pulmonary Atresia | 1(5) | |
| D-Transposition of Great Arteries | 9(33) | |
| Coarctation of the Aortab | 6(22) | |
| Tetralogy of Fallot | 5(19) | |
| Double Outlet Right Ventricle | 1(4) | |
| Interrupted Aortic Arch | 1(4) | |
| Total Anomalous Pulmonary Venous Return | 1(4) | |
| Truncus Arteriosus | 1(4) | |
| Valvular Aortic Stenosis | 1(4) | |
| Valvular Pulmonary Atresia | 1(3) | |
| Ventricular Septal Defect | 1(3) | |
Pre-operative diagnoses with post-operative physiology classification.
Total n = 44 infants with CHD; % of each diagnosis within the classification group.
Analyses are based on post-operative physiology classification groups.
Underwent Norwood procedure for palliative repair.
Four of the six underwent cardiopulmonary bypass for surgical repair.
Table 2.
Sample characteristics of healthy infants and infants with Congenital Heart Disease.
| Healthy Infants | Infants with Congenital Heart Disease | |||
|---|---|---|---|---|
|
| ||||
| n = 49(%) | All CHD n = 44(%) | Single Ventricle n = 17(%) | Biventricular n = 27(%) | |
| Gender | ||||
| Male | 31(63) | 27(61) | 12(71) | 15(56) |
| Female | 18(37) | 17(39) | 5(29) | 12(44) |
| Race | ||||
| African American | 14(29) | 3(7) | 1(6) | 2(7) |
| Asian | 1 (2) | 0 | 0 | 0 |
| Caucasian | 31(63) | 40(91)* | 16(94) | 24(89) |
| Other | 3 (6) | 1 (2) | 0 | 1(4) |
| Ethnicity | ||||
| Latin/Hispanic | 3(6) | 4(9) | 1(6) | 3(11) |
| Non Latin/Hispanic | 43(88) | 30(68) | 14(82) | 16(59) |
| Other | 3(6) | 10(23) | 2(12) | 8(30) |
| Birth Weight, kg | 3.4 ± 0.5 | 3.4 ± 0.4 | 3.5 ± 0.3 | 3.3 ± 0.5 |
| z-score | 0.2 ± 1.0 | 0.2 ± 1.0 | 0.5 ± 0.6 | 0.0 ±1.0 |
Significance level *p<0.05 all infants with CHD compared to healthy infants.
Table 3.
Growth, body composition and resting energy expenditure (means ± SD) in all subjects at 3 months of age.
| Healthy Infants | Infants with Congenital Heart Disease | |||
|---|---|---|---|---|
|
| ||||
| n = 49 | All n = 44 | Single Ventricle n = 17 | Biventricular n = 27 | |
| Age at visit, days | 95 ± 13 | 96 ± 13 | 99 ± 15 | 93 ± 11 |
| Weight | ||||
| kg | 6.1 ± 0.8 | 5.6 ± 0.9** | 5.6 ± 1.0** | 5.5 ± 0.7** |
| z-score | −0.3 ± 1.0 | −1.1 ± 1.1*** | −1.1 ± 1.1** | −1.0 ± 1.0** |
| Length | ||||
| cm | 61.4 ± 2.8 | 59.8 ± 2.3** | 60.1 ± 2.1 | 59.6 ± 2.5* |
| z-score | 0.0 ± 1.2 | −0.7 ± 1.1* | −0.8 ± 0.9* | −0.6 ± 1.2 |
| Head Circumference | ||||
| cm | 40.8 ± 1.3 | 39.7 ± 1.5*** | 39.2 ± 1.6*** | 40.0 ± 1.3** |
| z-score | 0.3 ± 0.9 | −0.6 ± 1.2*** | −1.1 ± 1.3*** | −0.2 ± 1.1*§ |
| Fat-free Mass, kg | 4.4 ± 0.5 | 4.3 ± 0.5 | 4.2 ± 0.5 | 4.3 ± 0.5 |
| Fat mass, kg | 1.7 ± 0.5 | 1.3 ± 0.4 | 1.4 ± 0.5 | 1.3 ± 0.3 |
| % Fat | 27.0 ± 5.0 | 23.1 ± 5.1** | 23.7 ± 5.5* | 22.7 ± 4.9** |
| REE Kcal/day | 328 ± 52 | 324 ± 55 | 325 ± 59 | 322 ± 54 |
| Schofield, % predicteda | 105 ± 13 | 112 ± 13* | 111 ± 14 | 112 ± 12* |
| WHO, % predictedb | 104 ± 13 | 115 ± 14** | 115 ± 17* | 114 ± 12* |
p<0.05,
p<0.01,
p<0.001 show healthy infants compared to all infants with CHD and with each CHD postoperative physiology group;
p<0.05 depicts significance comparing the SV and BV CHD groups.
Schofield prediction equation adjusts for age, gender, weight and length.(26)
WHO prediction equation adjusts for age, gender and weight.(27)
Figure 1.
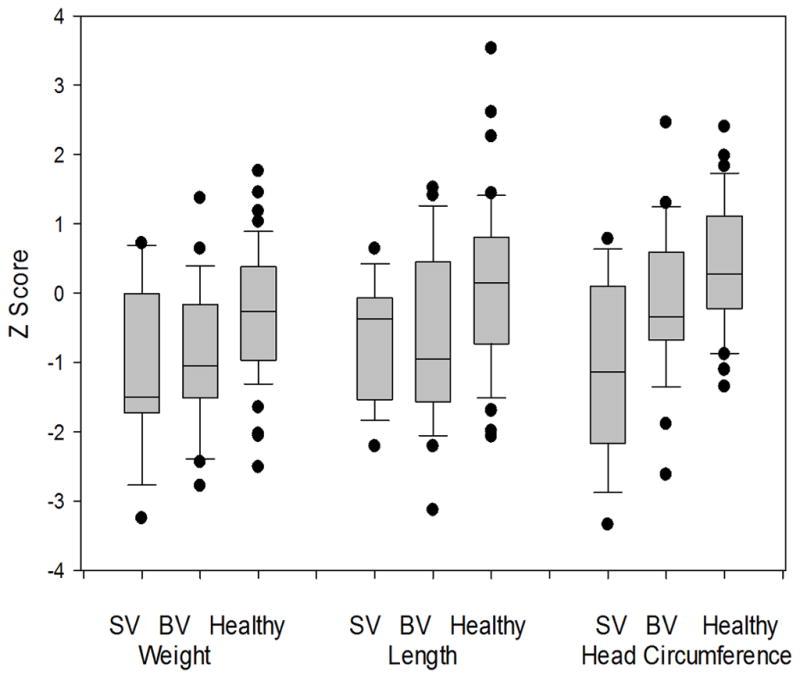
Box plot graph of growth measures at 3 months of age.
n for each group: SV = 17, BV = 27, Healthy= 49.
The individual group means for REE, %WHO REE, %Sch REE, FFM, FM, and %FAT are shown in Table 3. Compared to the healthy infants (27%), infants with SV (24%; p=0.04) or BV (23%; p<0.001) physiology had significantly lower %FAT. REE as %WHO predicted was significantly higher in infants with CHD (115, p=0.02) than in the healthy infants. REE as %Sch predicted was higher in the BV group (112, p=0.02) versus the healthy group. There was no difference in the %Sch predicted in the SV group (111, p=0.13), versus healthy infants. In addition, there were no differences in REE or body composition between the SV and BV physiology groups.
From Pearson correlation analysis, REE was significant and positively correlated with FFM (r=0.71, p<0.0001), FM (r=0.44, p<0.0001) and age in days (r=0.31, p=0.003). The multiple linear regression model that best predicts REE in this study sample includes FFM, age in days, SV and BV physiology and has an adjusted r2 =0.55 (Table 4). After adjusting for FFM and age in days, the differences in REE between infants with either SV or BV physiology and healthy infants (reference group) were not significant (Figure 2). A model including FFM, FM, age in days and physiology was examined, however FM was not significant in the presence of the other variables and did not contribute to the prediction of REE for infants with CHD.
Table 4.
Regression model of covariates with strongest contribution to REE kcal/day.
| Parameter | Standard Error | t value | p | r2 | |
|---|---|---|---|---|---|
| Intercept | −95.2 | 41.6 | −2.3 | 0.02 | 0.55 |
| FFM, kg | 72.7 | 7.3 | 9.8 | <0.0001 | |
| Age, days | 1.6 | 0.3 | 3.6 | 0.0006 | |
| SV | 7.6 | 10.3 | 0.7 | 0.5 | |
| BV | 8.3 | 8.7 | 1.0 | 0.3 | |
|
| |||||
| n = 93 infants | |||||
Model depicting contribution of FFM, age and cardiac physiology to REE kcal/day. Healthy infants are the reference group.
Figure 2.
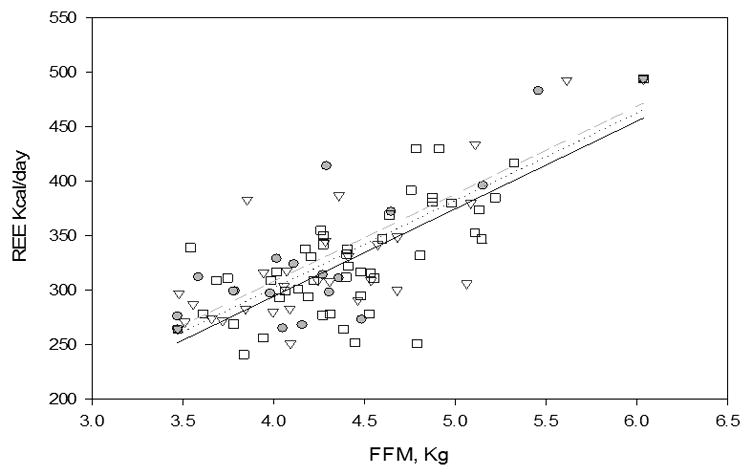
Regression line of REE kcal/day for fat-free mass (FFM), kg from TOBEC.
 Single Ventricle;
Single Ventricle;
 Bi-ventricle;
Bi-ventricle;
 Healthy Controls
Healthy Controls
The predicted line is calculated from an ANCOVA model using REE kcal/day as the dependent variable with FFM and physiology as independent variables.
Age was a significant covariate in each model tested. When age was removed, retaining FFM, and SV and BV physiology, there was a decrease in variance from r2=0.55 to r2=0.53 for predictors of REE. An interaction term for SV and BV physiology and FFM was tested but was not statistically significant, nor did these interactions contribute to the prediction of REE (data not shown). REE increased significantly as FFM increased in this study sample (Figure 2), with no significant difference found in the slope of this increase among the three groups (healthy, SV, BV).
Discussion
In this study, we evaluated REE at 3-months of age in infants who underwent neonatal surgery for CHD compared to a group of healthy infants. After adjusting for the strongest predictors of REE, FFM and infant age in days, there was no difference in REE among infants with CHD compared to healthy infants. These findings do not support the clinical premise that postoperative cardiac physiology is a primary factor causing increased REE and thus contributing to delayed growth in infants with CHD. Instead, these data demonstrate that body composition (specifically FFM) and infant age, were the strongest predictors of REE. In addition, 3-month old infants with CHD had inadequate weight-for-age primarily due to decreased accretion of body fat stores (%FAT).
As expected, the strongest contributor to REE in this study was FFM, the metabolically active component of body composition, consisting mostly of organs and muscles.(30, 31) This is the key predictor of REE across body size, age, sex and many diseases that affect child growth. An increased FFM leads to an increased REE per kg of body weight. In our study sample, there was no difference in the mean FFM between the study groups. Despite its strong positive correlation (r=0.44, p=<0.0001) to REE, total FM in kg, the most variable constituent of body composition in infancy was also not different between the groups, nor did FM significantly contribute to the model predicting REE after adjusting for FFM and age.
The typical, rapid pace of weight gain of early infancy is due in large part to an increase in FM, which does not contribute significantly to REE. This is likely related to the relatively lower metabolic activity of fat tissue.(32) Although total FM in kg did not differ between groups, %FAT was significantly lower in infants with CHD when compared to healthy infants. At birth, full term neonates have approximately 14 – 15% body fat. Fat accretion progresses rapidly in early infancy, and by 3 months of age, male infants have 25 – 30% body fat, and female infants have as much as 32%.(30) This rapid accretion of fat and growth seen in the typically developing infant is the result of a positive energy balance occurring over time. Our data show the infants with CHD had 23% body fat, far below the expected amount at 3-months of age for either males or females. The decreased weight-for-age z-scores in the infants with CHD are primarily due to reduced FM not FFM. Previous work reported poor weight gain at hospital discharge(6, 7), findings of the current study demonstrate that poor weight gain persists between hospital discharge and 3-months of age. This study suggests that inadequate energy intake continues following hospital discharge. Insufficient energy intake necessary to support a state of positive energy balance will lead to failure to increase fat stores or loss of FM and a decreased percentage of body fat. Since the accretion of FM is directly related to energy intake, inadequate caloric intake (either enteral or parenteral) in these infants may be responsible for a reduced accretion of fat and lower %FAT. With no demonstrated excessive energy requirement related to cardiac physiology and the decreased %FAT in our study sample, it seems a modest increase in caloric intake may improve growth.
Infant age was significantly associated with REE in the regression model (p=0.0006). Despite best efforts to schedule study visits within the three month birth date protocol window, an age range exists across all groups (71 – 140 days), and may account for the significance of age demonstrated in the model. Age and FFM are highly related, in that as age increases, FFM also increases. In multiple models tested, infant age was consistently a significant covariate. These findings are similar to those of Puhakka et al(16) who also found age to be a significant predictor of REE in their cohort of 25 subjects with CHD, with a large age range from 2 months to 10 years.
Numerous studies have examined REE preoperatively; fewer studies have examined REE following surgery. Nydegger and Bines(32) conducted a review of the literature on energy expenditure in infants with CHD from 1966 through 2004. Of the nine studies examined, four were postoperative and only two included control infants. All studies found poor growth to be a common occurrence.(32) In a cohort of 22 infants Leitch et al(17) demonstrated that pre-operative, post-prandial measured REE was higher than the control group, and by 2 weeks of age the infants with CHD had demonstrated a decrease in lean body mass. These findings were determined not to be a factor in growth delay, instead, the authors concluded the increase in total energy expenditure as measured by the doubly labeled water method, was a major contributing factor to growth delay compared to the control group.(17) In a follow-up study of the same cohort at 5 years of age, Leitch et al (33) found no difference in REE, total energy expenditure, weight or body composition between the children with CHD and the control group. Our study is unique in its approach to understanding energy expenditure in infants with CHD following neonatal surgery, in that we compared REE in infants with CHD who underwent surgical intervention in the neonatal period with a group of healthy infants at 3-months of age, examining growth parameters and body composition in all subjects. Another unique aspect of our study is that we compared infants with BV physiology to those with SV and separately to healthy infants. In the literature, there is much less attention given to the disparity in growth of infants with BV physiology compared to healthy infants. These data show that infants with BV physiology are also at risk for growth failure at 3-months of age when compared to healthy infants.
Energy requirements for healthy male and female infants at 3 months of age are approximately 95 kcal/kg/day across gender.(34) As a function of age, sex, body size and mode of feeding, energy requirement is the caloric intake necessary to result in a positive energy balance necessary to support growth and physical activity compatible with good health.(34) Following surgical intervention for CHD, alterations in feeding and mechanisms of swallow are a common complication which can lead to poor caloric intake and subsequently poor growth (8, 35 – 37). A potential solution to growth failure often seen in these infants following neonatal surgery would be to increase their daily intake volume and/or increase the caloric density, both aimed at raising the number of calories these infants ingest. Clinical experience demonstrates that often neither of these solutions are tolerated, resulting in further dysfunctional feeding such as emesis, gagging, choking, uncoordinated suck, delayed gastric emptying, dysphagia and oral aversion behaviors. (35, 36) Use of enteral feeding tubes and/or initiation of total parenteral nutrition as an adjunct to enteral feeding are strategies to be considered to promote increased caloric intake and support growth in this population of infants. Initiation of any of these methods to increase daily energy intake, requires close infant monitoring and meticulous care to prevent potential complications. Enteral feeding tubes may not be correctly placed or may become dislodged causing aspiration, high density formula may delay gastric emptying, increase the renal solute load and cause an increase in urine osmolarity and the use of total parental nutrition requires central venous access, in which the high glucose content carries the risk of infection.(2, 8–9)
Our data demonstrate significant reduction in weight and weight-for-age z-score at 3-months of age in infants with CHD compared to healthy infants, primarily due to reduced %FAT. This deficit in %FAT suggests that a modest increase in caloric intake may result in positive energy balance and support a normal accretion of FM necessary for growth. Optimizing growth for infants with CHD may decrease the risks of delayed neurobehavioral development, morbidity and mortality associated with poor growth in early infancy.
Conclusion
In our study, REE was not different between infants with CHD and healthy infants, nor was there a difference in REE between infants with SV or BV physiology. Growth z-scores and %FAT were lower in the CHD group which may be attributable to inadequate energy intake. Differences in body composition between the infants with CHD and healthy infants were demonstrated only in %FAT, and not the FM or FFM compartments. Our findings refute the clinical assertion that cardiac physiology causes elevated energy expenditure and increased energy requirements in infants with CHD. Intermittent measurements of body composition along with ongoing, accurate growth measures will document the infant’s pattern of growth. Nutrition support focused on increased caloric intake and close infant monitoring may decrease the occurrence of growth failure often seen in this population and diminish the long-term potential complications.
Acknowledgments
The authors would like to acknowledge Geoffrey Bird, MD, Gil Wernovsky, MD and the medical, nursing and ancillary staff of the CICU and the staff of the CTRC at CHOP for their assistance with this study. Mentorship during the dissertation process and manuscript development with the primary author from Martha Curley, RN, PhD is also acknowledged and very much appreciated.
Funding: NIH/NINR R01 NR002093; MO1-RR00240; UL1-RR-024134. This project was supported by the National Institutes of Health, National Institute of Nursing Research and the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Definition of Terms and Abbreviations
- BV
Biventricular Physiology
- CHD
Congenital Heart Disease
- %FAT
% Fat
- FM
Fat Mass, kg
- FFM
Fat Free Mass, kg
- REE
Resting Energy Expenditure, kcal/day
- %Sch
%Schofield REE prediction equation
- SV
Single Ventricle Physiology
- TOBEC
Total Body Electrical Conductivity
- %WHO
%World Health Organization REE prediction equation
Footnotes
Conflict of Interest: The authors have no any conflicts of interest related to the development of this manuscript, nor has there been any honorarium awarded for its development.
Author Contributions
Sharon Y Irving: Project manager for study, involved in subject recruitment, data collection, data analysis, primary manuscript author, manuscript review and edits
Barbara Medoff-Cooper, Virginia A Stallings: Study design, data analysis, contributed to manuscript development, manuscript review and edits
Nicole O Stouffer, Joan I Schall: Primary statisticians for data analysis, manuscript review and edits
Charlene W Compher, Bradley S Marino, Chitra Ravishankar: Study consultants, manuscript development, review and edits
References
- 1.Davis D, Davis S, Cotman K, Worley S, Londrico D, Kenny D, et al. Feeding difficulties and growth delay in children with hypoplastic left heart syndrome versus d-transposition of the great arteries. Pediatr Cardiol. 2008 Mar;29(2):328–33. doi: 10.1007/s00246-007-9027-9. [DOI] [PubMed] [Google Scholar]
- 2.Forchielli ML, McColl R, Walker WA, Lo C. Children with congenital heart disease: a nutrition challenge. Nutrition Review. 1994;52(10):348–53. doi: 10.1111/j.1753-4887.1994.tb01359.x. [DOI] [PubMed] [Google Scholar]
- 3.Dooley KJ, Bishop L. Medical management of the cardiac infant and child after surgical discharge. Crit Care Nurs Q. 2002;25(3):98–104. doi: 10.1097/00002727-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Black MM, Dubowitz H, Krishnakumar A, Starr RH. Early Intervention and Recovery Among Children With Failure to Thrive: Follow-Up at Age 8. Pediatr. 2007;120(1):59–69. doi: 10.1542/peds.2006-1657. [DOI] [PubMed] [Google Scholar]
- 5.Dykman RA, Casey PH, Ackerman PT, McPherson WB. Behavioral and Cognitive Status in School-Aged Children With a History of Failure to Thrive During Early Childhood. Clinical Pediatrics. 2001;40:63–70. doi: 10.1177/000992280104000201. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JB, Marino BS, Irving SY, Garcia-Espana JF, Ravishankar C, Stallings VA, et al. Poor post-operative growth in infants with two-ventricle physiology. Cardiol Young. 2011;21:421–9. doi: 10.1017/S1047951111000229. [DOI] [PubMed] [Google Scholar]
- 7.Medoff-Cooper B, Irving SY, Marino BS, Garcia-Espana JF, Ravishankar C, Bird GL, et al. Weight change in infants with a functionally univentricular heart: from surgical intervention to hospital discharge. Cardiol Young. 2011;21(2):136–44. doi: 10.1017/S104795111000154X. [DOI] [PubMed] [Google Scholar]
- 8.Schwalbe-Terilli C, Hartman DH, Nagel ML, Gallagher PR, Ittenbach RF, Burnham NB, et al. Enteral Feeding and Caloric Intake in Neonates After Cardiac Surgery. Am J Crit Care. 2009;18(1):52–7. doi: 10.4037/ajcc2009405. [DOI] [PubMed] [Google Scholar]
- 9.Pillo-Blocka F, Adatia I, Sharieff W, McCrindle BW, Zlotkin S. Rapid advancement to more concentrated formula in infants after surgery for congenital heart disease reduces duration of hospital stay: A randomized clinical trial. J Pediatr. 2004;145(6):761–6. doi: 10.1016/j.jpeds.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 10.Unger R, DeKleermaeker M, Gidding SS, Christoffel K. Improved Weight Gain With Dietary Intervention in Congenital Heart Disease. Am J Dis Child. 1992;146:1078–84. doi: 10.1001/archpedi.1992.02160210080026. [DOI] [PubMed] [Google Scholar]
- 11.Schwarz SM, Gewitz M, See CC, Berezin S, Glassman MS, Medow CM, et al. Enteral Nutrition in Infants with Congenital Heart Disease and Growth Failure. Pediatr. 1990;86(3):368–73. [PubMed] [Google Scholar]
- 12.Menon G, Poskitt EM. Why does congenital heart disease cause failure to thrive? Arch Dis Child. 1985;60(12):1134–9. doi: 10.1136/adc.60.12.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson M, Poskitt EM. The effects of high-energy feeding on energy balance and growth in infants with congenital heart disease and failure to thrive. Br J Nutr. 1991;65:131–143. doi: 10.1079/bjn19910075. [DOI] [PubMed] [Google Scholar]
- 14.Farrell AG, Schamberger MS, Olson IL, Leitch CA. Large left-to-right shunts and congestive heart failure increase total energy expenditure in infants with ventricular septal defect. Am J Cardiol. 2001;87(9):1128–31. doi: 10.1016/s0002-9149(01)01479-5. [DOI] [PubMed] [Google Scholar]
- 15.Ackerman IL, Karn CA, Denne SC, Ensing GJ, Leitch CA. Total But Not Resting Energy Expenditure is Increased in Infants with Ventricular Septal Defects. Pediatr. 1998;102(5):1172–7. doi: 10.1542/peds.102.5.1172. [DOI] [PubMed] [Google Scholar]
- 16.Puhakka K, Rasanen J, Leijala M, Peltola K. Metabolic Effects of Corrective Surgery in Infants and Children with Congenital Heart Defects. Br J Anaesth. 1993;70:149–53. doi: 10.1093/bja/70.2.149. [DOI] [PubMed] [Google Scholar]
- 17.Leitch CA, Karn CA, Peppard RJ, Granger D, Liechty EA, Ensing GJ, et al. Increased energy expenditure in infants with cyanotic congenital heart disease. Journal of Pediatrics. 1998;133:755–60. doi: 10.1016/s0022-3476(98)70146-5. [DOI] [PubMed] [Google Scholar]
- 18.Avitzur Y, Singer P, Dagan O, Kozer E, Abramovitch D, Dinari G, et al. Resting energy expenditure in children with cyanotic and noncyanotic congenital heart disease before and after open heart surgery. JPEN J Parenter Enteral Nutr 2003. 2003 Jan 1;27(1):47–51. doi: 10.1177/014860710302700147. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zhang G, Herridge J, Holtby H, Humpl T, Redington AN, et al. Energy expenditure and caloric and protein intake in infants following the Norwood procedure. Pediatr Crit Care Med. 2008;9(1):55–61. doi: 10.1097/01.PCC.0000298756.82286.23. [DOI] [PubMed] [Google Scholar]
- 20.Gebara BM, Gelmini M, Sarnaik A. Oxygen consumption, energy expenditure, and substrate utilization after cardiac surgery in children. Critical Care Medicine. 1992;20(11):1550–4. doi: 10.1097/00003246-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 21.De Witt B, Meyer R, Desai A, Macrae D, Pathan N. Challenge of predicting resting energy expenditure in children undergoing surgery for congenital heart disease. Pediatr Crit Care Med. 2010;11(4):496–501. doi: 10.1097/PCC.0b013e3181ce7465. [DOI] [PubMed] [Google Scholar]
- 22.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 23.World Health Organization. WHO Anthro (version 3.2.2, January 2011) and macros. Geneva, Switzerland: World Health Organization; 2011. [June 5, 2012]. [5/6/2011]; Available from: http://www.who.int/childgrowth/software/en/ [Google Scholar]
- 24.Reichman CA, Shepherd RW, Trocki O, Cleghorn G, Davies PSW. Comparison of measured sleeping metabolic rate and predicted basal metabolic rate during the first year of life: evidence of a bias changing with increasing metabolic rate. Eur J Clin Nutr. 2002;56:650–5. doi: 10.1038/sj.ejcn.1601372. [DOI] [PubMed] [Google Scholar]
- 25.Weir J. New methods for calculating metabolic rate with special reference to protein metabolism. The Journal of Physiology. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schofield WN. Predicting Basal Metabolic Rate, New Standards and Review of Previous Work. Hum Nutr Clin Nutr. 1985;39C(Suppl):1, 5–41. [PubMed] [Google Scholar]
- 27.World Health Organization. Energy and protein requirements: technical report series #724. Geneva: 1985. FAO/WHO/UNU Expert Consultation; pp. 71–112. [PubMed] [Google Scholar]
- 28.Fiorotto M, Klish W. Total body electral conductivity measurements in the neonate. Clinical Perinatology. 1991;18(3):611–27. [PubMed] [Google Scholar]
- 29.Robinson C, Schumaker RE. Interaction Effects: Centering, Variance Inflation Factor, and Interpretation Issues. Multiple Linear Regression Viewpoints. 2009;35(1):6–11. [Google Scholar]
- 30.Bechard LJ, Wroe E, Ellis K. Body Composition and Growth. In: Duggan C, Watkins JB, Walker WA, editors. Nutrition in Pediatrics. Shelton, Connecticut: People’s Medical Publishing House; 2009. pp. 27–37. [Google Scholar]
- 31.Illner K, Brinkmmann G, Heller M, Bosy-Westphal A, Muller MJ. Metabolically active components of fat free mass and resting energy expenditure in nonobese adults. Am J Endocrinol Metab. 2000;278:E308–E15. doi: 10.1152/ajpendo.2000.278.2.E308. [DOI] [PubMed] [Google Scholar]
- 32.Nydegger A, Bines J. Energy metabolism in infants with congenital heart disease. Nutrition. 2006;22:697–704. doi: 10.1016/j.nut.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Leitch CA, Karn CA, Ensing GJ, Denne SC. Energy expenditure after surgical repair in children with cyanotic congenital heart disease. The Journal of Pediatrics. 2000;137(3):381–5. doi: 10.1067/mpd.2000.107844. [DOI] [PubMed] [Google Scholar]
- 34.Butte NF. Energy requirements of infants. Public Health Nutrition. 2005;8(7A):953–67. doi: 10.1079/phn2005790. [DOI] [PubMed] [Google Scholar]
- 35.Medoff-Cooper B, Irving SY. Innovative Strategies for Feeding and Nutrition in Infants with Congenitally Malformed Hearts. Cardiol Young. 2009;19(Suppl 2):90–95. doi: 10.1017/S1047951109991673. [DOI] [PubMed] [Google Scholar]
- 36.Medoff-Cooper B, Naim M, Torowicz D, Mott A. Feeding, growth, and nutrition in children with congenitally malformed hearts. Cardiol Young. 2010;20(Suppl 3):149–153. doi: 10.1017/S1047951110001228. [DOI] [PubMed] [Google Scholar]
- 37.Kohr L, Dargan M, Hague A, Nelson SP, Duffy E, Backer CL, Mavroudis C. The Incidence of Dysphagia in Pediatric Patients After Open Heart Procedures With Tranesophageal Echocardiolgraphy. Ann Thorac Surg. 2003;76(5):1450–1456. doi: 10.1016/s0003-4975(03)00956-1. [DOI] [PubMed] [Google Scholar]


