Abstract
Studies suggest a protective relationship between Vitamin D and breast cancer risk. Several studies assessed the association of Vitamin D with mammographic breast density, a known and strong breast cancer risk factor. Understanding the potential role of Vitamin D in the modification of breast density might open new avenues in breast cancer prevention. This systematic review summarizes published studies that investigated the association between Vitamin D and mammographic breast density and offers suggestions for strategies to advance our scientific knowledge.
Keywords: Vitamin D, Breast density, Breast cancer, Cancer prevention
Introduction
Mammographic breast density is a well-established and very strong predictor of breast cancer risk [1–4]. Appearance of the breast on the mammogram is a reflection of the amount of fat, connective, and epithelial tissue in the breast [3]. Light (non-radiolucent) areas on the mammogram represent the fibrous and glandular tissues (“mammographically dense”), whereas the dark (radiolucent) areas are primarily fat. Women with 75% or greater percent density (proportion of the breast that appears dense on the mammogram out of the total breast area) are at 4–6 times greater risk of breast cancer compared with women with fatty breasts [3, 5–7]. On the tissue level, areas with greater breast density were reported to have greater total nuclear area, a greater proportion of collagen, and a greater area of glandular structures. Breast density is not a static characteristic and changes over time [8]. Use of postmenopausal hormones, especially combined therapy, increases breast density. However, this increase is reversible [9]. Breast density decreases with age, especially during the menopausal transition and during tamoxifen use [10]. An earlier study has shown a significant reduction in breast density during 1-year therapy with leuprolide acetate depot, conjugated estrogen, and medroxy-progesterone acetate [11].
Several studies examined the association between vitamin D and breast cancer risk. An inverse association between vitamin D intake and the risk of breast cancer was reported in both pre- and postmenopausal women [12], including large cohort and case–control studies [13–15]. A recent meta-analysis showed decrease in breast cancer risk for women with high vitamin D intake compared with those with low intake in a pooled analysis from six cohort studies (RR = 0.90; 95% CI 0.83–0.98) [12]. The pooled results from five case–control studies, however, found no significant associations (RR = 0.95; 95% CI 0.69–1.32), which was attributed to the heterogeneity between the studies [12]. Recently, several studies investigated the association between serum 25(OH)D levels and breast cancer. A significant inverse association was reported by a few authors, including the most recent meta-analysis that included 6,147 cases from 9 studies [16]. The increase in 25(OD)D levels by 20 ng/ml was associated with breast cancer risk reduction in the pooled analysis of case–control studies (RR = 0.59, 95% CI 0.48–0.73), but these findings were not confirmed in the pooled analysis from prospective studies where 25(OH)D levels were measured years before diagnosis (RR = 0.92, 95% CI 0.82–1.04) [16]. In 2008, the International Agency for Research on Cancer concluded that the evidence on the association between Vitamin D and breast cancer was “limited” [17].
The sources of Vitamin D include a limited number of foods and vitamin D-containing multivitamins and supplements (D2 or D3) and ultraviolet B (UV-B) radiation (D3) [13, 18]. Exposure to UV-B sunlight results in conversion of 7-dehydrocholesterol to previtamin D3 which is subsequently converted into 25-hydroxyvitamin D (25[OH]D) in the liver [13]. 25(OH)D is the main circulating form of Vitamin D with a half-life of 2–3 weeks [13]. Renal 1-α hydroxylase controlled by parathyroid hormone (PTH) further converts 25(OH)D into the active hormone 1,25(OH)2D with a half-life of only about 4 h [13]. 1,25(OH)2D interacts with its vitamin D receptor (VDR) and regulates the intestinal calcium absorption and bone homeostasis [13]. Due to the similar 1-α hydroxylase (CYP27B1) in the breast epithelium (and many other organs), the active form of vitamin D is also produced locally in the breast from circulating 25(OH)D [13, 19]. In contrast to renal synthesis of active vitamin D, the local (autocrine) production in the breast is regulated by the concentration of circulating 25(OH)D3 rather than systemic calcium levels and PTH [13]. The locally synthesized active form of vitamin D is not released into circulation and is utilized locally in the breast [13, 19].
Vitamin D has anticarcinogenic properties in in vitro and animal studies [12, 20–25]. Some of the suggested mechanisms include inhibition of cellular proliferation, induction of differentiation and apoptosis, and inhibition of angiogenesis in normal and malignant breast cells [13, 19, 26]. In animal studies, disruption of the vitamin D receptor signaling pathway resulted in abnormal ductal morphology, increased incidence of premalignant lesions, and more rapid mammary tumor development [27]. In experimental models, Vitamin D was reported to inhibit both the synthesis and the biological actions of estrogens [28], stimulators of breast epithelial, and stromal proliferation [29–37]. It has been suggested that such inhibition results from the suppression of aromatase responsible for the conversion of androgens into active estrogens [28, 38]. In addition, 1,25(OH)2D also down regulates estrogen receptor [28]. Vitamin D-related signaling is dysregulated in breast tumors due to decrease in functional VDR, as well as upregulation of 1,25(OH)2D catabolism leading to decreased levels of active vitamin D [13, 39].
Vitamin D could potentially influence breast density by direct and indirect mechanisms described above [40]. Vitamin D supplementation might be a potential approach for changing breast density and subsequently, breast cancer risk. The purpose of this review is to summarize published studies on associations between Vitamin D and mammographic breast density and to identify methodological issues that need to be addressed in future studies to fill the gaps in the existing knowledge.
Literature search
Published studies were identified using the PubMed Central (US National Institutes of Health [NIH]), BioMed Central, Embase, and Scopus literature search (through Washington University in St. Louis). We limit this review to studies published before March 30, 2011 that were accessible in full-text format and were published in English. Articles were searched using the terms “vitamin D and breast density”, “1,25(OH)2D and breast density”, and “25(OH)D and breast density”. Bibliography of the articles found through electronic searches helped to identify additional relevant references that were then hand-searched.
Statistical analysis
Mann–Whitney–Wilcoxon rank-sum test was used to combine the results of the studies by the type of exposure assessment (circulating Vitamin D levels, intake assessment with FFQs) as well as by menopausal status of participants (premenopausal, postmenopausal) [41]. In all the comparisons above, we had to exclude studies by Brisson et al. (investigation of the seasonal variations), Masala et al. (no reported density estimates), and Thompson et al. (results reported by race/ethnicity, but not for all women combined). The study by Tseng et al. was included only in one of the comparisons since this study did not report the findings separately by menopausal status. Premenopausal study participants were essentially the same in the studies by Diorio et al. and Berube et al., and the differences in breast density at the extreme Vitamin D levels were in the same direction in both studies; therefore, only one of the studies [42] was included in the meta-analysis. Significance of the test was assessed at the 0.05 significance level. All analyses were performed using SAS software (version 9.2, SAS Institute, Cary, NC).
Results
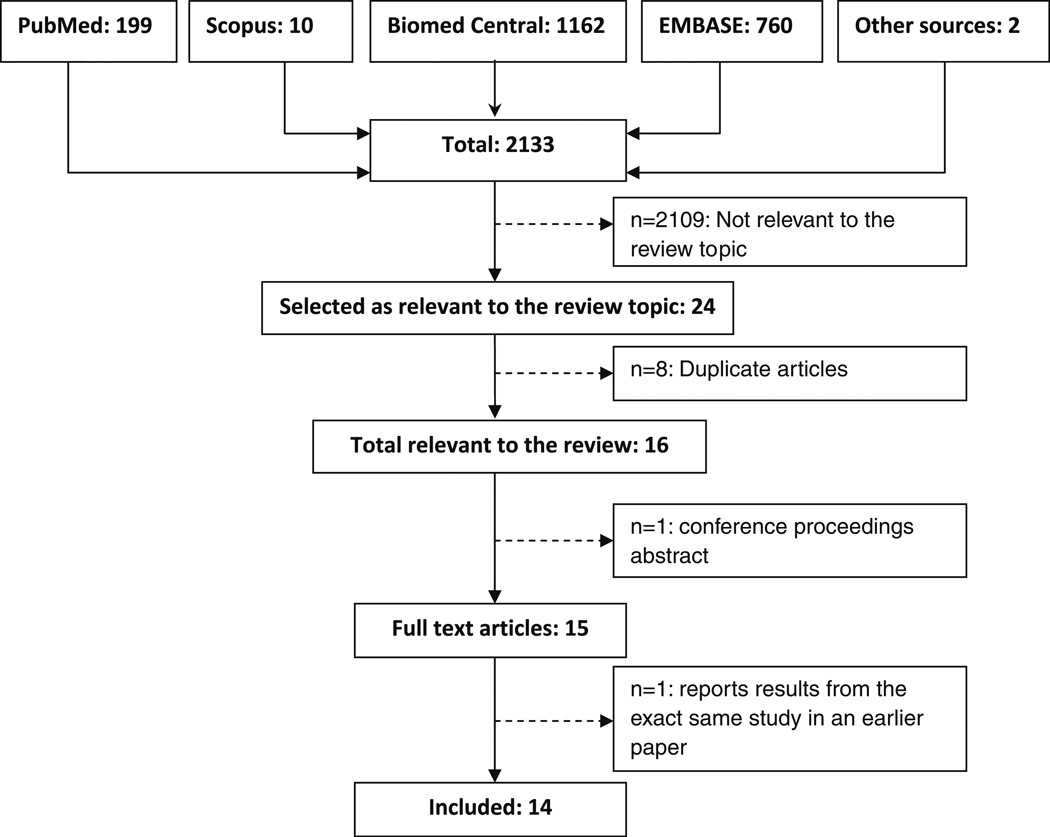
We identified 15 eligible studies (Fig. 1). However, two of the papers described the findings from the same study and were considered as one investigation for the purpose of this review. In Table 1, we summarize the key characteristics of the studies. The earliest study we found was published in 2000. The majority of the published studies originated in the United States (64%). Most of the studies (86%) were cross-sectional; two studies were longitudinal. All studies except one investigated associations in healthy (breast cancer-free) women; one study examined the association in postmenopausal breast cancer survivors.
Fig. 1.
Flow diagram of the literature search
Table 1.
Summary characteristics of the studies on association between Vitamin D and mammographic breast density
| Study haracteristic | n (%) | Sample size range | Total sample size across all studies |
|---|---|---|---|
| Study population | |||
| Breast cancer survivors (postmenopausal only) | 1 (7%) | 426 | 426 |
| Healthy women | 13 (93%) | 99–1,668 | 10,339 |
| Premenopausal/Perimenopausala | 11 (79%) | 38–777 | 3,739 |
| Postmenopausal* | 11 (79%) | 61–1,244 | 5,863 |
| Race/Ethnicity | |||
| Predominantly white | 9 (64%) | 157–1,668 | 9,013 |
| Hispanic | 1 (7%) | 99 | 1,471 |
| Mixed | 4 (29%) | 182–808 | 1,653 |
| Study design | |||
| Prospective | 2 (14%) | 1,161–1,668 | 2,829 |
| Cross-sectional | 12 (86%) | 99–1,560 | 7,936 |
| Exposure (Vitamin D) assessment | |||
| Food frequency questionnaire | 2 (14%) | 237–1,668 | 1,905 |
| Food frequency questionnaire + supplements | 7 (50%) | 99–1,560 | 5,329 |
| Dietary 5-day recording | 1 (7%) | 1,161 | 1,161 |
| Circulating 25(OH)D levels | 5 (36%) | 182–960 | 2,796 |
| Circulating 1,25(OH)2D levels | 1 (7%) | 960 | 960 |
| Breast density assessment approach | |||
| Percent breast density (continuous) | 12 (86%) | 99–1,560 | 8,940 |
| Categorical breast density | 2 (14%) | 157–1,668 | 1,825 |
One study did not report the exact number of pre- and postmenopausal women in the study population
Most studies (57%) had a large sample size (>700 participants): the mean number of participants was 769 (median 756, range 99–1,668) with the total number of 3,739 pre- or peri- and 5,863 postmenopausal women across all 14 studies. Study populations differed with respect to women’s menopausal status. Most studies (8 or 57%) included both pre- and postmenopausal women, 3 studies had only postmenopausal participants, and three studies focused only on premenopausal women. Among studies with mixed population by menopausal status, six have reported the results separately for pre- and postmenopausal women.
Most of the studies (9 or 64%) have assessed the Vitamin D intake using food frequency questionnaires, and one study estimated vitamin D intakes from 5-day food diaries. Five studies measured circulating 25(OH)D; one study has also measured circulating levels of 1,25(OH)2D. All of these studies, except one, reported assay precision (coefficient of variation) [43].
Nine studies (64%) had predominantly a white population. One study included only Hispanic women and four other studies had a mixed population with respect to race/ethnicity. Most of the studies (86%) evaluated breast density as a continuous outcome (percent breast density) using computerized techniques. Of those, four studies have also examined the associations with absolute dense area. Two of the studies used categorical breast density in the analysis (Wolfe’s density categories: combined P2 + DY [dense] and combined N1 + P1 [non-dense] or BI-RADS breast density classification). Previous studies, however, found a high agreement between qualitative and quantitative breast density estimation methods [44–46].
In Table 2, we present for each study the characteristics of study population as well as vitamin D and breast density assessment methods (see Table 2).
Table 2.
Summary description of the studies on association between Vitamin D and mammographic breast density
| Author, year | Country | Study design, N | Age (years) | N pre-or peri/ post- |
Exposure (Vitamin D) assessment |
Breast density assessment |
Comments |
|---|---|---|---|---|---|---|---|
| Prospective studies | |||||||
| Mishra [56] | UK | Prospective, 1,161 | Mean: 51.5 | NR/NRa | Dietary intakes 5-day food diaries, including supplements | Percent breast density, absolute areas of dense and non-dense tissue | No history of breast cancer |
| Mammogram closest to the age of 50 used for density assessment (for most women within 2 years) | |||||||
| Diet was assessed post-screening, habits might have changed since the mammogram | |||||||
| Supplement use assessed only at age 53 | |||||||
| No report on whether there were women who were premenopausal at exposure assessment and postmenopausal at mammogram | |||||||
| Foods and nutrient intakes calculated using the in-house suites of programs specific to the United Kingdom | |||||||
| Masala [55] | Italy | Prospective, 1,668 | Mean: 53.3 | 610/1055b | FFQ | Wolfe’s classification (P2 + DY and N1 + P1) | Mediterranean women |
| Mammograms taken 5 years after enrollment | |||||||
| Do not report if questionnaire collected data on supplement use | |||||||
| Low levels of vitamin D intake in this population | |||||||
| Nutrients’ intake estimated with specifically developed Italian food tables | |||||||
| Cross-sectional studies | |||||||
| Knight [52] | USA | Cross-sectional, 487 | Mean: 56.4 Range: 27–85 | 133/354 | Serum 25OHD measured with RIA | Percent breast density and total dense area | The time between blood sample and mammogram varied and the measure of vitamin D may not always have reflected the level at the time the mammogram. Of the 487 women included in this analysis, 356 (73%) had their blood drawn within 1 year of the mammogram used in this analysis and 131 (27%) had their blood drawn more than 1 year after the mammogram, but within 4 years. One woman had her blood taken 8 years after the mammogram |
| RIA Coefficient of Variation <10% | |||||||
| Brisson [50] | Canada | Cross-sectional, 741 | Mean: 46.8 | 741/0 | Plasma 25(OH)D measured with RIA | Percent breast density | 95% people of European descent |
| Green [54] | USA | Cross-sectional, 960 | Median: 61.0 | 0/960 | Plasma 25(OH)D and 1,25(OH)2D measured with RIA | Percent breast density | It is a cross-sectional analysis within nested case–control study Coefficient of variation for RIA between 7.3 and 17.6% |
| Chai [66] | USA | Cross-sectional, 182 | Mean: 42.6 | 182/0 | Serum 25(OH)D measured with double-antibody | Percent breast density and total dense area | 67 Caucasian, 74 Asian, 22 Hawaiian, and 19 women of mixed/other ethnicity |
| enzyme-linked immunosorbent assay kit | |||||||
| Neuhouser [57] | USA | Cross-sectional, 426 | Mean: 61.0 | 0/426 | Serum 25(OH)D measured with RIA | Percent breast density and total dense area | Breast cancer survivors within longitudinal cohort |
| 22.8% African-American, 11.3% Hispanic, and 62.8% non-Hispanic white | |||||||
| FFQ + supplement intake | Exposure assessment at 24 month of follow-up Mammogram (postdiagnosis) of contralateral breast done about 1.5 years after breast cancer diagnosis | ||||||
| High prevalence of vitamin D insufficiency/deficiency in this population (76.8%) | |||||||
| Season of mammogram (fall, summer, winter, and spring) was used as a surrogate of UV-B sunlight exposure | |||||||
| RIA Coefficient of Variation 3.7% | |||||||
| Vachon [53] | USA | Cross-sectional, 1,508 | Mean: 61.4 Range: 40–90 | 264/1,244c | FFQ + supplement intake | Percent breast density | Exclusion criteria: history of breast cancer |
| Mammograms within previous year or two or current, mediolateral oblique view only | |||||||
| Women returning mammograms differed on several dietary factors | |||||||
| The study group of women may have been somewhat more health conscious than the other women in the cohort | |||||||
| Berube [49]d | Canada | Cross-sectional, 1,560 | Mean: 46.7 (pre) and 61.4 (post) | 777/783 | FFQ + supplement intake | Percent breast density | Exclusion criteria: personal history of cancer, breast reduction or implants, diabetes mellitus, dwarfism/acromegaly, and thyroid, adrenal, or hepatic disease; history of tamoxifen or raloxifene; oral contraceptives or hormone replacement therapy use in the last 3 months before mammography; and pregnancy |
| Range: 31–81 | |||||||
| Diorio [42] | Canada | Cross-sectional, 771 | Mean: 46.8 | 771/0 | FFQ + supplement intake | Percent breast density | Eligibility criteria: women not taking hormonal derivatives within 3 months of the mammography, not pregnant, with no history of tamoxifen or raloxifene use, no history of cancer at any site, no breast reduction or implants, and no diabetes mellitus, dwarfism/acromegaly, thyroid, adrenal, or hepatic disease |
| Colangelo [47] | USA | Cross-sectional, 99 | Mean: 52.1 Range: 40–77 | 38/61 | FFQ + supplement intake | Percent breast density and total dense area | Hispanic women |
| Thompson [48] | USA | Cross-sectional, 237 | Mean: 51 | 137/101 | Food frequency questionnaire | Percent breast density | Hispanic or non-Hispanic White women |
| Exclusion criteria: body weight over 127 kg; history of chronic disease or other health issues that would alter bone health, such as autoimmune disorders, diabetes, cancer, hyper- or hypothyroidism, breast augmentation or reduction surgery, extensive surgical breast biopsy, use of menopausal hormone therapy or oral contraceptives within the past year, or pregnancy/breastfeeding in the past 2 years | |||||||
| Bertone-Johnson [51] | USA | Cross-sectional, 808 | Range: 50–79 | 0/808 | FFQ + supplement intake | Percent breast density | Asian/Pacific Islander, black, Hispanic, White non- Hispanic women |
| Low levels of dietary vitamin D and calcium intake in the study population | |||||||
| Tseng [40] | USA | Cross-sectional, 157 | Mean: 50 | 86/71c | FFQ + supplement intake | BI-RADS classification | High-risk population: women with at least one first-degree or second degree relative with breast or ovarian cancer |
| Exclusion criterial: a history of breast augmentation or reduction, a history of prophylactic mastectomy, a history of cancer except nonmelanoma skin cancer, a current or planned pregnancy, current breastfeeding, a weight change of at least 20 lb during the past year, a substantial change in diet over the past year, or no mammogram planned within the study timeframe | |||||||
FFQ Food frequency questionnaire, NR not reported; RIA radioimmunoassay
121 premenopausal and 166 postmenopausal women at age 53
Three women with unknown menopausal status
Estimated from reported percentages
The second article by Berube et al. [77] is based on the same study
Findings from the previous studies
Diversity of the studies with respect to study populations, vitamin D exposure assessment and modeling, density estimation, and presentation of the results makes direct comparison across the studies difficult. Those issues are discussed in details later. Below, we present findings across the studies related to the association between vitamin D and mammographic breast density as presented in Supplementary Table 1.
Vitamin D intake and breast density
Five out of nine studies have reported significant inverse associations between Vitamin D intake and breast density and one study reported a modest positive association. All significant findings were reported by cross-sectional studies that assessed Vitamin D intake and most of the findings were limited to premenopausal women.
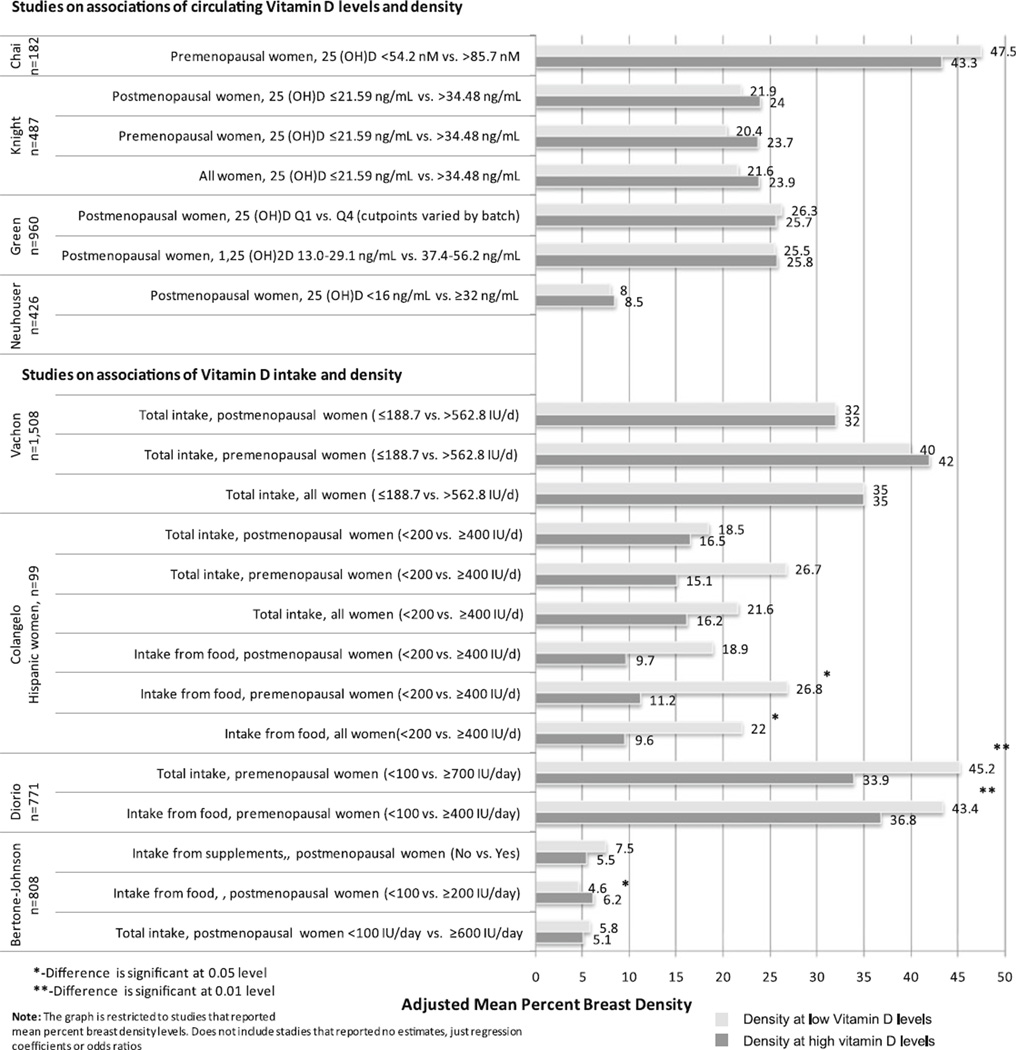
A small study by Colangelo et al. in 99 Hispanic women from Chicago Breast Health Project Phase II Pilot Study reported an inverse association between dietary Vitamin D intake and percent breast density (p = 0.009) [47]. When the analysis was stratified by menopausal status, the association remained marginally significant in premenopausal women, but was not significant in postmenopausal women. The difference in mean percent density between the lowest (<200 IU/d) and highest vitamin D intake from food (≥400 IU/d) was 12.4% in all women and 15.6% in premenopausal women (Fig. 2). No associations were found between total Vitamin D intake and breast density. Similarly, an inverse association in premenopausal Hispanic women from the Women‘s Breast and Bone Density Study was reported by Thompson et al. (p < 0.01) [48]. An increase in Vitamin D intake of one IU per 1,000 kcal resulted in 6.85% reduction in mean percent breast density.
Fig. 2.
Mean percent breast density at the lowest (upper bar) and highest (lower bar) Vitamin D intake/circulating levels across seven studies
Tseng et al. reported a marginally significant inverse association between vitamin D intake and density measured with BI-RADS density classification (odds ratio for third tertile versus first tertile of Vitamin D intake = 0.5; 95% CI 0.2–1.0) among 86 premenopausal and 71 postmenopausal women participating in the Family Risk Assessment Program (FRAP) at Fox Chase Cancer Center in Philadelphia [40]. The results, however, were not reported separately by menopausal status.
Three of the studies that reported significant inverse associations were conducted among women receiving screening mammograms at radiology clinics in Quebec City (Canada) [42, 49, 50]. One of the studies measured circulating Vitamin D levels and is described in the next section. Diorio et al. reported lower percent breast density in 771 premenopausal women with higher vitamin D intake from either food alone or food and supplements combined (p = 0.008 and 0.004) [42]. The difference in mean percent density between the lowest (<100 IU/day) and highest vitamin D intake categories (≥400 IU/day for food intake or ≥700 IU/day for food and supplements) was 6.6% for food and 11.3% for food and supplements combined (Fig. 2) [42].
In addition to the study by Diorio et al., investigation by Berube et al. included a slightly larger number of premenopausal women (n = 777) and 783 postmenopausal participants [49]. The recruitment period for this study (February 2001–March 2002) overlaps with the recruitment period for the study by Diorio et al. (February–December 2001); both studies included essentially the same set of premenopausal women. The study found an inverse association between total or dietary vitamin D intake and percent breast density among premenopausal women, but the strength of the association between vitamin D and breast density was weakened after adjustment for calcium intake. The negative association between dietary vitamin D intake and breast density tended to be stronger at higher levels of calcium intake (absolute mean decrease in breast density for increments of 100 IU vitamin D −1.1 at the calcium intake level 1,106.1–3,130.0 mg/d vs. −0.6 at the calcium intake level 198.1–725.2 mg/d). No associations were found among postmenopausal women [49].
A modest positive association between Vitamin D from food sources and breast density in postmenopausal women was reported by Bertone-Johnson in the Mammogram Density Ancillary Study of the Women’s Health Initiative [51]. Higher density at higher Vitamin D levels was also reported by a few authors [52, 53], but the difference in percent density at the extreme levels of Vitamin D was small and not significant [52]. The rest of the studies did not find significant associations [51–57].
No differences in breast density at the extreme Vitamin D intake levels were found in combined results across four studies that reported the overall results on association between breast density and total Vitamin D intake (p = 0.14) [40, 47, 53, 56]. Similarly, no differences in breast density at the extreme Vitamin D intake levels were found when we combined results across five studies that reported association between total Vitamin D intake and breast density in premenopausal women (p = 1.0) [42, 47, 49, 53, 56] as well as across five studies that reported associations in postmenopausal women (p = 0.61) [47, 49, 51, 53, 56]. However, most studies reported lower density at the higher Vitamin D intake levels in both pre- and postmenopausal women as well as in all women combined.
Circulating Vitamin D levels and breast density
Only five studies investigated associations between circulating Vitamin D levels and breast density. All studies were cross-sectional and four of them observed no associations.
The study by Brisson reported correlation between seasonal changes in circulating Vitamin D levels and corresponding changes in breast density over time [50]. Changes in blood vitamin D were inversely related to changes in breast density with a lag time of about 4 months [50]. This study from Quebec City used the same set of premenopausal women as the study by Diorio et al. who were recruited between February and December 2001.
Knight et al. investigated associations between circulating 25(OH)D levels and breast density among 133 premenopausal and 354 postmenopausal women in the Minnesota Breast Cancer Family Study. This study reported higher density in women with the higher 25(OH)D levels as compared to women with lowest concentrations. The difference in mean percent density between the lowest (≤21.59 ng/mL) and highest 25(OH)D levels (>34.48 ng/mL) was 2.3% in all women, 3.3% in premenopausal women, and 2.1% in postmenopausal women (Fig. 2). However, the time between blood sample and mammogram varied (range 1–8 years) and the 25(OH)D concentrations may not have always reflected the level at the time of the mammogram.
A study by Green et al. within the Nurses’ Health Study cohort included 960 postmenopausal women and found no associations of either 25(OH)D or 1,25(OH)2D with breast density. The difference in mean percent density between the lowest and highest quartiles of metabolite was 0.6% for 25(OH)D and 0.3% for 1,25(OH)2D (Fig. 2).
Chai et al. investigated the association of 25(OH)D levels with breast density among 182 premenopausal women from a nutritional trial who were enrolled through mammography clinics on the island of Oahu (Hawaii). The difference in mean percent density between the lowest (<54.2 nM) and highest (>85.7 nM) 25(OH)D levels was 4.2% (Fig. 2). Consistent with more of the studies on Vitamin D intake and density, breast density was higher in women with lowest circulating Vitamin D levels.
Neuhauser et al. examined the association between 25(OH)D levels and breast density among 426 postmenopausal breast cancer survivors from the Health, Eating, Activity, and Lifestyle Study. Breast density of contralateral breast was assessed about 1.5 years after breast cancer diagnosis, and blood samples were collected 24 months post diagnosis. The difference in mean percent density between the lowest (<16 ng/mL) and highest (≥32 ng/mL) 25(OH)D levels was 0.5% (Fig. 2). This study population, however, had a high prevalence of Vitamin D deficiency (76.8%).
When the results from four studies that measured serum vitamin D levels in postmenopausal women were combined, we observed no difference in percent density contrasting the extreme high and low vitamin D levels (p = 0.64). In premenopausal women, only two studies were available so we did not combine the results.
Discussion
The association of Vitamin D and mammographic breast density remains poorly understood. We have identified 14 published studies that investigated association between Vitamin D and breast density. Differences in study designs, including characteristics of the study population, exposure (Vitamin D) assessment and modeling, and breast density estimation make comparison of the results across the studies difficult. Below, we discuss some of those methodological issues, including vitamin D exposure assessment methods, timing of exposure assessment, associations in pre- and postmenopausal women, and lack of variability in exposure and outcome.
Vitamin D exposure assessment methods
The vast majority of the studies have estimated Vitamin D intake using a food frequency questionnaires (FFQ), a method that accurately estimates the dietary vitamin D exposure [58, 59]. However, Vitamin D levels estimated from FFQ do not account for additional Vitamin D produced in response to sunlight exposure. Thus, in the studies using FFQ for exposure assessment, it becomes important to collect additional information on season and lifestyle factors that might modify total Vitamin D levels and to use those covariates in the analysis. In addition, in the studies that assess the total Vitamin D intake from food and supplements, the detected associations could be confounded by other vitamins and minerals contained in multivitamins and could reflect a combined effect of those ingredients rather than the effect of Vitamin D alone. Finally, similar intakes could translate into different levels of biologically active Vitamin D due to differences in persons’ metabolism, including genetic polymorphisms, coexisting conditions, for example [60–63].
Measurement of circulating 25(OH)D appears to be the best biomarker reflecting the Vitamin D levels from different sources that also reflects the long-term Vitamin D exposure [64]. Controlling for the season of blood draw in the analysis is important to account for seasonal variation. It is, however, unclear how well the levels of circulating 25(OH)D translate into local levels of active 1,25(OH)2D in the breast tissue because of the additional (autocrine) synthesis of 1,25(OH)2D in the breast. Recent advances in application of liquid chromatography coupled with mass spectrometry for measurement of Vitamin D-related compounds in biological samples could open new avenues for measurement of Vitamin D metabolites in the breast tissue samples for epidemiologic studies [65].
Timing of the exposure assessment
The majority of the studies we identified were cross-sectional; however, in all of the studies, blood draws and mammograms did not occur on the same date. In some studies [52, 55], the time interval varied substantially (1–8 years). Moreover, sometimes the exposure assessment took place after the mammogram [52] in a substantial number of participants (27%), and some authors did not report on the timing of exposure assessment [66]. Most of the studies accounted for the difference in dates by adjusting the results for the time between the exposure assessment (blood draw or FFQ) and the mammograms. However, given the seasonal changes in Vitamin D levels, adjustment for the time interval alone might not be sufficient and should be supplemented by the adjustment for season to capture the seasonal variability in Vitamin D exposure.
Menopausal status of study participants
Studies with significant time between exposure and mammograms did not report on whether there were any women who were premenopausal at the time of the exposure assessment and became postmenopausal at the time of the mammogram. Some studies suggest that breast density–associated breast cancer risk is different in pre- and postmenopausal women [67]. Breast density declines around menopause due to the breast tissue involution [2, 68]. This decrease in density after menopause may not necessarily be reflective of Vitamin D influences but rather the changes in the tissue architecture. On the other hand, menopause also represents an important transition in vitamin D requirements that results from the loss of VDRs due to the decline in estrogen levels and increasing demand for vitamin D [69, 70]. Finally, the underlying biological mechanisms by which Vitamin D may affect breast density could also be different in pre- and postmenopausal women owing to the differences in the breast tissue proliferation rates [71, 72] and estrogen synthesis before and after menopause [38, 73, 74]. Due to these issues, reporting the results separately for pre- and postmenopausal women becomes important.
In the studies among premenopausal women, it is unclear if the density was estimated in the same phase of the menstrual cycle. A few authors have reported changes in breast density during the menstrual cycle [75, 76]. Thus, adjustment for the phase of the menstrual cycle in premenopausal women or consistent collection of the mammogram data in one phase is preferable.
Variability of the exposure and the outcome in the study population
Success in revealing true associations depends on the level of biomarker and its variability in the study population. As reported by some authors, the levels of Vitamin D in their study populations were very low [40, 51, 55, 57]. The lack of variation in vitamin D exposure may prevent detection of an association. Studies that modeled Vitamin D intake as quartile or tertiles based on the distribution in the study population were able to achieve a more balanced design with respect to the exposure. In contrast, studies that used pre-defined categories for Vitamin D levels ended up with a larger proportion of women in the lower Vitamin D intake categories [42, 47]. Such skewed distribution might have also contributed to either failure to detect associations or detecting associations by chance.
In a few studies, the mean percent breast density reported for the extreme levels of Vitamin D was low (<20%), indicating that the study population tended to have more women in the lowest density categories compared with the general population. Lack of variability in the outcome may have also contributed to the failure to detect true associations. Stratified sampling on the degree of density that approximates distribution of density categories in the general population would improve generalizability of the results.
Other considerations
Several studies have adjusted their estimates to the calcium intake, and some authors reported different strength of the association between Vitamin D and density in women with different calcium intake levels. Laboratory studies have also suggested that the effect of Vitamin D and calcium in the breast might result from its effect on the insulin growth factor signaling pathway [13, 49]. The effect of Vitamin D on density may be experienced only by a subgroup of women with certain calcium and/or IGF-I or IGFBP-3 levels [42]. Further studies are warranted to determine whether adjustment for calcium and IGF-I levels is essential while reporting the findings on associations between Vitamin D and breast density.
In the study with breast cancer survivors [57], the density was measured in the contralateral breast. In women diagnosed with breast cancer, the changes in the breast tissue microenvironment, including Vitamin D signaling pathway, might not necessarily be restricted to the immediately affected area and could be already present in the distant tissue in both ipsilateral and contralateral breast, perhaps to a lesser degree. Thus, the findings in women with a history of breast cancer might not be applicable to healthy (cancer-free) women.
The cross-sectional nature of the studies limits conclusions about causal relationship between Vitamin D and breast density. The studies reporting inverse associations had a very small difference in mean percent breast density between the extremes of vitamin D intake. Whether this small change in breast density could be sufficient to cause a long-term decrease in breast cancer risk in unclear. It is also unknown whether the effect of Vitamin D results in permanent changes in tissue leading to a long-term changes in breast density. In addition, exposure occurring during the most intensive breast tissue re-modeling and growth, such as puberty and pregnancy, might have a stronger influence on density changes later in life. Further studies are warranted to determine causal relationship between the exposure and density, to investigate long-term effects of Vitamin D on breast density, and to understand the timing of exposure that might be important for breast cancer prevention.
Conclusions
Whether Vitamin D modifies mammographic breast density remains unclear. Further studies are warranted to investigate the associations while addressing methodological issues discussed in this review. More recent advances in laboratory sciences may also result in application of modern analytical techniques to the exposure assessment and measurement of Vitamin D levels directly in the breast tissue, thus allowing investigation of the relationship between the tissue Vitamin D metabolites and breast density.
Supplementary Material
Acknowledgments
Dr. Graham Colditz is supported by ACS Clinical Research Professorship. Dr. Bettina Drake is supported by PC081669 from the Department of Defense. Dr. Yaghjyan is supported by Barnes-Jewish Hospital Foundation.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10552-011-9851-3) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no Conflict of interest.
Contributor Information
Lusine Yaghjyan, Email: yaghjyanl@wudosis.wustl.edu, Division of Public Health Sciences, Department of Surgery, School of Medicine, Washington University in St. Louis, Campus Box 8100, 660 S. Euclid Avenue, St. Louis, MO 63110, USA.
Graham A. Colditz, Email: colditzg@wudosis.wustl.edu, Division of Public Health Sciences, Department of Surgery, School of Medicine, Washington University in St. Louis, Campus Box 8100, 660 S. Euclid Avenue, St. Louis, MO 63110, USA; Institute for Public Health, Washington University in St. Louis, Campus Box 8100, 660 S. Euclid Avenue, St. Louis, MO 63110, USA.
Bettina Drake, Email: drakeb@wudosis.wustl.edu, Division of Public Health Sciences, Department of Surgery, School of Medicine, Washington University in St. Louis, Campus Box 8100, 660 S. Euclid Avenue, St. Louis, MO 63110, USA.
References
- 1.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6(10):798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg OM, Martin LJ, Boyd NF. Mammographic density, lobular involution, and risk of breast cancer. Br J Cancer. 2008;99(9):1369–1374. doi: 10.1038/sj.bjc.6604635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamimi RM, Byrne C, Colditz GA, Hankinson SE. Endogenous hormone levels, mammographic density, and subsequent risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 2007;99(15):1178–1187. doi: 10.1093/jnci/djm062. [DOI] [PubMed] [Google Scholar]
- 4.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Byng JW, Jong RA, Fishell EK, Little LE, Miller AB, Lockwood GA, Tritchler DL, Yaffe MJ. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst. 1995;87(9):670–675. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 6.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst. 1995;87(21):1622–1629. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 7.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 8.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N. The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):343–349. doi: 10.1158/1055-9965.EPI-04-0490. [DOI] [PubMed] [Google Scholar]
- 9.Rutter CM, Mandelson MT, Laya MB, Seger DJ, Taplin S. Changes in breast density associated with initiation, discontinuation, and continuing use of hormone replacement therapy. JAMA. 2001;285(2):171–176. doi: 10.1001/jama.285.2.171. [DOI] [PubMed] [Google Scholar]
- 10.Vachon CM, Pankratz VS, Scott CG, Maloney SD, Ghosh K, Brandt KR, Milanese T, Carston MJ, Sellers TA. Longitudinal trends in mammographic percent density and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2007;16(5):921–928. doi: 10.1158/1055-9965.EPI-06-1047. [DOI] [PubMed] [Google Scholar]
- 11.Spicer DV, Ursin G, Parisky YR, Pearce JG, Shoupe D, Pike A, Pike MC. Changes in mammographic densities induced by a hormonal contraceptive designed to reduce breast cancer risk. J Natl Cancer Inst. 1994;86(6):431–436. doi: 10.1093/jnci/86.6.431. [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 13.Khan QJ, Kimler BF, Fabian CJ. The relationship between vitamin D and breast cancer incidence and natural history. Curr Oncol Rep. 2010;12(2):136–142. doi: 10.1007/s11912-010-0081-8. [DOI] [PubMed] [Google Scholar]
- 14.Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):422–429. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- 15.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94(17):1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46(12):2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 17.IARC. Vitamin D and cancer. France: 2008. [Google Scholar]
- 18.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16(2):83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 19.Cui Y, Rohan TE. Vitamin D, calcium, and breast cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2006;15(8):1427–1437. doi: 10.1158/1055-9965.EPI-06-0075. [DOI] [PubMed] [Google Scholar]
- 20.Chouvet C, Vicard E, Devonec M, Saez S. 1, 25-Dihydroxyvitamin D3 inhibitory effect on the growth of two human breast cancer cell lines (MCF-7, BT-20) J Steroid Biochem. 1986;24(1):373–376. doi: 10.1016/0022-4731(86)90085-3. [DOI] [PubMed] [Google Scholar]
- 21.Simboli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1, 25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58(4):367–376. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 22.Narvaez CJ, Zinser G, Welsh J. Functions of 1alpha, 25-dihydroxyvitamin D(3) in mammary gland: from normal development to breast cancer. Steroids. 2001;66(3–5):301–308. doi: 10.1016/s0039-128x(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 23.Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. J Natl Cancer Inst. 1999;91(2):176–181. doi: 10.1093/jnci/91.2.176. [DOI] [PubMed] [Google Scholar]
- 24.Colston KW, Hansen CM. Mechanisms implicated in the growth regulatory effects of vitamin D in breast cancer. Endocr Relat Cancer. 2002;9(1):45–59. doi: 10.1677/erc.0.0090045. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee P, Chatterjee M. Antiproliferative role of vitamin D and its analogs–a brief overview. Mol Cell Biochem. 2003;253(1–2):247–254. doi: 10.1023/a:1026072118217. [DOI] [PubMed] [Google Scholar]
- 26.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 27.Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80(6 Suppl):1721S–1724S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan AV, Swami S, Feldman D. Vitamin D and breast cancer: inhibition of estrogen synthesis and signaling. J Steroid Biochem Mol Biol. 2010;121(1–2):343–348. doi: 10.1016/j.jsbmb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Pattarozzi A, Gatti M, Barbieri F, Wurth R, Porcile C, Lunardi G, Ratto A, Favoni R, Bajetto A, Ferrari A, Florio T. 17-beta-estradiol promotes breast cancer cell proliferation-inducing stromal cell-derived factor-1-mediated epidermal growth factor receptor transactivation: reversal by gefitinib pretreatment. Mol Pharmacol. 2008;73(1):191–202. doi: 10.1124/mol.107.039974. [DOI] [PubMed] [Google Scholar]
- 30.LaMarca HL, Rosen JM. Estrogen regulation of mammary gland development and breast cancer: amphiregulin takes center stage. Breast Cancer Res. 2007;9(4):304. doi: 10.1186/bcr1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo J, Fernandez SV, Russo PA, Fernbaugh R, Sheriff FS, Lareef HM, Garber J, Russo IH. 17-beta-estradiol induces transformation and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20(10):1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 32.Seeger H, Deuringer FU, Wallwiener D, Mueck AO. Breast cancer risk during HRT: influence of estradiol metabolites on breast cancer and endothelial cell proliferation. Maturitas. 2004;49(3):235–240. doi: 10.1016/j.maturitas.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Johansson CM, Anderson TJ, Bergstrom R, Lindgren A, IR P. Epithelial proliferation in the normal human breast in relation to endogenous hormones and oral contraceptive use. The Breast. 1998;7:162–167. [Google Scholar]
- 34.Sutherland RL, Prall OW, Watts CK, Musgrove EA. Estrogen and progestin regulation of cell cycle progression. J Mammary Gland Biol Neoplasia. 1998;3(1):63–72. doi: 10.1023/a:1018774302092. [DOI] [PubMed] [Google Scholar]
- 35.Foidart JM, Colin C, Denoo X, Desreux J, Beliard A, Fournier S, de Lignieres B. Estradiol and progesterone regulate the proliferation of human breast epithelial cells. Fertil Steril. 1998;69(5):963–969. doi: 10.1016/s0015-0282(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 36.Pike MC, Spicer DV, Dahmoush L, Press MF. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15(1):17–35. doi: 10.1093/oxfordjournals.epirev.a036102. [DOI] [PubMed] [Google Scholar]
- 37.Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–433. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
- 38.Zhu BTCA. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Lopes N, Sousa B, Martins D, Gomes M, Vieira D, Veronese LA, Milanezi F, Paredes J, Costa JL, Schmitt F. Alterations in Vitamin D signalling and metabolic pathways in breast cancer progression: a study of VDR, CYP27B1 and CYP24A1 expression in benign and malignant breast lesions. BMC Cancer. 2010;10:483. doi: 10.1186/1471-2407-10-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng M, Byrne C, Evers KA, Daly MB. Dietary intake and breast density in high-risk women: a cross-sectional study. Breast Cancer Res. 2007;9(5):R72. doi: 10.1186/bcr1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care. 1990;6(1):5–30. doi: 10.1017/s0266462300008916. [DOI] [PubMed] [Google Scholar]
- 42.Diorio C, Berube S, Byrne C, Masse B, Hebert-Croteau N, Yaffe M, Cote G, Pollak M, Brisson J. Influence of insulin-like growth factors on the strength of the relation of vitamin D and calcium intakes to mammographic breast density. Cancer Res. 2006;66(1):588–597. doi: 10.1158/0008-5472.CAN-05-1959. [DOI] [PubMed] [Google Scholar]
- 43.Tworoger S, Hankinson S. Use of biomarkers in epidemiologic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control. 2006;17(7):889–899. doi: 10.1007/s10552-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 44.Jeffreys M, Warren R, Smith GD, Gunnell D. Breast density: agreement of measures from film and digital image. Br J Radiol. 2003;76(908):561–563. doi: 10.1259/bjr/14999231. [DOI] [PubMed] [Google Scholar]
- 45.Lee-Han H, Cooke G, Boyd NF. Quantitative evaluation of mammographic densities: a comparison of methods of assessment. Eur J Cancer Prev. 1995;4(4):285–292. doi: 10.1097/00008469-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Muhimmah I, Zwiggelaar R. Mammographic density classification using multiresolution histogram information. The international special topic conference on information technology in biomedicine (ITAB’06); Ioannina, Greece. 2006. [Google Scholar]
- 47.Colangelo LA, Chiu BCH, Lopez P, Scholtens D, Willis LC, Hendrick RE, Gapstur SM. A pilot study of vitamin D, calcium, and percent breast density in Hispanic women. Nutrition Research. 2006;26(1):11–15. [Google Scholar]
- 48.Thomson CA, Arendell LA, Bruhn RL, Maskarinec G, Lopez AM, Wright NC, Moll CE, Aickin M, Chen Z. Pilot study of dietary influences on mammographic density in pre- and postmenopausal Hispanic and non-Hispanic white women. Menopause. 2007;14(2):243–250. doi: 10.1097/01.gme.0000235362.72899.7b. [DOI] [PubMed] [Google Scholar]
- 49.Berube S, Diorio C, Masse B, Hebert-Croteau N, Byrne C, Cote G, Pollak M, Yaffe M, Brisson J. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1653–1659. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- 50.Brisson J, Berube S, Diorio C, Sinotte M, Pollak M, Masse B. Synchronized seasonal variations of mammographic breast density and plasma 25-hydroxyvitamin d. Cancer Epidemiol Biomarkers Prev. 2007;16(5):929–933. doi: 10.1158/1055-9965.EPI-06-0746. [DOI] [PubMed] [Google Scholar]
- 51.Bertone-Johnson ER, Chlebowski RT, Manson JE, Wactawski-Wende J, Aragaki AK, Tamimi RM, Rexrode KM, Thomson CA, Rohan TE, Peck JD, Pisano ED, Martin CF, Sarto G, McTiernan A. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010;17(6):1152–1160. doi: 10.1097/gme.0b013e3181e102d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knight JA, Vachon CM, Vierkant RA, Vieth R, Cerhan JR, Sellers TA. No association between 25-hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1988–1992. doi: 10.1158/1055-9965.EPI-06-0241. [DOI] [PubMed] [Google Scholar]
- 53.Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota breast cancer family cohort. Cancer Epidemiol Biomarkers Prev. 2000;9(2):151–160. [PubMed] [Google Scholar]
- 54.Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127(3):667–674. doi: 10.1002/ijc.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masala G, Ambrogetti D, Assedi M, Giorgi D, Del Turco MR, Palli D. Dietary and lifestyle determinants of mammographic breast density. A longitudinal study in a Mediterranean population. Int J Cancer. 2006;118(7):1782–1789. doi: 10.1002/ijc.21558. [DOI] [PubMed] [Google Scholar]
- 56.Mishra G, McCormack V, Kuh D, Hardy R, Stephen A, dos Santos Silva I. Dietary calcium and vitamin D intakes in childhood and throughout adulthood and mammographic density in a British birth cohort. Br J Cancer. 2008;99(9):1539–1543. doi: 10.1038/sj.bjc.6604697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neuhouser ML, Bernstein L, Hollis BW, Xiao L, Ambs A, Baumgartner K, Baumgartner R, McTiernan A, Ballard-Barbash R. Serum vitamin D and breast density in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19(2):412–417. doi: 10.1158/1055-9965.EPI-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chlebowski RT, Johnson KC, Lane D, Pettinger M, Kooperberg CL, Wactawski-Wende J, Rohan T, O’Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. 25-hydroxyvitamin D concentration, vitamin D intake and joint symptoms in postmenopausal women. Maturitas. 2011;68(1):73–78. doi: 10.1016/j.maturitas.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu H, Gozdzik A, Barta JL, Wagner D, Cole DE, Vieth R, Parra EJ, Whiting SJ. The development and evaluation of a food frequency questionnaire used in assessing vitamin D intake in a sample of healthy young Canadian adults of diverse ancestry. Nutr Res. 2009;29(4):255–261. doi: 10.1016/j.nutres.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Signorello LB, Williams SM, Zheng W, Smith JR, Long J, Cai Q, Hargreaves MK, Hollis BW, Blot WJ. Blood Vitamin D Levels in Relation to Genetic Estimation of African Ancestry. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2325–2331. doi: 10.1158/1055-9965.EPI-10-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. Am J Clin Nutr. 2009;89(2):634–640. doi: 10.3945/ajcn.2008.26445. [DOI] [PubMed] [Google Scholar]
- 62.D’Alésio A, Garabédian M, Sabatier JP, Guaydier-Souquières G, Marcelli C, Lemaçon A, Walrant-Debray O, Jehan F. Two single-nucleotide polymorphisms in the human vitamin D receptor promoter change protein–DNA complex formation and are associated with height and vitamin D status in adolescent girls. Hum Mol Genet. 2005;14(22):3539–3548. doi: 10.1093/hmg/ddi382. [DOI] [PubMed] [Google Scholar]
- 63.Howard G, Nguyen T, Morrison N, Watanabe T, Sambrook P, Eisman J, Kelly P. Genetic influences on bone density: physiological correlates of vitamin D receptor gene alleles in premenopausal women. J Clin Endocrinol Metab. 1995;80(9):2800–2805. doi: 10.1210/jcem.80.9.7673427. [DOI] [PubMed] [Google Scholar]
- 64.Hofmann JN, Yu K, Horst RL, Hayes RB, Purdue MP. Long-term variation in serum 25-hydroxyvitamin D concentration among participants in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(4):927–931. doi: 10.1158/1055-9965.EPI-09-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higashi T, Shimada K, Toyo’oka T. Advances in determination of vitamin D related compounds in biological samples using liquid chromatography-mass spectrometry: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(20):1654–1661. doi: 10.1016/j.jchromb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 66.Chai W, Maskarinec G, Cooney RV. Serum 25-hydroxyvitamin D levels and mammographic density among premenopausal women in a multiethnic population. Eur J Clin Nutr. 2010;64(6):652–654. doi: 10.1038/ejcn.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerlikowske K, Cook AJ, Buist DS, Cummings SR, Vachon C, Vacek P, Miglioretti DL. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28(24):3830–3837. doi: 10.1200/JCO.2009.26.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghosh K, Hartmann LC, Reynolds C, Visscher DW, Brandt KR, Vierkant RA, Scott CG, Radisky DC, Sellers TA, Pankratz VS, Vachon CM. Association between mammographic density and age-related lobular involution of the breast. J Clin Oncol. 2010;28(13):2207–2212. doi: 10.1200/JCO.2009.23.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munir J, Birge S. Vitamin D Deficiency in Pre and Postmenopausal Women. Menopause Management. 2008;17(5):10–21. [Google Scholar]
- 70.Duque G, El Abdaimi K, Macoritto M, Miller MM, Kremer R. Estrogens (E2) regulate expression and response of 1, 25-dihydroxyvitamin D3 receptors in bone cells: changes with aging and hormone deprivation. Biochem Biophys Res Commun. 2002;299(3):446–454. doi: 10.1016/s0006-291x(02)02657-8. [DOI] [PubMed] [Google Scholar]
- 71.Talley LI, Grizzle WE, Waterbor JW, Brown D, Weiss H, Frost AR. Hormone receptors and proliferation in breast carcinomas of equivalent histologic grades in pre- and postmenopausal women. Int J Cancer. 2002;98(1):118–127. doi: 10.1002/ijc.10171. [DOI] [PubMed] [Google Scholar]
- 72.Misell L, Hwang E, Au A, Esserman L, Hellerstein M. Development of a novel method for measuring in vivo breast epithelial cell proliferation in humans. Breast Cancer Res Treat. 2005;89(3):257–264. doi: 10.1007/s10549-004-2228-5. [DOI] [PubMed] [Google Scholar]
- 73.Guillemette C, Belanger A, Lepine J. Metabolic inactivation of estrogens in breast tissue by UDP-glucuronosyltransferase enzymes: an overview. Breast Cancer Res. 2004;6(6):246–254. doi: 10.1186/bcr936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344(4):276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 75.Ursin G, Parisky YR, Pike MC, Spicer DV. Mammographic density changes during the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2001;10(2):141–142. [PubMed] [Google Scholar]
- 76.White E, Velentgas P, Mandelson MT, Lehman CD, Elmore JG, Porter P, Yasui Y, Taplin SH. Variation in mammographic breast density by time in menstrual cycle among women aged 40–49 Years. J Natl Cancer Inst. 1998;90(12):906–910. doi: 10.1093/jnci/90.12.906. [DOI] [PubMed] [Google Scholar]
- 77.Berube S, Diorio C, Brisson J. Multivitamin-multimineral supplement use and mammographic breast density. Am J Clin Nutr. 2008;87(5):1400–1404. doi: 10.1093/ajcn/87.5.1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.