Abstract
Recreational use of the drug 4-methylmethcathinone (mephedrone; 4-MMC) became increasingly popular in the United Kingdom in recent years, spurred in part by the fact it was not criminalized until April of 2010. Although several fatalities have been associated with consumption of 4-MMC and cautions for recreational users about its addictive potential have appeared on Internet forums, very little information about abuse liability for this drug is available. This study was conducted to determine if 4-MMC serves as a reinforcer in a traditional intravenous self-administration model. Groups of male Wistar and Sprague-Dawley rats were prepared with intravenous catheters and trained to self-administer 4-MMC in one hour sessions. Per infusion doses of 0.5 and 1.0 mg/kg were consistently self-administered resulting in greater than 80% discrimination for the drug-paired lever and mean intakes of about 2–3 mg/kg/hr. Dose-substitution studies after acquisition demonstrated that the number of responses and/or the total amount of drug self-administered varied as a function of dose. In addition, radiotelemetry devices were employed to show that self-administered 4-MMC was capable of increasing locomotor activity (Wistar) and decreasing body temperature (Sprague-Dawley). Pharmacokinetic studies found the T1/2 of 4-MMC was about an hour in vivo in rat plasma and 90 minutes using in vitro liver microsomal assays. This study provides evidence of stimulant-typical abuse liability for 4-MMC in the traditional preclinical self-administration model.
Keywords: cathinone, reinforcement, stimulant, thermoregulation
Introduction
Predictions about the abuse liability of 4-methylmethcathinone (4-MMC, mephedrone) are mixed; user experiences (Bluelight, 2008) suggest that 4-MMC is similar to both prototypical stimulants (cocaine, methamphetamine) and the entactogen 3,4-methylenedioxymethamphetamine (MDMA; “Ecstasy”) (Geezaman, 2009; MephTest, 2009; Psychonaut Webmapping Research Group, 2010). Of those individuals that have tried both cocaine and 4-MMC intranasally, 76% report the quality of the high of 4-MMC as being similar to or better than that of cocaine and 85% reported the high of 4-MMC as lasting as long or longer than that of cocaine (Winstock et al., 2011).
The single available report of 4-MMC intravenous self-administration (IVSA) in rats indicates that 4-MMC can support IVSA (Hadlock et al., 2011), however it was limited to only a single per-infusion dose and access conditions that likely increase drug intake. Specifically, access to drug was relatively long (4 hr) and under high (29°C) ambient temperature (TA). Escalation of d-methamphetamine (MA) intake occurs when daily drug access is 6 hr but not when it is 2 hr (Kitamura et al., 2006) and intake of cocaine, MA and MDMA increase under relatively high (30°C vs. ~22 °C) TA conditions (Cornish et al., 2008; Cornish et al., 2003); but also see Feduccia et al, 2010. Thus, the available data may overestimate the degree to which 4-MMC IVSA can be established in more traditional rodent laboratory conditions.
Also, available reports demonstrate that the physiological effects of 4-MMC distinguish it from MA and MDMA. Under 20–23°C TA, 4-MMC reduces body temperature in rats – similar to MDMA, while 4-MMC does not change body temperature under 27–30°C TA – unlike MDMA which increased body temperature under such conditions (Miller et al., 2012; Wright et al., 2012). As MDMA IVSA, as compared with MA IVSA, can be difficult to establish (De La Garza et al., 2007) – requiring high per-infusion doses (Schenk, 2009) as well as being produced in only a subset of rats (Colussi-Mas et al., 2010; Schenk et al., 2007) – these physiological distinctions may predict differences between the IVSA profile of 4-MMC and that of MDMA or MA (Cornish et al., 2003; Feduccia et al., 2010).
To further determine the capacity of 4-MMC to support IVSA and the relationship between 4-MMC intake and body temperature, we conducted IVSA experiments of varied per-infusion dose and reinforcement schedule in rats wherein body temperature or locomotor activity were recorded via radiotelemetry transmitters. Additionally, pharmacokinetic and in vitro metabolic assays were included to further elucidate the behavioral and physiological studies.
METHODS
Animals
Male Wistar rats (N=29; Charles River; New York) and Sprague-Dawley rats (N=53; Harlan; California) housed in a humidity and temperature-controlled (22 C ±1) vivarium on a reverse 12:12 hr light/dark cycle were used for the IVSA studies. Animals were 10–13 weeks old and 350–400 grams at the start of the experiment. They had ad libitum access to food (except for pellet training, see below) and water in their home cage. Pharmacokinetic studies used male Wistar (N=18) and Sprague-Dawley rats (N=15), Taconic; Germantown, NY. Procedures were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and the University of New England consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Clark et al., 1996).
Surgery
For the self-administration experiments, rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance) and prepared with chronic intravenous catheters as described elsewhere (Caine et al., 1993) with minor modifications (Emmett-Oglesby and Lane, 1992). Briefly, catheters consisted of silastic tubing (18 cm) fitted to a guide cannula (Plastics One, Roanoke, VA) bent 90° and encased in dental cement anchored to an~3 cm circle of mesh. Catheter tubing was passed subcutaneously from the animal’s back to the right shoulder, inserted into the right jugular vein and tied with suture thread. Liquid tissue adhesive was used to close incisions (3M™ Vetbond™ Tissue Adhesive; 1469SB). For two groups, radiotelemetry transmitters (Data Sciences International; CTA-F40) were also implanted during the catheterization procedure as previously described (Miller et al., 2012; Wright et al., 2012). For the first three days of the 7-day (minimum) recovery period, cephazolan (0.4 g/ml; 2.0 ml/kg sc; once daily) and flunixin (2.5 mg/ml; 2.0 ml/kg sc; once daily) were administered.
Catheters were flushed with saline containing either timentin (before sessions; 0.1 g/ml; 0.2–0.3 ml/rat) or heparin (after sessions; 10 USP units/ml; 0.2–0.3 ml/rat). Catheter patency was assessed nearly once a week after the last session of the week via administration ~0.2 ml of Brevital sodium (1% methohexital sodium; Eli Lilly, Indianapolis, IN). Animals with patent catheters exhibit prominent signs of anesthesia (pronounced loss of muscle tone) within 3 sec of i.v. injection. Animals that failed to display these signs were discontinued from the study.
Procedure
General Procedure
Subjects were transported to an experimental room (24±1 °C) and placed into operant cages (Med Associates) located inside sound-attenuating chambers beginning 0.5–4.0 h into the vivarium dark cycle. Catheters were connected to tubing inside a protective spring suspended into the chamber from a liquid swivel attached to a balance arm; drugs were delivered via syringe pump. Sessions started with the extension of two levers into the chamber. Following completion of each response-ratio, a white light above the reinforced lever signaled reinforcer delivery and remained on during a 20-sec post-reinforcement timeout during which responses had no scheduled consequences.
Radiotelemetry recordings of body temperature and activity were made every five minutes via a telemetry receiver plate placed inside the sound attenuating chamber but outside the operant box in two of the groups. Animals were recorded for 15 minutes prior to the start of self-administration to establish temperature and activity baselines.
Pre-acquisition training
Rats were food-restricted (20 g chow/rat/day) and trained to press the left lever for 45 mg food pellets (TestDiet; 1811156) under a FR1 schedule of reinforcement. Once stable responding was achieved (≥50 reinforcers per 60 min session) the ratio requirement was increased to FR2 (until ≥50 reinforcers per session were obtained) then to FR5 (until ≥ 50 reinforcers per session were obtained). On average, completing FR5 required 4±1 sessions. Rats were returned to ad libitum feeding conditions after completing FR5.
Drug Self-Administration Acquisition
After pellet training, animals were given one-hour, FR5 IVSA training sessions (M-F). The first experiments used a relatively high per-infusion dose (1.0 mg/kg) in Sprague-Dawley (N=12) and Wistar rats (N=13) because studies of locomotor responses to injections (sc) of 4-MMC indicated at least a 3-fold reduction in potency relative to methamphetamine (Wright et al., 2012) and similar doses have been found necessary to establish robust MDMA self-administration (Schenk et al., 2003; Schenk et al., 2007). The third and fourth experiments evaluated a lower (0.5 mg/kg/inf) training dose in groups of Sprague-Dawley (N=16) and Wistar (N=16) rats. The final experiment determined the self-administration of d-methamphetamine (0.05 mg/kg/inf; N=9) or vehicle (N=8) in groups of Sprague-Dawley rats.
To analyze telemetry measures as a function of cumulative self-administered dose, for Sprague-Dawley rats trained at the 1.0 mg/kg per-infusion dose, sessions were identified during which sufficient numbers of individuals self-administered cumulative doses of 1 mg/kg and 3–4 mg/kg per session. Out of this group of 12, three individuals did not have cumulative doses of 1 mg/kg and an additional three did not have cumulative doses of 3–4 mg/kg; thus, N=9 for each of these dose conditions. For the Wistar rats trained at the 0.5 mg/kg per-infusion dose, intake was sufficiently variable across individuals and stable within individuals such that it was necessary to group sessions during which cumulative intake (mg/kg) was 2.5 (N=4), 3.0 (N=3), 3.5 (N=4) or 4.0 (N=1) to end up with a total group of N=12 under this dose condition. For both groups, a control condition was derived from a day when only saline was available.
Fixed-Ratio, Dose-Response Testing
Upon completion of IVSA acquisition, Sprague-Dawley rats trained on 0.5 mg/kg/inf 4-MMC were given sessions wherein the per-infusion dose was varied within subjects. Each dose was given in a block of 3 sessions and the order of the blocks was pseudorandomized across subjects. After the completion of all 4 blocks of testing doses, rats were returned to the 0.5 mg/kg/inf 4-MMC training dose for at least three more sessions.
Progressive-Ratio, Dose-Response Testing
After acquisition, the Wistar rats trained on 0.5 mg/kg/inf 4-MMC were tested under a progressive-ratio schedule of reinforcement (PR) in which the response requirement increased after each reinforcer delivery (Hodos, 1961). The sequence of response ratios within each session was derived from the following equation (rounded to the nearest integer): Response Ratio = 5e^(injection # × 0.2) − 5 (Richardson and Roberts, 1996).
Animals were given 6 sessions of PR testing at the 0.5 mg/kg/inf training dose to achieve stability and thereafter the per-infusion dose was varied within subjects (3 doses: 1 above and 2 below the training dose). Each dose was administered in sequential daily sessions (order of doses pseudorandomized across subjects) until a stability criterion was met (no more than ±3-infusion difference between sessions and no consistent downward/upward trends). After reaching stability criteria for all three doses, vehicle-only session were given until stable responding was obtained.
Drug-Substitution Testing
An additional group of Sprague-Dawley rats (N = 8) previously trained to self-administer d-methamphetamine (0.1 mg/kg/inf) under an FR2 schedule of reinforcement was used to determine the effect of substituting 4-MMC. For this study, the per-infusion dose of 4-MMC was varied across subjects (5 doses). Doses were given in blocks of 3 sequential sessions (order of blocks pseudorandomized across subjects); the average of the three sessions was used for analysis.
Drugs
The racemic 4-methlymethcathinone used for this study was synthesized as previously described (Miller et al., 2012; Wright et al., 2012). d-Methamphetamine was provided by RTI International (Research Triangle Park, NC) under contract from the National Institute on Drug Abuse (Bethesda, MD). All drug doses are expressed as the HCl salts.
Distribution and Metabolism
Pharmacokinetic studies
Placement of jugular vein and carotid artery catheters was conducted at the vendor prior to delivery at the laboratory; the jugular catheter was employed for IV dosing. Blood samples were collected via the carotid artery catheters at times 0, 2, 5, 15, 30, 60, 120 and 240 min and 6, 8, 12, and 24 h after dosing in groupsof Sprague-Dawley (N=3) and Wistar (N=3) rats. Brains were collected from additional groups (N=3 per timepoint, per strain) 2, 15, 30 and 60 min after IV dosing by rapid decapitation; a second group of N=3 Wistar and Sprague-Dawley rats was used to generate plasma PK data for plasma/brain ratio calculations in this latter study. Deionized water was added to half the brain to achieve a tissue concentration of 0.1 g tissue/mL water. Tissue was homogenized for 3–5 minutes, and the homogenate was spun at 14000 g for 10 min at 4°C. The supernatant was decanted into a labeled vial. The pellet was again homogenized for 3–5 minutes in the same volume of deionized water used for the sample and again spun at 14000 g for 10 minutes at 4°C. The supernatant was decanted into the same vial. Plasma and brain supernatant samples were stored at −80°C until analysis. Brains from untreated animals were homogenized to provide blank matrix for preparation of calibration standards. Blank rat plasma was obtained from Valley Biomedical (Winchester, VA). Sample analysis was by LC/MS/MS with data analysis via the non-compartmental analysis model of PK Solutions (Summit Research Services; Montrose, CO).
Plasma Stability
The percent of 4-MMC remaining following incubation at 37 °C (0, 1, 3, 5 24 hr) in plasma derived from Sprague-Dawley and Wistar rats was determined at 1.0 μM analyte concentration. Samples were analyzed by LC/MS/MS and the half-life was calculated using regression analysis.
Microsomal Stability
Metabolic stability using liver microsome preparations from Sprague-Dawley and Wistar rats (BD Biosciences, San Diego CA) was determined at 0.125 mg/mL protein concentration (1.0 μM drug concentration) following incubation at 37 °C (0, 5, 15, 30 and 60 minutes). The samples were analyzed by LC/MS/MS.
LC/MS/MS analysis
4-MMC was quantified by LC/MS/MS; analysis was conducted using an Agilent (Santa Clara, CA) 6460 triple quadrupole mass spectrometer coupled with an Agilent liquid chromatography (LC) system. The LC system consists of a binary pump, degasser, column heater, and autosampler. Chromatographic separation was performed on an Agilent Zorbax Eclipse XDB C-18 Rapid Resolution (4.6 × 50 mm) analytical column using a ballistic gradient of mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) at a flow rate of 2.0 ml/min, with a 1:1 split. Mobile phase was heated to a temperature of 60°C. The gradient was as follows: 95% A for 1 minute, ramp 95→5% A over the two 3 minutes. The mass spectrometer source used nitrogen as the nebulizing and sheath gas. Detection of analyte and internal standard was by electrospray in the positive ion mode. Nebulizer gas was heated to a temperature of 350°C; and sheath gas was heated to 400°C. Both gas flows were maintained at 11 L/min. Capillary and nozzle voltages were set to 4000 V and 500 V respectively. 4-MMC and the internal standard were detected by multiple reaction monitoring (MRM). 4-MMC was detected by monitoring the transition 178.1→145.1, with a fragmentor voltage of 101 V and a collision energy of 38 V. Warfarin was monitored using the transition 309.1→162.9, with a fragmentor of 85 V and collision energy of 10 V. Retention times for 4-MMC and warfarin were 2.73 and 3.00 minutes respectively. A volume of 5.0 μL of sample extract was injected for analysis.
Samples were prepared by protein precipitation with acetonitrile containing internal standard (200 nM warfarin). Matrix to solvent ratio for the precipitation was 1:3 for plasma and 1:4 for brain homogenate. Calibration standards were prepared by serial dilution in the appropriate matrix. Analyte concentrations were determined by the interpolation of peak area ratio of analyte to internal standard from a calibration curve formed by matrix spiked with reference material. The calibration range in plasma was 5.0–10000 nM, and 10.0–5000 nM in brain homogenate, using 1/x2 weighting.
Data analysis and Exclusion Criteria
Statistical analyses employed repeated-measures analysis of variance (rmANOVA). When a factor reached statistical significance (p < 0.05), effect-pattern delineation was determined by paired-means comparisons using Fisher’s protected least significant difference (Fisher’s PLSD). Otherwise, post hoc paired-means comparisons used Tukey’s honest significant difference method (Tukey HSD). Analyses were conducted with GB-STATv7.0 (Dynamic Microsystems, Silver Spring MD).
Animals were excluded from the analyses of a particular phase if any one of the following exclusion criterion was met during that phase: 1) Failure of the Brevital test (described above); 2) Less than 80% discrimination for the reinforced lever across the analysis interval; 3) Unambiguous loss of catheter patency (e.g., unable to flush catheter). The discrimination criterion was omitted for the comparison of vehicle and d-methamphetamine self-administration since this outcome was predicted for the vehicle group.
Results
Acquisition of Drug Self-Administration
Sprague-Dawley rats; 1.0 mg/kg/inf
Rats (N=12) were given 9 sessions of 1.0 mg/kg/inf 4-MMC IVSA training during which body temperature and activity were recorded. Two rats’ catheters did not remain patent through acquisition and an additional three rats did not show reward-lever discrimination >80%; these individuals were therefore excluded from analyses. As is shown in Figure 1A, over the first 9 days, reward-lever discrimination averaged 95% (range: 84–100%) and reward-lever presses increased from an average of 12 to 28; an effect which was not confirmed by the analysis (F8,48 = 1.86; p>0.088).
Figure 1. Acquisition of 4-MMC self-administration: Reward-lever responses and reward-lever discrimination.
A–D) Mean number of reward-lever responses and reward-lever selectivity (% reward-lever presses ÷ total lever presses) for: A) Sprague-Dawley rats trained to self-administer at 1.0 mg/kg/inf 4 (N =7). B) Sprague-Dawley rats trained to self-administer at 0.5 mg/kg/inf (N = 12). C) Wistar rats trained to self-administer at 1.0 mg/kg/inf (N = 11). D) Wistar rats trained to self-administer at 0.5 mg/kg/inf (N = 16). E–F) Mean number of reward-lever responses as a function of rat strain and per-infusion dose available during acquisition: E) Rats trained at 1.0 mg/kg/inf. F) Rats trained at 0.5 mg/kg/inf. Statistically reliable differences from the first session are indicated by * and differences between groups are represented by #. Error bars represent ±SEM.
Wistar rats; 1.0 mg/kg/inf
Rats (N=13) were given 10 sessions of 1.0 mg/kg/inf 4-MMC IVSA training. All rats exhibited reward-lever discrimination >80%; however, two rats’ catheters did not remain patent through acquisition and were therefore excluded from analyses. As is shown in Figure 1C, over the first 10 days, reward-lever discrimination averaged 98% (range: 96–100%) and reward-lever presses averaged 21 (session 8) to 34 (session 7) per session; changes that did not attain statistical reliability (F9,90= 1.89; p>0.063).
Sprague-Dawley vs. Wistar rats at 1.0 mg/kg/inf
To examine an apparent strain difference in reward-lever presses observed early in acquisition on the 1.0 mg/kg/inf training dose (Figure 1E), a second analysis was done to compare Wistar to Sprague-Dawley rats that confirmed an interaction between strain and session (F8,128= 2.83; p>0.01). Post hoc tests confirmed that Wistar rats made significantly more reward-lever presses on sessions 1 thru 7 than Sprague-Dawley rats.
Sprague-Dawley rats; 0.5 mg/kg/inf
Rats (N=16) were given 10 sessions of 0.5 mg/kg/inf 4-MMC IVSA training (Figure 1B). Four rats did not exhibit reward-lever discrimination >80% and were excluded from analysis. For the 12 remaining rats, reward-lever discrimination was no lower than 90% across the first 10 sessions and reward-lever presses averaged from 16 (session 3) to 36 (session 1) per session. The analysis of reward-lever presses confirmed a main effect of session (F9,99= 2.29; p<0.05) and post hoc tests confirmed that there were fewer presses on sessions 2–5 with respect to the initial session.
Wistar rats; 0.5 mg/kg/inf
A group of Wistar rats (N=16) were given 10 sessions of self-administration training with a 0.5 mg/kg/inf dose of 4-MMC (Figure 1D) during which body-temperature and activity were recorded. All sixteen individuals made over 80% of their responses on the drug-paired lever. Reward-lever responding varied from 24 (session 10) to 34 (session 1). Analysis of reward-lever responses did not confirm a main effect of session (F9,135= 1.12; p>0.05).
Sprague-Dawley vs. Wistar rats at 0.5 mg/kg/inf
When 0.5 mg/kg/inf was the training dose, Sprague-Dawley rats omitted fewer reward-lever responses early in acquisition (sessions 2–5) than Wistar rats (Figure 1F). Paired-means comparisons using Tukey’s HSD confirmed these differences.
Body temperature and locomotor activity
Sprague-Dawley rats; 1.0 mg/kg/inf
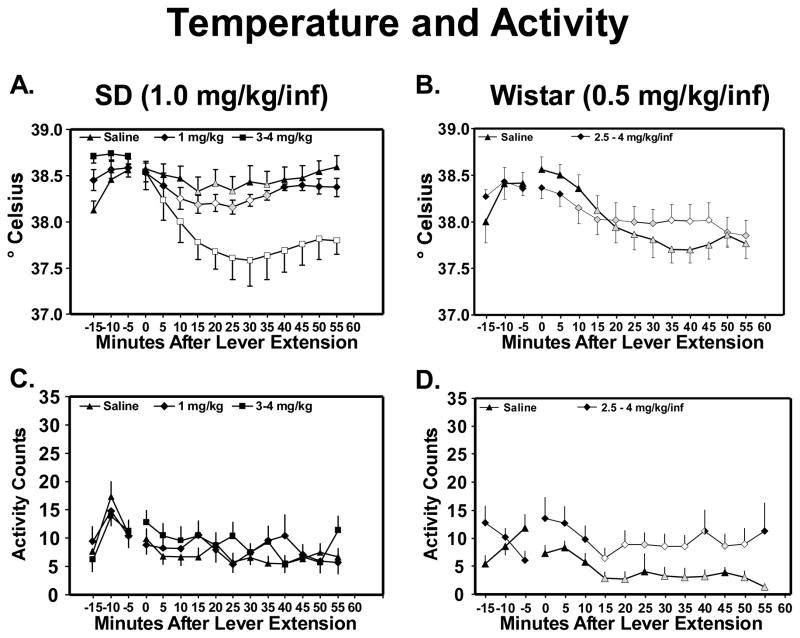
Self-administration of 4-MMC dose-dependently decreased body temperature but did not change activity counts (Figure 2A and 2C). Analysis of body temperature confirmedmain effects of cumulative dose (F2,22= 11.37; p<0.001) and time after lever extension (F11,121= 17.90; p<0.0001) as well as an interaction between dose and time (F22,242= 5.43; p<0.0001). Post hoc comparisons confirmed that, in comparison to vehicle, body temperature was lower after either self-administration of 3–4 mg/kg (5–55 min) or 1 mg/kg (10, 20 and 30 min) of 4-MMC. And, in comparison to the 0-time point, body temperature was lower over a larger range of time points after either self-administration of 3–4 mg/kg (5–55 min) or 1 mg/kg (10–35 min) of 4-MMC than after self-administration of vehicle alone (15–25 and 35 min). Analysis of the activity data did not confirm main effects of dose or time nor an interaction between dose and time.
Figure 2. Acquisition of 4-MMC self-administration: Body temperature and activity counts.
AB) Mean body temperature (°C) across self-administration sessions (5-min time bins) as a function of cumulative dose per session (mg/kg) for: A) Sprague-Dawley rats trained on 1.0 mg/kg/inf (N = 9–12). B) Wistar rats trained on 0.5 mg/kg/inf (N = 11). C–D) Mean activity counts across self-administration sessions as a function of cumulative dose per session for: C) Sprague-Dawley rats trained on 1.0 mg/kg/inf (N = 9–12). D) Wistar rats trained on 0.5 mg/kg/inf (N = 11). Symbols: shaded = significantly different from the 0 timepoint (at which levers were extended) within dose; open = significantly different from the 0 timepoint within dose and from vehicle at that time bin; half-open = significantly different from vehicle at that time bin. Error bars represent ±SEM.
Wistar rats; 0.5 mg/kg/inf
Compared to saline, self-administration of 4-MMC caused biphasic changes in body temperature; an initial decrease (1st 15 min) followed by an increase (25–45 min) (Figure 2B). Also, self-administration of 4-MMC increased activity counts across the session (Figure 2D).
Statistical analysis of body temperature confirmed a main effect of time after lever extension (F11,110= 15.20; p<0.0001) as well as an interaction between cumulative dose of drug per session and time after lever extension (F11,110= 3.97; p<0.0001), but no main effect of cumulative dose of drug per session (F1,10= 0.77; p>0.05). Post hoc comparisons confirmed that body temperature was lower 5 minutes after, and higher 35–45 minutes after, the start of 4-MMC self-administration sessions compared with the session when only saline was available. Additionally, relative to the 0 timepoint, body temperature was lower from 10–55 minutes for 4-MMC self-administration sessions and 15–55 minutes in the saline session.
The analysis of activity counts confirmed a significant main effect of time after lever extension (F11,110= 4.00; p<0.0001) and of cumulative dose of drug per session (F1,10= 13.57; p>0.005). Post hoc tests confirmed that activity was greater when 4-MMC was self-administered than when only saline was available (0–10 and 20–55 min from the 0 timepoint) and that activity decreased from the 0 timepoint for both conditions (4-MMC, 15–50 and 45–50 min; saline, 15–20, 30–40 and 50–55 min).
Dose-response analysis on a fixed-ratio schedule of reinforcement
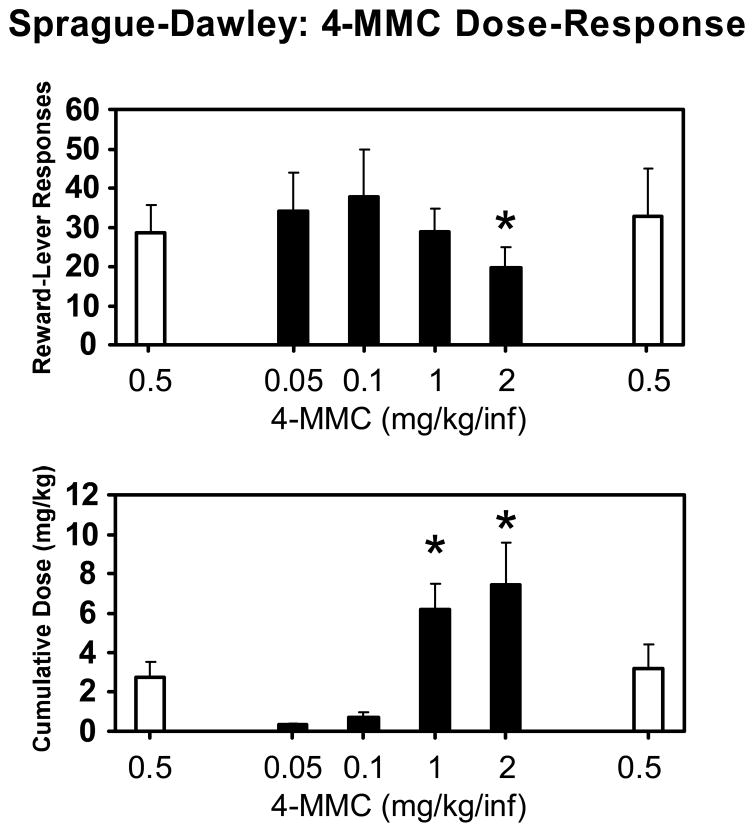
After acquisition of 4-MMC self-administration (0.5 mg/kg/inf), some of the Sprague-Dawley rats (N=12) were tested in a dose-response experiment wherein the per-infusion dose was varied within-subjects to 0.05, 0.1, 1.0 or 2.0 mg/kg (Figure 3). Five of the 12 rats were excluded from analysis due to loss of catheter patency. Analysis confirmed a main effect of per-infusion dose on both the number of reward-lever presses (F3,18= 3.35; p<0.05) and cumulative per-session dose (F3,18= 12.82; p<0.0001). Post hoc comparisonsconfirmed that reward-lever presses decreased as dose increased. Specifically, fewer reward-lever presses were emitted when the per-infusion dose was 2.0 mg/kg than when 0.05 or 0.1 mg/kg. Additionally, the cumulative per-session dose was lower when the per-infusion dose was 0.05 or 0.1 mg/kg than when it was 1.0 or 2.0 mg/kg.
Figure 3. Fixed-ratio dose-response.
Mean number of reward-lever responses (top) and cumulative drug intakes (mg/kg/session; bottom) for Sprague-Dawley rats (N = 7) (trained on 0.5 mg/kg/inf) as function of the per-infusion dose available. Data for sessions at the training dose three days before and three days after the dose-substitution sessions are included for comparison. A significant difference from both of the lower two dose-substitution conditions is indicated by the * symbol. Error bars represent ±SEM.
Dose-response analysis on a progressive-ratio schedule of reinforcement
After acquisition of 4-MMC self-administration, the 0.5 mg/kg/inf trained Wistar rats that remained patent (N=14) were tested on a progressive-ratio schedule of reinforcement (PR) for 6 sessions wherein the response requirement for each subsequent drug infusion was increased and the per-infusion dose was 0.5 mg/kg (the training dose) (Figure 4). Two animals’ data were excluded from analysis because their catheters did not remain patent. On PR at the training dose, all animals showed reward-lever discrimination >80% and the group averaged 49–146 reward-lever responses and 4.8–6.0 infusions across the six sessions. Analysis did not confirm any main effects of session on reward-lever discrimination (F5, 75= 1.83; p>0.05), number of reward-lever responses (F5, 75= 1.75; p>0.05) or number of infusions (F5, 75= 1.38; p>0.05).
Figure 4. Progressive-ratio baseline sessions.
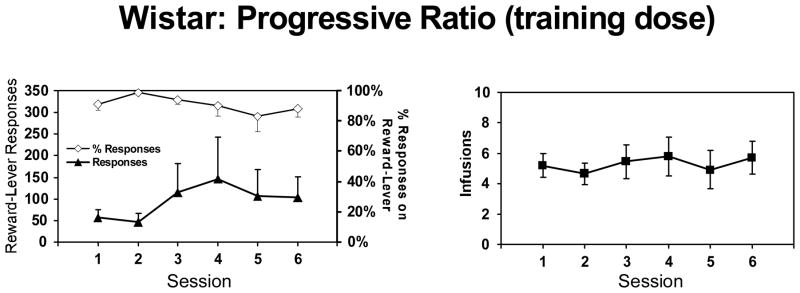
Mean number of reward-lever responses and percent of total lever responses on the drug-paired lever per session (left panel) and the number of infusions per session (right panel) from Wistar rats (N = 14) under a progressive-ratio schedule of reinforcement (after initial acquisition at the per-infusion dose of 0.5 mg/kg/inf). Error bars represent ±SEM.
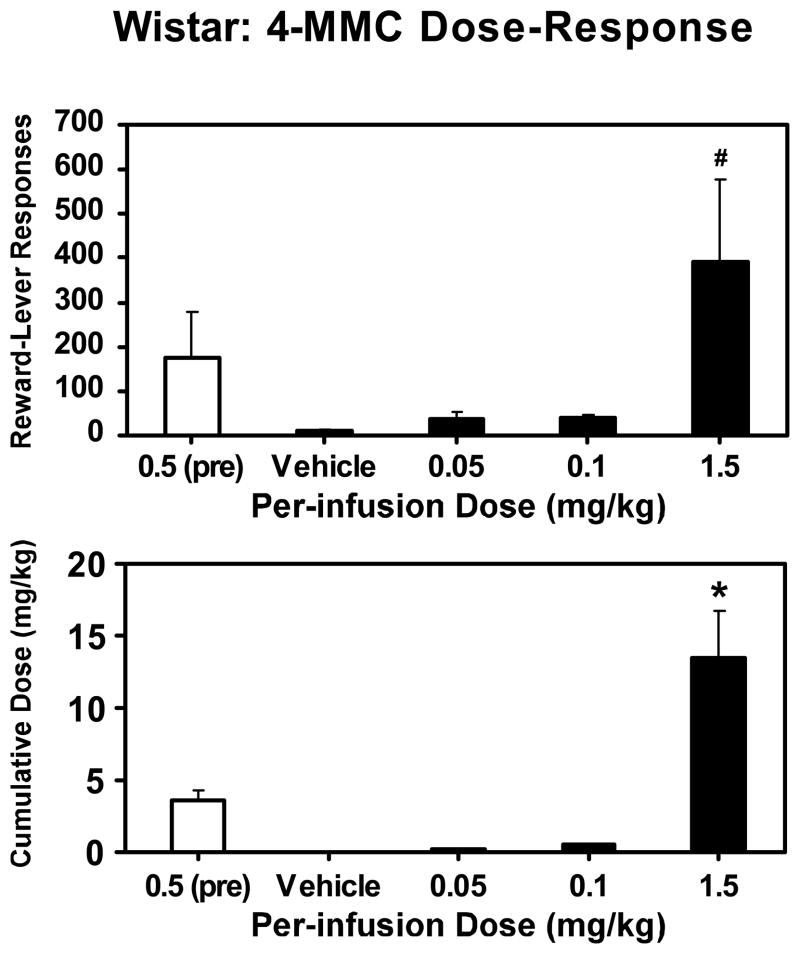
After these initial PR sessions, the rats were entered into a dose-response experiment wherein the per-infusion dose was varied within-subjects to 0.05, 0.1 or 1.5 mg/kg (Figure 5). Upon completing this dose-response series, rats were given vehicle-only PR sessions until stability criteria were reached. Catheter patency was maintained through dose-response and vehicle-only testing in nine rats.
Figure 5. Progressive-ratio dose-response.
Mean number of reward-lever responses and cumulative drug intakes for Wistar rats (N = 9) under a progressive-ratio schedule of reinforcement as a function of available per-infusion dose (mg/kg). Data for sessions at the training dose (0.5 mg/kg/inf) in the three days before dose-substitution sessions are included for comparison. A significant difference from both of the lower two dose conditions and vehicle is indicated by the * symbol. Error bars represent ±SEM.
Analysis included all 5 per-infusion doses; the training dose (average of the last three training-dose sessions; 0.5 mg/kg), the 3 per-infusion doses of the dose-response testing (0.05, 0.1 and 1.5 mg/kg) and the vehicle-only sessions (0 mg/kg). Analysis confirmed a main effect of per-infusion dose on the number of reward-lever presses (F4,32= 2.83; p<0.05) and the cumulative dose of drug per session (F3,24= 13.98; p<0.0001). Post hoc comparisons confirmed that as the per-infusion dose increased, the number of reward-lever presses and the cumulative dose per session increased. Specifically, more lever presses were emitted when the per-infusion dose was 1.5 mg/kg than when it was 0, 0.05 or 0.1 mg/kg. Lastly, a significantly higher cumulative dose was self-administered when 1.5 mg/kg/inf was available than under any other condition.
Substitution of 4-MMC for d-methamphetamine
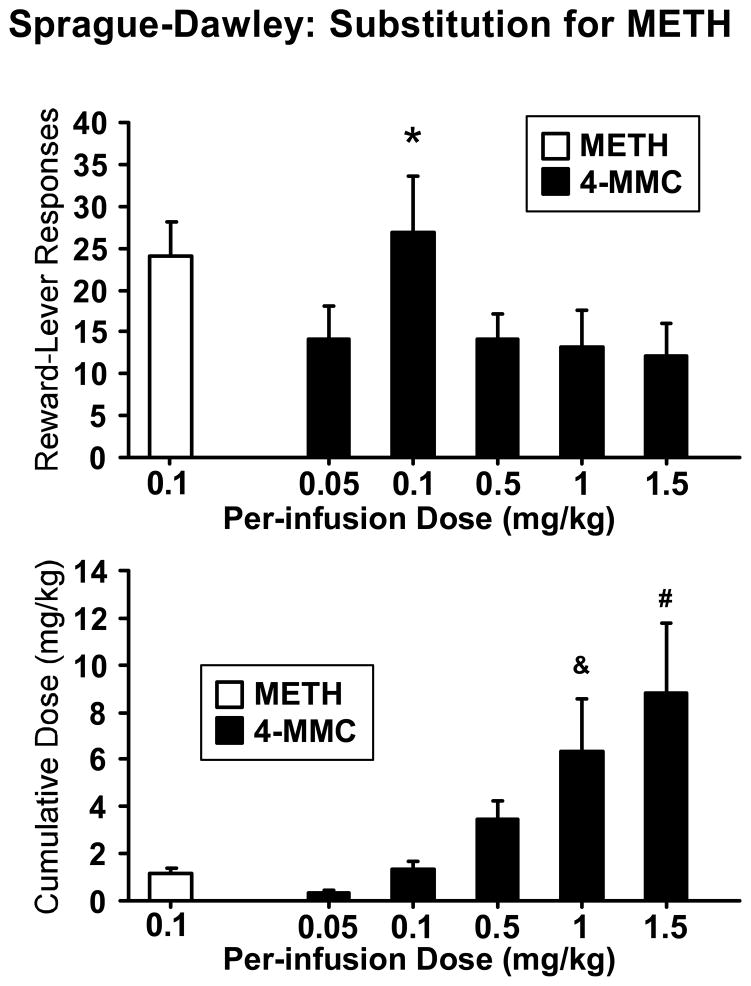
A group of Sprague-Dawley rats (N= 8) had been previously trained to self-administer d-methamphetamine (MA) at a per-infusion dose of 0.1 mg/kg in a different study under a FR2 schedule of reinforcement. These animals were entered into dose-response testing with 4-MMC wherein the per-infusion dose was varied within-subjects to 0.05, 0.1, 0.5, 1.0 or 1.5 mg/kg (Figure 6).
Figure 6. Drug Substitution: 4-MMC in d-methamphetamine (MA)-trained rats.
Mean number of reward-lever responses and cumulative drug intakes (mg/kg) of 4-MMC for a group of Sprague-Dawley rats (N = 8) trained to self-administer MA (0.1 mg/kg/inf; FR2). Data for the MA training dose in the three days before the dose-substitution are included for comparison. Statistically reliable differences from all other 4-MMC conditions are indicated by the * symbol, from 0.05–0.5 by the # symbol and from 0.05–0.1 by the & symbol. Error bars represent ±SEM.
Analysis confirmed a main effect of per-infusion dose on the number of reward-lever responses (F4,28= 5.82; p<0.05). Post hoc comparisons confirmed that more lever presses were emitted when the per-infusion dose was 0.1 mg/kg than when the per-infusion dose was any other value. Analysis also confirmed a main effect of per-infusion dose on the cumulative per-session dose (F4,28= 9.39; p<0.0001). Post hoc comparisons confirmed that more 4-MMC was self-administered when the per-infusion dose was 1.0 or 1.5 mg/kg than when the per-infusion dose was 0.05 or 0.1 mg/kg and more 4-MMC was self-administered when the per-infusion dose was 1.5 mg/kg than when 0.5 mg/kg.
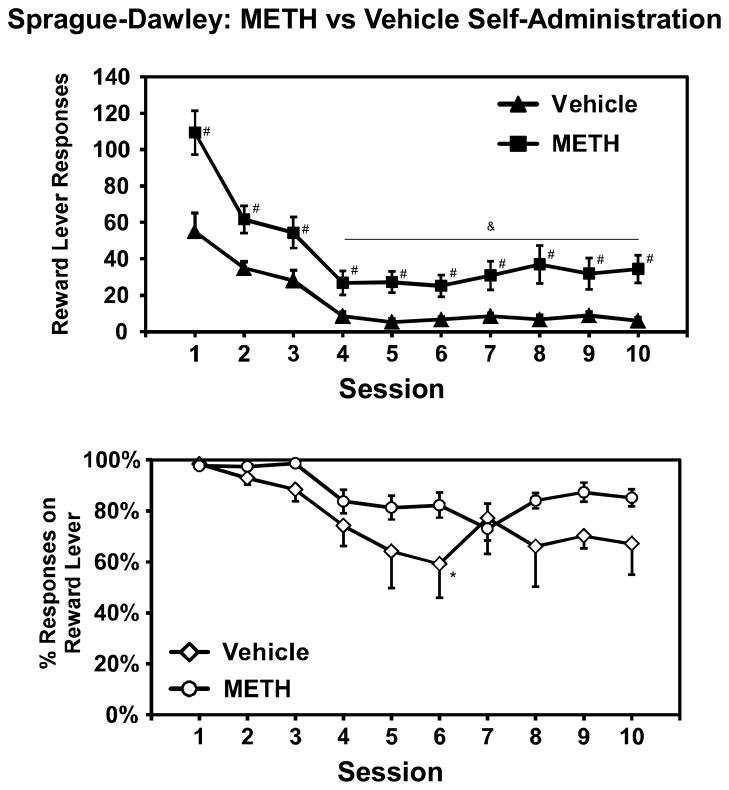
Self-administration of d-methamphetamine and vehicle
For comparison purposes, a group of Sprague-Dawley rats (N= 9) was trained to self-administer d-methamphetamine (MA) at a per-infusion dose of 0.05 mg/kg/inf and a second group of Sprague-Dawley rats (N= 8) was trained to respond for pellets and then permitted to self-administer saline for 10 sessions. Since the objective was to compare to presumed non-acquisition conditions, all patent animals were retained in the analysis regardless of lever discrimination. Two of the nine rats in the MA group failed to maintain over 80% reward lever responding across the entire 10 sessions and three failed to maintain an 80% discrimination ratio across the seven sessions after food restriction. Three of the eight animals in the vehicle group failed to maintain 80% reward lever responding in the entire 10 day interval and six of those eight rats failed in the post-restriction interval of seven sessions. The statistical analysis compared the two groups across the initial 10 sessions of training and confirmed a main effect of session (F9,135= 44.39; p<0.0001), of group (F1,15=13.01; p<0.005), and the interaction of group with session (F9,135= 2.64; p<0.01), on reward-lever presses. The post hoc analysis confirmed group differences for each session. Furthermore, fewer reward lever presses were obtained during sessions 4–10 relative to each of the first three sessions, and also in sessions 2–3 relative to the first session, for both groups. The analysis also confirmed that the percentage of reward lever presses was significantly affected by session (F9,135=4.59; p<0.0001), of group (F1,15=4.55; p<0.05) but there was no interaction. The post hoc test did not confirm differences between groups for any specific session and the only differences within group that were confirmed were between sessions 1 and 6 for the vehicle group.
The vehicle group was continued on vehicle self-administration for a total of 15 sessions. Analysis within group confirmed the main effects of session on reward lever presses (F14,98=17.64; p<0.0001) and percent reward lever presses (F14,98=2.49; p<0.005). The post hoc test confirmed that fewer reward lever presses were emitted in sessions 4–15 compared with any of the first three and in sessions 2–3 compared with session 1. The post hoc analysis also confirmed that percent reward lever presses in session 15 were significantly lower than in sessions 1 or 2.
After this, the group was switched to self-administer 0.5 mg/kg/inf 4-MMC for 18 sessions; one individual died unexpectedly after session 8. Analysis included the final vehicle session and used that as the comparison control in a Dunnett post-hoc analysis. Analysis confirmed main effects of session on reward lever presses (F18,126=1.8; p<0.05) and percent reward lever presses (F18,126=3.31; p<0.0001). The Dunnett procedure for both the reward lever presses and percent reward lever presses confirmed that all subsequent sessions differed significantly from the comparison vehicle session.
Distribution and Metabolism
Microsomal and Plasma Stability
As is reported in Table 1, 4-MMC is subject to hepatic metabolism with a half-life of 93–97 minutes when incubated with liver microsomal homogenates from Sprague-Dawley and Wistar rats, respectively. The 4-MMC was reasonably stable in Sprague-Dawley and Wistar plasma since over 60% of the compound remained after 3 hours of incubation.
Table 1.
Metabolism of 4-MMC in liver homogenates, and stability in plasma, derived from Sprague-Dawley and Wistar rats
| Strain | Metabolic Stability | Plasma Stability | ||
|---|---|---|---|---|
| Metabolic Rate (μM/min/mg) | Half-Life (min) | Half-Life (hr) | % Remaining at 3 hr | |
| Sprague-Dawley | 0.05 | 92.9 | 4.40 | 67.6 |
| Wistar | 0.05 | 97.4 | 3.67 | 62.4 |
Pharmacokinetic studies
The time course of elimination of 4-MMC from the plasma and brain of Sprague-Dawley and Wistar rats was similar, as is illustrated in Figure 7. The peak plasma concentrations observed following a 1 mg/kg intravenous dose were 318–269 ng/mL of plasma and the half-life was 0.8 h (Wistar) or 1.0 h (Sprague-Dawley) (Table 2). The ratios of AUC for plasma (expressed as ng*hr/ml) and the AUC for brain (expressed as ng*hr/g) were 6.81 (Wistar) and 8.20 (Sprague-Dawley).
Figure 7. Acquisition of METH and Vehicle self-administration: Reward-lever responses and reward-lever discrimination.
Sprague-Dawley rats were trained to self-administer d-methamphetamine (METH) at 0.05 mg/kg/inf 4 (N =9) or vehicle only (N=8). Statistically reliable differences from the first three sessions are indicated by &, from the first session by * and differences between groups are represented by #. Error bars represent ±SEM.
Table 2.
Pharmacokinetic parameters for male Sprague-Dawley (N=3) and Wistar (N=3) rats following intravenous administration of 1.0 mg/kg 4-MMC
| Tmax (hr) | Cmax (ng/mL) | AUC∞ (area) ng-hr/mL | T1/2 (hr) | Cl (mL/hr) | Vd (mL) | |
|---|---|---|---|---|---|---|
| Sprague-Dawley | 0.08 | 318 | 171 | 1.20 | 5863 | 9752 |
| Wistar | 0.08 | 269 | 173 | 0.80 | 5775 | 7729 |
Discussion
The results show that 4-MMC supports intravenous self-administration (IVSA) in both Wistar and Sprague-Dawley rats, resulting in consistent levels of drug intake from session to session and reward-lever selectivity well in excess of 80% for most rats. In this, 4-MMC differs from 3,4-methylenedioxymethamphetamine (MDMA) which is not readily self-administered by rats (De La Garza et al., 2007) and produces considerable inter-individual heterogeneity in acquisition (Bird and Schenk, 2012; Colussi-Mas et al., 2010; Schenk et al., 2007). In addition, these data show that self-administration of 4-MMC is sensitive to changes in the dose available for each infusion. This was a consistent feature across strain, original training dose and schedule of reinforcement. The apparent threshold of about 0.5 mg/kg/infusion contrasts with d-methamphetamine (MA), for which per-infusion doses of 0.05–0.1 mg/kg support consistent self-administration (Kitamura et al., 2006), suggesting that 4-MMC is less potent. These data are highly consistent with the neurochemical potency differences which have been reported (Baumann et al., 2012). A prior report (Hadlock et al., 2011) showed higher numbers of reinforcers obtained in 4-MMC IVSAversus MA during acquisition when using equal per-infusion doses but that study did not substitute the available dose; therefore, observed differences might have been due to the lower potency of 4-MMC. Furthermore, that prior study used 4 hour self-administration sessions and 29°C ambient temperature conditions, which might produce effects which interact with per-infusion dose or drug identity (Cornish et al., 2008; Kitamura et al., 2006). Here we show (Fig. 7) that under more traditional self-administration conditions, 0.05 mg/kg/inf MA produces an approximately similar amount of drug responding as did the 0.5 mg/kg/inf 4-MMC (Fig 1), whereas responding for intravenous vehicle infusions is considerably lower. It was also shown in Figure 8 that 4-MMC (0.5 mg/kg/inf) immediately increased reward lever responding and restored high lever discrimination ratios in animals trained on vehicle for 15 sessions.
Figure 8. Acquisition of 4-MMC following Vehicle self-administration: Reward-lever responses and reward-lever discrimination.
The group of Sprague-Dawley rats trained to self-administer vehicle (N=8) for 15 sessions (upper panel) were thereafter permitted access to 4-MMC (0.5 mg/kg/inf) for 18 sessions (lower panel). In the upper panel, statistically reliable differences from the first three sessions are indicated by &, from the first session by * and differences between groups are represented by #. Reward lever responses and discrimination ratios in all 4-MMC sessions differed significantly from the V15 session. Error bars represent ±SEM.
Locomotor stimulant effects were produced by self-administration of 4-MMC in Wistar rats. This extends prior reports on the effects of experimenter-administered 4-MMC on experimental-chamber activity and home-cage ambulation (Baumann et al., 2012; Kehr et al., 2011; Miller et al., 2012; Motbey et al., 2012; Wright et al., 2012) to the self-administration model. Interestingly wheel activity is monotonically suppressed by 4-MMC and MDMA, but increased biphasically by MA and 3,4-methylenedioxypyrovalerone, (Huang et al., 2012). The effect of 4-MMC self-administration on activity was not observed in Sprague-Dawley rats. However, in a prior report we showed that experimenter-administered 4-MMC increases activity in Sprague-Dawley rats more than in Wistar rats (Wright et al., 2012). Given the strain differences observed in the patterns of self-administration during acquisition (lower intake in Sprague-Dawley rats than Wistar rats for the 1st 5–7 sessions) but the lack of substantial strain differences in the pharmacokinetic data on experimenter-administered 4-MMC reported here (see below), it seems likely that this observed reversal of the strain difference in 4-MMC-induced activity is due to the kinetics of self-administered versus bolus dosing.
The thermoregulatory effect also differed between rat strains; in this case the Sprague-Dawley rats were most sensitive. Self-administration of 4-MMC reduced the body temperature of Sprague-Dawley rats when a cumulative dose of 3–4 mg/kg was self-administered, and to a much lesser extent when a cumulative dose of 1 mg/kg was self-administered, as compared to when only saline was available. This dose threshold for substantial thermoregulatory effect is consistent with evidence that 4-MMC reduces the body temperature of Wistar and Sprague-Dawley rats at a threshold of about 3.2 mg/kg by subcutaneous injection (Miller et al., 2012; Wright et al., 2012). As with the effect of 4-MMC on activity, these differences are likely attributable to the kinetics of self-administered versus bolus dosing. These data are the first to determine thermoregulatory effects in the self-administration setting and differ from our prior reports in route of administration, the fact that drug was self-administered and the dosing spaced at a self-selected rate. These features did not, however, produce a large qualitative difference which permits enhanced comparison across such models. In addition, this supports the conclusion that the subjective reports of cold feeling and/or tingling in users (Psychonaut_Webmapping_Research_Group, 2010; Winstock et al., 2011) may be related to body cooling in the rat model.
The pharmacokinetic study showed that 4-MMCiscleared rapidly from both Sprague-Dawley and Wistar rats and the in vitro metabolic assay confirmed that 4-MMC is subject to hepatic metabolism in rats, similar to the amphetamines (Fonsart et al., 2008; Zeng et al., 1999). Recent work confirms that cytochrome P450 2D6 is the main enzymatic contributor in humans (Pedersen et al., 2012). The drug also is rapidly taken up into the brain (peak levels were observed 2 min after drug injection) and is mostly cleared within an hour of injection. By way of comparison, Hadlock and colleagues (2011) reported plasma levels of 384 ng/ml when assessed one hour after the last of four sequential 10 mg/kg, s.c., 4-MMC doses administered at 2 hr intervals. Whole brain levels were 2.1 ng/mg when assessed at the same timepoint in that study. In the present work, peak brain concentrations were 4 ng/mg of tissue when assessed 2 min after a 1 mg/kg, i.v., dose of 4-MMC; this had fallen to under 1 ng/mg within 30 minutes of injection and under 0.4 ng/mg by 60 minutes after injection. Although the 4-MMC administration differed in dose, chronicity and route of administration, these findings are broadly consistent.
In total, the observed results are consistent with reinforcing properties much as have been produced by intravenous delivery of psychomotor stimulants such as methamphetamine, amphetamine, cathinone and methcathinone (Dalley et al., 2006; Gosnell et al., 1996; Kaminski and Griffiths, 1994; Kitamura et al., 2006; Woolverton and Johanson, 1984). Although the potency of 4-MMC as a reinforcer appeared low compared with methamphetamine, there was little evidence of the inconsistent self-administration that has been associated with 3,4-methylenedioxymethamphetamine (MDMA). MDMA IVSA results in low proportions of the subject pool meeting acquisition criteria, high session-to-session variability and difficulties in distinguishing low rates of responding for MDMA from vehicle (Dalley et al., 2006; De La Garza et al., 2007; Schenk et al., 2003; Schenk et al., 2007). This was a little unexpected since some subpopulations of human recreational users report 4-MMC to be subjectively similar to MDMA (Geezaman, 2009; MephTest, 2009). It is also the case that although 4-MMC inhibited 5-HT and DA uptake into rat synaptosomes, demonstrated micromolar affinity for serotonin and dopamine transporters and interacted with D2 dopamine and 5HT2A serotonin receptors with micromolar affinity, overall it demonstrated greater affinity for serotonin targets than for dopamine targets (Martínez-Clemente et al., 2012). Furthermore, neurochemical data suggest MDMA-like patterns of relatively greater serotonin versus dopamine accumulation in nucleus accumbens (Baumann et al., 2012; Kehr et al., 2011; Wright et al., 2012), Nevertheless, the present data are not consistent with the (modest) reinforcing effects of MDMA as reported in prior rat IVSAmodels. Indeed, the data are more similar to the stable self-administration of methamphetamine that is reported for 1–2 h access paradigms (Anker et al., 2012; Hadlock et al., 2011; Kitamura et al., 2006) and confirmed here using identical procedures. One possible reason for this is a report of a 17.5-fold higher DAT/SERT transport inhibition ratio, and 6 fold higher dopamine release advantage for mephedrone over MDMA that has been recently reported (Simmler et al., 2012). Mephedrone was also reported in that paper to have a 2-fold blood-brain barrier permeability advantage over both methamphetamine and MDMA. Such properties may produce relatively greater reinforcing effects.
One potential similarity with MDMA was the pronounced reduction in body temperature that occurred in the Sprague-Dawley group; this is an effect that occurs at normal laboratory temperature (~22–24 °C) following acute bolus challenge with MDMA in rats (Malberg and Seiden, 1998). One study did not observe a thermoregulatory effect of self-administered MDMA, however that study only measured rectal temperature at the conclusion of the session (Feduccia et al., 2010). As temperature effects in the present study were greatest about 20 minutes into the session and resolved by the end of the 1-hr session at low cumulative doses, and as removing animals from an experimental chamber and inserting a rectal thermometer can be activating, that prior report may have lacked the resolution to observe such thermic effects. In contrast to the hypothermic effect of 4-MMC IVSAin Sprague-Dawley rats, in Wistar rats 4-MMC IVSA was associated with a small attenuation of the body temperature decline observed when saline was substituted for the active drug. This strain difference may be due to the strain difference in locomotor activity (see above).
In summary, these data confirm a significant abuse liability of 4-MMC. This compound supported intravenous self-administration in two strains of rats, locomotor stimulation in Wistar rats and body temperature disruption in Sprague-Dawley rats. This extends and generalizes an initial report on 4-MMC self-administration in high ambient temperature conditions (Hadlock et al., 2011), thereby demonstrating that self-administration of 4-MMC can indeed be produced in a preparation more typical of prior stimulant self-administration studies (i.e., in short, 1-hr access sessions and under normal laboratory ambient temperature conditions). Thus, the potential for compulsive use of 4-MMC in humans is likely quite high, particularly in comparison with MDMA.
Figure 9. Pharmacokinetics.
Mean concentration of4-MMC in plasma following a bolus IV injection (1.0 mg/kg) in male Wistar and Sprague-Dawley rats (N =3 per strain). Error bars represent ±SEM.
Acknowledgments
This work was supported by USPHS grants DA018418, DA024105 and DA024705; the NIH/NIDA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. This is manuscript #21069 from The Scripps Research Institute.
Footnotes
Authors Contribution
S.M.A. and M.A.T. designed the behavioral studies which were implemented and refined by S.M.A., M.J.W., S.A.V. and K.M.C. under the direction of M.A.T. D.J.B. and K.L.H conducted the pharmacokinetic and metabolic stability studies. D.A. and T.J.D. synthesized and validated 4-methylmethcathinone.Analysis of the data and creation of figures was conducted by M.A.T., S.M.A., and K.L.H. Drafting of the manuscript was by S.M.A. and M.A.T. All authors have approved the manuscript.
References Cited
- Anker JJ, Baron TR, Zlebnik NE, Carroll ME. Escalation of methamphetamine self-administration in adolescent and adult rats. Drug Alcohol Depend. 2012;124:149–153. doi: 10.1016/j.drugalcdep.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird J, Schenk S. Contribution of impulsivity and novelty-seeking to the acquisition and maintenance of MDMA self-administration. Addict Biol. 2012 doi: 10.1111/j.1369-1600.2012.00477.x. [DOI] [PubMed] [Google Scholar]
- Bluelight 2011. (RC’s) Big mephedrone thread. 2008 [cited 1/11] Available from http://www.bluelight.ru/vb/showthread.php?t=400517.
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience: A Practical Approach. Oxford University Press; New York, NY: 1993. pp. 117–143. [Google Scholar]
- Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggarda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources, National Research Council; Washington D.C: 1996. p. 125. [Google Scholar]
- Colussi-Mas J, Wise RJ, Howard A, Schenk S. Drug seeking in response to a priming injection of MDMA in rats: relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. Int J Neuropsychopharmacol. 2010:1–13. doi: 10.1017/S1461145710000283. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Clemens KJ, Thompson MR, Callaghan PD, Dawson B, McGregor IS. High ambient temperature increases intravenous methamphetamine self-administration on fixed and progressive ratio schedules in rats. J Psychopharmacol. 2008;22:100–110. doi: 10.1177/0269881107082286. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Shahnawaz Z, Thompson MR, Wong S, Morley KC, Hunt GE, McGregor IS. Heat increases 3,4-methylenedioxymethamphetamine self-administration and social effects in rats. Eur J Pharmacol. 2003;482:339–341. doi: 10.1016/j.ejphar.2003.09.060. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW. Enduring Deficits in Sustained Visual Attention during Withdrawal of Intravenous Methylenedioxymethamphetamine Self-Administration in Rats: Results from a Comparative Study with d-Amphetamine and Methamphetamine. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301220. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Fabrizio KR, Gupta A. Relevance of rodent models of intravenous MDMA self-administration to human MDMA consumption patterns. Psychopharmacology. 2007;189:425–434. doi: 10.1007/s00213-005-0255-5. [DOI] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Lane JD. Tolerance to the reinforcing effects of cocaine. Behav Pharmacol. 1992;3:193–200. [PubMed] [Google Scholar]
- Feduccia AA, Kongovi N, Duvauchelle CL. Heat increases MDMA-enhanced NAcc 5-HT and body temperature, but not MDMA self-administration. Eur Neuropsychopharmacol. 2010;20:884–894. doi: 10.1016/j.euroneuro.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonsart J, Menet MC, Decleves X, Galons H, Crete D, Debray M, Scherrmann JM, Noble F. Sprague-Dawley rats display metabolism-mediated sex differences in the acute toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) Toxicol Appl Pharmacol. 2008;230:117–125. doi: 10.1016/j.taap.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Geezaman DF. Surprisingly like E. 2010;2009 [cited 9/29/2010] Available from http://www.erowid.org/experiences/exp.php?ID=77952. [Google Scholar]
- Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav Pharmacol. 1996;7:526–531. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science (New York, NY) 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Huang PK, Aarde SM, Angrish D, Houseknecht KL, Dickerson TJ, Taffe MA. Contrasting effects of d-methamphetamine, 3,4-methylenedioxymethamphetamine, 3,4-methylenedioxypyrovalerone, and 4-methylmethcathinone on wheel activity in rats. Drug Alcohol Depend. 2012;126:168–175. doi: 10.1016/j.drugalcdep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav. 1994;47:981–983. doi: 10.1016/0091-3057(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. British Journal of Pharmacology. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci. 1998;18:5086–5094. doi: 10.1523/JNEUROSCI.18-13-05086.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasac J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharm. 2012;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- MephTest. Good Alternative to MDMA. 2009 [cited 9/28/2010] Available from http://www.erowid.org/experiences/exp.php?ID=82321.
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, Taffe MA. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, ‘meow’): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2012 doi: 10.1002/dta.1369. [DOI] [PubMed] [Google Scholar]
- Psychonaut Webmapping Research Group. Mephedrone Report. Institute of Psychiatry, King’s College London; London, UK: 2010. [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Schenk S. MDMA self-administration in laboratory animals: a summary of the literature and proposal for future research. Neuropsychobiology. 2009;60:130–136. doi: 10.1159/000253549. [DOI] [PubMed] [Google Scholar]
- Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology (Berl) 2003;169:21–27. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC. MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci. 2007;26:3229–3236. doi: 10.1111/j.1460-9568.2007.05932.x. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction (Abingdon, England) 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-cathinone. Journal of the experimental analysis of behavior. 1984;41:35–43. doi: 10.1901/jeab.1984.41-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Zhang L, Chen YZ. Chiral gas chromatographic assay with flame ionization detection for amphetamine enantiomers in microsomal incubates. Biomed Chromatogr. 1999;13:33–36. doi: 10.1002/(SICI)1099-0801(199902)13:1<33::AID-BMC809>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]