Abstract
Objective
To compare growth and body composition of uninfected children exposed to HIV with a contemporary HIV-unexposed group and to US references.
Study design
Uninfected children exposed to HIV under 2 years were enrolled into a longitudinal observational study and unexposed children under 2 years in a cross-sectional evaluation. Weights, lengths, head circumferences, skinfold thicknesses, and arm and thigh circumferences were measured and adjusted for age using CDC and NHANES standards. Uninfected children exposed to HIV were compared with an unexposed nearest-neighbor matched comparison group. Uninfected children exposed to HIV were compared by age to CDC standards for growth measures and NHANES standards for body composition.
Results
One hundred eleven uninfected children exposed to HIV and 82 children not exposed to HIV were evaluated. For the matched comparison for both groups, the mean age was 10 months, 59% were male, and 73% were Black. No statistical differences were found in anthropometric measurements between uninfected children who were or were not exposed to HIV. Uninfected children exposed to HIV were smaller than US standards at birth with mean (SD) weight-for-age (WAZ) and weight-for-length (WLZ) of −0.39(1.06); P=0.002 and −0.35(1.04); P=0.005, respectively. Over the first 2-years of life, there was a trend toward increasing WAZ, length-forage (LAZ), and WLZ in uninfected children exposed to HIV. Subscapular and triceps skinfolds among uninfected children exposed to HIV were lower than national standards and there was a trend that mid-upper arm circumference (MAC) decreased over time.
Conclusions
Growth and body composition of uninfected children who were or were not exposed to HIV were similar. Uninfected children exposed to HIV are lighter at birth and show a pattern of slightly accelerated growth in the first 2 years. Uninfected children exposed to HIV had less subcutaneous fat and decreasing MAC over time when compared with US standards.
Keywords: HIV exposure, antiretroviral, anthropometrics
Effective preventive strategies during pregnancy have reduced the risk of mother-to-child transmission (MTCT) of the human immunodeficiency virus (HIV) in the United States to approximately 1 to 2% [1]. Preventive MTCT recommendations [2] include antiretroviral (ARV) therapy for all pregnant women with HIV regardless of CD4 lymphocyte count or viral load. Recommended ARV regimens include the use of two nucleoside reverse transcriptase inhibitors (NRTI), zidovudine (ZDV) and lamivudine being the preferred agents, in combination with a non-nucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI) [2].
Exposures to HIV and ARVs in utero are postulated to have adverse associations on infant development [3]. NRTIs cross the placenta and inhibit DNA polymerase, potentially interfering with fetal mitochondrial DNA (mtDNA) synthesis, resulting in short- and long-term mitochondrial depletion and/or dysfunction in some studies [3], although other studies have not demonstrated these effects [4–6]. Mitochondrial dysfunction is linked to growth abnormalities in other childhood disorders. The extent to which this is true in uninfected children exposed to HIV is not well-defined.
Pre- and postnatal growth can also be influenced by socioeconomic (SES) and lifestyle factors. Uninfected children exposed to HIV are often born to women of lower SES [7] with a greater prevalence of smoking and illicit drug use [7]. Few studies have compared uninfected children exposed to HIV with a socioeconomically similar group of infants. Although studies have shown little to no differences in growth among uninfected children exposed to HIV and the general population [4, 8, 9], these studies have not evaluated body composition, nor compared growth parameters to a socio-demographically similar group of children.
The objective of this study was to compare anthropometric outcomes of uninfected children exposed to HIV to an age, race, and sex-matched group of non-exposed infants from a similar geographical region. We compared serial growth and anthropometric trajectories of uninfected children exposed to HIV with US standards.
METHODS
Uninfected children exposed to HIV under 2 years of age were sequentially enrolled into a single-site and observational study on growth and body composition at the University of Miami HIV Screening Program between June 2006 and December 2009. Study visits followed the HIV screening protocol [10] where uninfected children exposed to HIV were evaluated at approximately 2 and 6 weeks and 4, 12, 18 and 24 months of age. However, there was some deviation in this proposed schedule due to individual variation in adhering to recommended timing of clinical visits. Laboratory protocol included serial virological testing with HIV-1 DNA polymerase chain reaction (PCR) assays during the first six months of life, and immunologic assays using enzyme-linked immunosorbent assays (ELISAs) and Western blot assay to confirm reactive screening test results after one year of age. Children were determined to be uninfected if they were HIV antibody-negative on two separate determinations after twelve months of age and HIV virus or antigen was never detected. Children with HIV were excluded from the analysis.
The HIV-unexposed group was a convenience sample of children, age birth to 2 years with similar demographic and SES characteristics. They were recruited from either an urban general pediatric outpatient practice at the University of Miami or from 5 local urban day-care centers in Miami-Dade County. Recruitment letters were sent to the parents explaining the study procedures and requesting their attendance for consenting process. This group was not known to be infected or exposed to HIV because there are well established and mandated screening programs in Miami-Dade County.
Children with known congenital, chromosomal, or metabolic anomalies were excluded from the study. The Human Subjects Research Office at the University of Miami approved the research protocol, and informed consent from the parent or legal guardian was obtained. For the uninfected children exposed to HIV, all data were collected during nutrition assessments as part of routine clinical care during the previously defined screening time points. Clinical data included birth weight, length, and gestational age. HIV-1 DNA PCR and ELISA results were collected to identify HIV infection status. Maternal information collected at enrollment included sociodemographic information, pre-pregnancy weight and height, and ARV prophylaxis during pregnancy. Maternal CD4 lymphocyte count nearest to delivery was abstracted from the newborn hospital discharge note. For the HIV-unexposed group, demographic data, including date of birth, race and ethnicity, was collected through parent or guardian interview. No clinical data or neonatal characteristics were available for this group.
Weight, recumbent length and head circumference were measured by standardized procedures [11]. Weight-for-age (WAZ), length-for-age (LAZ), weight-for-length (WLZ), and head circumference-for-age (HCZ) z-scores were generated according to Centers for Disease Control (CDC) standards [12]. Regional body measurements including mid-upper arm circumference (MAC), mid-thigh circumference (MTC), and skinfold thickness (SFT) were measured according to standardized procedures [11]. Triceps, biceps, subscapular and mid-thigh SFT were measured using Lange caliper (Cambridge Scientific, Cambridge, MD). All measurements, except SFT, were taken to the nearest 0.1 centimeter. SFT were taken to the nearest 0.1 millimeter. Body measurements were taken on the right side of the body. Triplicate measurements for all body measurements were taken and the mean was recorded. Triceps and MAC measurements were used to derive arm muscle circumference (AMC) [13]. Similarly, mid-thigh SFT and MTC measurements were used to derive thigh muscle circumference [14]. Z-scores for MAC, and triceps and subscapular SFT were generated [11, 15]. Weight, length, and head circumference z-scores for only uninfected premature infants exposed to HIV (less than 37 weeks gestation) were adjusted for prematurity and calculated using population-appropriate updated growth chart for prematurity [16]. Information regarding prematurity was not available for the infants not exposed to HIV. All regional body measurements (circumferences and skinfolds) were performed by one trained dietician (DN).
Statistical Analyses
The primary statistical methodology consisted of a comparison between the uninfected children exposed to HIV to a within study HIV-unexposed matched comparison group. Matches were determined individually for each unexposed case by choosing an uninfected children exposed to HIV that was closest to an unexposed individual (i.e., nearest-neighbor) over the multivariate space as defined by the matching variables. Euclidean distance was the metric chosen to quantify similarity. The HIV-unexposed comparison group, who were initially selected from the same socioeconomic demographic, were chosen so that the two groups had the same race/sex proportions with near identical age distributions. Of the 111 original uninfected children exposed to HIV, 82 matches were possible. When the dependent measure of interest was continuous, the two groups were compared using Student t-test. Fisher exact test was used when the dependent variable was dichotomous.
Uninfected children exposed to HIV were also compared with CDC growth charts for classical growth measures and to NHANES for body composition. Data were transformed to normative z-scores and plotted against age. Individual differences were described using spaghetti plots and the overall trend was modeled using a smoothed cubic spline. Statistical tests were generated to compare the difference between the subject’s observed z-score and the expected z-score of zero. This was accomplished using an unconditional mixed model as generally described by Singer and Willet [17]. This methodology accounted for subject-to-subject variation in both frequency and spacing of observations. Prior to analysis, the data were centered around the overall age mean and a single sample random intercept model was constructed in which the intercept and slope terms were used to test for changes in displacement around the zero reference line and trend, respectively. The parameters and variances were estimated using reduced estimate maximum likelihood (REML) assuming an unstructured variance-covariance structure. Although second order (i.e., quadratic) models were attempted, in most cases, the data were to sparse and ill conditioned and the solutions failed to converge. Given the observational nature of the study, results of statistical testing are given as exact P values and reflect the probability of the observed effect or one greater given the observed error structure of the data. Statistical calculations and graphics were generated with SAS (9.2) and SAS/JMP (9.0) statistical software (Cary, NC).
RESULTS
One hundred eleven uninfected children exposed to HIV and 82 children not exposed to HIV were enrolled in this study. A total of 264 anthropometric measurements were recorded between 2 weeks and 29.3 months of age. The number of visits ranged from 1 to 5 with a median of 3 visits. The earliest visits occurred at approximately 2 weeks of age with a high density of visits at approximately 12.5 to 15 months (Table I). The unexposed children had one evaluation each.
Table 1.
Median Age of uninfected children who were exposed to HIV by Study Visit Number (n=111)
| Visit Number | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of Subjects (%) | 1 | 2 | 3 | 4 | 5 | |
| Median Age (months) (Min, Max) at Visit | ||||||
|
| ||||||
| 25 (23) | 4.2 (0.5, 15.3) | |||||
| 29 (26) | 2.4 (0.4, 7.2) | 12.9 (4.1, 22.9) | ||||
| 45 (40) | 1.5 (0.4, 6.1) | 4.7 (1.7, 17.9) | 12.7 (5.2, 29.3) | |||
| 11 (10) | 1.5 (0.6, 1.6) | 4.4 (4.1, 13.9) | 12.4 (5.0, 14.9) | 15.1 (12.2, 20.9) | ||
| 1 (1) | 0.6 | 4.1 | 6.0 | 9.2 | 13.1 | |
Maternal and neonatal characteristics of the uninfected children exposed to HIV are presented in Table II. The average age of the mothers at the time of delivery was 28.6 years and 99% of the women were of a minority race or ethnicity. Fifty-six percent of women were overweight (BMI between 25.0 and 29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2) before pregnancy. Maternal CD4 cell counts close to delivery were available for 75 women. Of these, 8 (11%) had CD4 cell count below 200. Ninety-six percent of women received ART during pregnancy. Of these, ART was initiated before pregnancy in 25% of pregnant women, during the first trimester in 6%, during the second trimester in 50%, and during the third trimester in 8%. Time of initiation of treatment was not available for 7% of the sample. All infants received ZDV for 6 weeks after birth. Fourteen percent of the uninfected children exposed to HIV were premature. After correcting for prematurity, uninfected children exposed to HIV tended to be smaller than US standards at birth with a mean (SD) WAZ and WLZ of −0.39 (1.06); P=0.002 and −0.35 (1.04); P=0.005, respectively. Maternal age at delivery, maternal pre-pregnancy BMI and maternal CD4 cell count were not correlated with birth WAZ, LAZ and WLZ (data not shown).
Table 2.
Maternal and Neonatal Characteristics of the cohort exposed to HIV (n=111)
| Maternal Characteristics | |
| Age at delivery, years (SD) | 28.6 ± 6.04 |
| Race, n (%) | |
| Non-Hispanic, black | 90 (81%) |
| Hispanic | 19 (17%) |
| Non-Hispanic, white | 2 (2%) |
| Pre-pregnancy BMI, kg/m2 (n=94), n (%) | |
| < 18.5 | 1 (1%) |
| 18.5 – 24.9 | 37 (39%) |
| 25 – 29.9 | 30 (32%) |
| 30 – 44.9 | 23 (25%) |
| ≥45 | 3 (3%) |
| CD4 cell count nearest to delivery, cells/mm3 (n=75), n (%) | |
| > 350 | 44 (59%) |
| 200–349 | 23 (30%) |
| <200 | 8 (11%) |
| Neonatal Characteristics | |
| Preterm birth (<37 weeks gestation), n (%) | 15 (14%) |
| Gestational age (n=108), n (%) | |
| < 34 weeks | 4 (4%) |
| 34 – 37 weeks | 11 (10%) |
| ≥ 37 | 93 (86%) |
| Birth Anthropometrics, mean (SD) | |
| Birth weight kg (n=110)a | 3.09 (0.64) |
| Birth weight z-score* | −0.39 (1.06) |
| Birth length cm (n=89)b | 49.13 (2.93) |
| Birth length z-score | −0.028 (1.07) |
| Weight/length z-score**† (n=74)c | −0.35 (1.04) |
Significant different from zero P=0.0002.
Significant different from zero P=0.005.
Does not include premature infants.
Note:
Birth weight information was missing for 1 infant.
Birth length was missing for 22 infants.
Birth weight/length z-scores could not be generated for 36 infants due to the following: missing length (22), prematurity (11), and lack of reference data to generate weight/length for full term infants with length < 44 cm at birth (4).
Table III shows a comparison of 82 uninfected children exposed to HIV with 82 children not exposed to HIV, matched by sex, race and age. The average age was 10.4 months for uninfected children exposed to HIV and 10.13 months for children not exposed to HIV; 59% were males and 73% were African American (for both groups). There were no statistically significant differences in any of the anthropometric measures. Weight, length and head circumference z-scores of premature uninfected children exposed to HIV were adjusted for age. When prematurity was not adjusted for in the uninfected children exposed to HIV (data not shown), we still did not find significant statistical differences in growth between the groups.
Table 3.
Comparison of Anthropometry by HIV Exposure
| Variable, mean (SD) | Uninfected HIV exposed n=82 | HIV Unexposed n=82 | P-value |
|---|---|---|---|
| Age, months | 10.04 (6.8) | 10.13 (6.9) | 0.94 |
| Male, n (%) | 48 (59%) | 48 (59%) | 0.99 |
| Black, n (%) | 60 (73%) | 60 (73%) | 0.99 |
| Weight, kg | 8.71 (3.04) | 8.61 (2.98) | 0.83 |
| Weight z-score | 0.08 (1.12) | −0.14 (1.06) | 0.22 |
| Length, cm | 70.47 (11.54) | 70.55 (11.53) | 0.97 |
| Length z-score | 0.11 (0.93) | −0.01 (1.23) | 0.50 |
| Weight/Length z-score | 0.34 (0.94) | 0.11 (1.52) | 0.23 |
| Head circumference, cm | 43.88 (4.69) | 44.11 (4.69) | 0.75 |
| Head circumference z-score | 0.18 (0.97) | 0.23 (1.03) | 0.75 |
| Mid-upper arm circumference, cm | 14.92 (2.32) | 14.57 (2.07) | 0.31 |
| Mid-upper arm circumference z-score | 0.02 (0.80) | −0.09 (0.48) | 0.34 |
| Mid-upper arm muscle circumference, cm | 12.07 (2.08) | 11.87 (1.74) | 0.52 |
| Biceps skinfold, mm | 5.96 (1.99) | 5.70 (2.12) | 0.42 |
| Triceps skinfold, mm | 9.18 (2.81) | 8.57 (2.69) | 0.17 |
| Triceps skinfold z-score | −0.07 (0.70) | −0.15 (0.63) | 0.47 |
| Subscapular skinfold, mm | 7.10 (3.23) | 6.48 (2.01) | 0.17 |
| Subscapular skinfold z-score | 0.01 (1.15) | −0.21 (0.51) | 0.17 |
| Mid-thigh circumference, cm | 24.54 (4.92) | 23.91 (4.97) | 0.42 |
| Mid-thigh skinfold, mm | 16.63 (5.66) | 16.64 (6.11) | 0.99 |
| Mid-thigh muscle circumference, cm | 19.38 (4.06) | 18.75 (4.10) | 0.33 |
Note: When the non-z score measures were adjusted by age, sex, and race there was no difference in the means and P-values.
T-tests for independent samples were conducted to compare uninfected children exposed to HIV and controls.
Weight, length, and head circumference z-scores were adjusted for prematurity for only uninfected children exposed to HIV. Unadjusted comparisons produced similar results.
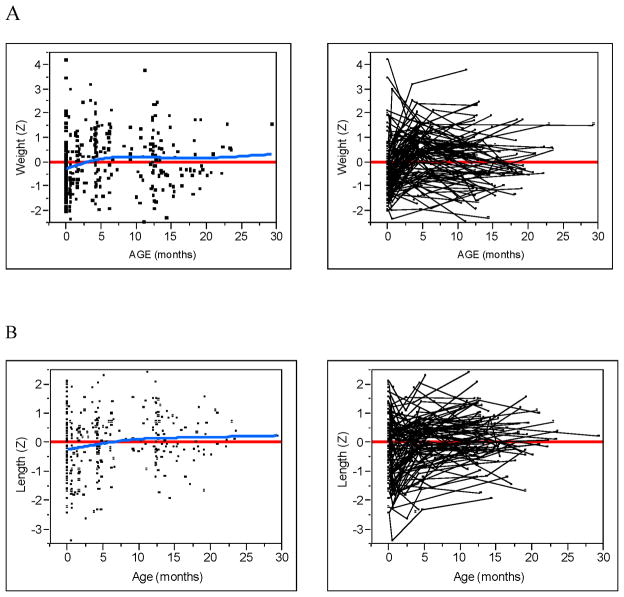
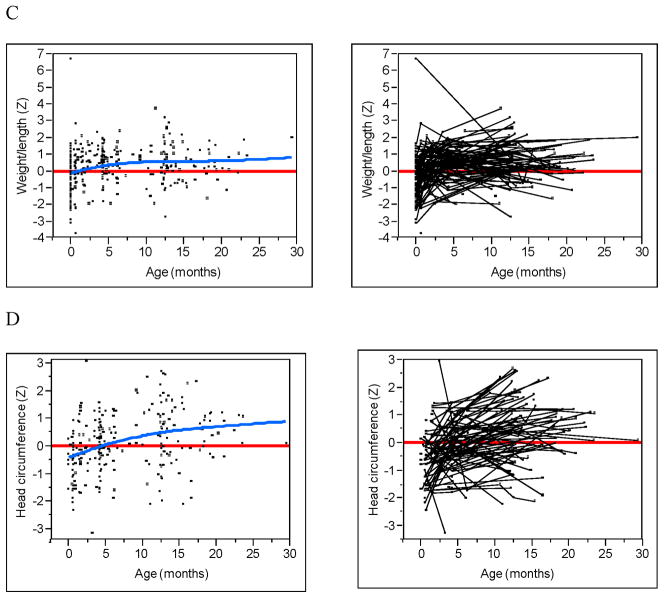
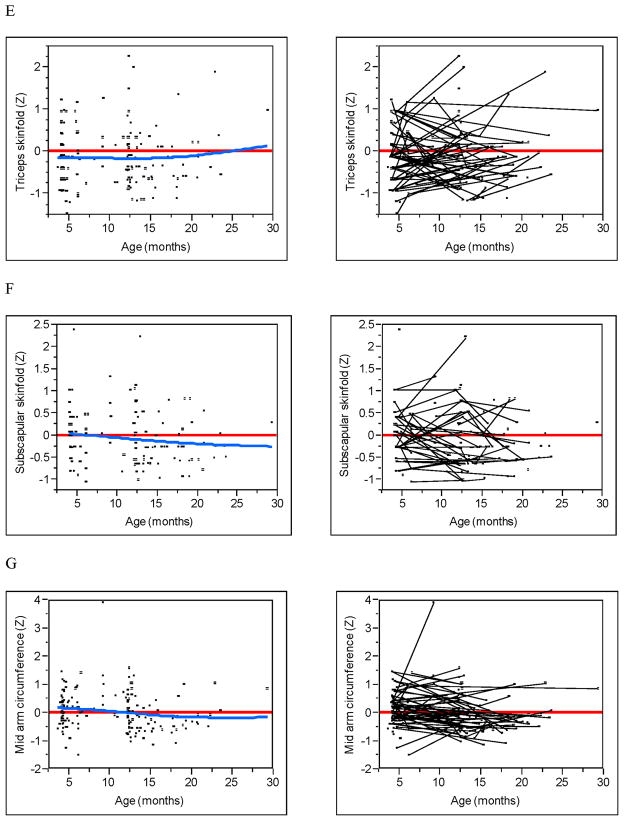
The Figure (available at www.jepds.com) shows WAZ, LAZ, WLZ, HCZ, triceps, subscapular SFT, and MACby age in the uninfected children exposed to HIV. The fit of the smoothed cubic spline (blue line) and a zero reference line (red) are given as well as spaghetti plots that identify subject-specific measurements (ie, trajectories). For WAZ, LAZ, WLZ, and HCZ, all four dependent effects showed small but statistically significant (all P<0.003) increasing trends over time (blue line), despite large individual differences between subjects. Much of the trend was a result of change from negative z-scores shortly after birth to positive z-scores at approximately 4 months of age. For triceps and subscapular SFT, no discernable trend was detected (all P>0.175). However, the trajectories of both SFTs demonstrated an overall displacement below the zero reference line (all P<0.006). This can be most easily seen from the spaghetti plots as the majority of the trajectories occur below the zero reference line with no apparent increasing or decreasing trend. The trajectories of MAC tended to be centered around the zero reference line (P=0.80) with a small decreasing trend (P=0.023).
Figure.
Growth and body composition z-scores by age. (A) Weight. (B) Length. (C) Weight-for-length. (D) Head Circumference. (E) Triceps skinfold. (F) Subscapular skinfold. (G) Mid-upper arm circumference. Raw data (left) with zero z-score reference line (red) and smoothed spline fit (blue). Subject linked spaghetti plots (right).
DISCUSSION
We evaluated the differences in growth and body composition between uninfected children exposed to HIV and a group of demographically-matched children who were not exposed to HIV. We did not find statistical differences in classical and regional growth measures between the two groups in a cross-sectional analysis. We also compared our uninfected children exposed to HIV with US growth standards and found that although WTZ and WLZ were below standards at birth, growth increased with time, yet was within national norms. Skinfolds were stable over time, but were significantly lower than national standards and MAC showed a decreasing trend.
Our compared postnatal growth and body composition of uninfected children exposed to HIV with a contemporary demographically and socioeconomically matched control group in the US. Ross found no difference in growth between 88 uninfected children exposed to HIV and 174 demographically and socioeconomically matched healthy controls from birth to 3 years of age in the United Kingdom [18]. The Pediatric AIDS Clinical Trials Group (PACTG) Protocol 219/076 compared growth of uninfected children exposed to HIV receiving ZDV or placebo, and they did not observe differences between the groups [4]. A large prospective European study investigating growth patterns of uninfected children exposed to HIV and those infected with HIV in the first 10 years of life that were compared with general British standards, reported no substantial differences between the uninfected children exposed to HIV and the reference group [8]. The European Collaborative Study [19] concluded that there was no association between ZDV monotherapy exposure and growth in uninfected children exposed to HIV up to 18 months of age. Jacobson et al [20] found similar total fat percentage assessed by dual x-ray absorptiometry between uninfected children exposed to HIV and NHANES ages 8–11 years, but did find lower body fat in the uninfected children exposed to HIV ages 12–15 years, especially males. Siberry studied the effects of in utero exposure to tenofovir compared with other combination regimens without tenofovir in uninfected infants exposed to HIV and found significantly lower mean LAZ and lower HCZ at age one year but not at birth for those exposed to tenofovir [9]. Nielsen-Saines found an association between higher viral load and shorter length at 3, 6, and 18 months of age among uninfected African children exposed to HIV [21].
We compared classical growth measures with US standards and found that uninfected children exposed to HIV had lower birth WAZ and birth WLZ. Lower birth weight has been associated with ARV exposure [18, 22], although contradictory results have been published [9, 23, 24]. Over the first two years of life, the uninfected children exposed to HIV showed a trend towards increasing WAZ, LAZ, HCZ, and WLZ but all of these measures were within the US norms after birth. These findings of lower but normal birth weights and subsequent trends toward accelerated growth for this population of uninfected children exposed to HIV suggest that lifestyle factors may influence growth more than HIV and ARVs. We were unable to determine if these trends persist given our short follow up. In HIV-unexposed populations, infants typically cross centiles in early infancy as their size at birth is often determined by maternal characteristics, yet post-natal growth is, in part, dictated by genetic predisposition [25]. Feeding patterns are also established during the post-natal period and can influence growth. For this socially disadvantaged population, poverty and food insecurity may contribute to rapid weight gain and risk for childhood obesity, as has been described elsewhere [26]. Food insecurity is prevalent in the US, and it is interlinked with HIV [26]. Jacobson showed higher prevalence of obesity in uninfected children exposed to HIV when compared with children with HIV and BMI z-scores of uninfected children exposed to HIV were higher than the general population [20].
In a separate analysis using longitudinal data, we compared body composition measures of uninfected children exposed to HIV with national standards. Subcutaneous fat as measured by subscapular and triceps SFT among uninfected children exposed to HIV were slightly, but significantly, lower than NHANES standards, and there was a trend that mid-upper arm circumference decreased over time. Our finding of lower subcutaneous fat in our cohort of uninfected children exposed to HIV is similar to that observed in patients with HIV who have received only NRTIs with concomitant lactic acidemia [27]. NRTI exposure is associated with altered fat distribution including peripheral lipoatrophy in a number of adult studies [27], and there is only limited information on the same effects in children [28]. Although uninfected children exposed to HIV rarely exhibit clinically apparent mitochondrial toxicity it is possible that our findings of lower subcutaneous fat could be mediated, in part, through mitochondrial changes induced by HIV or ART exposures. Patterns of abnormal fat distribution related to HIV infection vary from peripheral fat wasting alone or in combination with central fat accumulation [29]. These effects are of clinical importance as fetal and early postnatal, and childhood [30] periods are critical windows in the development of adipose depots.
Our two analyses may be perceived as demonstrating conflicting results. However, the results shed light on potential important factors that should be considered when evaluating growth and body composition of uninfected children exposed to HIV. Observed differences in the longitudinal analyses may result from the fact that NHANES and CDC standards are generated from a general and heterogeneous population which most likely differs on a number of key variables, including important socioeconomic covariates. Controlling for these factors, indirectly through recruitment of a socioeconomically similar cohort of infants, appeared to negate differences found between uninfected children exposed to HIV and national standards.
Our report augments the literature on growth in this expanding, yet under studied, uninfected children exposed to HIV. Our study has some limitations. The assessment of body composition may be more accurately measured through dual-energy X-ray absorptiometry than through anthropometry. However, the effects of inter-examiner variability were minimized because one dietician performed all measurements in a standard fashion. Due to the various phases in the HIV screening process, infants were not recruited at the same age. It would be a preferred design to enroll all children at a similar age. The cross-sectional study design prevented a comparison of changes in growth and body composition in the uninfected children exposed to HIV to the contemporary HIV-unexposed group. We also did not control for psychosocial or lifestyle factors that are known to affect growth. However, we enrolled the HIV-unexposed group with similar socio-demographics from Miami-Dade County with similar access to healthcare and income level. Finally, we did not have access to the neonatal characteristics of the HIV-unexposed group and could not adjust for these covariates in the analyses.
Clinicians and investigators who care for and study this expanding population of children exposed to HIV should be careful not to attribute changes in growth and body composition to HIV-specific factors without first carefully evaluating non-HIV covariates. Longitudinal studies with greater follow-up and with appropriate comparison groups are needed to understand the effects of prenatal exposures to HIV and ARV on growth and body composition throughout childhood and adolescence.
Acknowledgments
Supported by the National Institutes of Health (NHLBI 1 R01 HL095127 and NICHD 1 R01 HD060325), the Micah Batchelor Award for Research Excellence, the Coulter Jones Foundation, and the DHHS HRSA (H 12HA 00028).
ABBREVIATIONS
- AMC
arm muscle circumference
- ARV
Antiretroviral
- HCZ
Head Circumference-for-age z-score
- HIV
Human Immunodeficiency Virus
- LAZ
Length-for-age z-score
- MAC
mid-upper arm circumference
- MTC
mid-thigh circumference
- SFT
skinfold thickness
- WAZ
Weight-for-age z-score
- WLZ
Weight-for-length z-score
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers of Disease Control. Achievements in public health. Reduction in perinatal transmission of HIV infection--United States, 1985–2005. MMWR Morb Mortal Wkly Rep. 2006;55:592–7. [PubMed] [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2011. [Google Scholar]
- 3.Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–38. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 4.Culnane M, Fowler M, Lee SS, McSherry G, Brady M, O’Donnell K, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281:151–7. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- 5.European Collaborative Study. Combination antiretroviral therapy and duration of pregnancy. AIDS. 2000;14:2913–20. doi: 10.1097/00002030-200012220-00013. [DOI] [PubMed] [Google Scholar]
- 6.Funk MJ, Belinson SE, Pimenta JM, Morsheimer M, Gibbons DC. Mitochondrial disorders among infants exposed to HIV and antiretroviral therapy. Drug Saf. 2007;30:845–59. doi: 10.2165/00002018-200730100-00004. [DOI] [PubMed] [Google Scholar]
- 7.Tassiopoulos K, Read JS, Brogly S, Rich K, Lester B, Spector SA, et al. Substance use in HIV-Infected women during pregnancy: self-report versus meconium analysis. AIDS Behav. 2010;14:1269–78. doi: 10.1007/s10461-010-9705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newell ML, Borja MC, Peckham C. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111:e52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- 9.Siberry GK, Williams PL, Mendez H, Seage GR, 3rd, Jacobson DL, Hazra R, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–9. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read JS. Diagnosis of HIV-1 infection in children younger than 18 months in the United States. Pediatrics. 2007;120:e1547–62. doi: 10.1542/peds.2007-2951. [DOI] [PubMed] [Google Scholar]
- 11.National Health and Nutrition Examination Survey. Anthropometry Procedures Manual. 2002. [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2010 Feb 12;11:1–190. 2002. [PubMed] [Google Scholar]
- 13.Frisancho AR. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr. 1981;34:2540–5. doi: 10.1093/ajcn/34.11.2540. [DOI] [PubMed] [Google Scholar]
- 14.Knapik JJ, Staab JS, Harman EA. Validity of an anthropometric estimate of thigh muscle cross-sectional area. Med Sci Sports Exerc. 1996;28:1523–30. doi: 10.1097/00005768-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 15.McDowell MA, Fryar CD, Ogden CL. Anthropometric reference data for children and adults: United States, 1988–1994. Vital Health Stat. 2009 Feb 12;11 2010. [PubMed] [Google Scholar]
- 16.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer JDWJ. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 18.Ross A, Raab GM, Mok J, Gilkison S, Hamilton B, Johnstone FD. Maternal HIV infection, drug use, and growth of uninfected children in their first 3 years. Arch Dis Child. 1995;73:490–5. doi: 10.1136/adc.73.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankin C, Thorne C, Newell ML. Does exposure to antiretroviral therapy affect growth in the first 18 months of life in uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2005;40:364–70. doi: 10.1097/01.qai.0000162417.62748.cd. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson DL, Patel K, Siberry GK, Van Dyke RB, DiMeglio LA, Geffner ME, et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr. 2011;94:1485–95. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen-Saines K, Komarow L, Cu-Uvin S, Jourdain G, Klingman KL, Shapiro DE, et al. Infant Outcomes After Maternal Antiretroviral Exposure in Resource-Limited Settings. Pediatrics. 2012;129:e1525–e32. doi: 10.1542/peds.2011-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briand N, Mandelbrot L, Le Chenadec J, Tubiana R, Teglas JP, Faye A, et al. No relation between in-utero exposure to HAART and intrauterine growth retardation. AIDS. 2009;23:1235–43. doi: 10.1097/QAD.0b013e32832be0df. [DOI] [PubMed] [Google Scholar]
- 23.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and Infant Outcomes among HIV-Infected Women Taking Long-Term ART with and without Tenofovir in the DART Trial. PLoS medicine. 2012;9:e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert JS, Watts DH, Mofenson L, Stiehm ER, Harris DR, Bethel J, et al. Risk factors for preterm birth, low birth weight, and intrauterine growth retardation in infants born to HIV-infected pregnant women receiving zidovudine. Pediatric AIDS Clinical Trials Group 185 Team. AIDS. 2000;14:1389–99. doi: 10.1097/00002030-200007070-00012. [DOI] [PubMed] [Google Scholar]
- 25.Smith DW, Truog W, Rogers JE, Greitzer LJ, Skinner AL, McCann JJ, et al. Shifting linear growth during infancy: illustration of genetic factors in growth from fetal life through infancy. J Pediatr. 1976;89:225–30. doi: 10.1016/s0022-3476(76)80453-2. [DOI] [PubMed] [Google Scholar]
- 26.Chilton M, Chyatte M, Breaux J. The negative effects of poverty & food insecurity on child development. Indian J Med Res. 2007;126:262–72. [PubMed] [Google Scholar]
- 27.Carr A, Miller J, Law M, Cooper DA. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS. 2000;14:F25–32. doi: 10.1097/00002030-200002180-00001. [DOI] [PubMed] [Google Scholar]
- 28.Hartman K, Verweel G, de Groot R, Hartwig NG. Detection of lipoatrophy in human immunodeficiency virus-1-infected children treated with highly active antiretroviral therapy. Pediatr Infect Dis J. 2006;25:427–31. doi: 10.1097/01.inf.0000215003.32256.aa. [DOI] [PubMed] [Google Scholar]
- 29.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 30.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]