Abstract
Mechanical forces are essential to the development and progression of fibrosis, and are likely to be as important as soluble factors. These forces regulate the phenotype and proliferation of myofibroblasts and other cells in damaged tissues, the activation of growth factors, the structure and mechanics of the matrix, and, potentially, tissue patterning. Better understanding of the variety and magnitude of forces, the characteristics of those forces in biological tissues, and their impact on fibrosis in multiple tissues is needed and may lead to identification of important new therapeutic targets.
Keywords: tissue stiffness, myofibroblast, tension, stretch, shear, hydrostatic pressure
Introduction
Mechanical forces are essential to the development, progression, and (potentially) regression of tissue fibrosis. Although often ignored in studies and models of fibrosis, particularly in the era of genomics and proteomics, mechanical signals are similar to chemical signals in their range of effects and are likely to be equally important. The mechanical forces that act in fibrosis are highly varied, and may mediate individual cell phenotypes as well as global architectural changes. Understanding the role of mechanics in fibrosis is key to understanding the basic pathophysiological mechanisms of fibrotic diseases as well as developing new therapies.
Forces
There are multiple forces at work in tissues. These include tension and compressive forces (forces which pull or push perpendicular to the surface of an object) and shear forces (which are parallel to the surface) (Fig. 1A). These forces exert stress on objects, defined as force (in Newtons (N)) normalized to the area over which it acts and expressed in units of pascals (1 Pa = 1 pN/μm2). Forces in tissues result from cell-generated tension, fluid flow, stretch, and hydrostatic/osmotic pressure, which are resisted to variable extents by tissue stiffness. These forces collectively regulate the phenotype and proliferation of myofibroblasts and other cells in damaged tissues, the activation of growth factors, and the structure and mechanics of the matrix – all of which are central to fibrosis.
Figure 1.
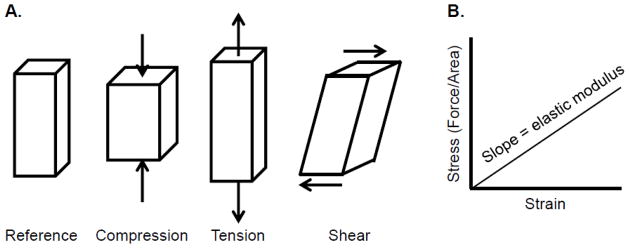
Forces affecting tissues. A) Forces acting on tissues. B) The elastic modulus of a material is slope of the stress (force per unit area) plotted against the strain (deformation). The diagram demonstrates linear elasticity, where the stress/strain relationship is constant. In reality, most biological materials demonstrate non-linear elasticity, such that the elastic modulus changes as strain increases.
There are important differences between signaling from mechanical (force-generated) stimuli and signaling from soluble (chemical) stimuli [1]. Soluble signals, such as growth factors, diffuse radially and provide limited directional information, while mechanical signals can be highly directional and thereby convey complex information in three dimensions. This is particularly important for cells of the same type, which can communicate over long ranges via mechanical signals; for autocrine soluble signals, cells cannot build up concentration gradients relative to their neighbors. Mechanical signals, which decay as a function of 1/r (where r is the radius) when they are transmitted though an elastic continuum and decay even more gradually when transmitted directly through filamentous elements of the matrix, are also communicated over longer length scales than soluble signals, which decay as 1/r2. For example, some strains (deformations caused by forces) can be transmitted over distances of hundreds of microns [2]. Additionally, mechanical signals can be regulated rapidly. While chemical signals require translation into second messenger cascades, mechanical signals are often transmitted directly, without the need for diffusible intermediates. Thus, force-mediated signals can be started and stopped rapidly compared to soluble signals, allowing increased control in time.
It is important to note that changes in the mechanical properties of tissues, like changes in the level or distribution of soluble factors, can both cause and result from fibrosis. In the same way that a profibrogenic growth factor like transforming growth factor-β (TGF-β) stimulates myofibroblast activation and is then produced by those same myofibroblasts, thereby perpetuating fibrosis, myofibroblasts can be activated in response to mechanical forces and then perpetuate fibrosis by altering the mechanical environment.
Tissue stiffness and stiffness sensing
The best-studied force in tissue fibrosis is tension generated in response to tissue stiffness. Tissue stiffness is measured as the elastic modulus, defined as the resistance to deformation, and is expressed as the magnitude of a stress (compression, elongation, or shear force, normalized to area) divided by the strain (deformation) induced by the stress (Fig. 1B). Young’s elastic modulus (E) describes the resistance to a compressive or elongating force, while the shear elastic modulus (G) describes the resistance to a shear force. E and G are both expressed in units of Pa; for a perfectly elastic material that conserves volume (one that returns to its original shape when the stress is removed), E is three times G. Tissues, however, are not perfectly elastic but are viscoelastic, meaning that, like liquids, they have a viscosity, and that the strain in response to a stress changes with time [3, 4]. Although the role of the elastic modulus in regulating cell behavior is the subject of increasing study, the role of the viscous component of tissues is poorly understood [5]. Tissues are also structurally heterogeneous and resist deformation to different extents depending on the direction in which a force is applied. Additionally, neither the elastic nor the viscous stress of most biological tissues varies linearly with strain; although this can be important in maintaining the mechanical characteristics and integrity of a given tissue, it is difficult to model and study [6].
Tissue stiffness is sensed when cells adhere to matrix proteins and apply tension, meeting resistance that reflects the stiffness of the tissue. The cellular actin-myosin cytoskeleton exerts tension on extracellular matrix proteins via integrin attachments located within focal adhesions; stiffer tissues result in increased resistance to the pulling force exerted by cells, contributing to strengthening that force [7, 8]. Whether the mechanical force originating at the cell boundary is transmitted directly to the nucleus or to nuclear proteins (Yap/Taz, for example) [9], or whether signaling cascades are activated (potentially via focal adhesion kinase (FAK) or other focal adhesion proteins) as a result of tension at the site of the focal adhesion, are issues that have stimulated extensive investigation [10].
Normal tissues vary in their stiffness when measured over the same strains and time scales. Brain is very soft, with an elastic modulus around 100 Pa; liver, while also soft, is slightly stiffer at 400–600 Pa, and muscle and bone are stiffer still (104 and 106 Pa, respectively) [1]. It is clear from clinical practice that tissue stiffness changes in disease states. In the same way that we can easily tell by touch that a steel bar is stiffer than gelatin, palpation as part of the routine physical exam enables detection of differences in skin or liver stiffness and suggests that fibrotic tissues are stiffer than normal tissues. Multiple studies have shown that fibrotic lungs become stiffer in fibrosis, with elastic modulus values ranging from approximately 2 kPa for normal tissue to approximately 17 kPa for fibrotic tissue [11–13]. We have found normal livers ex vivo to have a shear modulus less than 1 kPa, while fibrotic livers range from 3 kPa to 22 kPa [14]. Transient elastography, which measures the elastic modulus, is widely used in clinical practice outside of the U.S. to assess the liver stiffness in patients with liver disease; although values vary from one study to the next, elastic moduli (measured at time scales that are shorter than those generally used for ex vivo studies) are typically less than 5 kPa for normal livers and greater than 12 kPa for cirrhotic livers [15].
Organs with established fibrosis are thought to be stiffer as a result of their increased quantity of extracellular matrix, in particular fibrillar collagens. It appears, however, that increased matrix alone is unlikely to account for increases in tissue stiffness in fibrosis and that stiffness and matrix quantity are not linearly related. Our studies suggest that increases in collagen and elastin cross-linking account for some of the increase in elastic modulus in liver fibrosis and that the mechanical properties of the injured liver change significantly early after injury, before significant matrix deposition has occurred [14, 16]. This crosslinking appears to be initiated by lysyl oxidase family crosslinking enzymes; the changes in stiffness are consistent with the effects of lysyl oxidases in isolated collagen cushions, the vasculature, and different cancers (see below) [17–21]. The contribution of altered cell (as opposed to matrix) stiffness to tissue mechanics in fibrosis has not been established but may be considerable [12].
Other forces acting on tissues
Forces other than tension generated in response to tissue stiffness may also contribute to the development and progression of fibrosis. Tissues are subject to shear stress caused by fluid flow through the vasculature, ducts, and interstitium. Of these, vascular flow and the effects of shear stress on the vascular endothelium are best understood, in particular in the context of cardiovascular disease and remodeling (a form of injury and fibrosis, although not strictly speaking tissue fibrosis). Alterations in vessel geometry, flow rate, and fluid viscosity contribute to changes in shear stress, regulating the release by endothelial cells of growth factors, vasodilators like nitric oxide, and other soluble factors, and leading to long-term changes in gene and protein expression [22]. Mechanotransduction results from cell surface deformation (affecting ion channel function, cell surface receptors, the glycocalyx, the primary cilia, and the physical properties of the membrane) as well as the transmission of signals from the cell surface to distant regions of the cell, affecting cell-substratum and cell-cell interactions which in turn lead to changes in chemical signals [22, 23]. Tissues such as kidney, liver, and lung have significant amounts of flow through specialized vessels including the glomerulus in the kidney, sinusoids in liver, and pulmonary vessels in lung. Altered flow through these vessels may be both the cause and the result of tissue remodeling and fibrosis, and may also result in pathologic angiogenesis [24].
Fluid flow through ducts such as the bile duct, pancreatic duct, and renal tubules represents another source of shear stress in tissues that may be relevant to fibrosis [25]. The primary cilia appear to be particularly important for mechanotransduction in these settings [26]. with genetic disorders of the primary cilia (including polycystic kidney disease and nephronophthisis) leading to epithelial pathology and fibrosis in the liver, kidney, and pancreas. Experimental data suggest that altered shear stress in the renal tubules increases inflammation [27], while altered shear stress in the glomerulus alters the actin cytoskeleton [28], suggesting that the effects of shear stress in tissue fibrosis may be complex.
Interstitial fluid flow, the extremely slow flow between capillaries and lymphatics (0.1–2 μm/sec, compared to 10–20 cm/sec for flow in blood vessels), is another important source of shear stress in tissue fibrosis. Interstitial flow varies with inflammation and edema, the amount and quality of the matrix (especially collagens and glycosaminoglycans), the composition of the fluid, and the size of lymphatics [29]; altered flow results in changes in growth factor release (including TGF-β), collagen alignment, and myofibroblast differentiation [30].
Other forces potentially operative in fibrosis include hydrostatic pressure, osmotic pressure, and stretch. These forces may be related to flow (pulsatile flow, for example, causes stretch; elevated hydrostatic pressure results from obstructed flow; and interstitial flow is driven by gradients in hydrostatic and osmotic pressure). Obstruction of the bile ducts, pancreatic duct, and ureters leads to fibrosis, likely due at least in part to changes in shear stress (stasis) and hydrostatic pressure [31]. The development of cardiac cirrhosis in response to high central venous pressures suggests that elevations in hydrostatic pressure are highly relevant to fibrosis in vivo. Stretch is particularly important in the lung, which is subject to cyclical stretch during respiration [32]. The pathways responsible for transducing these different forces are still under investigation, although there is evidence that cation channels in the transient receptor potential (TRP) family, the actin-interacting protein zyxin, and G protein-coupled receptors are activated in response to stretch [33, 34] while ion channel activation and alterations in cytoskeletal stability are part of the response to hydrostatic pressure [35].
Integration of soluble and mechanical signals
Mechanical and soluble signals are often interdependent. Mechanical forces can act both directly and indirectly on soluble factors. TGF-β, which is arguably the most important soluble factor in fibrosis, undergoes activation as the direct result of mechanical tension (Fig. 2). TGF-β is secreted as part of a latent complex and stored in the ECM; one component of this latent complex, the latency-associated peptide (LAP), binds directly to certain integrins, linking it to cells. Hinz and co-workers, in a series of elegant experiments, demonstrated that cells exert tension on the LAP through this integrin attachment. If the matrix to which the cells and TGF-β complex are attached is soft, it deforms in response to tension and the complex remains intact. If the matrix is stiff, however, resistance to cell-generated tension results in deformation of the LAP and release of active TGF-β [36, 37]. For myofibroblasts, this increased TGF-β results in increased α-smooth muscle actin (α-SMA), which interacts with cellular myosin to contract and produce increased tension – effectively a feed-forward loop incorporating both soluble and mechanical signals [37]. Thus, the stiffness of injured and fibrotic tissues may perpetuate fibrosis via mechanically-regulated increases in the amount of active TGF-β present. TGF-β is a common factor downstream of many mechanical forces: in addition to tension, other forces including interstitial fluid flow and stretch have been implicated in TGF-β activation and release [30, 38]. Similar mechanisms have not yet been identified for other growth factors but many are also stored in the matrix and may be activated or released in response to cell-generated tension and matrix deformation [39].
Figure 2.
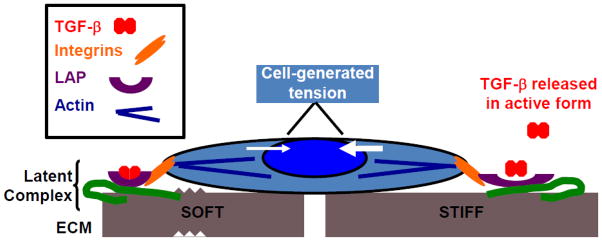
The role of mechanics in TGF-β activation. TGF-β is released in latent form, enclosed within the latency-associated peptide (LAP) as part of the TGF-β latent complex. Cell surface integrins, which connect to cytoplasmic actins at the site of focal adhesions, bind to LAP. As shown by Hinz and colleagues [36, 37], on soft surfaces (left) there is minimal resistance to cell generated tension and the complex remains latent. On stiff surfaces (right), there is significant resistance to cell-generated tension, this tension increases, and the LAP is pulled open, releasing active TGF-β.
In addition to acting directly on growth factors, mechanical signals can be converted to biochemical signals that intersect with or are part of soluble factor signaling pathways [40–42]. In lung fibroblasts, increased stiffness causes inhibition of prostaglandin E(2), which promotes fibrosis [11]. Stiffness also enhances the response to exogenous TGF-β [43]. NF-κB, which is downstream of many critical soluble factor pathways in fibrosis [44, 45], is also downstream of some mechanical forces. Wnt/β-catenin [46, 47], interleukins, and (as discussed above) G protein-coupled receptors and ion channels are common to both mechanical and soluble signaling pathways [41, 42]. Integrins and their downstream effector FAK are part of the cellular mechanotransduction apparatus but they also transduce signals from soluble factors. In some cases, integrins interact directly with soluble factors (for example, vascular endothelial growth factor (VEGF)) and growth factor receptors [48, 49]. Specific interactions between mechanical and soluble factors in fibrosis need to be defined.
The effects of mechanical forces on myofibroblasts
Myofibroblasts are the major fibrogenic cells in all forms of tissue fibrosis. These cells, which are derived from precursor cells including pericytes and fibroblasts, express α-SMA de novo after injury, develop stress fibers, and generate contractile force, exerting tension on the surrounding matrix. The importance of mechanical forces to myofibroblast activation and matrix deposition was demonstrated first for skin and mucosal wounds [50–52], and has since been shown for myofibroblasts in multiple tissues including heart [53], lung [11, 54, 55], liver [56–58], and kidney [59, 60], although different amounts of force may be required in different tissues and different contexts. In the case of matrix stiffness, increased stiffness results in increased α-SMA expression and matrix deposition, potentially as part of a positive feedback loop. Whether the reverse occurs is not clear: some in vitro studies, including studies with fibroblasts from patients with idiopathic pulmonary fibrosis, show that substrate softening results in the reversion of myofibroblasts to non-fibrogenic cells [61, 62], while other work suggests that myofibroblasts have a “mechanical memory” and retain their phenotype even after changes in the stiffness of their surroundings [54]. Most studies of the role of stiffness in myofibroblast differentiation have been carried out using artificial two-dimensional substrates; neither the effects of using three-dimensional systems nor the effects of viscoelastic or non-ideal elastic substrates are well characterized. A recent intriguing study of mesenchymal stem cells in culture showed that, when the elastic modulus was held constant, changes in substrate viscosity had a significant impact on cell behavior, including morphology, proliferation, and α-SMA expression [5]. Given that tissues are viscoelastic, it will be important to carry out similar studies exploring the effects of viscosity on myofibroblasts and their precursors.
Tension in response to matrix stiffness is not the only force the causes precursor cells to become myofibroblastic and fibrogenic. Hydrostatic pressure, which can occur within a tumor or edematous tissue, for example, enhances myofibroblast differentiation. Relevant to fibrosis, increased hydrostatic pressure resulted in the myofibroblastic activation of pancreatic and hepatic stellate cells in vitro [63, 64].
Stretch also regulates myofibroblast behavior [51]. Mouse skin, which was stretched, showed increased numbers of myofibroblasts [51]. In lung fibroblasts, stretch induced production of hyaluronic acid, which led to activation of the innate immune response [65]. A new method enabling the simultaneous study of stiffness and stretch suggested that stretch could overcome the effects of softness and that stiffness and stretch sensing employs similar mechanotransduction pathways to similar effect [66]. Interestingly, however, cyclic stretch, which is typical of the normal lung and which is decreased in fibrosis, inhibited the differentiation of lung myofibroblasts [67].
Finally, fluid flow, including vascular flow and interstitial fluid flow, can also regulate the myofibroblast phenotype [23, 30, 68]. The time course over which flow-related shear forces act on myofibroblast precursors is not known, nor is it clear whether there is signal attenuation or whether persistent changes in flow are required. The observation that alterations in both vascular and interstitial flow result in a similar myofibroblastic phenotype, however, suggests that cells constitutively sample the mechanics of their environment and may be sensitive to changes in flow rather than to absolute flow rates.
The effects of mechanical forces on non-fibrogenic cells in fibrosis
Although myofibroblasts are the primary matrix-depositing cells in tissue fibrosis, other cells also participate in the development and progression of fibrosis and are similarly responsive to mechanical forces. Chief among these are cells of the vasculature. Angiogenesis and fibrosis often progress in parallel, and may positively regulate each other. Two- and three-dimensional angiogenesis assays show that stiffness regulates the dynamics of tube formation [69–71] and more specifically regulates transcriptional pathways that control angiogenesis [72]. Liver sinusoidal endothelial cells, which are key cells in the development of liver fibrosis, demonstrate stiffness-dependent changes in podosomes [73], which regulate adhesion and migration, and alterations in the stiffness of glomerular podocytes are associated with renal disease [74]. In in vitro models, vascular endothelial cell contractility and permeability increased with increasing matrix stiffness, enhancing leukocyte extravasation; this could be important to fibrosis-associated inflammation (although it was not studied directly) [75–77].
Mechanical forces regulate other cell types as well. Macrophages, which mediate fibrosis in multiple organs, demonstrate phenotypic, transcriptional, and functional changes in response to altered matrix stiffness [78]. Stem cells, which are an important part of the response to injury, are also increasingly recognized as being mechanosensitive [79–81].
The effects of forces on architectural remodeling
Most work on mechanics in fibrosis has focused on the effects of forces on single cells. Not yet studied in detail is the role of mechanics in the large-scale architectural remodeling associated with fibrosis. Pioneering in vitro work by Harris [82, 83] and Grinnell [84] provides potential mechanical explanations for large-scale architectural arrangements in tissues and may be applicable to fibrosis – for example, to explain the development of bridging fibrosis in the liver or of the similarly complex reticular pattern of fibrosis in idiopathic pulmonary fibrosis [85]. Harris and Grinnell found that embedding stiff, fibroblast-containing implants in soft collagen gel resulted in realignment of collagen fibrils along the axes connecting implants, with shortening of the axes and migration of cells across the newly aligned collagen fibril bridges [82–84]. Mechanistically, the investigators suggested that the stiffness of the implants led to enhanced contractility of embedded fibroblasts, and that this cellular contractility enabled alignment of intervening collagen fibrils, with cell-generated tension leading to shortening of the implant-to-implant distance. More recently, Janmey’s group has shown that the non-linear elastic properties of certain matrix proteins (including fibrin) enable cells to influence neighboring cells hundreds of microns away [2]. Collectively, these observations suggest that extracellular matrix (ECM) remodeling and reciprocal interactions between cells and the remodeled ECM, even over long ranges, may be central to the progression of fibrotic disease.
Key features of these models are that myofibroblasts are contractile in stiff environments and that tissue stiffness is heterogeneous. This has been shown experimentally for lung and liver. Myofibroblasts from both tissues demonstrate increased contractility on stiffer substrates in vitro [43] (and unpublished work), and both tissues are mechanically heterogeneous in the normal and fibrotic states. Atomic force microscopy measurements of bleomycin-treated lung tissue demonstrated marked overall increases in stiffness after injury, focal areas of significantly higher stiffness, and increased heterogeneity [11], while microindentation methods demonstrated similar increases in mechanical heterogeneity in carbon tetrachloride-treated livers [86]. A challenge of future research will be to incorporate these observations into regional and tissue-scale models of fibrosis and to similarly incorporate other forces (including those from fluid flow and hydrostatic pressure) into the models.
In one approach to using mechanics to model tissue behavior in fibrosis, Bates and Suki proposed that the concept of “percolation” – transmission of events across networks – might be important in understanding lung fibrosis [87, 88]. They highlight the concept of a “percolation threshold,” which occurs when isolated fibrotic lesions connect to form a contiguous septum, resulting in a sudden increase in macroscopic stiffness (and in symptoms). Although this work was based on computational models and was highly simplified, examination of human tissues provided support for the model, which, akin to the work of Harris and Grinnell, emphasizes the importance of geographical variation and heterogeneity in fibrosis progression [88]. The authors also proposed that limited but targeted antifibrotic therapy might be an effective treatment for tissue fibrosis, a so-called “reverse percolation” effect [87].
Matrix proteins and tissue mechanics
The matrix and mechanics are inextricably intertwined in injured and fibrotic tissues. The matrix determines the mechanical tension and stretch sensed by cells, regulates tissue resistance to hydrostatic pressure, and mediates interstitial fluid flow. Specific matrix molecules, in particular the load-bearing matrix proteins, have specific roles in the mechanical environment. The collagens (with non-linear stress-strain properties) provide strength, the elastins (with linear stress-strain properties) resilience, and proteoglycans resistance to compression and shear [6, 89]. Importantly, however, we do not yet understand what specifically causes altered mechanics (especially altered stiffness) in diseased tissue – whether cells, specific matrix proteins, or specific protein modifications are responsible.
Increased deposition of the fibrillar collagens (especially collagens I and III) is typical of tissue fibrosis, and these collagens add stiffness to tissues. Given their rigid, rod-like shape, they can also undergo alignment (to form parallel arrays) when subject to various forces; this may be important to cell migration, angiogenesis, and the exposure of cells to flow, and may permit long-distance transmission of forces [2, 30, 90]. Data from the cancer literature suggest that aligned collagen fibrils serve as “tracks” for cell metastases [91], and it is possible that in fibrosis these fibrils have a similar role in facilitating migration of myofibroblasts or vascular cells.
As noted above, collagen cross-linking enzymes appear to play a critical role in fibrosis, likely through their effects on mechanics. In vitro analyses demonstrate that collagen cross-linked through the actions of lysyl oxidase is stiffer than non-cross-linked collagen [17]. Increased liver stiffness early after injury is associated with increases in lysyl oxidase-mediated collagen cross-linking and with tissue mechanical properties typical of a cross-linked matrix; inhibiting lysyl oxidase activity blunts changes in stiffness, reduces myofibroblast differentiation, and partially prevents fibrosis [14, 16, 92, 93]. Tissue transglutaminases also cross-link collagens. Although some investigators suggest that tissue transglutaminases are responsible for the protease-resistant cross-links of liver cirrhosis [94], others have found no role for these cross-linking enzymes in the progression of advanced liver fibrosis [95]. Thus, the role of transglutaminase-mediated cross-links in tissue mechanics and fibrosis remains to be determined. [Lysyl oxidases in fibrosis are discussed in detail in the previous chapter.]
The other major structural proteins upregulated in fibrosis, the elastins, contribute resilience, as opposed to rigidity, to tissues [96, 97]. Like the collagens, elastins undergo cross-linking initiated by lysyl oxidases, although the mechanical ramifications of this are not known. The relevance of elastin cross-linking is likely to be particularly important in lung fibrosis.
Proteoglycans, which make up most of the “ground substance” between cells, also influence the mechanical properties of normal and fibrotic tissues. The glycosaminoglycan chains attached to the core proteins of proteoglycans are characterized by closely-packed negative charges, enabling them to generate electrostatic repulsive forces and to increase their hydration. In the lung (and likely other tissues as well), the resulting resistance to compression contributes to tissue mechanics by stabilizing the network of collagen and elastin fibrils [98]. The small leucine-rich proteoglycans, including lumican, fibromodulin, and decorin, regulate the assembly and alignment of collagen fibrils [99, 100] and may alter collagen fibril mechanics [99, 101, 102]. Expression of the small leucine-rich proteoglycan biglycan was significantly correlated with changes in lung mechanics, including resistance and compliance, in a bleomycin model of lung fibrosis, with mechanical changes identified before collagen deposition [103]. Liver fibrosis in response to injury was reduced in both lumican and fibromodulin null mice, although the mechanics of the knockout livers were not examined [104, 105]. Larger proteoglycans such as versican, as well as the glycosaminoglycan hyaluronic acid, resist compressive forces and regulate interstitial flow. Additionally, the glycocalyx, of which proteoglycans are a part, may mediate mechanotransduction [23].
The fibronectins are a final major component of the fibrotic matrix. Fibronectins are among the first matrix proteins upregulated after injury and are highly mechanosensitive. Fibronectin is remarkably extensible, with a highly non-linear stress-strain curve, and is able to tolerate very high strains without breaking [106]. On stiff substrates, it becomes very rigid, with the expression of cryptic epitopes [107]; rigidity may therefore lead to changes in integrin binding and signaling. Cell-generated tension, which increases in fibrosis, is critical to fibronectin unfolding and self assembly [108]. Additionally, cellular fibronectin splice variants, which have insertions, are dramatically upregulated in fibrosis [109]. Although these have not been studied in detail, the insertion of these extra domains may alter the mechanical properties of the fibronectins and thereby the mechanical milieu of a given tissue.
Summary
Mechanical forces are increasingly appreciated to play a role in fibrosis on a par with soluble factors. Matrix stiffness is so far the best-appreciated mechanical stimulus in fibrosis, and liver and lung are the tissues best studied. Even for these tissues and stimuli, our understanding of forces, their effects, and mechanotransduction in fibrosis is rudimentary. Future work will need to expand our understanding of the variety and magnitude of forces, the characteristics of those forces in biological tissues, and their impact on fibrosis in multiple tissues. Ultimately, the mechanical features of fibrosis may prove to be attractive targets for antifibrotic therapies.
Highlights.
Mechanical forces play a critical role in fibrosis.
There are multiple forces acting on tissues.
Matrix stiffness is the best appreciated mechanical stimulus in fibrosis.
Mechanical forces determine the activation of myofibroblast s.
Acknowledgments
This work was funded in part by a grant from the National Institutes of Health (R01-DK-058123).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janmey PA, Miller RT. Mechanisms of mechanical signaling in development and disease. Journal of cell science. 2011;124:9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winer JP, Oake S, Janmey PA. Non-linear elasticity of extracellular matrices enables contractile cells to communicate local position and orientation. PloS one. 2009;4:e6382. doi: 10.1371/journal.pone.0006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z, Bilston L. On the viscoelastic character of liver tissue: experiments and modelling of the linear behaviour. Biorheology. 2000;37:191–201. [PubMed] [Google Scholar]
- 4.Navajas D, Maksym GN, Bates JH. Dynamic viscoelastic nonlinearity of lung parenchymal tissue. J Appl Physiol. 1995;79:348–356. doi: 10.1152/jappl.1995.79.1.348. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AR, Frith JE, Cooper-White JJ. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 7.Chen CS. Mechanotransduction - a field pulling together? Journal of cell science. 2008;121:3285–3292. doi: 10.1242/jcs.023507. [DOI] [PubMed] [Google Scholar]
- 8.Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. Journal of cell science. 2012;125:3025–3038. doi: 10.1242/jcs.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 10.Martins RP, Finan JD, Guilak F, Lee DA. Mechanical regulation of nuclear structure and function. Annual review of biomedical engineering. 2012;14:431–455. doi: 10.1146/annurev-bioeng-071910-124638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. The Journal of cell biology. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. American journal of respiratory and critical care medicine. 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown AC, Fiore VF, Sulchek TA, Barker TH. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. The Journal of pathology. 2013;229:25–35. doi: 10.1002/path.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. American journal of physiology. Gastrointestinal and liver physiology. 2007;293:G1147–1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 15.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study) Journal of hepatology. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 16.Perepelyuk M, Terajima M, Wang AY, Georges PC, Janmey PA, Yamauchi M, Wells RG. Hepatic stellate cells and portal fibroblasts are the major cellular sources of collagens and lysyl oxidases in normal liver and early after injury. American Journal of Physiology Gastrointestinal and Liver Physiology. 2013 Jan 17; doi: 10.1152/ajpgi.00222.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elbjeirami WM, Yonter EO, Starcher BC, West JL. Enhancing mechanical properties of tissue-engineered constructs via lysyl oxidase crosslinking activity. Journal of biomedical materials research. Part A. 2003;66:513–521. doi: 10.1002/jbm.a.10021. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1993;7:1208–1218. [PubMed] [Google Scholar]
- 19.Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. American journal of physiology. Heart and circulatory physiology. 2010;299:H1–9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- 20.Kothapalli D, Liu SL, Bae YH, Monslow J, Xu T, Hawthorne EA, Byfield FJ, Castagnino P, Rao S, Rader DJ, Pure E, Phillips MC, Lund-Katz S, Janmey PA, Assoian RK. Cardiovascular Protection by ApoE and ApoE-HDL Linked to Suppression of ECM Gene Expression and Arterial Stiffening. Cell reports. 2012;2:1259–1271. doi: 10.1016/j.celrep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice. Cardiovascular medicine. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Annals of biomedical engineering. 2011;39:1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim MY, Baik SK, Lee SS. Hemodynamic alterations in cirrhosis and portal hypertension. The Korean journal of hepatology. 2010;16:347–352. doi: 10.3350/kjhep.2010.16.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rohatgi R, Flores D. Intratubular hydrodynamic forces influence tubulointerstitial fibrosis in the kidney. Current opinion in nephrology and hypertension. 2010;19:65–71. doi: 10.1097/MNH.0b013e32833327f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. American journal of physiology. Renal physiology. 2010;299:F1220–1236. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miravete M, Dissard R, Klein J, Gonzalez J, Caubet C, Pecher C, Pipy B, Bascands JL, Mercier-Bonin M, Schanstra JP, Buffin-Meyer B. Renal tubular fluid shear stress facilitates monocyte activation toward inflammatory macrophages. American journal of physiology. Renal physiology. 2012;302:F1409–1417. doi: 10.1152/ajprenal.00409.2011. [DOI] [PubMed] [Google Scholar]
- 28.Friedrich C, Endlich N, Kriz W, Endlich K. Podocytes are sensitive to fluid shear stress in vitro. American journal of physiology. Renal physiology. 2006;291:F856–865. doi: 10.1152/ajprenal.00196.2005. [DOI] [PubMed] [Google Scholar]
- 29.Swartz MA, Fleury ME. Interstitial flow and its effects in soft tissues. Annual review of biomedical engineering. 2007;9:229–256. doi: 10.1146/annurev.bioeng.9.060906.151850. [DOI] [PubMed] [Google Scholar]
- 30.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. Journal of cell science. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 31.Guyot C, Combe C, Desmouliere A. The common bile duct ligation in rat: A relevant in vivo model to study the role of mechanical stress on cell and matrix behaviour. Histochemistry and cell biology. 2006;126:517–523. doi: 10.1007/s00418-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 32.Tschumperlin DJ, Boudreault F, Liu F. Recent advances and new opportunities in lung mechanobiology. Journal of biomechanics. 2010;43:99–107. doi: 10.1016/j.jbiomech.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suresh Babu S, Wojtowicz A, Freichel M, Birnbaumer L, Hecker M, Cattaruzza M. Mechanism of stretch-induced activation of the mechanotransducer zyxin in vascular cells. Science signaling. 2012;5:ra91. doi: 10.1126/scisignal.2003173. [DOI] [PubMed] [Google Scholar]
- 34.Kuipers AJ, Middelbeek J, van Leeuwen FN. Mechanoregulation of cytoskeletal dynamics by TRP channels. European journal of cell biology. 2012;91:834–846. doi: 10.1016/j.ejcb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Myers KA, Rattner JB, Shrive NG, Hart DA. Hydrostatic pressure sensation in cells: integration into the tensegrity model. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2007;85:543–551. doi: 10.1139/o07-108. [DOI] [PubMed] [Google Scholar]
- 36.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Current biology: CB. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 37.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. The Journal of cell biology. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. European journal of clinical investigation. 2004;34:129–136. doi: 10.1111/j.1365-2362.2004.01302.x. [DOI] [PubMed] [Google Scholar]
- 39.Wells RG, Discher DE. Matrix elasticity, cytoskeletal tension, and TGF-beta: the insoluble and soluble meet. Science signaling. 2008;1:pe13. doi: 10.1126/stke.110pe13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendez MG, Janmey PA. Transcription factor regulation by mechanical stress. The international journal of biochemistry & cell biology. 2012;44:728–732. doi: 10.1016/j.biocel.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Archives of dermatological research. 2012;304:589–597. doi: 10.1007/s00403-012-1278-5. [DOI] [PubMed] [Google Scholar]
- 42.Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Current opinion in rheumatology. 2013;25:92–100. doi: 10.1097/BOR.0b013e32835b13cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marinkovic A, Mih JD, Park JA, Liu F, Tschumperlin DJ. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-beta responsiveness. American journal of physiology. Lung cellular and molecular physiology. 2012;303:L169–180. doi: 10.1152/ajplung.00108.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- 45.Luedde T, Schwabe RF. NF-kappaB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nature reviews. Gastroenterology & hepatology. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valbuena A, Vera AM, Oroz J, Menendez M, Carrion-Vazquez M. Mechanical properties of beta-catenin revealed by single-molecule experiments. Biophysical journal. 2012;103:1744–1752. doi: 10.1016/j.bpj.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JH, Chen WL, Sider KL, Yip CY, Simmons CA. beta-catenin mediates mechanically regulated, transforming growth factor-beta1-induced myofibroblast differentiation of aortic valve interstitial cells. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:590–597. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- 48.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 49.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (VEGF)-A and contributes to VEGF-A-induced angiogenesis. The Journal of biological chemistry. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 50.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. The American journal of pathology. 2001;159:1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Squier CA. The effect of stretching on formation of myofibroblasts in mouse skin. Cell and tissue research. 1981;220:325–335. doi: 10.1007/BF00210512. [DOI] [PubMed] [Google Scholar]
- 52.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. The American journal of pathology. 1999;154:871–882. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galie PA, Westfall MV, Stegemann JP. Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2011;20:325–333. doi: 10.1016/j.carpath.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balestrini JL, Chaudhry S, Sarrazy V, Koehler A, Hinz B. The mechanical memory of lung myofibroblasts. Integrative biology: quantitative biosciences from nano to macro. 2012;4:410–421. doi: 10.1039/c2ib00149g. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. American journal of respiratory cell and molecular biology. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- 57.Olsen AL, Bloomer SA, Chan EP, Gaca MD, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. American journal of physiology. Gastrointestinal and liver physiology. 2011;301:G110–118. doi: 10.1152/ajpgi.00412.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lorena D, Darby IA, Reinhardt DP, Sapin V, Rosenbaum J, Desmouliere A. Fibrillin-1 expression in normal and fibrotic rat liver and in cultured hepatic fibroblastic cells: modulation by mechanical stress and role in cell adhesion. Laboratory investigation; a journal of technical methods and pathology. 2004;84:203–212. doi: 10.1038/labinvest.3700023. [DOI] [PubMed] [Google Scholar]
- 59.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-beta1-induced apoptosis and epithelial-mesenchymal transition. Molecular biology of the cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cortes P, Zhao X, Riser BL, Narins RG. Role of glomerular mechanical strain in the pathogenesis of diabetic nephropathy. Kidney international. 1997;51:57–68. doi: 10.1038/ki.1997.8. [DOI] [PubMed] [Google Scholar]
- 61.Gaca MD, Zhou X, Issa R, Kiriella K, Iredale JP, Benyon RC. Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix biology: journal of the International Society for Matrix Biology. 2003;22:229–239. doi: 10.1016/s0945-053x(03)00017-9. [DOI] [PubMed] [Google Scholar]
- 62.Marinkovic A, Liu F, Tschumperlin DJ. Matrices of physiologic stiffness potently inactivate IPF fibroblasts. American journal of respiratory cell and molecular biology. 2012 doi: 10.1165/rcmb.2012-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe S, Nagashio Y, Asaumi H, Nomiyama Y, Taguchi M, Tashiro M, Kihara Y, Nakamura H, Otsuki M. Pressure activates rat pancreatic stellate cells. American journal of physiology. Gastrointestinal and liver physiology. 2004;287:G1175–1181. doi: 10.1152/ajpgi.00339.2004. [DOI] [PubMed] [Google Scholar]
- 64.Okada Y, Tsuzuki Y, Hokari R, Miyazaki J, Matsuzaki K, Mataki N, Komoto S, Watanabe C, Kawaguchi A, Nagao S, Itoh K, Miura S. Pressure loading and ethanol exposure differentially modulate rat hepatic stellate cell activation. Journal of cellular physiology. 2008;215:472–480. doi: 10.1002/jcp.21329. [DOI] [PubMed] [Google Scholar]
- 65.Heise RL, Stober V, Cheluvaraju C, Hollingsworth JW, Garantziotis S. Mechanical stretch induces epithelial-mesenchymal transition in alveolar epithelia via hyaluronan activation of innate immunity. The Journal of biological chemistry. 2011;286:17435–17444. doi: 10.1074/jbc.M110.137273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Throm Quinlan AM, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PloS one. 2011;6:e23272. doi: 10.1371/journal.pone.0023272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blaauboer ME, Smit TH, Hanemaaijer R, Stoop R, Everts V. Cyclic mechanical stretch reduces myofibroblast differentiation of primary lung fibroblasts. Biochemical and biophysical research communications. 2011;404:23–27. doi: 10.1016/j.bbrc.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Shi ZD, Ji XY, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. American journal of physiology. Heart and circulatory physiology. 2009;297:H1225–1234. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stephanou A, Meskaoui G, Vailhe B, Tracqui P. The rigidity in fibrin gels as a contributing factor to the dynamics of in vitro vascular cord formation. Microvascular research. 2007;73:182–190. doi: 10.1016/j.mvr.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. American journal of physiology. Cell physiology. 2009;297:C179–187. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 71.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Experimental cell research. 2004;297:574–584. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 72.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Juin A, Planus E, Guillemot F, Horakova P, Albiges-Rizo C, Genot E, Rosenbaum J, Moreau V, Saltel F. Extracellular matrix rigidity controls podosome induction in microvascular endothelial cells. Biology of the cell/under the auspices of the European Cell Biology Organization. 2013;105:46–57. doi: 10.1111/boc.201200037. [DOI] [PubMed] [Google Scholar]
- 74.Tandon R, Levental I, Huang C, Byfield FJ, Ziembicki J, Schelling JR, Bruggeman LA, Sedor JR, Janmey PA, Miller RT. HIV infection changes glomerular podocyte cytoskeletal composition and results in distinct cellular mechanical properties. American journal of physiology. Renal physiology. 2007;292:F701–710. doi: 10.1152/ajprenal.00246.2006. [DOI] [PubMed] [Google Scholar]
- 75.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Science translational medicine. 2011;3:112ra122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011;118:1632–1640. doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krishnan R, Klumpers DD, Park CY, Rajendran K, Trepat X, van Bezu J, van Hinsbergh VW, Carman CV, Brain JD, Fredberg JJ, Butler JP, van Nieuw Amerongen GP. Substrate stiffening promotes endothelial monolayer disruption through enhanced physical forces. American journal of physiology. Cell physiology. 2011;300:C146–154. doi: 10.1152/ajpcell.00195.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel NR, Bole M, Chen C, Hardin CC, Kho AT, Mih J, Deng L, Butler J, Tschumperlin D, Fredberg JJ, Krishnan R, Koziel H. Cell elasticity determines macrophage function. PloS one. 2012;7:e41024. doi: 10.1371/journal.pone.0041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greenbaum LE, Wells RG. The role of stem cells in liver repair and fibrosis. The international journal of biochemistry & cell biology. 2011;43:222–229. doi: 10.1016/j.biocel.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yim EK, Sheetz MP. Force-dependent cell signaling in stem cell differentiation. Stem cell research & therapy. 2012;3:41. doi: 10.1186/scrt132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McNulty K, Janes SM. Stem cells and pulmonary fibrosis: cause or cure? Proceedings of the American Thoracic Society. 2012;9:164–171. doi: 10.1513/pats.201201-010AW. [DOI] [PubMed] [Google Scholar]
- 82.Harris AK, Stopak D, Wild P. Fibroblast traction as a mechanism for collagen morphogenesis. Nature. 1981;290:249–251. doi: 10.1038/290249a0. [DOI] [PubMed] [Google Scholar]
- 83.Stopak D, Harris AK. Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Developmental biology. 1982;90:383–398. doi: 10.1016/0012-1606(82)90388-8. [DOI] [PubMed] [Google Scholar]
- 84.Miron-Mendoza M, Seemann J, Grinnell F. Collagen fibril flow and tissue translocation coupled to fibroblast migration in 3D collagen matrices. Molecular biology of the cell. 2008;19:2051–2058. doi: 10.1091/mbc.E07-09-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cool CD, Groshong SD, Rai PR, Henson PM, Stewart JS, Brown KK. Fibroblast foci are not discrete sites of lung injury or repair: the fibroblast reticulum. American journal of respiratory and critical care medicine. 2006;174:654–658. doi: 10.1164/rccm.200602-205OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Levental I, Levental KR, Klein EA, Assoian R, Miller RT, Wells RG, Janmey PA. A simple indentation device for measuring micrometer-scale tissue stiffness. Journal of physics. Condensed matter: an Institute of Physics journal. 2010;22:194120. doi: 10.1088/0953-8984/22/19/194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suki B, Majumdar A, Nugent MA, Bates JH. In silico modeling of interstitial lung mechanics: implications for disease development and repair. Drug discovery today. Disease models. 2007;4:139–145. doi: 10.1016/j.ddmod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. American journal of respiratory and critical care medicine. 2007;176:617–623. doi: 10.1164/rccm.200611-1739OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. Springer-Verlag; New York: 1993. [Google Scholar]
- 90.Vader D, Kabla A, Weitz D, Mahadevan L. Strain-induced alignment in collagen gels. PloS one. 2009;4:e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Conklin MW, Eickhoff JC, Riching KM, Pehlke CA, Eliceiri KW, Provenzano PP, Friedl A, Keely PJ. Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American journal of pathology. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fiume L, Favilli G. Inhibition of experimental cirrhosis by carbon tetrachloride following treatment with aminoacetonitrile. Nature. 1961;189:71–72. doi: 10.1038/189071a0. [DOI] [PubMed] [Google Scholar]
- 93.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, Smith V. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nature medicine. 2010;16:1009–1017. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 94.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, Arthur MJ, Benyon RC, Iredale JP. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 95.Popov Y, Sverdlov DY, Sharma AK, Bhaskar KR, Li S, Freitag TL, Lee J, Dieterich W, Melino G, Schuppan D. Tissue transglutaminase does not affect fibrotic matrix stability or regression of liver fibrosis in mice. Gastroenterology. 2011;140:1642–1652. doi: 10.1053/j.gastro.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoff CR, Perkins DR, Davidson JM. Elastin gene expression is upregulated during pulmonary fibrosis. Connective tissue research. 1999;40:145–153. doi: 10.3109/03008209909029110. [DOI] [PubMed] [Google Scholar]
- 97.Pellicoro A, Aucott RL, Ramachandran P, Robson AJ, Fallowfield JA, Snowdon VK, Hartland SN, Vernon M, Duffield JS, Benyon RC, Forbes SJ, Iredale JP. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 98.Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS, Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol. 2005;98:672–679. doi: 10.1152/japplphysiol.00619.2004. [DOI] [PubMed] [Google Scholar]
- 99.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconjugate journal. 2002;19:287–293. doi: 10.1023/A:1025348417078. [DOI] [PubMed] [Google Scholar]
- 100.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. The Journal of cell biology. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jepsen KJ, Wu F, Peragallo JH, Paul J, Roberts L, Ezura Y, Oldberg A, Birk DE, Chakravarti S. A syndrome of joint laxity and impaired tendon integrity in lumican- and fibromodulin-deficient mice. The Journal of biological chemistry. 2002;277:35532–35540. doi: 10.1074/jbc.M205398200. [DOI] [PubMed] [Google Scholar]
- 102.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. Journal of cellular biochemistry. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 103.Ebihara T, Venkatesan N, Tanaka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Temporal aspects. American journal of respiratory and critical care medicine. 2000;162:1569–1576. doi: 10.1164/ajrccm.162.4.9912011. [DOI] [PubMed] [Google Scholar]
- 104.Krishnan A, Li X, Kao WY, Viker K, Butters K, Masuoka H, Knudsen B, Gores G, Charlton M. Lumican, an extracellular matrix proteoglycan, is a novel requisite for hepatic fibrosis. Laboratory investigation; a journal of technical methods and pathology. 2012;92:1712–1725. doi: 10.1038/labinvest.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mormone E, Lu Y, Ge X, Fiel MI, Nieto N. Fibromodulin, an oxidative stress-sensitive proteoglycan, regulates the fibrogenic response to liver injury in mice. Gastroenterology. 2012;142:612–621. e615. doi: 10.1053/j.gastro.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klotzsch E, Smith ML, Kubow KE, Muntwyler S, Little WC, Beyeler F, Gourdon D, Nelson BJ, Vogel V. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kubow KE, Klotzsch E, Smith ML, Gourdon D, Little WC, Vogel V. Crosslinking of cell-derived 3D scaffolds up-regulates the stretching and unfolding of new extracellular matrix assembled by reseeded cells. Integrative biology: quantitative biosciences from nano to macro. 2009;1:635–648. doi: 10.1039/b914996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. The Journal of cell biology. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. The Journal of pathology. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]


