Abstract
The association of posttraumatic stress disorder (PTSD) with cardiovascular disease risk may be mediated by inflammation. Our objective was to examine the association between PTSD and measures of inflammation and to determine whether these associations are due to shared familial or genetic factors. We measured lifetime history of PTSD using the Structured Clinical Interview for DSM-IV in 238 male middle-aged military veteran twin pairs (476 individuals), selected from the Vietnam Era Twins Registry, who were free of cardiovascular disease at baseline. We assessed inflammation using levels of high-sensitivity C-reactive protein (hsCRP), interleukin 6 (IL-6), fibrinogen, white blood cells, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 (ICAM-1). Geometric mean levels and percent differences by PTSD were obtained from mixed-model linear regression analyses with adjustment for potential confounders. Within-pair analysis was conducted to adjust for shared family environment and genetics (monozygotic pairs). Overall, 12.4% of participants had a lifetime history of PTSD. Adjusted mean levels of hsCRP and ICAM-1 were significantly higher among those with vs. without PTSD [hsCRP: 1.75 vs. 1.31 mg/l (33% difference); ICAM-1: 319 vs. 293 ng/ml (9% difference)]. Adjustment for depression rendered the association of PTSD with hsCRP non-statistically significant. For IL-6, no consistent association was seen. Within-pair analysis produced associations that were similar in direction for all three markers but lesser in magnitude for hsCRP and IL-6. There was no evidence of interaction by zygosity. Elevated hsCRP and ICAM-1 are associated with PTSD, and these associations may be confounded by shared non-genetic, antecedent familial and environmental factors.
Keywords: posttraumatic stress disorder, inflammation, cardiovascular disease, twins, Vietnam veterans
Introduction
Posttraumatic stress disorder (PTSD), a disabling anxiety disorder that is secondary to severe psychological stress, is common among military veterans with combat exposure (Friedman et al., 1994). The lifetime prevalence of PTSD is 15–19% in Vietnam veterans (Dohrenwend et al., 2006), and it is even higher among those who served in the recent Iraq and Afghanistan conflicts (Hoge et al., 2004). PTSD is also common in the general population, with a lifetime prevalence of 10–12% in women and 5–6% in men (Kessler et al., 1994, Yehuda, 2002b). Increasing evidence points to PTSD as risk factor for the development of atherosclerotic cardiovascular disease (Bedi and Arora, 2007, Boscarino, 2008, Player and Peterson, 2011, Kubzansky and Koenen, 2009, Coughlin, 2011). While the proposed mechanisms for this association between PTSD and cardiovascular disease remain primarily speculative (Boscarino, 2011), inflammation may play a role; the inflammatory process is central to the development of atherosclerosis (Libby, 2006, Libby and Theroux, 2005, Rozanski et al., 1999) and the stress response may trigger an inflammatory response (Song et al., 1999, Bierhaus et al., 2003). The establishment of an association between PTSD and inflammatory processes might provide a target for intervention and possibly prevent subsequent cardiovascular morbidity and mortality in those with PTSD.
Thus far, the evidence for an association of PTSD with inflammation is mostly from small studies (n=15–30) in populations with different PTSD etiologies. For example, PTSD was associated with higher levels of the pro-inflammatory markers C-reactive protein (CRP) and interleukin 6 (IL-6) in some studies (Spitzer et al., 2010, von Kanel et al., 2010b, Sutherland et al., 2003, Tucker et al., 2010, Gill et al., 2008), but this association was absent in others (von Kanel et al., 2010b, Sondergaard et al., 2004, McCanlies et al., 2011, von Kanel et al., 2007, Sutherland et al., 2003, Vidovic et al., 2011, Baker et al., 2001). Other potential inflammatory response markers, including fibrinogen (Robicsek et al., 2011, von Kanel et al., 2006), white blood cell (WBC) count (Boscarino and Chang, 1999), and adhesion molecules---including vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) (von Kanel et al., 2010a, von Kanel et al., 2008)---were associated with PTSD in some previous studies, but not consistently. Also, few of these studies (von Kanel et al., 2010b, von Kanel et al., 2006, von Kanel et al., 2008, Gill et al., 2008) controlled for depression, and some did not adjust for any potential confounders (Vidovic et al., 2011, Baker et al., 2001). In addition, it remains possible that observed associations between inflammatory markers and PTSD are at least partially due to common familial and genetic factors that influence both the inflammation response and PTSD. These shared factors, which are potentially confounding but difficult to measure, can nonetheless be controlled in twin analyses, since all twins share early maternal, familial, and environmental factors, and monozygotic (MZ) twins share all genetic factors as well. Our objective was to determine whether lifetime history of PTSD was associated with inflammation. Further, we describe the confounding influence of familial and environmental factors on the association between PTSD and inflammation.
Methods
Study Population
The Emory Twin Studies include samples recruited in two companion studies: the Twins Heart Study (THS) and the Stress and Vascular Evaluation in Twins (SAVEIT). The studies were designed to explore psychological, in addition to behavioral and biologic, risk factors for subclinical cardiovascular disease (Vaccarino et al., 2008a, Vaccarino et al., 2008b, Rooks et al., 2012, Shah et al., 2011). For both studies, male monozygotic (MZ) and dizygotic (DZ) twin pairs born between 1946 and 1956 were recruited from the Vietnam Era Twin (VET) Registry (Goldberg et al., 2002). Identical study protocols were followed. The sample (pooled across studies) included twin pairs where at least one member had PTSD or depression, and control twin pairs with both members free of PTSD and depression. Our sample included 562 twins or 281 twin pairs (170 monozygotic and 111 dizygotic pairs) recruited and tested between 2002 and 2010. Of these, 86 individuals were excluded due to previous cardiovascular disease history (coronary heart disease, myocardial infarction, coronary artery bypass graft, percutaneous coronary angioplasty, cerebrovascular accident, or peripheral vascular disease), leaving 476 individuals or 238 twin pairs. An additional 5–30 individuals were excluded due to missing inflammatory marker information, resulting in a final analysis sample between 446 and 471 individuals, depending on the inflammatory biomarker. The number of complete pairs (both twins without previous history of cardiovascular disease and with available inflammatory marker levels) ranged from 200 to 210.
Twin pairs were examined on the same date at the Emory University General Clinical Research Center and medical history was obtained at the time of examination. The institutional review board at Emory University approved the protocol, and informed consent was obtained from all study participants.
Lifetime History of PTSD
A lifetime history of PTSD was based on a PTSD diagnosis from the Structured Clinical Interview for DSM-IV (SCID) (First MB, 1995). The PTSD-specific module of the SCID contains items assessing: (i) exposure to traumatic events (a major disaster, very serious accident, or fire; being physically assaulted or raped; seeing another person killed or dead, or badly hurt, or hearing about something horrible that has happened to someone you are close to); (ii) reported nightmares, flashbacks, or persistent thoughts in response to the (worst listed) traumatic event; (iii) emotional upset in situations that remind the participant of the event; (iv) ≥1-month duration of these symptoms; and (v) clinically significant distress or impairment in social, occupational, or other important areas of functioning due to the disturbance. Following the PTSD diagnostic algorithm, PTSD is classified as either current (met criteria in month prior to study visit) or past (did not meet criteria in month prior to study visit), and both current and past diagnoses were included in the definition of lifetime history of PTSD.
Inflammatory Markers
All inflammatory markers were measured in plasma from a single blood draw at the time of study examination, and all biochemical assays for each twin pair were processed in the same analytical run. Levels of high-sensitivity CRP (hsCRP) were measured with the high-sensitivity Beckman Coulter assay (Beckman Coulter; Brea, CA). IL-6, VCAM-1, and ICAM-1 were assessed using commercially available enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). White blood cell count was measured with the Beckman Coulter LH 750 hematology analyzer (Beckman Coulter Diagnostics), and fibrinogen was measured by using the Dade Behring BCS coagulation analyzer (Dade Behring Inc., Newark, DE).
Potential Confounders
Potential confounding factors considered in this study included demographics (age), behavioral factors (smoking, alcohol consumption, and physical activity), medications (statins, aspirin), cardiovascular risk factors (hypertension, diabetes, BMI), and lifetime history of depression, measured with the SCID. Physical activity was assessed by means of the Baecke global physical activity score, which summarizes activity related to work, sports, and leisure (Richardson et al., 1995). Smoking status and total alcohol consumption were determined using standardized questionnaires from population studies (Howard et al., 1998, Demirovic et al., 1993). Smoking status was categorized as current, past, and never smoking. Total alcohol consumption (number of drinks within a typical week) included the number of alcoholic (wine, beer, or cocktail) beverages consumed per week. Current medication use was obtained by a trained research nurse during the study interview. Diabetes was defined by a plasma fasting glucose of ≥126 mg/dl or the use of insulin or oral hypoglycemic medications. Hypertension was defined by an average blood pressure ≥140/≥90 mmHg (two measurements 5 minutes apart in seated position after 10 minutes of rest) or the use of antihypertensive medications. Body mass index (BMI) was calculated as: (weight in kg)/(height in m)2. The SCID provided a lifetime diagnosis of major depression. Military service factors (Vietnam theater and combat exposure) were determined from military records via the VET Registry. Zygosity information on the twin pairs was assessed with DNA samples as described previously (Forsberg et al., 2010).
Statistical Analysis
Initial descriptive analyses were performed treating twins as individuals. Characteristics were compared by lifetime history of PTSD using t and χ2 tests according to variable distribution. The association between PTSD and each inflammatory marker was assessed at the individual level, accounting for clustering by twin pair. All inflammatory markers were log-transformed to normalize their right-skewed distributions and pairwise correlations were assessed. The association between PTSD and inflammatory markers was analyzed using mixed-model linear regression analyses modeling inflammatory markers (dependent variables) and PTSD (independent variable), with a random intercept for each pair (Carlin et al., 2005). Geometric mean levels and percent differences by PTSD were obtained from the models. Analyses were conducted before and after sequentially adjusting for groups of variables as described above. Additional analyses were performed to compare geometric mean levels of inflammatory markers and perform tests for trend with PTSD classified into three levels as current, past, or no PTSD. Results were further examined stratified by service in the Vietnam theater to determine whether the associations of inflammatory marker levels with PTSD differed by this exposure. Sensitivity analyses excluding potential outliers (values >3 SD from the geometric mean) and defining depression as ordinal (none, past, and current) were also performed.
Further models separately estimated the PTSD and inflammation associations within twin pairs in complete twin pairs. Within-pair analyses control for shared but unmeasured familial and antecedent environmental factors; when within-pair effects are smaller than the effects seen when twins are analyzed as separate individuals, this points to confounding by factors shared by twin pairs (Carlin et al., 2005). Due to power concerns with small numbers of pairs, these models were adjusted for a smaller set of confounders (age, physical activity, smoking, statins, and BMI), including those that were considered confounders by a priori consideration or by being associated with the exposure (PTSD), as well as with the outcome (P <0.10 in fully-adjusted individual-level models for at least two inflammatory markers). The within-pair analyses were also stratified by zygosity and interaction terms (zygosity × PTSD) were tested to determine whether the relationship between PTSD and inflammatory markers was different between MZ and DZ twins, in whom genetic factors are accounted for completely and partially, respectively. All within-pair analyses were performed in PTSD-discordant pairs (in which one twin has PTSD and the co-twin does not). The association is likely confounded by genetic factors if the within-pair effect is smaller in MZ than in DZ twins (McGue et al., 2010). All statistical analyses were performed using SAS software version 9.3 (SAS Institute, Cary, NC), and the statistical significance threshold was set at two-sided α=0.05.
Results
Characteristics of twins
Overall, 12.4% (59/476) of the study population of twins without previous cardiovascular disease history had a lifetime history of PTSD. Of the 59 twins with lifetime history of PTSD, 25 had current PTSD while 34 had past PTSD. Discordant pairs, in which one twin had PTSD and the other did not, comprised 17.7% of the pairs (42/238, 16.0% of DZ and 20.1% of MZ). The number of discordant pairs with complete information on both co-twins for the inflammatory markers ranged from 29 to 33. There were only 10 pairs (4.2%) where both co-twins had PTSD. Twins were predominantly white (96.2% of pairs). Other characteristics of participating twins are shown in Table 1. Those with PTSD were older by about 2 years, on average; however, other demographics, including educational attainment and marital status did not differ by PTSD status. Those with a lifetime history of PTSD were more likely to smoke and reported about twice as many alcoholic drinks per week, on average, than those without PTSD. Those with PTSD were also more likely to have hypertension and a lifetime history of depression and to have served in the Vietnam theater and to have had exposure to combat than their PTSD-free counterparts. Pairwise correlations between the inflammatory markers were generally weakly to moderately positive and statistically significant (Supplemental Table 1), with the correlations between hsCRP and fibrinogen (ρ=0.53), hsCRP and IL-6 (ρ=0.45), hsCRP and WBC count (ρ=0.41), and IL-6 and ICAM-1 (ρ=0.41) having the largest magnitude (all P<0.001).
Table 1.
Characteristics of Emory Twin Study participants, according to lifetime history of PTSD
| Characteristic | N | No PTSD | PTSD | P* |
|---|---|---|---|---|
| Demographics | ||||
| Mean age (SD), years | 476 | 55.1 (3.2) | 57.4 (2.0) | <0.001 |
| % married | 466 | 98.1% | 96.6% | 0.358 |
| % high school graduate | 476 | 72.4% | 64.4% | 0.202 |
| Behaviors | ||||
| Mean no. of drinks per week (SD) | 473 | 4.6 (8.4) | 8.6 (12.8) | 0.002 |
| Mean Baecke physical activity score (SD) | 474 | 7.4 (1.7) | 7.3 (1.9) | 0.668 |
| % smoking | 475 | 0.021 | ||
| % current smoking | 21.2% | 37.3% | ||
| % past smoking | 43.5% | 32.2% | ||
| Medications | ||||
| % on aspirin | 476 | 19.9% | 20.3% | 0.938 |
| % on statins | 476 | 20.9% | 15.3% | 0.315 |
| Cardiovascular risk factors | ||||
| Mean BMI | 475 | 29.4 (4.9) | 29.9 (4.4) | 0.462 |
| % with diabetes | 476 | 8.6% | 8.5% | 0.968 |
| % with hypertension | 476 | 45.8% | 64.4% | 0.007 |
| Psychiatric conditions | ||||
| % with history of depression | 474 | 20.0% | 54.2% | <0.001 |
| Military service | ||||
| % in Vietnam theater | 476 | 39.6% | 86.4% | <0.001 |
| % with combat exposure | 476 | 29.3% | 86.4% | <0.001 |
By t test (continuous variables) and χ2 or Fisher’s exact test (categorical variables).
Overall association of PTSD with inflammatory markers
Among twins treated as separate individuals, age-adjusted levels of hsCRP and ICAM-1 were significantly higher among twins with a history of PTSD compared to those without a history of PTSD (Table 2), with the age-adjusted percent difference being 50.9% for hsCRP and 11.4% for ICAM-1 (Figure 1). Additionally, those with PTSD tended to have lower age-adjusted levels of IL-6 than their counterparts without PTSD (percent difference, −13.2%; Figure 1), but the difference was not statistically significant. Of the remaining inflammatory markers examined, none had a substantial or statistically significant difference in age-adjusted levels by PTSD status (Table 2).
Table 2.
Adjusted mean* level of inflammatory markers, according to lifetime history of PTSD
| Marker (units) | N | Age-Adjusted | Fully Adjusted** | Fully Adjusted + Depression*** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean, No PTSD (95% CI) | Mean, PTSD (95% CI) | P | Mean, No PTSD (95% CI) | Mean, PTSD (95% CI) | P | Mean, No PTSD (95% CI) | Mean, PTSD (95% CI) | P | ||
| hsCRP (mg/l) | 471 | 1.30 (1.14–1.50) | 1.97 (1.46–2.65) | 0.008 | 1.31 (1.16–1.49) | 1.77 (1.34–2.34) | 0.041 | 1.31 (1.16–1.49) | 1.75 (1.31–2.34) | 0.060 |
| IL-6 (pg/ml) | 468 | 1.60 (1.47–1.74) | 1.39 (1.12–1.71) | 0.212 | 1.61 (1.48–1.74) | 1.26 (1.01–1.56) | 0.032 | 1.62 (1.49–1.75) | 1.25 (1.00–1.56) | 0.031 |
| Fibrinogen (mg/dl) | 445 | 340 (331–349) | 345 (323–369) | 0.993 | 340 (332–349) | 339 (317–361) | 0.564 | 341 (332–350) | 333 (312–356) | 0.346 |
| WBC (103/μ1) | 446 | 6.19 (6.01–6.38) | 6.26 (5.83–6.71) | 0.790 | 6.21 (6.04–6.38) | 6.14 (5.74–6.56) | 0.754 | 6.22 (6.05–6.39) | 6.03 (5.62–6.46) | 0.398 |
| VCAM-1 (ng/ml) | 470 | 589 (567–611) | 598 (546–654) | 0.898 | 586 (567–606) | 609 (557–667) | 0.795 | 589 (567–612) | 602 (549–660) | 0.596 |
| ICAM-1 (ng/ml) | 470 | 292 (284–303) | 324 (300–349) | 0.004 | 294 (285–303) | 314 (292–337) | 0.040 | 294 (285–303) | 315 (292–340) | 0.024 |
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; WBC, white blood cell; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
Geometric means.
Adjusted for age, physical activity, current smoking, alcohol intake, aspirin use, statin use, diabetes, hypertension, and body mass index.
Adjusted for age, physical activity, current smoking, alcohol intake, aspirin use, statin use, diabetes, hypertension, body mass index, and lifetime history of depression.
Figure 1.
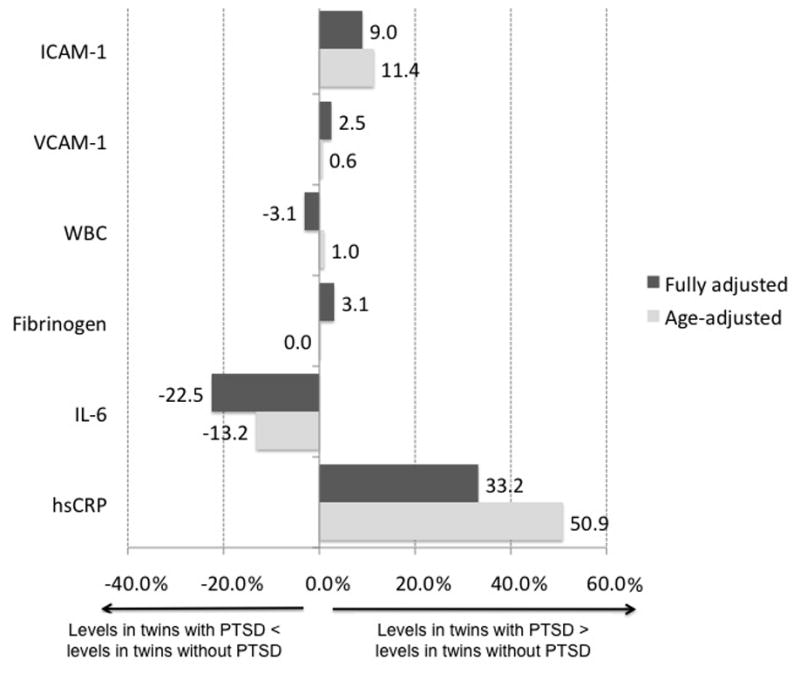
Percent difference in mean value of inflammatory marker for twins with PTSD vs. without PTSD, with twins treated as individuals. Values are plotted for age-adjusted and fully-adjusted (including depression) differences by PTSD. P values can be found in Table 2. hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; WBC, white blood cell; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
With full adjustment, the association of hsCRP with PTSD became less pronounced (percent difference, 33.2%); inclusion of depression in the model attenuated the association between PTSD and hsCRP (Table 2). ICAM-1, however, remained positively associated with PTSD with a slightly lower percent difference with full adjustment (9.0%; Figure 1). In contrast, lower levels of IL-6 were significantly associated with PTSD (−22.5% difference) in fully adjusted models. Significant or marginally significant potential confounders in fully adjusted models (including depression) included: smoking (positively associated with all inflammatory markers), physical activity (negatively associated with hsCRP and fibrinogen), statin use (negatively associated with hsCRP and ICAM-1), BMI (positively associated with IL-6, fibrinogen, and WBC count), and age (negatively associated with IL-6 and ICAM-1). Sensitivity analyses excluding outliers showed nearly identical fully adjusted levels of inflammatory markers for no PTSD vs. PTSD (data not shown). Similarly, sensitivity analyses examining depression as an ordinal rather than dichotomous variable made no difference in the fully adjusted levels of inflammatory markers comparing no PTSD to PTSD (data not shown).
When we examined a three-level ordinal indicator of PTSD (current, past, and never), we found a statistically significant increasing trend of association of PTSD with hsCRP, such that those with current PTSD had higher levels of hsCRP than those with past PTSD, and those with past PTSD had higher levels than those who had never had PTSD. These results remained significant after adjusting for behavioral and cardiovascular risk factors and depression (Table 3). In contrast, current and past PTSD showed similar levels of ICAM-1, although levels in those with current and past PTSD were higher than those who never had PTSD (Table 3). The ordinal PTSD measure was not associated with IL-6.
Table 3.
Adjusted mean level of inflammatory markers, according to PTSD status (no PTSD, past PTSD, and current PTSD).
| Marker | N | Mean (95% CI) | ||
|---|---|---|---|---|
| Age-Adjusted | Fully Adjusted** | Fully Adjusted*** | ||
| hsCRP (mg/l) | 471 | |||
| No PTSD | 1.30 (1.14, 1.49) | 1.31 (1.16, 1.48) | 1.31 (1.16, 1.49) | |
| Past PTSD | 1.53 (1.07, 2.19) | 1.42 (1.01, 1.99) | 1.40 (1.00, 1.98) | |
| Current PTSD | 2.87 (1.86, 4.41) | 2.47 (1.64, 3.71) | 2.45 (1.61, 3.71) | |
| Ptrend | <0.001 | 0.007 | 0.010 | |
| IL-6 (pg/ml) | 468 | |||
| No PTSD | 1.59 (1.47, 1.73) | 1.61 (1.48, 1.74) | 1.61 (1.49, 1.75) | |
| Past PTSD | 1.24 (0.95, 1.63) | 1.12 (0.85, 1.47) | 1.11 (0.85, 1.47) | |
| Current PTSD | 1.61 (1.17, 2.21) | 1.48 (1.07, 2.03) | 1.47 (1.06, 2.04) | |
| Ptrend | 0.470 | 0.131 | 0.136 | |
| Fibrinogen (mg/dl) | 445 | |||
| No PTSD | 340 (331, 350) | 341 (333, 350) | 341 (333, 350) | |
| Past PTSD | 331 (306, 358) | 326 (302, 352) | 323 (299, 349) | |
| Current PTSD | 355 (324, 388) | 348 (318, 380) | 343 (313, 375) | |
| Ptrend | 0.647 | 0.921 | 0.651 | |
| WBC (103/μ1) | 446 | |||
| No PTSD | 6.19 (6.01, 6.38) | 6.21 (6.04, 6.38) | 6.22 (6.05, 6.39) | |
| Past PTSD | 6.21 (5.68, 6.8) | 6.08 (5.59, 6.62) | 6 (5.51, 6.53) | |
| Current PTSD | 6.31 (5.71, 6.97) | 6.21 (5.65, 6.84) | 6.07 (5.51, 6.69) | |
| Ptrend | 0.735 | 0.880 | 0.488 | |
| VCAM-1 (ng/ml) | 470 | |||
| No PTSD | 589 (567, 612) | 588 (566, 611) | 588 (566, 611) | |
| Past PTSD | 601 (541, 669) | 595 (537, 659) | 601 (541, 667) | |
| Current PTSD | 578 (509, 658) | 595 (525, 675) | 605 (533, 688) | |
| Ptrend | 0.952 | 0.806 | 0.599 | |
| ICAM-1 (ng/ml) | 470 | |||
| No PTSD | 292 (283, 302) | 293 (284, 302) | 293 (284, 302) | |
| Past PTSD | 325 (298, 354) | 314 (288, 342) | 317 (290, 345) | |
| Current PTSD | 327 (294, 364) | 320 (288, 355) | 324 (292, 360) | |
| Ptrend | 0.007 | 0.045 | 0.027 | |
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; WBC, white blood cell; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
Geometric means.
Adjusted for age, physical activity, current smoking, alcohol intake, aspirin use, statin use, diabetes, hypertension, and body mass index.
Adjusted for age, physical activity, current smoking, alcohol intake, aspirin use, statin use, diabetes, hypertension, body mass index, and lifetime history of depression.
Finally, the age-adjusted geometric mean levels of all inflammatory markers by PTSD were examined in models stratified by Vietnam theater service and combat exposure. The associations with PTSD did not differ by Vietnam theater service or combat exposure, and tests for interactions (Vietnam service × PTSD and combat exposure × PTSD) were non-statistically significant for all markers.
Within-twin pair association of PTSD with inflammatory markers
When the twin pairs who were discordant for lifetime history of PTSD were analyzed comparing each twin with PTSD to his brother without PTSD, the associations of most markers with PTSD were generally similar in direction but smaller in magnitude, compared to those seen in all twins (Table 4). hsCRP was not significantly associated with PTSD among these discordant twins (Table 4). Only ICAM-1 was significantly and positively associated with PTSD in the within-pair analysis, and the magnitude of this association did not differ substantially from that found in the analysis of twins as individuals. None of the remaining markers, including IL-6, were associated with PTSD in within-pair analyses. Models that were stratified by zygosity (Supplemental Table 2) showed that, in general, effects did not appear to differ in MZ and DZ twin pairs, and there was no evidence of statistically significant zygosity-mediated effect modification for any of the inflammatory markers, although sample sizes were small (12–19 pairs) for these analyses.
Table 4.
Mean* level of inflammatory markers, by PTSD status, within twin pairs discordant for PTSD
| Inflammatory Marker | N** | Age-adjusted | Adjusted*** | ||||
|---|---|---|---|---|---|---|---|
| Mean, No PTSD (95% CI) | Mean, PTSD (95% CI) | P | Mean, No PTSD (95% CI) | Mean, PTSD (95% CI) | P | ||
| hsCRP (mg/l) | 33 | 1.46 (1.01–2.11) | 1.88 (1.30–2.72) | 0.18 | 1.58 (1.12–2.23) | 1.76 (1.25–2.49) | 0.55 |
| IL-6 (pg/ml) | 33 | 1.27 (0.98–1.63) | 1.29 (1.00–1.66) | 0.89 | 1.30 (1.02–1.66) | 1.21 (0.95–1.54) | 0.45 |
| Fibrinogen (mg/dl) | 29 | 335 (305–369) | 347 (305–370) | 0.40 | 336 (305–370) | 344 (312–379) | 0.48 |
| WBC (103/μ1) | 29 | 6.34 (5.71–7.04) | 6.51 (5.86–7.23) | 0.64 | 6.57 (6.02–7.18) | 6.23 (5.7–6.81) | 0.29 |
| VCAM-1 (ng/ml) | 33 | 619 (554–693) | 609 (554–691) | 0.67 | 619 (554–691) | 588 (527–657) | 0.17 |
| ICAM-1 (ng/ml) | 33 | 271 (244–300) | 305 (248–296) | 0.002 | 271 (248–296) | 298 (273–325) | 0.005 |
hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; WBC, white blood cell; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
Geometric means.
Number of discordant twin pairs.
Adjusted for age, physical activity, current smoking, statin use, and body mass index.
Discussion
We found that both hsCRP and ICAM-1 were higher among twins with PTSD than among those without PTSD. hsCRP was particularly high in twins with current PTSD, with a gradation of effects across levels of PTSD classified as never, past, or current PTSD. Adjustment for depression rendered the association between PTSD and hsCRP less statistically significant, although it remains unknown whether depression has a confounding effect or is, in fact, in the causal pathway between PTSD and inflammation. Conversely, no consistent association was found between PTSD and IL-6 in our analyses, and no meaningful trend in IL-6 was observed across categories of never, past, or current PTSD. There was no evidence of effect modification by Vietnam theater service for hsCRP, IL-6, or ICAM-1. The remaining examined markers---fibrinogen, WBC count, and VCAM-1---were not associated with PTSD.
When we examined the within-twin pair associations of PTSD with the inflammatory markers, we found that these effects were smaller in magnitude than the overall effects for most markers, with the exception of ICAM-1, providing evidence of familial confounding in the associations of these inflammatory markers with PTSD. Since DZ twins, like any full siblings, share, on average, 50% of their genes, while MZ twins share 100% of their genes, we would expect that the magnitude of the associations would be lessened among MZ compared to DZ twins if genetic factors were confounders. However, zygosity-stratified analyses showed no evidence of interaction for any of the markers and estimates were generally similar or even higher among DZ than MZ twin pairs. Thus, it is likely that most of the shared familial factors that confound the associations are not genetic in nature.
Of all the markers we examined, the association of hsCRP with PTSD had the largest magnitude, with levels being about one-third higher in those with vs. without PTSD after adjustment for several potential confounders, including depression. Accounting for both measured confounders and unmeasured early-life shared factors, levels remained >11% higher in those with PTSD. These results confirm previous data from a population-based study in Germany, which found greater odds of elevated hsCRP among those with PTSD, although this association was not adjusted for depression (Spitzer et al., 2010). Other previous studies found that PTSD was associated with lower, rather than higher hsCRP levels, or that hsCRP was not associated with PTSD at all (von Kanel et al., 2010b, Sondergaard et al., 2004, Sutherland et al., 2003, McCanlies et al., 2011, von Kanel et al., 2007). However, these studies tended to be small and none adjusted for a full array of potential confounding factors.
We also found that IL-6 was negatively associated with lifetime history of PTSD, although the result was not confirmed in within-twin pair analyses. Previous studies have been inconsistent regarding an association between IL-6 and PTSD (von Kanel et al., 2010b, Sutherland et al., 2003, Tucker et al., 2010, Gill et al., 2008, McCanlies et al., 2011, Vidovic et al., 2011, Baker et al., 2001). One possible explanation may be related to the complex biological properties of IL-6: IL-6 can activate the immune system but it also has anti-inflammatory properties in the acute phase of the stress reaction (Scheller et al., 2011, Xing et al., 1998). We observed that IL-6 tended to be lower in twins with past, but not current, PTSD. Although the reasons for this association are unknown, the low levels of IL-6 may reflect long-term suppression of the anti-inflammatory IL-6 response due to a hypersensitive hypothalamus-pituitary-adrenal axis (Yehuda, 2002a). It may also be that those who recover from PTSD, or who are exposed to a single traumatic event rather than multiple events (Handwerger, 2009), are more resilient, and thus better able to counteract PTSD-related immune dysfunction, than those who do not. Another possible explanation for these inconsistencies is that men may be more likely to inhibit the pro-inflammatory response of IL-6 than women (Rohleder et al., 2001). Four of six studies that included women (Gill et al., 2008, von Kanel et al., 2010b, Sutherland et al., 2003, Tucker et al., 2010, McCanlies et al., 2011, Vidovic et al., 2011, Baker et al., 2001) found a positive association of PTSD with IL-6, whereas one prior study including only men (Vidovic et al., 2011) showed no association. Clearly, this unexpected but potentially spurious finding needs to be confirmed in other samples, and, if the finding is confirmed, the role of IL-6 in the neuroimmunology of PTSD may need further clarification.
ICAM-1 is elevated in response to inflammatory cytokines and may directly contribute to the atherosclerotic process over time via the binding of leukocytes to endothelial cells. We found that, after adjustment for potential confounders and even after accounting for shared early-life familial and environmental factors in within-twin pair analyses, levels of ICAM-1 were 9% higher among those with PTSD compared to their PTSD-free counterparts. To our knowledge, this observed association was only reported once before (von Kanel et al., 2010a), in a study of persons with PTSD secondary to myocardial infarction. Another study by the same group (von Kanel et al., 2008), however, found no association of ICAM-1 with PTSD, although the study was limited by its small size (14 cases and 14 controls).
Fibrinogen, a coagulation factor that is also involved in the inflammation response, has thus far shown no evidence of being associated with PTSD (Robicsek et al., 2011, von Kanel et al., 2006), and our results confirmed this lack of association. In our study, WBC count and VCAM-1 were also not associated with lifetime history of PTSD. Few previous studies have examined these inflammatory biomarkers in relation to PTSD (Boscarino and Chang, 1999, von Kanel et al., 2010a). While these studies reported significant associations, their results are difficult to compare with ours due to differing etiologies and definitions of PTSD.
There are several study limitations worthy of mention. First, the cross-sectional design precludes assessment of a temporal relationship between PTSD and subsequent inflammation and also does not exclude the possibility that inflammation may lead to increased susceptibility to PTSD. There is also the possibility of misclassification in both PTSD and the inflammatory markers. A dichotomous diagnosis of PTSD may not capture the severity, duration, and/or recency of PTSD symptoms, and, importantly, elevations of inflammatory markers at a single time point may not represent chronic inflammation. Furthermore, although we excluded individuals with extant CVD to minimize inflammation due to active disease, we cannot be sure that individuals with subclinical CVD were not included in the study. Also, our study population included only male middle-aged veterans and the results may not be generalizable to female or to civilian populations of different age groups. Sample sizes in within-twin analyses, particularly those stratified by zygosity, were small, which limited our power to detect differences by PTSD and our ability to make inferences regarding the influence of genetic and non-genetic familial factors. The small sample sizes of PTSD-discordant twin pairs also precluded the examination of the ordinal PTSD variable in the within-pairs analyses and, consequently, potential differential familiar influences between past and current PTSD. Additionally, many of the inflammatory markers were weakly to moderately positively correlated; thus, it is possible that the chosen alpha of 0.05 may be too high and that borderline statistically significant results should be interpreted with caution. Finally, as with any observational study, there may be residual confounding; however, the ability to control for confounding due to unknown familial factors is one of the greatest strengths of our twin study design. Other strengths of our study include the relatively large sample size for individual-level analysis, the examination of several inflammatory biomarkers, and available data on a wide variety of potential confounders, including lifetime history of depression. Additionally, our results suggesting that the effects of PTSD may be independent of familial factors mirror those seen with twin studies of PTSD and inflammatory conditions such as rheumatoid arthritis (Boscarino et al., 2010) and asthma (Goodwin et al., 2007).
In conclusion, using an array of inflammatory biomarkers, we were able to discover that individuals with PTSD, compared with those without, have elevated levels of hsCRP and ICAM-1, while IL-6, fibrinogen, VCAM-1, and WBC are not similarly elevated in PTSD. Inflammatory abnormalities in PTSD may be at least partially due to unknown familial, possibly non-genetic, factors. Our results indicate that the link between PTSD and inflammation is complex and is in part influenced by shared environmental substrates that predispose individuals to both PTSD and alterations in inflammation.
Supplementary Material
Supplemental Table 1. Pairwise correlations between log-transformed inflammatory marker levels in Emory Twin Studies participants
Supplemental Table 2. Age-adjusted mean* inflammatory marker levels, by PTSD, within twin pairs discordant for PTSD
Figure 2.
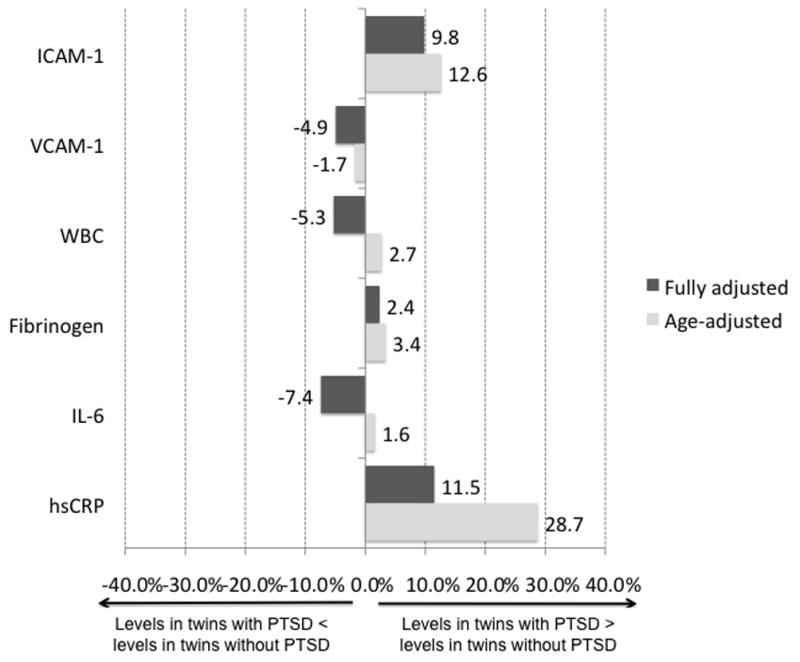
Within-twin pair percent difference in mean value of inflammatory marker in PTSD-discordant pairs. Values are plotted for age-adjusted and fully-adjusted differences by PTSD. P values can be found in Table 3. hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; WBC, white blood cell; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
Acknowledgments
We thank the continued cooperation and participation of the members of the VET Registry. This study was supported by K24HL077506, R01 HL68630, R01 AG026255, and National Institutes of Health (NIH)/National Institute of General Medical Sciences Institutional Research and Academic Career Development Awards Grant 5K12 GM000680 from the NIH; by the Emory University General Clinical Research Center MO1-RR00039; and by Grant 0245115N from the American Heart Association. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry. Numerous organizations have provided invaluable assistance, including VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University.
Footnotes
Conflict of Interest Statement
All authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- BAKER DG, EKHATOR NN, KASCKOW JW, HILL KK, ZOUMAKIS E, DASHEVSKY BA, CHROUSOS GP, GERACIOTI TD., JR Plasma and cerebrospinal fluid interleukin-6 concentrations in post-traumatic stress disorder. Neuroimmunomodulation. 2001;9:209–17. doi: 10.1159/000049028. [DOI] [PubMed] [Google Scholar]
- BEDI US, ARORA R. Cardiovascular manifestations of post-traumatic stress disorder. J Natl Med Assoc. 2007;99:642–9. [PMC free article] [PubMed] [Google Scholar]
- BIERHAUS A, WOLF J, ANDRASSY M, ROHLEDER N, HUMPERT PM, PETROV D, FERSTL R, VON EYNATTEN M, WENDT T, RUDOFSKY G, JOSWIG M, MORCOS M, SCHWANINGER M, MCEWEN B, KIRSCHBAUM C, NAWROTH PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSCARINO JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–76. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSCARINO JA. Post-traumatic stress disorder and cardiovascular disease link: time to identify specific pathways and interventions. Am J Cardiol. 2011;108:1052–3. doi: 10.1016/j.amjcard.2011.07.003. [DOI] [PubMed] [Google Scholar]
- BOSCARINO JA, CHANG J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med. 1999;61:378–86. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- BOSCARINO JA, FORSBERG CW, GOLDBERG J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–6. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- CARLIN JB, GURRIN LC, STERNE JA, MORLEY R, DWYER T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–99. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- COUGHLIN SS. Post-traumatic Stress Disorder and Cardiovascular Disease. Open Cardiovasc Med J. 2011;5:164–70. doi: 10.2174/1874192401105010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMIROVIC J, NABULSI A, FOLSOM AR, CARPENTER MA, SZKLO M, SORLIE PD, BARNES RW. Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Circulation. 1993;88:2787–93. doi: 10.1161/01.cir.88.6.2787. [DOI] [PubMed] [Google Scholar]
- DOHRENWEND BP, TURNER JB, TURSE NA, ADAMS BG, KOENEN KC, MARSHALL R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313:979–82. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIRST MBSR, WILLIAMS JBW, GIBBON M. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1995. Patient Edition (SCID-P) ed. [Google Scholar]
- FORSBERG CW, GOLDBERG J, SPORLEDER J, SMITH NL. Determining zygosity in the Vietnam era twin registry: an update. Twin Res Hum Genet. 2010;13:461–4. doi: 10.1375/twin.13.5.461. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN MJ, SCHNURR PP, MCDONAGH-COYLE A. Posttraumatic stress disorder in the military veteran. Psychiatr Clin North Am. 1994;17:265–77. [PubMed] [Google Scholar]
- GILL J, VYTHILINGAM M, PAGE GG. Low cortisol, high DHEA, and high levels of stimulated TNF-alpha, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–9. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG J, CURRAN B, VITEK ME, HENDERSON WG, BOYKO EJ. The Vietnam Era Twin Registry. Twin Res. 2002;5:476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- GOODWIN RD, FISCHER ME, GOLDBERG J. A twin study of posttraumatic stress disorder symptoms and asthma. Am J Respir Crit Care Med. 2007;176:983–7. doi: 10.1164/rccm.200610-1467OC. [DOI] [PubMed] [Google Scholar]
- HANDWERGER K. Differential patterns of HPA activity and reactivity in adult posttraumatic stress disorder and major depressive disorder. Harv Rev Psychiatry. 2009;17:184–205. doi: 10.1080/10673220902996775. [DOI] [PubMed] [Google Scholar]
- HOGE CW, CASTRO CA, MESSER SC, MCGURK D, COTTING DI, KOFFMAN RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- HOWARD G, WAGENKNECHT LE, BURKE GL, DIEZ-ROUX A, EVANS GW, MCGOVERN P, NIETO FJ, TELL GS. Cigarette smoking and progression of atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. JAMA. 1998;279:119–24. doi: 10.1001/jama.279.2.119. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, MCGONAGLE KA, ZHAO S, NELSON CB, HUGHES M, ESHLEMAN S, WITTCHEN HU, KENDLER KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- KUBZANSKY LD, KOENEN KC. Is posttraumatic stress disorder related to development of heart disease? An update. Cleve Clin J Med. 2009;76(Suppl 2):S60–5. doi: 10.3949/ccjm.76.s2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIBBY P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- LIBBY P, THEROUX P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- MCCANLIES EC, ARAIA SK, JOSEPH PN, MNATSAKANOVA A, ANDREW ME, BURCHFIEL CM, VIOLANTI JM. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–8. doi: 10.1016/j.cyto.2011.03.025. [DOI] [PubMed] [Google Scholar]
- MCGUE M, OSLER M, CHRISTENSEN K. Causal Inference and Observational Research: The Utility of Twins. Perspect Psychol Sci. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLAYER MS, PETERSON LE. Anxiety disorders, hypertension, and cardiovascular risk: a review. Int J Psychiatry Med. 2011;41:365–77. doi: 10.2190/PM.41.4.f. [DOI] [PubMed] [Google Scholar]
- RICHARDSON MT, AINSWORTH BE, WU HC, JACOBS DR, JR, LEON AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- ROBICSEK O, MAKHOUL B, KLEIN E, BRENNER B, SARIG G. Hypercoagulation in chronic post-traumatic stress disorder. Isr Med Assoc J. 2011;13:548–52. [PubMed] [Google Scholar]
- ROHLEDER N, SCHOMMER NC, HELLHAMMER DH, ENGEL R, KIRSCHBAUM C. Sex differences in glucocorticoid sensitivity of proinflammatory cytokine production after psychosocial stress. Psychosom Med. 2001;63:966–72. doi: 10.1097/00006842-200111000-00016. [DOI] [PubMed] [Google Scholar]
- ROOKS C, VELEDAR E, GOLDBERG J, BREMNER JD, VACCARINO V. Early trauma and inflammation: role of familial factors in a study of twins. Psychosom Med. 2012;74:146–52. doi: 10.1097/PSY.0b013e318240a7d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROZANSKI A, BLUMENTHAL JA, KAPLAN J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- SCHELLER J, CHALARIS A, SCHMIDT-ARRAS D, ROSE-JOHN S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- SHAH AJ, SU S, VELEDAR E, BREMNER JD, GOLDSTEIN FC, LAMPERT R, GOLDBERG J, VACCARINO V. Is heart rate variability related to memory performance in middle-aged men? Psychosom Med. 2011;73:475–82. doi: 10.1097/PSY.0b013e3182227d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONDERGAARD HP, HANSSON LO, THEORELL T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342:93–8. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- SONG C, KENIS G, VAN GASTEL A, BOSMANS E, LIN A, DE JONG R, NEELS H, SCHARPE S, JANCA A, YASUKAWA K, MAES M. Influence of psychological stress on immune-inflammatory variables in normal humans. Part II. Altered serum concentrations of natural anti-inflammatory agents and soluble membrane antigens of monocytes and T lymphocytes. Psychiatry Res. 1999;85:293–303. doi: 10.1016/s0165-1781(99)00012-8. [DOI] [PubMed] [Google Scholar]
- SPITZER C, BARNOW S, VOLZKE H, WALLASCHOFSKI H, JOHN U, FREYBERGER HJ, LOWE B, GRABE HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- SUTHERLAND AG, ALEXANDER DA, HUTCHISON JD. Disturbance of pro-inflammatory cytokines in post-traumatic psychopathology. Cytokine. 2003;24:219–25. doi: 10.1016/j.cyto.2003.09.004. [DOI] [PubMed] [Google Scholar]
- TUCKER P, JEON-SLAUGHTER H, PFEFFERBAUM B, KHAN Q, DAVIS NJ. Emotional and biological stress measures in Katrina survivors relocated to Oklahoma. Am J Disaster Med. 2010;5:113–25. doi: 10.5055/ajdm.2010.0013. [DOI] [PubMed] [Google Scholar]
- VACCARINO V, BRENNAN ML, MILLER AH, BREMNER JD, RITCHIE JC, LINDAU F, VELEDAR E, SU S, MURRAH NV, JONES L, JAWED F, DAI J, GOLDBERG J, HAZEN SL. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008a;64:476–83. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VACCARINO V, LAMPERT R, BREMNER JD, LEE F, SU S, MAISANO C, MURRAH NV, JONES L, JAWED F, AFZAL N, ASHRAF A, GOLDBERG J. Depressive symptoms and heart rate variability: evidence for a shared genetic substrate in a study of twins. Psychosom Med. 2008b;70:628–36. doi: 10.1097/PSY.0b013e31817bcc9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIDOVIC A, GOTOVAC K, VILIBIC M, SABIONCELLO A, JOVANOVIC T, RABATIC S, FOLNEGOVIC-SMALC V, DEKARIS D. Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation. 2011;18:199–211. doi: 10.1159/000322869. [DOI] [PubMed] [Google Scholar]
- VON KANEL R, ABBAS CC, BEGRE S, SANER H, GANDER ML, SCHMID JP. Posttraumatic stress disorder and soluble cellular adhesion molecules at rest and in response to a trauma-specific interview in patients after myocardial infarction. Psychiatry Res. 2010a;179:312–7. doi: 10.1016/j.psychres.2009.06.005. [DOI] [PubMed] [Google Scholar]
- VON KANEL R, BEGRE S, ABBAS CC, SANER H, GANDER ML, SCHMID JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010b;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- VON KANEL R, HEPP U, BUDDEBERG C, KEEL M, MICA L, ASCHBACHER K, SCHNYDER U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom Med. 2006;68:598–604. doi: 10.1097/01.psy.0000221229.43272.9d. [DOI] [PubMed] [Google Scholar]
- VON KANEL R, HEPP U, KRAEMER B, TRABER R, KEEL M, MICA L, SCHNYDER U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–52. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- VON KANEL R, HEPP U, TRABER R, KRAEMER B, MICA L, KEEL M, MAUSBACH BT, SCHNYDER U. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158:363–73. doi: 10.1016/j.psychres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- XING Z, GAULDIE J, COX G, BAUMANN H, JORDANA M, LEI XF, ACHONG MK. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–20. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YEHUDA R. Post-traumatic stress disorder. N Engl J Med. 2002a;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- YEHUDA R. Post-traumatic stress disorder. New England Journal of Medicine. 2002b;346:108–114. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Pairwise correlations between log-transformed inflammatory marker levels in Emory Twin Studies participants
Supplemental Table 2. Age-adjusted mean* inflammatory marker levels, by PTSD, within twin pairs discordant for PTSD


