Abstract
The adrenal steroid, dehydroepiandrosterone sulfate (DHEAS), is generally regarded as being a reliable endocrine marker of aging, because in humans and nonhuman primates its circulating concentrations are very high during young adulthood, and the concentrations then decline markedly during aging. Despite promising results from early studies, we were recently surprised to find that caloric restriction (CR) did little to prevent or delay the decline of DHEAS concentrations in old rhesus macaques. Here we summarize the use of circulating DHEAS concentrations as a biomarker of aging in CR studies and suggest reasons for its limited value. Although DHEAS can reliably predict aging in animals maintained on a standard diet, dietary manipulations may affect liver enzymes involved in the metabolism of steroid hormones. Consequently, in CR studies the reliability of using DHEAS as a biomarker of aging may be compromised.
Keywords: Adrenal gland, Biomarker, Cortisol, DHEAS, Rhesus macaque
1. Caloric Restriction and aging
According to the US Census Bureau, almost 20% of the US population is expected to be 65 years of age and older by 2030. Clearly, this demographic change will have a major impact on society’s future health care needs and on the economy of the country. Consequently, there is an immediate need for a better understanding of mechanisms that underlie normal and pathological human aging, and for the development of safe and effective therapies. To date, however, dietary caloric restriction (CR) remains the only reproducible, non-genetic intervention that has been shown to limit or delay deleterious aging processes in mammals. A moderate (30–50%) reduction in caloric intake, without malnutrition, has been shown to extend average and maximal lifespans, to reduce the incidence of age-related diseases, and to maintain healthy physiological function later into life in short-lived species (Weindruch and Sohal, 1997; Weindruch and Walford, 1988).
The first report of the lifespan extending effects of CR appeared over 75 years ago (McCay et al., 1935). Since then, numerous studies have advanced CR research, and have generated progress toward elucidating possible mechanisms underlying the anti-aging benefits of CR (Bartke et al., 2001; Gredilla and Barja, 2005; Heilbronn and Ravussin, 2003). Despite the large body of evidence describing the benefits of CR in short-lived species, including yeast, nematode worms, flies, fish, dogs, and rodents (Barrows and Kokkonen, 1982; Weindruch and Walford, 1988), it remains to be determined whether CR is relevant to human or non-human primate aging (Colman et al., 2009; Lane et al., 1997b, 2001; Masoro, 2000; Mattison et al., 2005, 2012; Ramsey et al., 2000; Roth et al., 2004; Weindruch and Sohal, 1997; Lane, 2000; Mattison et al., 2003; Roth et al., 2001).
2. The Rhesus macaque as a translational animal model for biogerontological studies
Like humans, rhesus macaque monkeys (Macaca mulatta) are long-lived primates, and they show many similarities in their anatomy, physiology behavior, and genetics (Gibbs et al., 2007; Messaoudi et al., 2011). Consequently, rhesus monkeys are regarded as pragmatic animal models for studying mechanisms that underlie normal and pathological human development and aging. Their use as translational animal models has many advantages. For example, rhesus monkeys can be maintained under carefully controlled environmental conditions (e.g., photoperiod, temperature, diet, and medication). In addition, animals of a specific age, size, sex, and genetic characteristic can be selected, thereby eliminating extraneous variables and self-selection bias that are typically associated with human clinical trials. Moreover, because the timing of necropsies can be carefully controlled in rhesus macaques, high quality postmortem RNA samples can be collected for gene expression profiling.
Cross-sectional and longitudinal studies of the effects of CR on longevity and aging in the rhesus monkey began in 1987 (Ingram et al., 1990). However, with a maximum lifespan of ~40 years (Bliwise, 2000; Bodkin et al., 2003; Roth et al., 2004), several more years of study will be necessary before a consensus is reached on possible CR effects on rhesus lifespan. On the one hand, ongoing studies at the Wisconsin National Primate Research Center (WNPRC) and at the National Institute on Aging (NIA) have both demonstrated health benefits associated with CR in adult rhesus monkeys (Colman et al., 2009; Mattison et al., 2012). On the one hand, some discrepancies exist between the findings of these two studies; improved survival was reported for the CR monkeys in the WNPRC study (Colman et al., 2009) but not in the NIA study (Mattison et al., 2012), suggesting that there may be separation between health benefits, morbidity, and mortality. Consequently, the availability of relevant biomarkers of aging are critical to furthering the understanding of the effects of CR on the aging process, especially to assess effects in long lived species like primates (Ingram et al., 2001; Nakamura et al., 1994, 1998).
3. Adrenal steroids as biomarkers of aging
The adrenal steroids -- cortisol, dehydropiandrosterone (DHEA) and DHEA sulfate (DHEAS) -- play an important role in regulating responses to stress. Elevated cortisol suppresses the immune system, breaks down tissues and has a general catabolic effect; whereas, DHEA and DHEAS counter-balance the effects of cortisol by activating the immune system and building up tissues. Importantly, cortisol and DHEAS are two of the most abundant steroids in the circulation of adult humans and rhesus monkeys. Furthermore, DHEAS shows a profound age-related decrease, while cortisol levels remain constant or increase (Downs et al., 2008; Labrie, 1997; Lane et al., 1997a; Orentreich et al., 1992; Roth et al., 2004; Urbanski et al., 2004; Urbanski and Sorwell, 2012; Sorwell and Urbanski, 2010). Based on longitudinal DHEAS measurements across the life span of adult Japanese macaques, this age-related decline appears to be gradual rather than occurring precipitously during old age (Sorwell et al., 2012); consequently, it is difficult to link it causally to a specific age-associated physiological event. Nevertheless, there is evidence that DHEA and DHEAS play an important role in attenuating some of the deleterious effects of elevated cortisol levels that are associated with chronic stress. For example, in aged animals, the DHEA:cortisol ratio shows a marked age-associated decline, and it has been suggested that the unopposed high cortisol levels play a key role in age-associated cognitive decline, impaired attention span, and loss of long-term memory (van Niekerk et al., 2001; Karishma and Herbert, 2002). In contrast, in young adults the highly elevated DHEA and DHEAS levels help to moderate these negative effects of cortisol.
Because of the profound age-related decline in circulating DHEAS levels, in both humans and rhesus monkeys, there has been interest in using DHEAS as a potential biomarker of aging progression in calorie-restriction studies (Heilbronn et al., 2006). Early efforts were extremely promising. A major study, initiated by the National Institute on Aging (NIA, Baltimore, USA), found that over a 3-year period circulating DHEAS levels declined by over 30% in control rhesus monkeys but by only 5% in age-matched CR animals (Lane et al., 1997a; 1999; 2001). Although the animals were only young adults at the time of the report, there was great optimism that prolonged CR would significantly block or delay the marked age-related attenuation of DHEAS levels that is characteristic of old age in humans and rhesus monkeys. Unfortunately, these studies were conducted before it was well established that circulating DHEAS levels in rhesus monkeys have a well-defined 24-hours pattern, with a peak early in the morning and a nadir in the late evening. Consequently, there was concern that blood samples collected at a single time-point during the day from anesthetized monkeys might not adequately control for the diurnal variation in release of DHEA and DHEAS from the adrenal glands. Furthermore, it remained to be determined if long-term CR resulted in sustained elevation of circulating DHEAS levels.
4. Recent updates on DHEAS as a biomarker of aging in calorie restriction studies
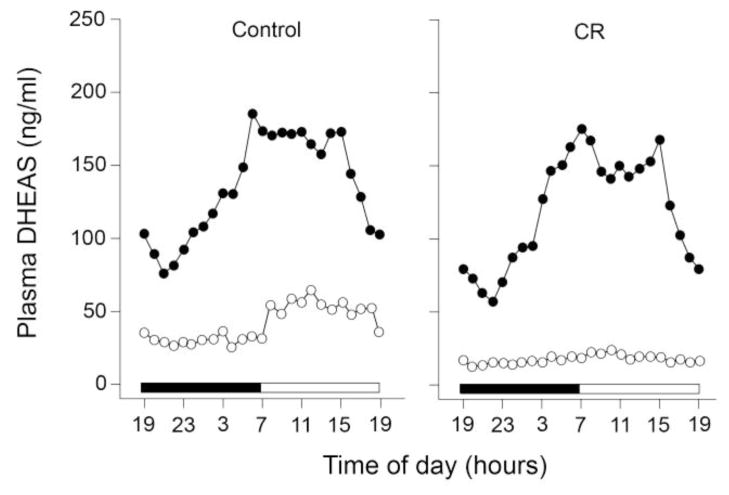
Two important studies were performed recently to test the hypothesis that circulating DHEAS levels reliably reflect the degree of aging in CR studies. In the first study, young (~11 years old) and old (~27 years old) male rhesus monkeys were either subjected to 30% calorie restriction for 4–5 years or were maintained on a standard diet (Downs et al., 2008; Urbanski et al., 2004). Importantly, each animal was fitted with a subclavian vein catheter, which enabled remote serial collection of blood without disturbing the animals (Urbanski, 2011). Measurement of DHEAS in plasma samples, collected hourly across the day and night, showed a clear-cut 24-hour rhythm. Peak levels were detected in the morning soon after the animals woke up and nadir levels occurred in the evening a few hours into the dark (Fig. 1). As expected, overall mean DHEAS levels were markedly lower in the old animals. Importantly, however, there was no significant difference between the CR and the age-matched control animals. Moreover, contrary to expectations, short-term CR did not cause an elevation of plasma DHEAS levels in the old CR. Overall, these results are consistent with the findings from a recent human study in which 6 months of CR had no obvious effect on circulating DHEAS levels (Heilbronn et al., 2006). It should be noted, however, that the CR paradigm in our monkey study was initiated when the animals were already old (i.e., approximately 22 years old) and was carried out for only 4–5 years. Therefore, the results of this experiment, as well as the human study, do not rule out the possibility that an earlier initiation of CR, or a longer duration of treatment, might have exerted a more beneficial influence on plasma DHEAS levels.
Fig. 1.
Effect of age and dietary manipulation on the circulating 24-h patterns of DHEAS in male rhesus macaques maintained at the ONPRC. Left panel: mean plasma DHEAS profiles from young (~11 years old, n = 5) and old (~27 years old, n = 6) controls are represented by black and white symbols, respectively. Right panel: mean plasma DHEAS profiles from age-matched young (n = 5) and old (n = 4) monkeys subjected to short-term calorie-restriction (4–5 years; CR) represented by black and white symbols, respectively. The serial blood samples (1 ml) were collected remotely each hour via a subclavian vein catheter, as described (Urbanski 2011). The horizontal black and white bars on the abscissas correspond to the 12L:12D lighting schedule. Two-way analysis of variance (ANOVA) followed by the Newman–Keuls test was used to evaluate group differences (using age and diet as variables). In both the controls and CR monkeys there is a significant (P<0.01) age-related decrease in overall mean DHEAS levels. Importantly, DHEAS levels in the old CR monkeys were not higher than in the age-matched controls. Data adapted from Downs et al., 2008.
Therefore, the aim of the second study was to examine if the same CR monkeys from the original NIA study (Lane et al., 1997a; 1999; 2001) would continue to show more highly elevated circulating DHEAS levels than their age-matched controls, even after long-term (14–15 years) 30% calorie restriction (Urbanski et al., 2003). CR had been initiated when the animals were either juvenile (J), adolescent (A) or old (O), and now at the time of the subsequent study they were 17, 19, and 32 years of age, respectively. A single blood sample was collected by venipuncture from each animal 3–4 hours after lights on (i.e. at the expected time of the DHEAS and cortisol peak); the animals were sedated and the blood samples were collected within 10–15 minutes. The serum was stored frozen and was subsequently assayed for DHEAS and cortisol, as previously described (Ducsay et al., 1991; Downs et al, 2008; Urbanski et al., 2004). The results (Fig. 2) show that from middle age (17–19 years) to old age (32 years) serum DHEAS levels continued to decline significantly. It is unclear if the rate of this decline was different between the CR and control monkeys, but at the measured time-points (i.e., 17, 19 and 32 years of age) there appeared to be no significant effect of diet on DHEAS levels, even though beneficial effects of CR were reported in these animals (Mattison et al., 2012). Mean serum cortisol levels were similar in the middle-aged and old animals, regardless of diet; cortisol served as an important reference because a decline in the ratio of DHEA:cortisol has been linked to age-associated cognitive decline (van Niekerk et al. 2001; Karishma and Herbert, 2002). It is important to emphasize that these negative DHEAS findings do not necessarily imply that CR is ineffective or bad for primate aging physiology. DHEAS is involved in glucose metabolism, and so extreme manipulation of the diet may itself lead to attenuation of circulating DHEAS levels, independently of aging. For example, intense glycemic control in patients with Type 2 diabetes mellitus has been shown to significantly attenuate circulating DHEAS levels (Kanazawa et al., 2012). Also, sulfotransferase enzyme gene expression appears to be affected by glucose levels (Runge-Morris and Vento, 1995; Yard et al., 2002), and so the synthesis of DHEAS from DHEA may be disrupted by CR, again, independently of aging. In the present study, however, none of the animals were diabetic and all showed normal serum glucose levels (i.e., below 80 mg/dL). Consequently, it is unlikely that the highly attenuated serum DHEAS levels of the old CR animals were caused by abnormally high levels of circulating glucose. Overall, the data from the short-term and long-term nonhuman primate studies suggest that DHEAS may be a less reliable biomarker of aging in studies that involve dietary manipulation.
Fig. 2.
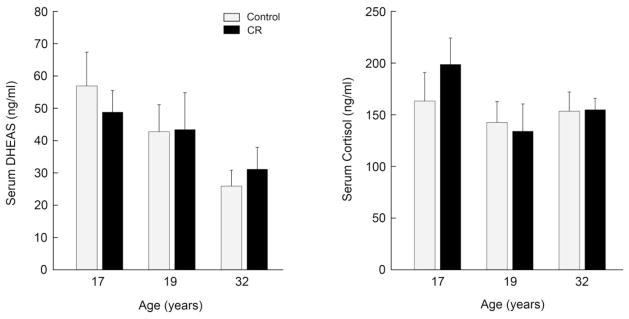
Effect of long-term calorie restriction (14–15 years; CR) on mean (± SEM) serum concentrations of DHEAS and cortisol in middle-aged (17–19 years) and old (~32 years) male rhesus macaques, maintained by the NIA at the NIH Animal Center. Single blood samples (3 ml) were collected by venipuncture 3–4 hours after lights on (i.e., at the time of expected peak) from sedated control and age-matched CR monkeys (gray and black bars, respectively); n = 6–10 animals per group. The main difference between the 17-year-old and the 19-year-old groups is that in the former the caloric restriction was initiated while the monkeys were still prepubertal. Statistical analysis (2-way ANOVA) detected a significant (P<0.05) effect of aging on serum DHEAS concentrations but no effect of diet. There was no obvious effect of age or diet on mean serum cortisol concentrations. These data suggest that CR has no long-term effect on maintaining elevated serum DHEAS concentrations; the DHEAS levels were already highly attenuated by middle age (i.e., considerably lower than the DHEAS levels of the young monkeys depicted in Fig. 1), and declined further during old age.
5. Conclusions
In both humans and rhesus monkeys, circulating levels of DHEAS are extremely high during early adulthood and then show a marked decrease during middle to old age. This is true for both males and females (Downs et al., 2008), although in the case of the latter, DHEAS levels are generally lower and begin showing a significant age-related decline well before menopause (Sorwell et al., 2012). Taken together, the rhesus monkey studies suggest that DHEAS has the potential to be a good biomarker of aging. On the other hand, the results also show that circulating DHEAS levels may be of limited value when used in studies that involve dietary manipulation. Although early observations on the NIA CR animals looked promising, suggesting that CR may sustain or significantly delay the age-related attenuation of DHEAS levels, these benefits were not obvious when CR was maintained for an extended period of 14–15 years. Similarly, no obvious benefits were observed when short-term (4–5 years) CR was initiated in older animals.
Several factors could account for the attenuated DHEAS levels of old CR animals. First, the timing of initiation and duration of CR intervention may be critical in determining the hormonal responses of a long-lived species, like the rhesus monkey. Typically, studies involving short-lived species have initiated CR very early in life and have continued it throughout life into old age (Weindruch and Sohal, 1997; Weindruch and Walford, 1988), and it has been suggested that longer intervention or CR initiated earlier in life may reveal similar benefits in the monkey. In the context of circulating DHEAS levels, however, neither short-term nor long-term CR showed any beneficial effects on this biomarker of aging; similarly the timing of the initiation of the CR intervention appeared to have no obvious effect either. Second, DHEAS may be an inappropriate biomarker of aging under CR conditions. This possibility is supported by studies showing that short-term changes in diet alone can affect serum and urinary DHEAS levels in humans (Remer and Manz, 1999; Remer et al., 1998; Remer, 2000). Consequently, it is plausible that the CR manipulation itself may have directly caused a dampening of DHEAS levels. This idea is supported by the finding of a recent study in which real-time PCR was used to profile gene expression in the liver of young and old rhesus monkeys, either exposed to short-term CR or maintained on a standard diet. Interestingly, expression of 3BHSD (the gene that encodes 3β hydroxy sulfate decarboxylase, a key enzyme in the metabolism of DHEA), was significantly elevated in old CR monkeys relative to their age-matched controls (K. Thiel, K. Sorwell, and H.F. Urbanski, unpublished observations). Given that caloric restriction can influence the expression of liver enzymes that are involved in steroid metabolism, circulating DHEAS levels may be difficult to interpret in aging studies that also involve dietary manipulation, thus undermining their reliability as a biomarker of aging.
Highlights.
Circulating DHEAS levels are highly elevated in young adults and subsequently show a marked age-related decline.
In rhesus monkeys, plasma DHEAS has a well-defined diurnal rhythm, with peaks in the morning.
Despite early promise, recent findings suggest that circulating DHEAS levels may not be good biomarkers of aging in calorie restriction studies.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants AG-019914, AG-036670 and OD-011092. Support was also provided in part by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrows CH, Kokkonen GC. Dietary restriction and life extension, biological mechanisms. In: Moment GB, editor. Nutritional approaches to aging research. Boca Raton, FL: CRC Press Inc; 1982. pp. 219–43. [Google Scholar]
- Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646. [DOI] [PubMed] [Google Scholar]
- Bliwise DL. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3. WB Saunders; Philadelphia: 2000. pp. 26–42. [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J Gerontol. 2003;58A:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducsay CA, Hess DL, McClellan MC, Novy MJ. Endocrine and morphological maturation of the fetal and neonatal adrenal cortex in baboons. J Clin Endocrinol Metab. 1991;73:385–395. doi: 10.1210/jcem-73-2-385. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csürös M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’brien WE, Prüfer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–3717. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E Pennington CALERIE Team. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. Erratum in: JAMA 2006 295, 2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. Dietary restriction and aging: the initiation of a primate study. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Sugimoto T. Effects of intensive glycemic control on serum levels of insulin-like growth factor-I and dehydroepiandrosterone sulfate in Type 2 diabetes mellitus. J Endocrinol Invest. 2012;35:469–472. doi: 10.3275/8033. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons, and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- Lane MA. Nonhuman primate models in biogerontology, Exp. Gerontol. 2000;35:533–541. doi: 10.1016/s0531-5565(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997a;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Beyond the rodent model: calorie restriction in rhesus monkeys. Age. 1997b;20:45–56. doi: 10.1007/s11357-997-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Roth GS. Nutritional modulation of aging in nonhuman primates. J Nutr Health Aging. 1999;3:69–76. [PubMed] [Google Scholar]
- Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Caloric restriction in primates, Ann. N Y Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol Aging. 2005;26:1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Alison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Messaoudi I, Urbanski HF, Kohama SG. Integrative models of aging in the nonhuman primate. In: Wiliams RM, editor. Monkeys: Biology, Behavior and Disorders. Chapter 1. Nova Science Publishers; Hauppauge, NY: 2011. pp. 1–54. [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: Further evaluation of hematology and blood chemistry data from a caloric restriction study in rhesus monkeys. Exp Gerontol. 1998;35:421–443. doi: 10.1016/s0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Cutler RG, Ingram DK. Evaluating measures of hematology and blood chemistry in male rhesus monkeys as biomarkers of aging. Exp Gerontol. 1994;29:151–177. doi: 10.1016/0531-5565(94)90048-5. [DOI] [PubMed] [Google Scholar]
- van Niekerk JK, Huppert JA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/s0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Remer T, Manz F. Role of nutritional status in the regulation of adrenarche. J Clin Endocrinol Metab. 1999;84:3936–3944. doi: 10.1210/jcem.84.11.6093. [DOI] [PubMed] [Google Scholar]
- Remer T, Pietrzik K, Manz F. Short-term impact of a lactovegetarian diet on adrenocortical activity and adrenal androgens. J Clin Endocrinol Metab. 1998;83:2132–2137. doi: 10.1210/jcem.83.6.4883. [DOI] [PubMed] [Google Scholar]
- Remer T. Adrenarche and nutritional status. J Pediatr Endocrinol Metab. 2000;13(Suppl 5):1253–1255. [PubMed] [Google Scholar]
- Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocrinol Metab. 2001;86:3292–3295. doi: 10.1210/jcem.86.7.7655. [DOI] [PubMed] [Google Scholar]
- Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Vento C. Effects of streptozotocin-induced diabetes on rat liver sulfotransferase gene expression. Drug Metab Dispos. 1995;23:455–459. [PubMed] [Google Scholar]
- Sorwell K, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age. 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell K, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33:1487.e1–1487.e13. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal Models of Behavioral Analysis, Neuromethods. Vol. 50. Humana Press; NY: 2011. pp. 217–235. Chapter 9. [Google Scholar]
- Urbanski HF, Sorwell K. Age-related changes in neuroendocrine rhythmic function in the rhesus macaque. Age. 2012;34:1111–1121. doi: 10.1007/s11357-011-9352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Lane MA, Roth GS, Ingram DK. Effect of aging and caloric restriction on the 24-hour release pattern of DHEAS and cortisol in male rhesus macaques. Proc. 10th Congress International Association of Biomedical Gerontology; 2003. p. Abstract 171. [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-h plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci. 2004;1019:1–5. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield, Il: Charles C. Thomas; 1988. [Google Scholar]
- Yard B, Feng Y, Keller H, Mall C, van Der Woude F. Influence of high glucose concentrations on the expression of glycosaminoglycans and N-deacetylase/N-sulphotransferase mRNA in cultured skin fibroblasts from diabetic patients with or without nephropathy. Nephrol Dial Transplant. 2002;17:386–391. doi: 10.1093/ndt/17.3.386. [DOI] [PubMed] [Google Scholar]