Abstract
Most patients with multidrug-resistant tuberculosis (MDR-TB) in South Africa are HIV-infected, but the safety and tolerability of co-treatment is unknown. We reviewed all adverse events (AEs) for MDR-TB patients in a home-based treatment program in rural KwaZulu-Natal. Of 91 MDR-TB patients, 74 (81%) were HIV-positive and receiving antiretroviral therapy (ART). AEs were common but most were mild and did not require therapy modification. The most common severe AEs were hypothyroidism (36%) and psychosis (5%). Patients receiving concurrent ART did not experience AEs more frequently than those on MDR-TB therapy alone. Concurrent treatment for MDR-TB/HIV can be safely administered in a home-based care setting.
Keywords: HIV, multidrug-resistant tuberculosis, side effects, adverse events, resource-limited settings
INTRODUCTION
The emergence of multidrug-resistant tuberculosis (MDR-TB; resistance to at least isoniazid and rifampin) has posed numerous challenges for TB-endemic countries worldwide. MDR-TB is associated with high mortality rates, particularly in the setting of HIV co-infection.1 Second-line TB medications are more toxic and less potent than treatment for drug-susceptible TB, and most countries have adopted centralized, inpatient models of care for MDR-TB, in part due to concerns about monitoring and treating adverse drug events. However, with limited numbers of hospital beds and specialists, some countries have recently adopted community-based, or ambulatory treatment for MDR-TB.2-4
Adverse events (AEs) associated with MDR-TB treatment have been well-described,2,5-11 and play an important role in treatment because they impact regimen choice, medication adherence and retention in care. Among patients co-infected with MDR-TB and HIV, there is the additional concern of additive or synergistic toxicities when second-line TB medications are given concurrently with antiretroviral therapy (ART). To date, however, there have been few data addressing this issue—the majority of AE reports have been in HIV-negative populations.5-11 As the HIV and drug-resistant TB epidemics converge, establishing the safety of co-administration of these treatments is essential. Moreover, as a greater proportion of MDR-TB patients are treated in outpatient or community-based settings, it is important to establish that ambulatory treatment can be carried out safely and effectively.
The objective of this study was to examine the frequency and severity of AEs in patients with MDR-TB and HIV co-infection treated at an integrated MDR-TB/HIV home-based treatment program in KwaZulu-Natal (KZN), South Africa.
METHODS
Setting
Tugela Ferry is a resource-limited, rural area in KZN province, South Africa, with a high incidence of MDR-TB.12 More than 80% of MDR-TB patients are co-infected with HIV.1 The details of the ambulatory MDR-TB/HIV treatment program in Tugela Ferry have been previously described.13 Patients with suspected or proven MDR-TB are referred to the local Specialized MDR-TB Treatment hospital and clinic. All patients are tested for HIV and, if positive, are initiated on ART as soon as possible. Following a brief admission, patients are treated at home by a visiting injection team (intensive phase) or lay treatment-supporter (continuation phase) with family support. Patients undergo clinical evaluation and laboratory testing monthly with full blood count and chemistries. Thyroid stimulating hormone (TSH) is tested six-monthly. Sputum smear, culture, and drug-susceptibility testing (DST) are performed monthly.
Treatment
Patients are treated with a standardized MDR-TB regimen consisting of kanamycin (15 mg/kg, max 1000mg daily), ofloxacin (800mg daily), cycloserine (10-20 mg/kg, max 750mg daily, divided BID), ethionamide (10-20 mg/kg, max 750mg daily, divided BID), pyrazinamide (20-30mg/kg, max 1600mg daily) and ethambutol (15-20mg/kg, max 1200mg daily). No patients received capreomycin or para-aminosalicylic acid (PAS). The intensive phase, when patients receive kanamycin, lasts 4 months following culture conversion (six months minimum). Total duration of treatment is a minimum of 24 months. The South African first-line ART regimen consists of: efavirenz, lamivudine, and either stavudine (40mg BID) or tenofovir (beginning April 2010).
Adverse Event Monitoring and Management
Patients are screened at baseline for any pre-existing symptoms. Following hospital discharge, they are seen monthly at the clinic by a clinician. At each visit, they are screened for any AEs, using a standardized screening instrument which includes 15 common complaints, such as peripheral neuropathy (numbness, burning or pain), nausea, and rash. The clinician evaluates positive findings and grades them in severity using a modified DAIDS toxicity table.14 Modifications in therapy are at the discretion of the treating clinician. All patients undergo formal audiometry at baseline and every 1-2 months while receiving kanamycin.
Analysis
We reviewed medical records for culture-confirmed MDR-TB patients who initiated treatment at the MDR-TB center between 11/1/2008 and 4/15/2011. Patients were excluded if they had resistance to kanamycin, amikacin, capreomycin or a fluoroquinolone.
We identified any reported AEs as of 11/15/2011. Hospital/clinic records were manually reviewed to corroborate self-reports of confusion, psychosis, depression, and insomnia and to identify any interventions. We defined psychosis as severe if cycloserine or terizidone was stopped, even if a grade was not provided. Laboratory results for hemoglobin, potassium, creatinine, and alanine aminotransferase (ALT) were graded using the DAIDS toxicity table. TSH was graded as: normal (0.34-5.6 mIU/L), elevated (5.6-8.0 mIU/L) and severely elevated (>8.0 mIU/L).
AEs were pooled to determine the proportion of patients who experienced at least one AE. We then calculated the proportion experiencing each specific AE at least once, as well as those experiencing severe AEs (grade ≥3). Finally, we analyzed the proportion of patients experiencing each AE during 6-month time blocks. Intra-patient trends over time were analyzed using generalized estimating equations. AE proportions and trends were compared among those who did and did not receive concurrent ART.
The study was approved by the ethics committees at the University of KwaZulu-Natal, Albert Einstein College of Medicine, Yale University, and by the KwaZulu-Natal Department of Health.
RESULTS
During the study period, 101 patients with culture-confirmed MDR-TB initiated treatment at the decentralized clinic. Of these, 10 either died or were transferred during their initial hospitalization and had no AE data available. The remaining 91 patients were included in this analysis.
Fifty (55%) patients were female and the median age was 34 (IQR 29-41) years. Seventy-six (84%) were HIV co-infected, with a median CD4 count of 207 cells/mm3 (IQR 89-411) at MDR-TB treatment initiation. Sixty-six (87%) HIV-infected patients were already receiving ART at MDR-TB treatment initiation, and 8 of the remaining 10 patients started ART a median of 45 days (IQR 19-90) later. One patient defaulted treatment before initiating ART, and one was an elite controller. At the time of analysis, patients had been followed for a median of 652 days (IQR 476-728).
Clinical Adverse Events
Clinical AEs were extremely common, with 90 patients (99%) reporting at least one AE. The most common were peripheral neuropathy (73%), injection site pain (66%), rash (53%), and nausea/vomiting (42%) (Table 1). Clinician grading was inconsistent, with only 38% of AEs receiving a grade. The most common severe AEs (SAEs) were psychosis (5%), hearing loss (8%), and peripheral neuropathy (5%). Cycloserine or terizidone was stopped in all cases of psychosis. Four patients became severely depressed (two were suicidal) and required discontinuation of cycloserine or terizidone. Nine patients (10%) required dose-reductions of kanamycin for hearing loss, and six (7%) changed from stavudine to zidovudine or tenofovir because of peripheral neuropathy. Formal audiometry results were available for 35 patients, of whom 24 (69%) had some degree of hearing loss and 4 (11%) had grade 3 or higher (defined as >55 dB threshold shift in 2 or more contiguous frequencies).
Table 1.
Frequency of adverse events reported at any point during MDR-TB treatment
| All patients (n=91) | HIV-positive receiving concomitant MDR-TB treatment and ART (n=74)* | HIV-negative (n=15) | |
|---|---|---|---|
| Adverse Event | Patients reporting event, N (%) | Patients reporting event, N (%) | Patients reporting event, N (%) |
| Clinical Adverse Events † | |||
| Peripheral neuropathy | 66 (73) | 55 (74) | 10 (67) |
| Injection site pain | 57 (66) | 47 (64) | 8 (53) |
| Rash | 48 (53) | 39 (53) | 7 (47) |
| Arthralgia | 39 (43) | 32 (43) | 6 (40) |
| Nausea/vomiting | 38 (42) | 30 (41) | 7 (47) |
| Insomnia | 33 (36) | 26 (35) | 6 (40) |
| Abdominal pain | 32 (35) | 25 (34) | 6 (40) |
| Myalgia | 32 (35) | 26 (35) | 6 (40) |
| Hearing Loss | 31 (34) | 24 (32) | 6 (40) |
| Generalized weakness | 27 (30) | 22 (30) | 4 (27) |
| Tinnitus | 26 (29) | 22 (30) | 3 (20) |
| Depression | 20 (22) | 18 (24) | 1 (7) |
| Diarrhea | 20 (22) | 14 (19) | 4 (27) |
| Confusion/psychosis | 18 (20) | 16 (22) | 2 (13) |
| Seizure | 3 (3) | 3 (4) | 0 (0) |
| Laboratory Adverse Events † | (n=88) | (n=72) | (n=14) |
| Elevated ALT | 43 (49) | 36 (50) | 6 (43) |
| Hypothyroidism‡ | 38 (51) | 34 (57) | 4 (33) |
| Hypokalemia | 35 (40) | 29 (40) | 6 (43) |
| Hyperkalemia | 30 (34) | 24 (33) | 5 (36) |
| Anemia | 29 (33) | 28 (39) | 1 (14) |
| Elevated creatinine | 21 (24) | 18 (25) | 3 (21) |
Does not include 2 HIV-infected patients who did not receive ART.
comparisons between HIV+/ART and HIV-negative groups non significant (p>0.05) for all clinical and lab AEs.
n=76 with a TSH result (n=60 HIV+/ART+; n=12 HIV-negative; n=2 HIV+/ART-)
ALT=alanine aminotransferase; ART=antiretroviral therapy; MDR-TB: multidrug-resistant TB
Laboratory Adverse Events
Eighty-nine (98%) patients had at least one laboratory result available during treatment. Laboratory AEs were common, but rarely clinically significant (Table 1). Forty-six (52%) patients had a grade ≥1 elevation in ALT, of whom 6 (7%) had a grade 3 or 4 elevation. All 6 of these elevations resolved on repeat testing without any change in regimen. Similarly, 32 (36%) patients experienced grade ≥1 elevation in potassium and 11 (12%) experienced grade 3 or 4 elevation but all were normal on repeat testing. Mild hypokalemia was also common (37 [42%] grade ≥1) but only 3 (3%) patients had severe hypokalemia (K+ ≤2.4 mEq/L); all improved with supplemental oral potassium. Hypothroidism was very common. Among 73 (80%) patients with at least one TSH result, 37 (51%) had an elevated TSH and 26 (36%) had a level >8.0 mIU/L, requiring initiation of levothyroxine. For each AE, there was no significant difference in frequency or severity between patients who were and were not receiving concurrent ART.
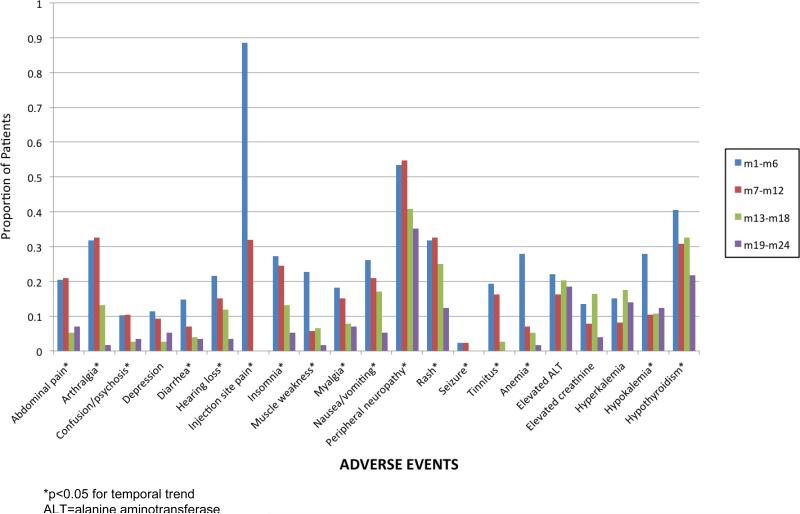
Changes in Adverse Events Over Time
Examining 6-month, within-treatment time blocks, we found that all clinical AEs decreased in frequency over the course of treatment (Figure 1). These temporal decreases were statistically significant (p<0.05), except for depression. Anemia improved over the course of treatment, as did hypokalemia and elevations in creatinine; the latter two may be related to discontinuation of kanamycin in the continuation phase. Hypothyroidism also improved over time, possibly due to levothyroxine therapy. The frequency of ALT elevations or hyperkalemia did not change over the course of treatment. There was no significant difference in temporal trends between patients receiving and not receiving concurrent ART. At the end of the study period, 78 patients (86%) were either cured (n=37) or still on treatment (n=41).
Figure 1.
Proportion of patients experiencing clinical adverse events, by within-treatment 6-month time blocks.
DISCUSSION
This study demonstrates the safety of concurrent treatment of MDR-TB and HIV in an ambulatory setting with home-based care. Overall, we found very high rates of AEs, but the majority were mild and did not require discontinuation of either MDR-TB treatment or ART. Interestingly, we found no significant differences in AE frequency between subjects who were and were not treated with concomitant ART, even among AEs with a plausible mechanism of additive or synergistic toxicity (e.g., peripheral neuropathy and neuropsychiatric effects).
Eighteen subjects (20%) in our study either reported, or were found to have confusion/psychosis, but on chart review, only 5 (5%) were considered sufficiently severe by the treating clinician to discontinue cycloserine or terizidone. Neuropsychiatric toxicity from cycloserine is well-known,15 and there has been concern about co-administration with efavirenz. Four of the 5 patients with severe psychosis in our cohort received concurrent efavirenz, but this was not stopped and psychosis resolved in all patients. In Lesotho, 16% of MDR-TB patients experienced psychosis from cycloserine but the authors did not note how many discontinued cycloserine or how many were receiving concurrent efavirenz.2 In Istanbul, 21% of patients experienced psychiatric symptoms but this was a combined category which included psychosis, depression and anxiety, making it difficult to compare with our results.
Hypothyroidism was very common in our study and 36% of patients required levothyroxine therapy. Although hypothyroidism was previously believed to be rare in MDR-TB treatment, recent reports suggest that it is more common.16-18 In Lesotho, 69% of MDR-TB patients treated with a combination regimen including both ethionamide and PAS had a TSH level >10 mIU/L. Although hypothyroidism was less common in our cohort, none of our patients were treated with PAS, suggesting that PAS and ethionamide have an additive effect in causing hypothyroidism. Some studies reporting lower rates of hypothyroidism only measured TSH in symptomatic patients, thus missing subclinical cases.5,8
Irreversible hearing loss from kanamycin or amikacin is perhaps the most devastating AE associated with MDR-TB treatment. In our study, audiometry results were not available for most patients, and consequently, our results of self-report are a minimal estimate. The available audiometry results, however, confirm high rates of subclinical hearing loss. Further study is needed to determine if changes in dose or frequency prevent ongoing hearing loss without affecting TB outcomes. Reported rates of ototoxicity vary widely in the literature, depending largely on the availability of routine audiometry testing rather than symptom-based testing or clinical self-report. In the cohort from Istanbul, 50% of patients receiving amikacin discontinued injections prematurely because of ototoxicity.8
Nearly all AEs were reported less frequently over time, even though patients were actively asked about specific AEs at each visit throughout treatment. Although certain AEs are related to specific therapies (e.g., ototoxicity), the improvement of most over time despite continuation of therapy suggests either that they represent the general discomfort of being ill (improving as the underlying illness is treated), or that patients become accustomed to medication side effects and learn to tolerate them until the end of treatment. In either case, our results are encouraging, and demonstrate that patients continue treatment with favorable outcomes.13
This study has several important limitations. First, patients were not routinely screened for AE-related symptoms while they were admitted to the hospital. This may have missed AEs shortly after initiating therapy, when they are likely to be more common. Second, with the exception of psychosis and confusion, AEs were primarily identified by self-report and, given the inconsistent severity grading by the clinicians, it is difficult to determine their clinical significance. Third, although we did not find a difference in the incidence of AEs between HIV co-infected subjects receiving concomitant ART and HIV-uninfected subjects, our study only included 15 HIV-negative subjects and may not have had adequate power to detect a small difference.
With a growing case burden and limited hospital beds, South Africa has recently moved to a community-based treatment model for MDR-TB nationwide.19 We have found that, although AEs are frequent, patients with MDR-TB—with or without HIV co-infection—can be safely treated at home with supportive care. Our data support the recent WHO treatment guidelines for MDR-TB calling for ambulatory models of care.20 With intensive patient/family education, close clinical follow-up, and rapid assessment and management of AEs, patients remain in treatment and achieve successful outcomes even in rural, resource-limited settings with high HIV-prevalence.21
ACKNOWLEDGEMENTS
The authors thank Dr. Alois Mngadi and Sister (Prof. Nurse) Lee-Megan Larkan for their outstanding clinical management of the study subjects.
Source of Funding: JCMB is supported by the National Institutes of Health (K23 AI083088). NRG and NSS are both recipients of the Doris Duke Charitable Foundation Clinical Scientist Development Award (NRG 2007070, NSS 2007071). GHF is also supported by the Doris Duke Charitable Foundation (2007018), the Gilead Foundation, and The Irene Diamond Fund (R05130). Additional funding for the program was provided by the US President's Emergency Plan for AIDS Relief. Statistical support was provided by the Center for AIDS Research at Albert Einstein College of Medicine/Montefiore Medical Center (P30 AI051519).
Footnotes
Conflicts of Interest
All remaining authors have no funding or conflict to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. American journal of respiratory and critical care medicine. 2010 Jan 1;181(1):80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 2.Seung KJ, Omatayo DB, Keshavjee S, Furin JJ, Farmer PE, Satti H. Early outcomes of MDR-TB treatment in a high HIV-prevalence setting in Southern Africa. PLoS One. 2009;4(9):e7186. doi: 10.1371/journal.pone.0007186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. The New England journal of medicine. 2003 Jan 9;348(2):119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 4.Heller T, Lessells RJ, Wallrauch CG, et al. Community-based treatment for multidrug-resistant tuberculosis in rural KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. Apr;14(4):420–426. [PubMed] [Google Scholar]
- 5.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001 Jul;5(7):648–655. [PubMed] [Google Scholar]
- 6.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007 Dec;11(12):1314–1320. [PubMed] [Google Scholar]
- 7.Bloss E, Kuksa L, Holtz TH, et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000-2004. Int J Tuberc Lung Dis. 2010 Mar;14(3):275–281. [PubMed] [Google Scholar]
- 8.Torun T, Gungor G, Ozmen I, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005 Dec;9(12):1373–1377. [PubMed] [Google Scholar]
- 9.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004 Nov;8(11):1382–1384. [PubMed] [Google Scholar]
- 10.Baghaei P, Tabarsi P, Dorriz D, et al. Adverse effects of multidrug-resistant tuberculosis treatment with a standardized regimen: a report from Iran. Am J Ther. 2011 Mar-Apr;18(2):e29–34. doi: 10.1097/MJT.0b013e3181c0806d. [DOI] [PubMed] [Google Scholar]
- 11.Vega P, Sweetland A, Acha J, et al. Psychiatric issues in the management of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2004 Jun;8(6):749–759. [PubMed] [Google Scholar]
- 12.Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brust JC, Shah NS, Scott M, et al. Integrated, home-based treatment for MDR-TB and HIV in rural South Africa: an alternate model of care. Int J Tuberc Lung Dis. 2012 Aug;16(8):998–1004. doi: 10.5588/ijtld.11.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Division of AIDS (DAIDS) [August 1, 2011];Table for grading the severity of adult and pediatric adverse events. http://rcc.tech-res.com/safetyandpharmacovigilance.
- 15.Lewis WC, Calden G, Thurston JR, Gilson WE. Psychiatric and neurological reactions to cycloserine in the treatment of tuberculosis. Dis Chest. 1957 Aug;32(2):172–182. doi: 10.1378/chest.32.2.172. [DOI] [PubMed] [Google Scholar]
- 16.Dutta BS, Hassan G, Waseem Q, Saheer S, Singh A. Ethionamide-induced hypothyroidism. Int J Tuberc Lung Dis. 2012 Jan;16(1):141. doi: 10.5588/ijtld.11.0388. [DOI] [PubMed] [Google Scholar]
- 17.Satti H, Mafukidze A, Jooste PL, McLaughlin MM, Farmer PE, Seung KJ. High rate of hypothyroidism among patients treated for multidrug-resistant tuberculosis in Lesotho. Int J Tuberc Lung Dis. 2012 Feb 8; doi: 10.5588/ijtld.11.0615. [DOI] [PubMed] [Google Scholar]
- 18.Isaakidis P, Varghese B, Mansoor H, et al. Adverse Events among HIV/MDR-TB Co-Infected Patients Receiving Antiretroviral and Second Line Anti-TB Treatment in Mumbai, India. PloS one. 2012;7(7):e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Department of Health, South Africa Multi-Drug Resistant Tuberculosis: A policy framework on decentralized and deinstitutionalized management for South Africa. 2011.
- 20.Falzon D, Jaramillo E, Schunemann HJ, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011 Sep;38(3):516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 21.Brust JC, Lygizos M, Chaiyachati K, et al. Culture Conversion Among HIV Co-Infected Multidrug-Resistant Tuberculosis Patients in Tugela Ferry, South Africa. PLoS One. 2011;6(1):e15841. doi: 10.1371/journal.pone.0015841. [DOI] [PMC free article] [PubMed] [Google Scholar]