Abstract
The TOO MANY MOUTHS receptor may be responsible for the organ-specific effects of brassinosteroids.
During the past few years, our understanding of stomatal development and patterning has been advanced by the cloning of a large number of genes in Arabidopsis (Arabidopsis thaliana). These genes encode peptide ligands (epidermal patterning factor, or EPF), and receptor proteins (TOO MANY MOUTHS [TMM] and the ERECTA family [ERf] of receptor-like kinases [ER, ERL1, and ERL2]; Nadeau and Sack, 2002; Shpak et al., 2005; Torii, 2012). Genes functioning downstream of these receptors include those encoding the mitogen-activated protein kinase (MAPK) module, which consists of the MAPK kinase kinase YODA (YDA); the MAPK kinases MKK4, MKK5, MKK7, and MKK9; and the MAPKs MPK3 and MPK6 (Bergmann et al., 2004; Wang et al., 2007; Lampard et al., 2009). MPK3 and MPK6 phosphorylate and inactivate the basic helix-loop-helix transcriptional factor SPEECHLESS (SPCH), blocking the initiation of stomatal development (MacAlister et al., 2007; Pillitteri et al., 2007; Lampard et al., 2008). It is well known that the interaction of signaling pathways permits the fine tuning of cellular activities needed to carry out complex developmental processes. However, despite the progress made in studies on the stomatal signaling pathway, our understanding of its interaction with other signaling pathway remains very limited.
One of the most well characterized signal transduction pathways in plant biology is the pathway regulated by brassinosteroids (BRs; Kim and Wang, 2010). These plant regulators are perceived by the Leu-rich-repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1 (BRI1; He et al., 2000). Direct binding of BRs to BRI1 results in the activation of a small family of kinases known as BRASSINOSTEROID SIGNALING KINASEs (Tang et al., 2008). Downstream components of bacterial spore kinases include the GLYCOGEN SYNTHASE KINASE3 (GSK3)-like BRASSINOSTEROID INSENSITIVE2 (BIN2) kinase (Li and Nam, 2002) and two protein phosphatases, BRI1 SUPPRESSOR1 (BSU1; Mora-García et al., 2004) and PROTEIN PHOSPHATASE2A (PP2A; Tang et al., 2011), which control the phosphorylation states of a family of transcription factors including BRI1-EMS-SUPPRESSOR1 (BES1; Yin et al., 2002) and BRASSINAZOLE RESISTANT1 (BZR1; He et al., 2005). BR signaling leads to the inactivation of BIN2, and PP2A-mediated dephosphorylation and the activation of BZR1 and BES1 (He et al., 2002; Kim and Wang, 2010; Tang et al., 2011).
Two works have revealed that BRs control stomatal production through regulating proteins that function in the stomatal pathway (Gudesblat et al., 2012a; Kim et al., 2012). However, while one of these studies showed that BRs repress stomatal development by alleviating the BIN2-mediated inhibition of YDA (Kim et al., 2012; Fig. 1A), the other study concluded that these plant regulators promote stomatal formation by inhibiting the BIN2-mediated phosphorylation of SPCH (Gudesblat et al., 2012a; Fig. 1B). A number of recent papers (Casson and Hetherington, 2012; Kong et al., 2012), including one published by the authors of one of the original articles (Gudesblat et al., 2012b), highlighted this apparent discrepancy and looked for its possible sources. In this article, I examine data from the cotyledon and hypocotyl separately and propose that BRs have opposite effects on the formation of stomata in these plant organs. I also look for the reasons behind such responses and propose that the TMM action, dampening excessive signal to ERf from the peptide ligands CHALLAH (CHAL) and EPF-LIKE5 (EPFL5) in hypocotyls, may be responsible for the organ-specific effects of BRs.
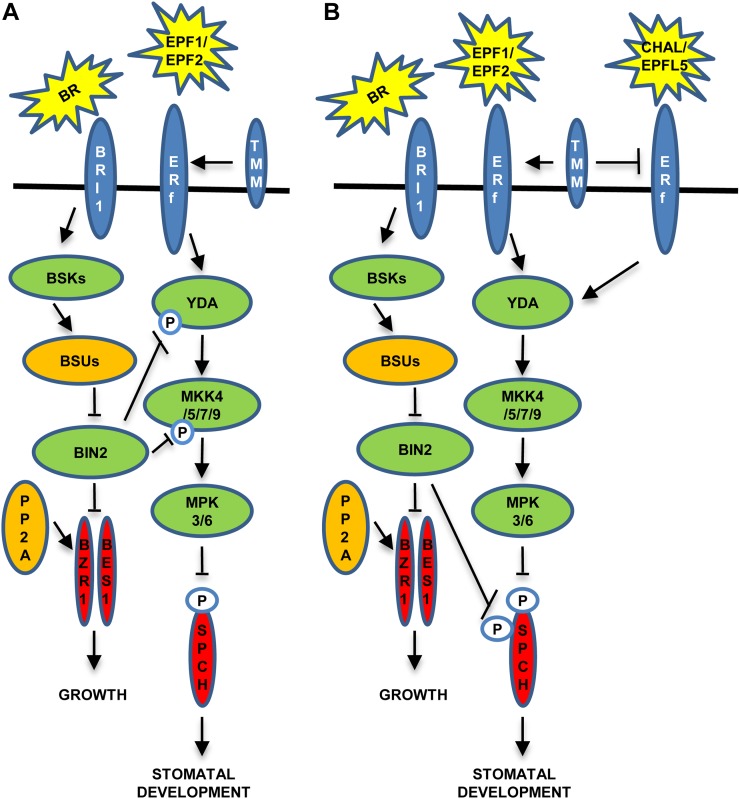
Figure 1.
Proposed cross talk between BRs and stomatal development. A, Regulation of stomatal formation in the cotyledon. TMM enhances EPF1 and EPF2 signaling through ERf. At low BR levels, BIN2 phosphorylates and inactivates both YDA and MKK4, switching off the signaling that inactivates SPCH by phosphorylating residues in the MPKTD, which triggers stomatal production. Genetic data also suggest that ERf and TMM regulate BIN2 (or upstream components). B, Stomatal formation in the hypocotyl. TMM enhances EPF1 and EPF2 signaling, but also dampens CHAL and EPFL5 signaling. TMM action reduces EPF1 and EPF2 signaling, leading to a reduction of MAPK activity. Under this scenario, in the absence of BRs, BIN2 inactivates SPCH by phosphorylating residues in the N terminus and in the MPKTD. These phosphorylation events repress stomatal development.
BRS REPRESS STOMATAL DEVELOPMENT IN COTYLEDONS
Plants with quadruple loss of function of BSU-related phosphatases (bsu-q) displayed a striking phenotype, producing a cotyledon surface consisting almost entirely of stomata (Kim et al., 2012). BR-deficient plants (deetiolated2-1 [det2-1]), or those that lack sensitivity to BRs (bri1-116, dominant bin2-1, and plants overexpressing BIN2), also developed an increased number of stomata in their cotyledons (Kim et al., 2012). These mutants also developed stomatal clusters (Kim et al., 2012). By contrast, plants lacking BR-signaling GSK3-like kinases (bin2-3 bil1 bil2 loss-of-function mutant) and det2-1 plants overexpressing a member of the BSU subfamily (BSU1-LIKE2) developed a reduced number of stomata (Kim et al., 2012). These results support the notion that BRs repress stomatal development in cotyledons (Kim et al., 2012). In agreement with this interpretation, the pharmacological activation of BR signaling by brassinolide (BL), the most active natural BR, or bikinin, an inhibitor of BIN2 activity, reduced stomatal production in cotyledons (Kim et al., 2012). Bikinin also fully suppressed the stomatal clustering of bin2-1 and partially suppressed the severe phenotypes of bsu-q (Kim et al., 2012).
Interestingly, gain-of-function mutants of BZR1 (bzr1-1D mutant) exhibited the wild-type phenotype, and bzr1-1D also failed to suppress the stomatal phenotypes of bri1-116, bsu-q, and bin2-1 (Kim et al., 2012). In light of these results, Kim et al. (2012) proposed that BRs repress stomatal development independent of the BIN2 substrate BZR1.
But does the BR pathway interact with the signaling cascade that controls stomatal development? When CA-YDA, a constitutively active YDA variant that probably acts through activation of the MAPK pathway, which phosphorylates and inactivates SPCH (Wang et al., 2007; Lampard et al., 2008), was expressed in bri1-116, bsu-q, or bin2-1 mutants, stomatal development was blocked (Kim et al., 2012). Consistent with these data, the bsu-q spch-3 mutant exhibited a phenotype similar to that of spch-3, that is, lacked stomata (Kim et al., 2012). Therefore, BR signaling components function upstream of YDA (Kim et al., 2012; Fig. 1A). Bikinin treatment suppressed the weak stomatal clustering of tmm and partially rescued the er erl1 erl2 phenotype, but it had no effect on the yda epidermis (Kim et al., 2012). Brassinazole, an inhibitor of BR biosynthesis, enhanced the stomatal phenotype of tmm, but it did not further increase the number of stomata in er erl1 erl2, probably because almost all of the cells in this mutant were already stomatal cells (Kim et al., 2012). Together, these results suggest that BIN2 functions downstream of ERf and TMM, but upstream of YDA (Kim et al., 2012).
In contrast with the repressive role of BRs in stomatal production, Gudesblat et al. (2012a) found that BR-deficient plants (constitutive photomorphogenesis and dwarfism [cpd]) and those lacking BR sensitivity (bri1-116) had cotyledons that appeared normal. In addition, cotyledons of plants overexpressing the BR biosynthesis gene DWARF4 (DWF4) also displayed a normal phenotype, while those of plants overexpressing BRI1 slightly increased in the stomatal production (Gudesblat et al., 2012a). This disparity may have arisen from the use of different experimental growth conditions (Gudesblat et al., 2012b). Thus, in the conditions employed by Gudesblat et al. (2012a), an unknown factor may have masked the effect of BRs on stomatal production in this plant organ.
BRS PROMOTE STOMATAL DEVELOPMENT IN HYPOCOTYLS
Plants that either lacked BRs (cpd, det2-1) or had reduced sensitivity to BRs (bri1-1, bri1-4, bri1-116, and dominant bin2-1) developed a very low number of stomata in the hypocotyl, and those with enhanced BR responses (plants overexpressing either DWF4 or BRI1, bin2-3 bil1 bil2, and bin2-3) displayed the opposite phenotype, that is, a strong increase in stomatal production (Fuentes et al., 2012; Gudesblat et al., 2012a). Indeed, BL increases stomatal production, while brassinazole and triadimefon, two inhibitors of BR biosynthesis, reduce the number of stomata (Fuentes et al., 2012; Gudesblat et al., 2012a). Mutant backgrounds responded to these treatments as expected, with bri1-1 and bri1-4 displaying absolute insensitivity to BL, while det2-1 increased stomatal production in response to this plant regulator (Fuentes et al., 2012). Interestingly, the gain-of-function mutants bes1-d and bzr1-d had normal numbers of stomata in their hypocotyls (Gudesblat et al., 2012a). Therefore, BIN2 represses stomatal development in the hypocotyl in a BZR1- and BES1-independent manner (Gudesblat et al., 2012a).
The observation that bsu-q plants produced large stomatal clusters on hypocotyls is unexpected (Kim et al., 2012). Because BRs promote stomatal development in the embryonic stem, the lack of sensitivity to BRs in bsu-q would be expected to block stomatal production. The appearance of these clusters could be explained by the notion that some of the genes mutated in bsu-q might play a role in preventing adjacent cells from developing into stomata.
To investigate the possible interaction between BR signaling and genes that function in the stomatal pathway, Gudesblat et al. (2012a) examined mutants with impaired stomatal pathway genes grown in the presence of exogenous BL (Gudesblat et al., 2012a). They found that BL increased stomatal production in the hypocotyls of epf1, epf2, er-105, erl1-2, erl2-1, and yda-5 (Gudesblat et al., 2012a). This indicates that BRs act downstream or independent of the genes impaired in these mutants (Gudesblat et al., 2012a). The fact that BL failed to promote stomatal production in spch-3 is consistent with SPCH as a downstream transcriptional factor initiating stomatal cell lineages.
TMM AND BRS FUNCTION DURING DIFFERENT STEPS OF STOMATAL DEVELOPMENT
The hypocotyl epidermis is arranged in files that run parallel to the long axis of this organ, and only some of these files develop stomata (Berger et al., 1998; Hung et al., 1998). The first step of stomatal development in the hypocotyl is a cell division along its longitudinal axis, which is parallel to the longitudinal axis of the organ (Berger et al., 1998). This cell division produces a small and often triangular or rectangular cell named the meristemoid (Berger et al., 1998). Meristemoids are self-renewing cells that undergo a sequence of asymmetric cell divisions before stomata formation (Berger et al., 1998). The tmm mutant hypocotyl lacked stomata, but it exhibited arrested meristemoids (Bhave et al., 2009). Interestingly, BL not only failed to reverse the lack of stomata in the tmm-1 hypocotyl (Fuentes et al., 2012; Gudesblat et al., 2012a) but also increased the production of meristemoids (Fuentes et al., 2012). In light of these results, Fuentes et al. (2012) proposed that BRs promote the initiation of the stomatal pathway, and TMM is required in later stages of stomatal development for the progression of meristemoids toward stomata. Consistent with these results, bri1-1, bri1-4, and det2-1 mutants and wild-type plants treated with the inhibitor of BR biosynthesis triadimefon did not display arrested meristemoids (Fuentes et al., 2012).
Stomata-forming cell files have more cells than their neighboring nonstomatal cell files (Hung et al., 1998). Gudesblat et al. (2012a) found that BL increased the number of cells in stomata-forming cell files, with tmm mutants displaying a response similar to that found in wild-type plants. Then, BL increases the number of nonstomatal cells and promotes the initiation of the stomatal pathway in a TMM-independent manner.
BIN2 PHOSPHORYLATES SEVERAL PROTEINS IN STOMATAL SIGNALING CASCADE
In vitro and in vivo experiments have demonstrated that YDA binds to BIN2 (Kim et al., 2012). In addition, CA-YDA, which lacks 23 putative GSK3 phosphorylation sites, binds to BIN2 in yeast two-hybrid assays (Kim et al., 2012). As might be expected, BIN2 phosphorylates the region deleted in CA-YDA, which inhibits YDA activity (Kim et al., 2012; Fig. 1A). In consonance with these data, the kinase activities of MPK3 and MPK6 were reduced when BIN2 was activated (det2 mutants) but were increased when BIN2 was repressed (bikinin or BL treatments in wild-type plants; Kim et al., 2012). Because BRs increase MAPK activity by repressing BIN2, and MAPKs repress SPCH activity, it is expected that BL or bikinin treatments would decrease SPCH activity, repressing stomatal development in cotyledons.
In vitro kinase assays have shown that BIN2 also phosphorylates both MKK4 and MKK5 (Khan et al., 2013). In addition, BIN2 phosphorylation of MKK4 represses its activity against MPK6 in vitro (Khan et al., 2013; Fig. 1A). BIN2 phosphorylates two residues of MKK4 (Khan et al., 2013): Ser-230 and Thr-234. Since mutations in Thr-234 abolished the activity of MKK4 against MPK6 in vitro, Khan et al. (2013) proposed that Thr-234 may be the main phosphorylation site of MKK4 by BIN2. Moreover, the overexpression of a mutant version of MKK4 in Thr-234, by replacing Thr with Ala to prevent phosphorylation, conferred a cotyledon epidermis composed almost entirely of stomata (Khan et al., 2013). This phenotype mirrors those in which the activities of either MKK4 and MKK5 or MPK3 and MPK6 are lost (Wang et al., 2007), which suggests that the MKK4 mutant version favorably competes with the endogenous one in binding MPK3 and MPK6, interfering with the activation of MPK3 and MPK6 (Khan et al., 2013). This would prevent SPCH repression, triggering stomatal development. It will be interesting to determine the significance of BIN2-mediated Ser-230 phosphorylation.
Gudesblat et al. (2012a) investigated whether BIN2 phosphorylates SPCH. They found that BIN2 phosphorylates SPCH in vitro, and such phosphorylation is abolished after incubation with bikinin (Gudesblat et al., 2012a). BIN2 phosphorylates five residues of SPCH located within the MAPK target domain (MPKTD; Gudesblat et al., 2012a). BIN2 also phosphorylates five residues of SPCH located at the N terminus of the protein (Gudesblat et al., 2012a). Two of the five in vitro phosphorylated MPKTD residues, and one of the five in vitro phosphorylated N-terminal residues, were also found to be phosphorylated in vivo (Gudesblat et al., 2012a). The differences in results obtained in in vitro and in vivo phosphorylation experiments can easily be explained by the fact that kinases have broader substrate specificity in vitro than in vivo. BL reduced the phosphorylation of two of the three SPCH residues that were phosphorylated by BIN2 in vitro and in vivo (Gudesblat et al., 2012a; Fig. 1B): Ser 65 at the N-terminal domain and Ser 171 at the MPKTD. BRs may therefore promote SPCH activity, and thus stomatal development in the hypocotyl, by inhibiting the phosphorylation of these two BIN2 target residues. The expression of mutant versions of SPCH in these residues, by replacing them with Ala, rescued stomatal production in the spch-3 cotyledon (Gudesblat et al., 2012a). The expression of this mutant protein also increased the proliferation of nonstomatal epidermal cells, suggesting a role of BIN2 in regulating cell division through SPCH (Gudesblat et al., 2012a).
BL increased the amount of SPCH protein but not the transcript level, and brassinazole reduced the concentration of SPCH (Gudesblat et al., 2012a). In addition, the proteosome inhibitor MG132 also increased the level of SPCH (Gudesblat et al., 2012a). These data are consistent with a model in which BRs control stomatal production by regulating SPCH levels. Given that the expression of mutant versions of SPCH protein with mutations in its BIN2-specific phosphorylation sites (Ser 65 and others at the N-terminal domain) increased the level of SPCH in spch-3, it is likely that SPCH degradation depends on the phosphorylation of these sites (Gudesblat et al., 2012a).
But, how can BIN2 regulate different proteins depending on the organ where it is acting (YDA and MKK4 in cotyledons and SPCH in hypocotyls)? The simplest explanation is that organ type affects the levels of the components of the MAPK module, with high levels in cotyledon epidermal cells and low ones in hypocotyl cells. High levels of the MAPK components in cotyledon cells could allow that BIN2-mediated repression of both YDA and MKK4 was the dominant effect, promoting stomatal formation. In contrast, low MAPK levels in hypocotyl cells could allow BIN2 regulation of SPCH to repress stomata development. Under this scenario, the repression of stomatal cluster development in the leaves of brassinazole-treated MKK4 or MKK5 overexpressing plants (Khan et al., 2013), suggests the existence of an excess of MKK4 and MKK5 that cannot be phosphorylated by BIN2, repressing SPCH activity.
It is known that BIN2 controls the output of BR signaling through the control of the subcellular localization of BES1 (Ryu et al., 2010a, 2010b) and BZR1 (Ryu et al., 2007). In a similar way to the BIN2-mediated regulation of BES1 or BZR1, this hypothetical scenario, with different MAPK activities depending on the organ, does not exclude that BIN2 may regulate the output of BR signaling also through the control of the subcellular localization of YDA and SPCH. This does not seem to be the case of MKK4, whose nucleocytoplasmic localization unchanged after treatment with BR or brassinazole (Khan et al., 2013).
TMM ACTION AND LEVELS OF MAPKS
Mutations in stomatal regulators confer patterning defects that are uniform across all organs that produce stomata. Unlike most stomatal mutants, tmm exhibits contrasting phenotypes in different organs (Geisler et al., 1998; Bhave et al., 2009); for example, tmm hypocotyls produce no stomata, but tmm cotyledons develop stomatal clusters. How can TMM play opposite roles depending of the organ where it acts? Mutations in the CHAL putative peptide ligand gene, which is expressed in internal tissues of the hypocotyl and stem (Abrash and Bergmann, 2010; Abrash et al., 2011), restored stomata to tmm hypocotyl without significantly affecting cotyledon stomata development (Abrash and Bergmann, 2010). Interestingly, overexpression experiments reveal that CHAL needs ERf to inhibit stomatal development but that, in striking contrast to the putative secretory peptide genes EPF1 and EPF2, whose repression of stomatal development depends on both ERf members and TMM, the effects of CHAL overexpression are dramatically enhanced in absence of TMM (Hara et al., 2007, 2009; Hunt and Gray, 2009; Abrash and Bergmann, 2010). In contrast to CHAL, EPF1 and EPF2 are expressed across all aerial organs examined, in overlapping but also in different stomatal precursor cells (Hara et al., 2007, 2009; Hunt and Gray, 2009). This allows proposing that TMM permits or enhances EPF1 and EPF2 signaling through ERf receptors and across all organs that produce stomata, but dampens CHAL signaling, also through ERf receptors, in the hypocotyl and stem (Abrash and Bergmann, 2010; Torii, 2012; Fig. 1B). Then, in the tmm mutant, CHAL signaling would prevent stomatal formation in the hypocotyl and stem, and the lack of the EPF1 and EPF2 signaling would trigger stomatal cluster formation in cotyledons. TMM also dampens the signaling mediated by the CHAL paralog EPFL5, whose promoter also is induced in the hypocotyl, although in a different pattern from that of CHAL (Abrash et al., 2011). Consistent with this model, tmm erl1 resumed stomatal formation in stems (Shpak et al., 2005).
Disruption of BR biosynthesis, perception, or signaling displays phenotypes that mirror those found in the tmm mutant. Does the TMM action explain the fact that BRs play opposite roles in cotyledons and hypocotyls? In the embryonic stem, TMM not only potentiates the transduction of EPF1 and EPF2 but also dampens the excessive signal from CHAL and EPFL5 to the ERf. Consequently, one could speculate that this mechanism limits the availability of TMM and/of ERf for EPF1 and EPF2, reducing EPF1 and EPF2 signaling. This would lead to a reduction of MAPK activity, triggering an accumulation of SPCH. Under this scenario, BIN2 would predominantly regulate SPCH, negatively regulating stomatal formation. In the cotyledon, the absence of CHAL and EPFL5 signaling would increase EPF1 and EPF2 signaling, leading to a robust activation of MAPK and, consequently, allowing the repression of both YDA and MKK4 by BIN2 to be the dominant effect. This would promote stomata formation. Thus, it is likely that the TMM action, dampening excessive signal to ERf from CHAL and EPFL5 in hypocotyls, is responsible for the organ-specific effects of BRs.
Loss of CHAL increased meristemoids in tmm hypocotyl (Abrash and Bergmann, 2010), in the same way as BL (Fuentes et al., 2012), most likely because of the repression of both YDA and MKK4 by BIN2 in this double mutant. Some of these meristemoids developed into stomata in tmm chal (Abrash and Bergmann, 2010), indicating that CHAL is required for a full repression of meristemoid progression in the tmm background.
BRS AND CELL-FATE SPECIFICATION IN THE HYPOCOTYL
The MYBs-BHLHs-TTG-GL2 network controls stomatal cell-fate specification in the hypocotyl (Serna, 2004). Although BL did not affect the expression pattern of either GLABRA2 (GL2) or CAPRICE (CPC), whose promoters are induced in nonstomata-forming cell files, the treatment with the BR biosynthesis inhibitor triadimefon resulted in a random induction of the GL2 promoter and a loss of induction of the CPC promoter in the hypocotyl epidermis (Fuentes et al., 2012). This suggests that BRs control stomatal cell fate by regulating the expression of at least two members of this network (Fuentes et al., 2012). Supporting this hypothesis, the cpc mutant failed to respond to triadimefon, while try cpc (TRIPTYCHON [TRY] shares high sequence similarity with CPC) exhibited insensitivity to BL. In addition, try cpc det2-1 phenocopied the stomatal phenotype of try cpc, also exhibiting insensitivity to the phytohormone (Fuentes et al., 2012). Unexpectedly, gl2-1 responded to both BL and triadimefon, suggesting that other genes might be masking this absence of gl2-1 response. In contrast to this interpretation, and based on the number of cells per file, Gudesblat et al. (2012a) proposed that BRs do not affect cell fate in the hypocotyl epidermis. However, because no study has been reported that demonstrates a causal relationship between the number of cells per file and stomatal cell fate, an alternative interpretation would suggest that BRs control separately two aspects of the hypocotyl epidermis: stomatal cell fate and cell division.
CONCLUSION
In line with Casson and Hetherington (2012), this paper supports the hypothesis that cotyledons and hypocotyls respond differently to BRs, resulting in a decrease in stomatal production in cotyledons and an increase in hypocotyls. I propose that the TMM action, dampening excessive signal to ERf from CHAL and EPFL5 in hypocotyls, may be responsible for a reduction of MAPK activity in this plant organ, triggering, under low BR levels, BIN2 regulation of SPCH to repress stomatal development. In contrast, in cotyledons, high MAPK levels could allow the inhibition of both YDA and MKK4 to be the dominant effect, promoting stomatal formation. Interestingly, BR-deficient and BR-insensitive Arabidopsis mutants not only produced more stomata in their cotyledons, but they were also more sensitive to abscisic acid than wild-type plants (Xue et al., 2009), suggesting that plants coordinate both development and physiology to optimize photosynthesis and water use.
Glossary
- BR
brassinosteroid
- BL
brassinolide
- MPKTD
MPAK target domain
References
- Abrash EB, Bergmann DC. (2010) Regional specification of stomatal production by the putative ligand CHALLAH. Development 137: 447–455 [DOI] [PubMed] [Google Scholar]
- Abrash EB, Davies KA, Bergmann DC. (2011) Generation of signaling specificity in Arabidopsis by spatially restricted buffering of ligand-receptor interactions. Plant Cell 23: 2864–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Linstead P, Dolan L, Haseloff J. (1998) Stomata patterning on the hypocotyl of Arabidopsis thaliana is controlled by genes involved in the control of root epidermis patterning. Dev Biol 194: 226–234 [DOI] [PubMed] [Google Scholar]
- Bhave NS, Veley KM, Nadeau JA, Lucas JR, Bhave SL, Sack FD. (2009) TOO MANY MOUTHS promotes cell fate progression in stomatal development of Arabidopsis stems. Planta 229: 357–367 [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. (2004) Stomatal development and pattern controlled by a MAPKK kinase. Science 304: 1494–1497 [DOI] [PubMed] [Google Scholar]
- Casson SA, Hetherington AM. (2012) GSK3-like kinases integrate brassinosteroid signaling and stomatal development. Sci Signal 5: pe30. [DOI] [PubMed] [Google Scholar]
- Fuentes S, Cañamero RC, Serna L. (2012) Relationship between brassinosteroids and genes controlling stomatal production in the Arabidopsis hypocotyl. Int J Dev Biol 56: 675–680 [DOI] [PubMed] [Google Scholar]
- Geisler M, Yang M, Sack FD. (1998) Divergent regulation of stomatal initiation and patterning in organ and suborgan regions of the Arabidopsis mutants too many mouths and four lips. Planta 205: 522–530 [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Schneider-Pizoń J, Betti C, Mayerhofer J, Vanhoutte I, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, et al. (2012a) SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat Cell Biol 14: 548–554 [DOI] [PubMed] [Google Scholar]
- Gudesblat GE, Betti C, Russinova E. (2012b) Brassinosteroids tailor stomatal production to different environments. Trends Plant Sci 17: 685–687 [DOI] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. (2007) The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes Dev 21: 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. (2009) Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves. Plant Cell Physiol 50: 1019–1031 [DOI] [PubMed] [Google Scholar]
- He Z, Wang Z-Y, Li J, Zhu Q, Lamb C, Ronald P, Chory J. (2000) Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science 288: 2360–2363 [DOI] [PubMed] [Google Scholar]
- He J-X, Gendron JM, Yang Y, Li J, Wang Z-Y. (2002) The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc Natl Acad Sci USA 99: 10185–10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J-X, Gendron JM, Sun Y, Gampala SSL, Gendron N, Sun CQ, Wang Z-Y. (2005) BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science 307: 1634–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C-Y, Lin Y, Zhang M, Pollock S, Marks MD, Schiefelbein J. (1998) A common position-dependent mechanism controls cell-type patterning and GLABRA2 regulation in the root and hypocotyl epidermis of Arabidopsis. Plant Physiol 117: 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Gray JE. (2009) The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr Biol 19: 864–869 [DOI] [PubMed] [Google Scholar]
- Khan M, Rozhon W, Bigeard J, Pflieger D, Husar S, Pitzschke A, Teige M, Jonak C, Hirt H, Poppenberger B. (2013) Brassinosteroid-regulated GSK3/shaggy-like kinases phosphorylate mitogen-activated protein (MAP) kinase kinases, which control stomata development in Arabidopsis thaliana. J Biol Chem 288: 7519–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-W, Wang Z-Y. (2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61: 681–704 [DOI] [PubMed] [Google Scholar]
- Kim T-W, Michniewicz M, Bergmann DC, Wang Z-Y. (2012) Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482: 419–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Pan J, Cai G, Li D. (2012) Recent insights into brassinosteroid signaling in plants: its dual control of plant immunity and stomatal development. Mol Plant 5: 1179–1181 [DOI] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC. (2008) Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322: 1113–1116 [DOI] [PubMed] [Google Scholar]
- Lampard GR, Lukowitz W, Ellis BE, Bergmann DC. (2009) Novel and expanded roles for MAPK signaling in Arabidopsis stomatal cell fate revealed by cell type-specific manipulations. Plant Cell 21: 3506–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nam KH. (2002) Regulation of brassinosteroid signaling by a GSK3/SHAGGY-like kinase. Science 295: 1299–1301 [DOI] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. (2007) Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445: 537–540 [DOI] [PubMed] [Google Scholar]
- Mora-García S, Vert G, Yin Y, Caño-Delgado A, Cheong H, Chory J. (2004) Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev 18: 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296: 1697–1700 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. (2007) Termination of asymmetric cell division and differentiation of stomata. Nature 445: 501–505 [DOI] [PubMed] [Google Scholar]
- Ryu H, Kim K, Cho H, Park J, Choe S, Hwang I. (2007) Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell 19: 2749–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Kim K, Cho H, Hwang I. (2010a) Predominant actions of cytosolic BSU1 and nuclear BIN2 regulate subcellular localization of BES1 in brassinosteroid signaling. Mol Cells 29: 291–296 [DOI] [PubMed] [Google Scholar]
- Ryu H, Cho H, Kim K, Hwang I. (2010b) Phosphorylation dependent nucleocytoplasmic shuttling of BES1 is a key regulatory event in brassinosteroid signaling. Mol Cells 29: 283–290 [DOI] [PubMed] [Google Scholar]
- Serna L. (2004) A network of interacting factors triggering different cell fates. Plant Cell 16: 2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. (2005) Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309: 290–293 [DOI] [PubMed] [Google Scholar]
- Tang W, Kim T-W, Oses-Prieto JA, Sun Y, Deng Z, Zhu S, Wang R, Burlingame AL, Wang Z-Y. (2008) BSKs mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 321: 557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Yuan M, Wang R, Yang Y, Wang C, Oses-Prieto JA, Kim TW, Zhou HW, Deng Z, Gampala SS, et al. (2011) PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat Cell Biol 13: 124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU. (2012) Mix-and-match: ligand-receptor pairs in stomatal development and beyond. Trends Plant Sci 17: 711–719 [DOI] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue LW, Du JB, Yang H, Xu F, Yuan S, Lin HH. (2009) Brassinosteroids counteract abscisic acid in germination and growth of Arabidopsis. Z Naturforsch C 64: 225–230 [DOI] [PubMed] [Google Scholar]
- Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. (2002) BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 109: 181–191 [DOI] [PubMed] [Google Scholar]