Terminal oxidases are essential for survival under high light/dark changes but not under diurnal conditions.
Abstract
Cyanobacteria perform photosynthesis and respiration in the thylakoid membrane, suggesting that the two processes are interlinked. However, the role of the respiratory electron transfer chain under natural environmental conditions has not been established. Through targeted gene disruption, mutants of Synechocystis sp. PCC 6803 were generated that lacked combinations of the three terminal oxidases: the thylakoid membrane-localized cytochrome c oxidase (COX) and quinol oxidase (Cyd) and the cytoplasmic membrane-localized alternative respiratory terminal oxidase. All strains demonstrated similar growth under continuous moderate or high light or 12-h moderate-light/dark square-wave cycles. However, under 12-h high-light/dark square-wave cycles, the COX/Cyd mutant displayed impaired growth and was completely photobleached after approximately 2 d. In contrast, use of sinusoidal light/dark cycles to simulate natural diurnal conditions resulted in little photobleaching, although growth was slower. Under high-light/dark square-wave cycles, the COX/Cyd mutant suffered a significant loss of photosynthetic efficiency during dark periods, a greater level of oxidative stress, and reduced glycogen degradation compared with the wild type. The mutant was susceptible to photoinhibition under pulsing but not constant light. These findings confirm a role for thylakoid-localized terminal oxidases in efficient dark respiration, reduction of oxidative stress, and accommodation of sudden light changes, demonstrating the strong selective pressure to maintain linked photosynthetic and respiratory electron chains within the thylakoid membrane. To our knowledge, this study is the first to report a phenotypic difference in growth between terminal oxidase mutants and wild-type cells and highlights the need to examine mutant phenotypes under a range of conditions.
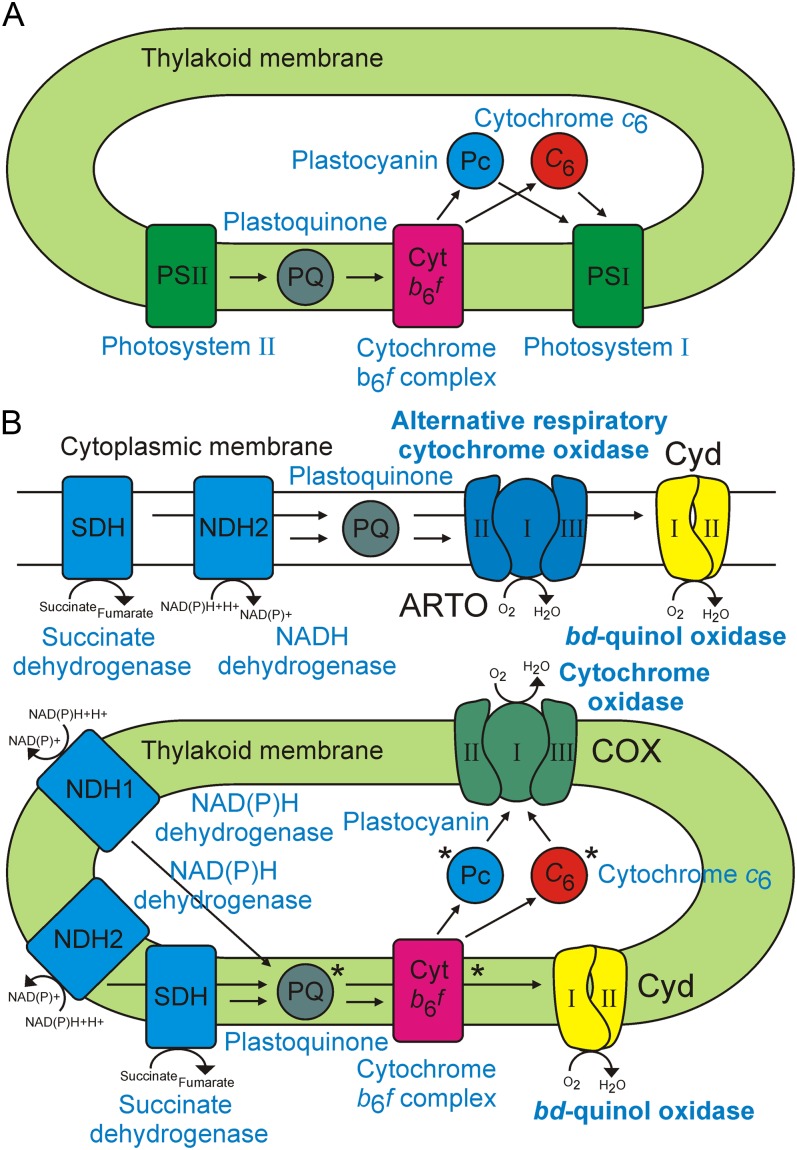
Cyanobacteria (oxygenic photosynthetic bacteria) represent an important lineage that diverged from others early in evolution and today play a key role in global ecology, most notably in marine systems (Zwirglmaier et al., 2008). With the exception of the anomalous cyanobacterium Gloeobacter violaceus, they are unusual among prokaryotes in possessing two distinct membrane systems: the thylakoid, containing an electron transfer chain capable of both respiratory and photosynthetic functions, and the cytoplasmic membrane, similar to that of other Gram-negative bacteria, and the site of an additional electron transfer chain, solely involved in respiration (Smith and Howe, 1993; Vermaas, 2001). Respiration and photosynthesis share many components in the thylakoid membrane, including plastoquinone, the cytochrome b6f complex (cyt b6f), and the soluble redox carriers plastocyanin and cytochrome c6, suggesting that these two processes are interlinked (Peschek et al., 2004). In the model species Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis), plastoquinone is the electron acceptor from either PSII or one of several dehydrogenase complexes, which are linked to the oxidation of NADPH, NADH, or succinate (Cooley and Vermaas, 2001; Ohkawa et al., 2001). Electrons are transferred directly from plastoquinone to a cytochrome bd-quinol oxidase complex (Cyd), encoded by cydAB (Berry et al., 2002), or via cyt b6f and the soluble redox carriers to either PSI or an aa3-type cytochrome c oxidase complex (COX), encoded by ctaCIDIEI (Howitt and Vermaas, 1998; Fig. 1). In addition, there is a third terminal oxidase, the alternative respiratory terminal oxidase (ARTO), encoded by ctaCIIDIIEII. The absence of a CuA- and Mg2+-binding site in CtaCII (Howitt and Vermaas, 1998) and similarity to the subunits of the cytochrome bo3 ubiquinol oxidase complex from Escherichia coli, which has been shown to lack a cytochrome c-binding site and to undergo reduction by a quinol molecule (Abramson et al., 2000), suggests that ARTO is reduced by plastoquinol. A fourth terminal oxidase, the plastoquinol or plastid terminal oxidase (PTOX), has been identified in the chloroplasts of red and green algae, higher plants, and some cyanobacterial strains but is not present in Synechocystis (McDonald et al., 2011).
Figure 1.
Schematic diagrams of the photosynthetic electron transport chain (A) and respiratory electron chains (B). Components shared by both processes are indicated with asterisks, and terminal oxidase complexes are highlighted in boldface type. Individual subunits are only shown for terminal oxidase complexes. The localization of NAD(P)H dehydrogenase (NDH2), succinate dehydrogenase, and Cyd in the cytoplasmic membrane has not been confirmed. [See online article for color version of this figure.]
Original studies suggested the presence of a respiratory chain in the Synechocystis cytoplasmic membrane consisting of dehydrogenase complexes donating electrons to plastoquinone, followed by transfer to cyt b6f, plastocyanin, and/or cytochrome c6, and terminating in COX. This was based on the detection of COX subunits via immunogold labeling using an anti-COX antibody in salt-stressed cells (Peschek et al., 1994). However, these results have been questioned due to the possible cross reactivity of the antibody with ARTO subunits as well as a lack of confirmation of membrane purity (Schultze et al., 2009). In the latter studies, cyt b6f subunits were only detected in the thylakoid membrane (Schultze et al., 2009). The purity of these membrane fractions was confirmed by probing with thylakoid- and cytoplasmic membrane-specific antibodies. In addition, proteomic studies have detected cyt b6f subunits in purified thylakoid membrane fractions (Srivastava et al., 2005; Agarwal et al., 2010) but not in cytoplasmic membranes from Synechocystis cultured under normal (Huang et al., 2002) and salt-stressed (Huang et al., 2006) conditions. This suggests the presence of a simpler respiratory chain in the cytoplasmic membrane, in which electrons donated to plastoquinone by dehydrogenase complexes are transferred directly to plastoquinol-reduced terminal oxidases. The main electron donors are likely to be either one or more of three different type 2 NAD(P)H dehydrogenases (Howitt et al., 1999) or succinate dehydrogenase, since antibodies raised against various subunits of type 1 NAD(P)H dehydrogenase have detected these proteins only in the thylakoid membrane and not the cytoplasmic membrane (Ohkawa et al., 2001; Zhang et al., 2004). Synechocystis has multiple type 1 NAD(P)H dehydrogenase complexes with distinct roles in respiration, preferentially utilizing NADPH as a substrate, cyclic electron flow via the oxidation of reduced ferredoxin, or CO2 fixation (Mi et al., 1995; Ohkawa et al., 2000).
COX has been confirmed as a terminal oxidase in the thylakoid membrane, because subunits of this complex cannot be deleted in a PSI-deficient strain (Howitt and Vermaas, 1998). Cyd has also been localized to the thylakoid membrane based on inhibitor studies. The addition of 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone, which inhibits cyt b6f and pentachlorophenol, a Cyd inhibitor, results in complete suppression of plastoquinol oxidation (Berry et al., 2002). Deletion of Cyd and addition of 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone also results in the same effect. In the same study, ARTO was unable to oxidize plastoquinol in a COX/Cyd mutant, suggesting that it cannot substitute for Cyd in the thylakoid membrane. Proteomics studies on purified membrane fractions have confirmed the presence of the CtaCII subunit of ARTO in the cytoplasmic membrane (Huang et al., 2002, 2006; Pisareva et al., 2007) but not in the thylakoid membrane (Srivastava et al., 2005; Agarwal et al., 2010) of Synechocystis. However subunits of the other terminal oxidases were not identified in these same studies (Srivastava et al., 2005; Agarwal et al., 2010), suggesting that terminal oxidases are present in low abundance in both membranes. It has also been proposed that Cyd is localized to the cytoplasmic membrane (Howitt and Vermaas, 1998). In Synechococcus sp. PCC 7002 (hereafter referred to as Synechococcus), which lacks Cyd, a faster PSI reduction rate was observed in an ARTO-deficient mutant, supporting localization in the thylakoid membrane and a role as a possible Cyd substitute (Nomura et al., 2006).
The functions of the terminal oxidases have been investigated in Synechocystis and Synechococcus (Howitt and Vermaas, 1998; Pils and Schmetterer, 2001; Hart et al., 2005; Nomura et al., 2006). The activity of Cyd increased when cyt b6f was inhibited in Synechocystis, implicating a role for Cyd in preventing overreduction of the plastoquinone pool (Berry et al., 2002). COX possibly functions as a terminal electron sink under conditions of low PSI activity or to prevent overreduction of the soluble redox carriers (Howitt and Vermaas, 1998). This was further supported by investigations in Synechococcus, in which deletion of COX and COX/ARTO resulted in 1.7- and 2.2-fold increases, respectively, in the reduction rate of PSI (Nomura et al., 2006). Alternative electron sinks, such as the Flv2/Flv4 complex, which accepts electrons directly from PSII (Zhang et al., 2012), the hydrogenase complex, nitrate reductase, or carbon fixation, may compensate for the loss of terminal oxidase activity, as demonstrated by increased hydrogen production in a Synechocystis COX/Cyd-deficient strain (Gutthann et al., 2007). Energization of both the thylakoid and cytoplasmic membrane electron transfer chains is important under conditions of salt stress in Synechocystis (Jeanjean et al., 1990, 1993; Peschek et al., 1994) and Synechococcus sp. PCC 6311 (Fry et al., 1986), possibly to provide a proton gradient for Na+/H+ transporters localized in the cytoplasmic membrane under conditions when photosynthetic activity is low (Hagemann, 2011). The role and membrane localization of PTOX, which in cyanobacteria is predominantly found in marine strains, have not been investigated but may provide an additional electron sink under iron-deprived conditions, when cyt b6f and PSI are limited (Bailey et al., 2008). Deletion of PTOX in Arabidopsis (Arabidopsis thaliana) results in a variegated phenotype, with leaves characterized by distinct green and white patches containing either normal chloroplasts or pigment-deficient chloroplasts (Carol et al., 1999). This phenotype was observed under continuous light at 50, 150, and 450 µmol photons m−2 s−1 and in an 8-h-light/16-h-dark cycle at 150 and 450 µmol photons m−2 s−1 (Rosso et al., 2009). The variegated phenotype was exacerbated at higher light intensities. No phenotypic difference was observed between the mutant strain and the wild type under an 8-h-light (50 µmol photons m−2 s−1)/16-h-dark cycle when the mutant was grown at 25°C, but variegation was apparent when the temperature was lowered to 12°C. High light, continuous illumination, and/or low temperature were all observed to increase the proportion of closed PSII reaction centers and, therefore, the excitation pressure in the mutant and led to impaired thylakoid membrane biogenesis (Rosso et al., 2009). In several plant species, up-regulation of PTOX has been observed in response to adverse conditions, such as exposure to high-light stress in low or elevated temperature environments (Streb et al., 2005) or under salt stress (Stepien and Johnson, 2009). Deletion of the PTOX2 gene in Chlamydomonas reinhardtii, encoding PTOX, confirmed a role in preventing overreduction of the plastoquinone pool in the light (Houille-Vernes et al., 2011). The mutant demonstrated reduced fitness compared with the wild-type under illumination, although growth was not examined under varying environmental conditions.
Despite the effects of loss of terminal oxidases on electron transfer, no significant effect on growth or viability has been observed when these complexes are lost in Synechocystis (Howitt and Vermaas, 1998; Pils and Schmetterer, 2001) or Synechococcus (Nomura et al., 2006). However, in contrast to the studies of PTOX in plants, the cyanobacterial mutant strains were cultured only under constant light levels, and there has been no investigation during dark periods or light/dark transitions. Therefore, we examined the growth of terminal oxidase mutants of Synechocystis under conditions more representative of those seen in the natural environment, where light conditions may rapidly change due to altering cloud cover or obstruction by objects or other organisms. Our results showed that loss of the thylakoidal terminal oxidases was lethal when cells were rapidly exposed to high light. This indicates a fundamental role for these complexes in responding to sudden light stress.
RESULTS
Conservation of Terminal Oxidases in Cyanobacteria
As a first step to understand the role of terminal oxidases in cyanobacteria, the sixty sequenced cyanobacterial genomes in the National Center for Biotechnology Information database were examined for the presence of genes encoding terminal oxidases (Table I; Supplemental Table S1). All strains contained at least one set of COX genes, suggesting that this is the major terminal oxidase. Seven strains contained only COX and none of the plastoquinol-reduced terminal oxidases. Four of these were Prochlorococcus spp. with a high ratio of chlorophyll b to divinyl chlorophyll a (a2). These species are typically located in deep marine environments and are exposed to low levels of illumination, in contrast to Prochlorococcus spp. with a low ratio of chlorophyll b to a2, which require higher light intensities for growth (Rocap et al., 2002). Another cyanobacterium, UCYN-A, lacks PSII (Zehr et al., 2008). The remaining two, Cyanothece spp. PCC 8801 and 8802, were isolated from rice (Oryza sativa) fields in Taiwan, and it is not known to what light levels they would routinely be exposed (Huang and Chow, 1986). The remaining strains contained genes for COX and at least one copy of ARTO (16), Cyd (nine), or PTOX (nine). These data suggest that the presence of COX and at least one plastoquinol-reduced terminal oxidase is of physiological importance in cyanobacteria. The majority of the strains (15 out of 19) that encode COX and more than one plastoquinol-reduced terminal oxidase are either halotolerant, as indicated by the presence of one or more of the nhaP genes, encoding a cytoplasmic localized Na+/H+ transporter (Hagemann, 2011), and/or nitrogen fixers, identified by the presence of nifH, encoding the iron-containing component of nitrogenase (Ben-Porath and Zehr, 1994). In each case, it is possible that additional terminal oxidases are required to provide sufficient ATP for Na+ export under conditions where photosynthesis is reduced (Hagemann, 2011) and to remove oxygen that may inhibit nitrogenase activity (Berman-Frank et al., 2001; Staal et al., 2003).
Table I. Terminal oxidases present in 60 cyanobacteria species.
A BLAST comparison was performed to identify terminal oxidases and the nifH and nhaP genes, an indication of nitrogen fixation (except in the case of Microcoleus chthonoplastes PCC 7420 and Synechococcus sp. JA-3-3Ab) and halotolerance, respectively.
| No. of Strains | COX | ARTO | Cyd | PTOX | Nitrogen Fixation | Halotolerant |
|---|---|---|---|---|---|---|
| 7 | + | 3 | 2 | |||
| 16 | + | + | 3 | 8 | ||
| 9 | + | + | 1 | 3 | ||
| 9 | + | + | 0 | 0 | ||
| 11 | + | + | + | 9 | 10 | |
| 3 | + | + | + | 0 | 0 | |
| 2 | + | + | + | 0 | 1 | |
| 3 | + | + | + | + | 3 | 3 |
Generation of Recombinant Strains of Synechocystis
Unmarked mutants of Synechocystis lacking COX, ARTO, or Cyd were constructed by disruption of ctaCIDIEI, ctaCII, or cydAB, respectively, via a two-step homologous recombination protocol. Plasmids in which the genes of interest were disrupted with an npt1/sacRB cassette were introduced into Synechocystis, and transformants were selected in the presence of kanamycin. Following complete segregation of strains, markerless constructs containing deleted copies of the target genes and lacking the npt1/sacRB cassette were introduced, and cells were cultured in the presence of Suc to select for recombination-mediated removal of the cassette. Complete segregation was confirmed by PCR assays with primers upstream and downstream of the respective genes (Supplemental Fig. S1). Double and triple mutants were generated and confirmed by the same methods. The double mutants, COX/ARTO and ARTO/Cyd, were generated from the ARTO single knockout. The COX/Cyd double mutant was generated from the COX single knockout. The triple mutant was generated from the ARTO/Cyd double mutant. The strains generated in this study are listed in Supplemental Table S2. Unmarked mutants were generated in order to minimize effects on expression of downstream genes and to pyramid mutations. Reverse transcription (RT)-PCR analysis confirmed that trxA, encoding thioredoxin and located 152 bp downstream of ctaEI, is not cotranscribed with this gene. Likewise, Sll0814, encoding a hypothetical protein and located 214 bp downstream of ctaCII, is also not cotranscribed (Supplemental Fig. S2). Therefore, polar effects on downstream genes are unlikely. MiSeq genome sequencing of the wild-type and ΔCOX/Cyd strains confirmed that apart from the targeted deletions, no further differences existed between the strains (Supplemental Table S3). Dark respiratory rates, as measured by oxygen uptake, were severely reduced in the COX/Cyd mutant and the triple mutant, both of which lack thylakoid-localized terminal oxidases, but were similar to the wild type in the other strains (Supplemental Table S2). This is consistent with results from previous studies (Howitt and Vermaas, 1998).
Growth of the Terminal Oxidase-Deficient Strains
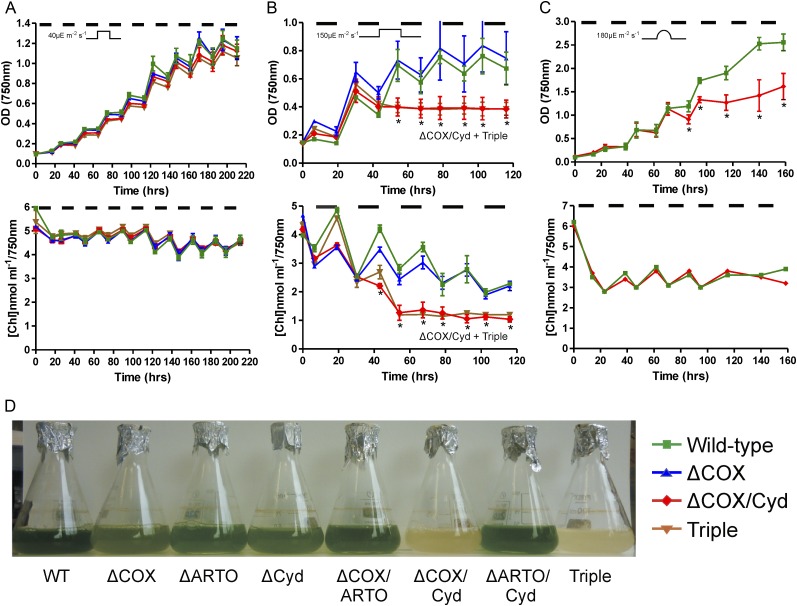
Synechocystis strains were cultured under two light intensities: moderate (40 µmol photons m−2 s−1), whereby wild-type cells do not suffer light stress, and high (150 µmol photons m−2 s−1), which results in light stress and photobleaching of wild-type cells. The growth of all strains cultured under continuous moderate light was similar, as reported previously (Howitt and Vermaas, 1998; Supplemental Fig. S4A). The amount of chlorophyll per cell remained essentially constant for all strains, the lack of photobleaching indicating the absence of light stress. Exposure to continuous high light resulted in similar growth and a gradual increase in photobleaching in all strains (Supplemental Fig. S4B). To test whether terminal oxidases are important for dark respiration, strains were cultured under 12-h moderate-light/dark square-wave cycles. No statistically significant difference in growth was observed between strains, and no evidence of photobleaching was seen in any strain (Fig. 2A; Supplemental Fig. S4C). This suggests that the respiratory electron chain is not essential for survival in unstressed cells under 12-h moderate-light/dark cycles. In contrast, when strains were exposed to 12-h high-light/dark square-wave cycles, the two strains deficient in thylakoid-localized terminal oxidases (COX/Cyd and the triple mutant) demonstrated statistically significant increases in photobleaching by the beginning of the second 12-h light period (approximately 42 h; Fig. 2B; Supplemental Fig. S4D). Visually, cells appeared completely photobleached by the end of this period, although some residual chlorophyll, measured following methanol extraction, remained in the COX/Cyd mutant (1.52 nmol mL−1 at optical density [OD] of 750 nm) versus the wild type (3.09 nmol mL−1 at OD of 750 nm; P < 0.005). However, cells demonstrated no recovery from this state, indicating that they were dead (Fig. 2, B and D; Supplemental Fig. S4D). Such rapid loss of viability indicates a requirement for at least one active terminal oxidase in the thylakoid membrane under these conditions. These results also show that ARTO cannot compensate for the loss of COX and Cyd. Growth of the wild-type and double mutant strains was then measured under 12-h sinusoidal light/dark cycles (Supplemental Fig. S4F), in which light was gradually increased to a maximum level of 180 µmol photons m−2 s−1 to simulate natural diurnal conditions (Fig. 2C; Supplemental Fig. S4E). Growth of the COX/Cyd and triple mutant strains was reduced compared with the wild type and the other double terminal oxidase mutants. In some circumstances, photobleaching was observed, although this was not consistent.
Figure 2.
A to C, Growth of triplicate cultures was measured at OD of 750 and 680 nm under 12-h moderate-light (40 µmol photons m−2 s−1)/dark square-wave cycles (A), 12-h high-light (150 µmol photons m−2 s−1)/dark square-wave cycles (B), and 12-h sinusoidal light (maximum light level of 180 µmol photons m−2 s−1)/dark cycles (diurnal; C). Dark periods are indicated by black bars. The amount of chlorophyll was determined by 680:750-nm ratios and is an indication of photobleaching in cells. For ease of visualization, only results from the wild-type (green), ΔCOX (blue), ΔCOX/Cyd (red), and triple mutant (brown) strains are shown. Results from all strains are shown in Supplemental Figure S4, C to E. Asterisks indicate significant differences between wild-type and ΔCOX/Cyd samples (P < 0.05). D, Strains exposed to 50 h of 12-h high-light (150 µmol photons m−2 s−1)/dark square-wave cycles. The thylakoid-localized terminal oxidase-deficient strains appear completely photobleached in marked comparison with the other six strains. Results for the other biological replicates are shown in Supplemental Figure S4. WT, Wild type. [See online article for color version of this figure.]
Photosynthesis Measurements in the Wild Type and the Thylakoid Terminal Oxidase Mutant
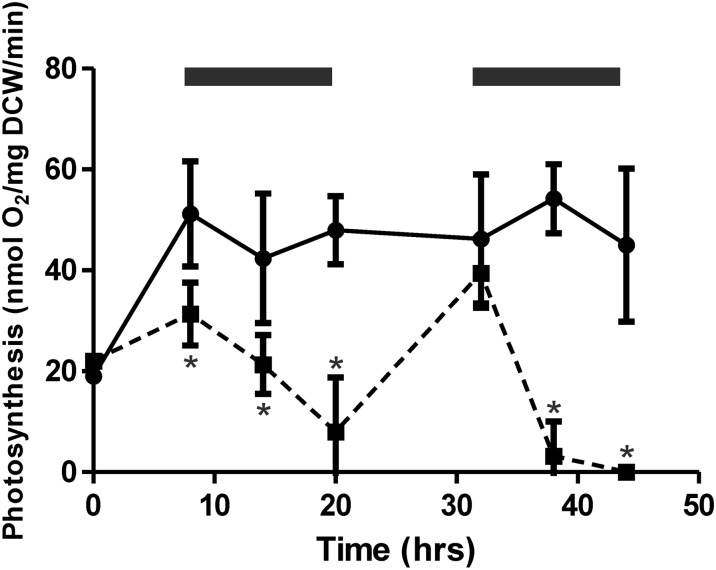
Oxygen evolution measurements were performed on the wild type and the COX/Cyd mutant to investigate whether rapid photobleaching observed in this strain upon exposure to 12-h high-light/dark square-wave cycles coincides with a loss of photosynthetic activity. Oxygen evolution was measured at the beginning and end of the light periods (150 µmol photons m−2 s−1) and in the middle of the dark period for a total of 44 h, by which time the COX/Cyd mutant showed no further growth (Fig. 3). A significant drop in oxygen evolution activity was observed in the COX/Cyd strain in the first dark period (Fig. 3) from 31.3 ± 6.2 to 8.0 ± 10.8 nmol oxygen mg−1 dry cell weight (DCW) min−1. In contrast, the rate was not significantly different in the wild type between the beginning and end of the dark period (from 51.2 ± 10.4 to 48.0 ± 6.7 nmol oxygen mg−1 DCW min−1). Photosynthetic activity recovered in the COX/Cyd mutant to a similar level as in the wild type by the end of the second light period (wild type, 46.2 ± 12.8 nmol oxygen mg−1 DCW min−1; ΔCOX/Cyd, 39.4 ± 6.8 nmol oxygen mg−1 DCW min−1). During the second dark period, the rate of oxygen evolution in ΔCOX/Cyd rapidly decreased to zero (Fig. 3). Similar to the first dark period, oxygen production in the wild type remained relatively constant between the beginning and end of the dark period (from 46.2 ± 12.8 to 45.0 ± 15.1 nmol oxygen mg−1 DCW min−1).
Figure 3.
Oxygen evolution rates of wild-type (solid line) and ΔCOX/Cyd (dashed line) samples cultured under 12-h high-light (150 µmol photons m−2 s−1)/dark square-wave cycles. Asterisks indicate significant differences between samples (P < 0.05). Dark periods are indicated by black bars. Results are from three biological replicates.
Glycogen Measurements in the Wild Type and the Thylakoid Terminal Oxidase Mutant
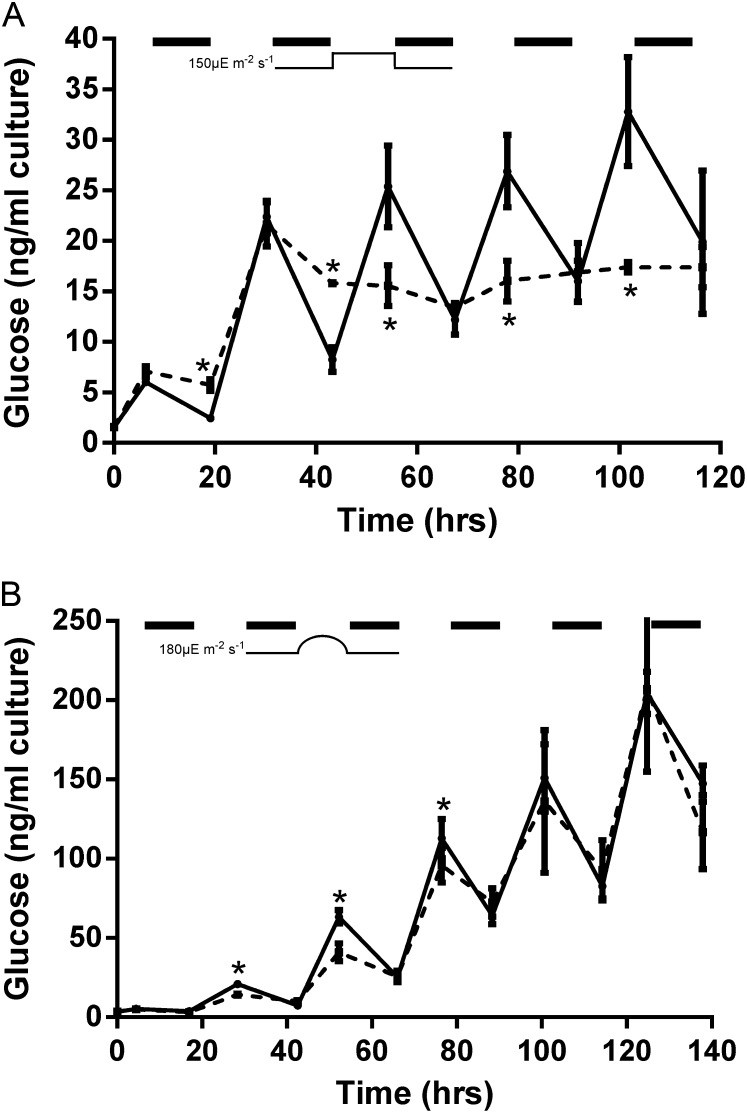
The main energy storage molecule in cyanobacteria is glycogen (Lindberg et al., 2010; Suzuki et al., 2010), which can be determined by assaying the amount of Glc following acid hydrolysis. Glycogen levels in the wild type and the COX/Cyd mutant cultured under 12-h high-light/dark square-wave cycles (Fig. 4A) or 12-h sinusoidal light/dark cycles (Fig. 4B) were measured at the beginning and end of the 12-h dark periods. In the 12-h high-light/dark square-wave cycles, the COX/Cyd strain accumulated similar levels of glycogen during the first two light periods compared with the wild type but demonstrated a significantly lower utilization of glycogen in the first two dark periods. Following the second dark period, glycogen levels were constant, presumably due to death of the cells. By contrast, in 12-h sinusoidal light/dark cycle samples, the COX/Cyd mutant accumulated and utilized significantly less glycogen than the wild type for the first 3 d, but levels were similar between the two strains after this period.
Figure 4.
Glc measurements of wild-type (solid line) and ΔCOX/Cyd (dashed line) samples cultured under 12-h high-light (150 µmol photons m−2 s−1)/dark square-wave cycles (A) and 12-h sinusoidal light (maximum light level of 180 µmol photons m−2 s−1)/dark cycles (diurnal; B). Asterisks indicate significant differences between samples (P < 0.05). Dark periods are indicated by black bars. Results are from three biological replicates. Glc was measured from 1-mL samples and is dependent on cell amounts.
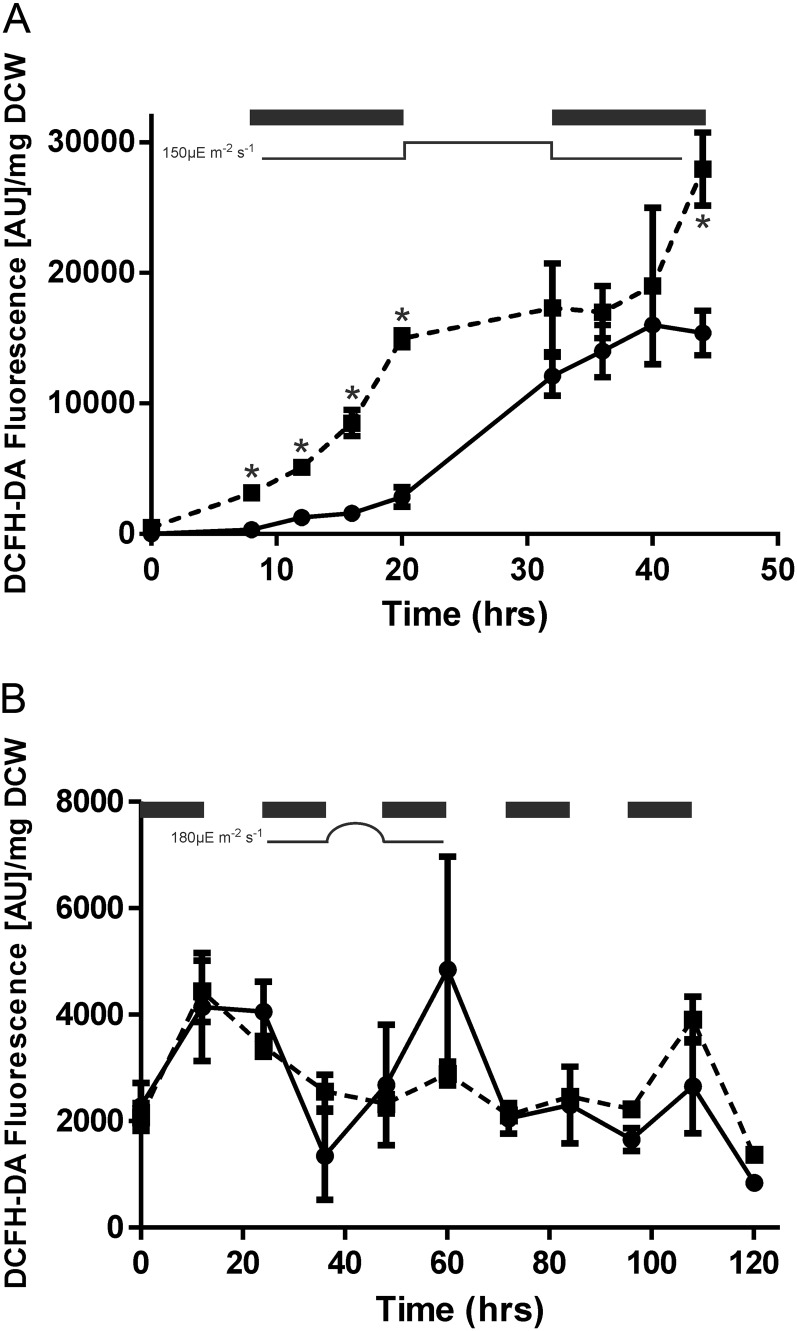
Measurement of Reactive Oxygen Species in the Wild Type and the Thylakoid Terminal Oxidase Mutant
We examined the production of reactive oxygen species (ROS) in the wild type and the COX/Cyd mutant cultured under 12-h high-light/dark square-wave cycles for 44 h via 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) fluorescence (Fig. 5A). DCHF-DA is a cell-permeable dye that is hydrolyzed to the nonfluorescent 2′,7′-dichlorodihydrofluorescein in vivo and in turn is oxidized to the highly fluorescent dichlorofluorescein (DCF) by hydrogen peroxide, other peroxides, and peroxynitrite (Crow, 1997; Ding et al., 1998; He and Häder, 2002; Rastogi et al., 2010). DCHF-DA was confirmed to be a valid marker for measuring oxidative damage in Synechocystis by measuring fluorescence in wild-type cells exposed to methyl viologen (MV). MV outcompetes endogenous electron acceptors of PSI and is reoxidized by oxygen, giving superoxide, which is rapidly converted to hydrogen peroxide (Thomas et al., 1998). Upon exposure to light, wild-type cells demonstrated a large increase in fluorescence in the presence of MV (24,240 ± 2,034 versus 6,771 ± 195 relative fluorescence units [RFU] at OD of 750 nm; Supplemental Fig. S5). The effect of adding MV was noticeably smaller when cells were maintained in the dark (4,731 ± 314 versus 3,714 ± 135 RFU at OD of 750 nm).
Figure 5.
DCF fluorescence of wild-type (solid line) and ΔCOX/Cyd (dashed line) samples cultured under 12-h high-light (150 µmol photons m−2 s−1)/dark square-wave cycles (A) and 12-h sinusoidal light (180 µmol photons m−2 s−1)/dark cycles (B). Asterisks indicate significant differences between samples (P < 0.05). Dark periods are indicated by black bars. Results are from three biological replicates. AU, Absorbance units.
For cells grown under square-wave illumination, DCF fluorescence increased significantly in both the wild-type (from 331 ± 97 to 2,836 ± 750 RFU mg−1 DCW) and ΔCOX/Cyd (from 3,167 ± 277 to 14,970 ± 653 RFU mg−1 DCW) strains throughout the first dark period. However the level of fluorescence was 5-fold less in wild-type compared with ΔCOX/Cyd strains. By the end of the second light period, the level of fluorescence was not significantly different between the two strains. This demonstrates that the deletion of thylakoid-localized terminal oxidases does not contribute to the generation of ROS under periods of illumination. In the second dark period, some increase in DCF fluorescence was observed in the ΔCOX/Cyd strain, leading to a final level of fluorescence that was significantly higher in ΔCOX/Cyd compared with the wild type (27,960 ± 2,794 versus 15,400 ± 1,712 RFU mg−1 DCW; Fig. 5A). In contrast, growth of the wild-type and ΔCOX/Cyd strains under 12-h sinusoidal light/dark cycles resulted in no significant increase in ROS production over a 5-d period (Fig. 5B).
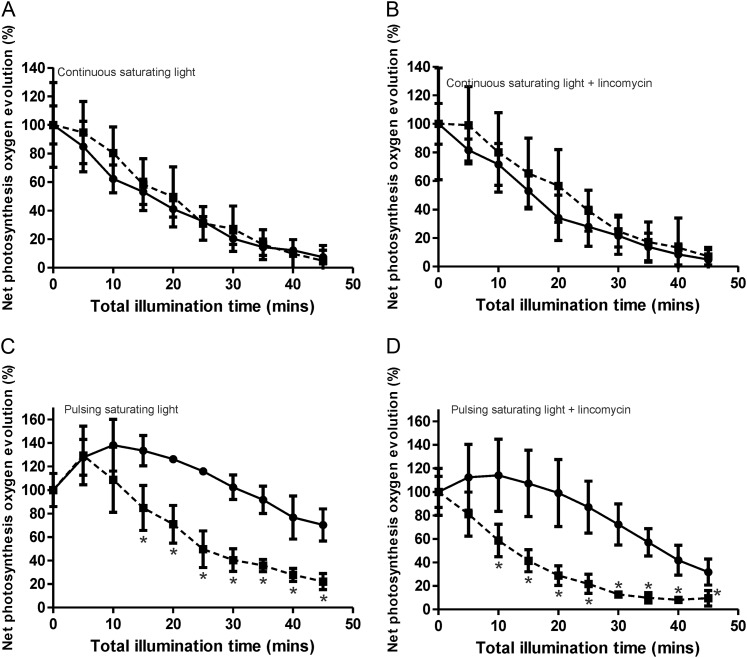
Measurements of Photoinhibition in the Wild Type and the Thylakoid Terminal Oxidase Mutant
To test for photoinhibition, the wild-type and COX/Cyd mutant strains were grown under continuous moderate light to mid log phase followed by incubation in the dark for 20 min. Cells were then incubated under constant saturating light at 1,500 µmol photons m−2 s−1 for 50 min in the absence and presence of lincomycin, during which time oxygen evolution was measured. No difference in the rate of oxygen evolution was observed between the wild-type and COX/Cyd mutant strains under continuous light (Fig. 6, A and B). When cells were incubated under pulsing light of 5 min on (1,500 µmol photons m−2 s−1)/5 min off, photoinhibition of the wild type was reduced compared with growth under continuous light conditions (Fig. 6, C and D). However, compared with the wild type, the COX/Cyd mutant strain showed a significant decrease in photosynthesis, exacerbated by lincomycin, indicating a greater level of photoinhibition (Fig. 6, C and D).
Figure 6.
Characterization of wild-type (solid line) and ΔCOX/Cyd (dashed line) strains. Photoinhibition was measured under constant light and in the absence (A) and presence (B) of lincomycin or under pulsing light of 5 min on/5 min off in the absence (C) and presence (D) of lincomycin. Only measurements from light periods are shown. Light was at an intensity of 1,500 µmol photons m−2 s−1. Asterisks indicate significant differences between samples (P < 0.05). Results are from three separate biological replicates.
DISCUSSION
The results presented here show that at least one thylakoid membrane-localized terminal oxidase is required for the survival of Synechocystis in periods of alternating high light and dark, when the light/dark transition is rapid. Under such conditions, the loss of COX and Cyd is lethal and cannot be compensated for by the presence of ARTO. This suggests that ARTO either is not present in the thylakoid membrane or is expressed at levels insufficient to cope with these conditions and that the roles of the cytoplasmic and thylakoid respiratory chains are different. A less severe effect on cell survival was seen when light levels changed sinusoidally (i.e. mimicking diurnal conditions), but there was still a significant impairment of growth in the absence of both complexes. The sinusoidal changes in light levels are more representative of diurnal conditions, but it is likely that rapid changes would also be experienced under natural environmental conditions, for example, as a result of cloud movement.
The similar growth of all strains observed under continuous light suggests a limited role for terminal oxidases when conditions remain unchanged, a state that never occurs in natural environments. However, it was notable that comparable growth was observed between all strains exposed to square-wave cycles of moderate light/dark, under which conditions photobleaching was limited. This would suggest that respiration is not essential for dark maintenance in unstressed cells, an unexpected finding given the assumption that the metabolic energy required for dark periods is predominantly provided by respiration, utilizing products broken down from glycogen via glycolysis and the citric acid cycle (Matthijs and Lubberding, 1988). It is possible that the energy requirements of unstressed cells during dark periods are low enough that other processes such as fermentation can compensate for the loss of respiration (Stal and Moezelaar, 1997). Metabolic pathways involved in the fermentation of sugars to pyruvate, followed by conversion to lactate, acetate, or ethanol, have been identified in Synechocystis (Kaneko et al., 1996), but their role in cellular physiology has not been investigated.
The lethality of the COX/Cyd mutant under high-light/dark square-wave illumination is probably due to a range of factors. First, the observation that wild-type and mutant strains showed similar levels of photoinhibition under constant illumination, while the mutant strain showed more photoinhibition under alternating light/dark cycles (Fig. 6, C and D), suggests that the COX/Cyd proteins allow the cells to avoid overreduction of photosynthetic components and consequent damage, including photoinhibition, immediately after an increase in light level that is too rapid for the cells to compensate for through other mechanisms. For example, protein diffusion is relatively slow in the thylakoid membrane, as recently demonstrated by the 10- to 20-min half-time redistribution of type 1 NAD(P)H dehydrogenase and succinate dehydrogenase in cells transferred from low to moderate light (Liu et al., 2012). Second, the COX/Cyd mutant cells suffer increased generation of ROS in the dark compared with wild-type cells (Fig. 5A). This may result from the respiratory chain becoming overreduced in cells lacking two of the terminal oxidase activities, and this presumably also accounts for the drop in activity of the photosynthetic machinery of the mutant seen in the dark (Fig. 3). In addition, the absence of thylakoid-localized terminal oxidases would limit the cell’s ability to reduce oxygen, thereby potentially increasing the generation of ROS. Third, the lack of respiration in the dark (indicated by the lower rate of glycogen breakdown) may lead to insufficient ATP being available for the replacement or repair of PSII and other complexes, compounding the damage. In contrast, there is significant mobilization of stored carbon reserves in wild-type cells during the dark after subjection to high-light/dark square-wave illumination (Fig. 4A).
Despite the initially low remaining rate of photosynthesis observed in the COX/Cyd mutant after the first dark period (Fig. 3B), this strain demonstrated photosynthesis rates similar to the wild type after 12 h of illumination. This, together with the facts that all strains exposed to continuous high light (Supplemental Fig. S4B) showed similar growth and that comparable levels of ROS were seen between the wild type and the COX/Cyd mutant during light periods (Fig. 5A), suggests that increased oxidative damage does not occur in the mutants on continuous illumination. This would be expected, both because PSI could function as an alternative electron acceptor in the light and because the respiratory chain would not be required during light periods. However, during the second dark period, there is a complete loss in photosynthetic activity in the COX/Cyd mutant. As a result of these effects, the mutant cells die after two light/dark cycles.
Under sinusoidal illumination, mutant cells show some reduction in glycogen synthesis and breakdown, but they are able to adapt to this in some way, possibly by up-regulation of fermentation, so that the difference from the wild-type cells becomes less marked with time (Fig. 4B). This may be because the lower levels of photodamage, due to stable levels of ROS production (Fig. 5B), under these illumination conditions result in less physiological stress, in part due to a period of low light at the start of the day, allowing the photosystem to adapt before damaging light levels are reached. Furthermore, in the middle of the day, the period over which light conditions are high enough to damage the cells is reduced (approximately 4.5 h).
The demonstration here of the importance of COX and Cyd contrasts with previous studies indicating little effect if any on growth or viability as a result of the loss of these complexes (Howitt and Vermaas, 1998; Pils and Schmetterer, 2001; Nomura et al., 2006), and indicates the importance of characterizing mutant phenotypes under a range of environmental conditions.
CONCLUSION
The results in this study show that the thylakoid-located terminal oxidases have an essential and dual role in allowing Synechocystis to respond to rapid changes in light levels. While the role of terminal oxidases in preventing overreduction of either cyt b6f (Berry et al., 2002), in the case of the plastoquinol-accepting terminal oxidases, and PSI, in the case of COX (Howitt and Vermaas, 1998), has been indicated before, we demonstrate the conditions under which this function is critical for survival. The thylakoid-located terminal oxidases help to reduce the extent of photoinhibition initially, limit oxidative damage to the cell during dark periods, and promote the use of reserves to provide energy for repair in the dark. The presence of COX and at least one plastoquinol-accepting terminal oxidase in 53 of the 55 cyanobacterial strains that have both PSI and PSII and may be exposed to high light (or 53 out of 53 if the Cyanothece spp. PCC 8801 and 8802 that were isolated from a paddy field are not exposed to high light) implies that this combination is of widespread importance among cyanobacteria.
MATERIALS AND METHODS
Bacterial Strains, Media, and Growth Conditions
Synechocystis sp. strain PCC 6803 (Williams, 1988) and strains derived from it were routinely cultured in BG-11 medium, supplemented with 10 mm sodium bicarbonate (Castenholz, 1988) at 30°C under moderate light (40 µmol photons m−2 s−1), and shaken at 160 rpm unless otherwise indicated. A total of 15 g L−1 agar was used for the preparation of solid medium, supplemented with 30 µg mL−1 kanamycin and 5% (w/v) Suc when necessary.
Bioinformatics
FASTA BLAST comparisons (Altschul et al., 1990) were performed using the Synechocystis ctaC1, ctaCII, cydA, and nhaP3 genes and Nostoc sp. PCC 7120 nifH and Arabidopsis (Arabidopsis thaliana) PTOX sequences against the 60 genomes listed in the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi). The presence of a C[AT]ELC motif in CtaC1 and a D[AS]X[FY]S motif in CtaCII was used to differentiate between these two sequences (Howitt and Vermaas, 1998).
Plasmid Construction
Primers used in this study are listed in Supplemental Table S4. PCR was performed by standard procedures using Phusion high-fidelity DNA polymerase (New England Biolabs). The genome sequence of Synechocystis (Kaneko et al., 1996) was consulted via Cyanobase (http://genome.kazusa.or.jp/cyanobase) for primer design. Gene deletion of ctaC1D1E1 was performed by amplifying a 932-bp fragment upstream of ctaC1 using primers COXleftfor and COXleftrev and a 928-bp fragment downstream of ctaE1 using primers COXrightfor and COXrightrev, followed by insertion of the respective fragments into the XbaI/BamHI and SacI/EcoRI sites of pUC19 to generate pCOX1. Gene deletion of ctaII was performed by amplifying a 1,007-bp fragment upstream of ctaCII using primers Ctaleftfor and Ctaleftrev and a 1,050-bp fragment downstream of ctaCII using primers Ctarightfor and Ctarightrev, followed by insertion of the respective fragments into the XbaI/BamHI and SacI/EcoRI sites of pUC19 to generate pCta1. Gene deletion of cydAB was performed by amplifying a 1,029-bp fragment upstream of cydA using primers Cydleftfor and Cydleftrev and a 1,036-bp fragment downstream of cydB using primers Cydrightfor and Cydrightrev, followed by insertion of the respective fragments into the XbaI/BamHI and SacI/EcoRI sites of pUC19 to generate pCyd1. The BamHI-digested npt1/sacRB cassette from pUM24Cm (Ried and Collmer, 1987) was inserted into the BamHI site between the upstream and downstream fragments in pCOX1, pCta1, and pCyd1 to generate pCOX2, pCta2, and pCyd2, respectively.
Construction of Terminal Oxidase Mutant Strains
To generate marked mutants, approximately 1 µg of plasmids pCOX2, pCta2, and pCyd2 were mixed with Synechocystis cells for 6 h in liquid medium, followed by incubation on BG11 agar plates for approximately 24 h. An additional 3 mL of agar containing kanamycin was added to the surface of the plate followed by further incubation for approximately 1 to 2 weeks. Transformants were subcultured to allow the segregation of mutant alleles. Segregation was confirmed by PCR using primers COXf/COXr, Ctar/Ctar, or Cydf/Cydr, which flank the deleted region. Generation of unmarked mutants was carried out according to Xu et al. (2004). To remove the npt1/sacRB cassette, mutant lines were transformed with 1 µg of the markerless pCOX1, pCta1, and pCyd1 constructs. Following incubation in BG-11 liquid medium for 4 d and on agar plates containing Suc for a further 1 to 2 weeks, transformants were patched on kanamycin and Suc plates. Suc-resistant, kanamycin-sensitive strains containing the unmarked deletion were confirmed by PCR using primers flanking the deleted region (Supplemental Fig. S1). Single mutant lines were then used to generate double mutant lines, followed by the triple mutant via the same method. MiSeq genome sequencing of wild-type and ΔCOX/Cyd strains was performed using the Illumina MiSeq personal sequencer and analyzed using Artemis.
RT-PCR
RNA was isolated from wild-type Synechocystis cells and stored in Trizol reagent (Invitrogen) prior to homogenization for 1 min using a Biospec minibead beater. RNA was purified according to the Trizol reagent protocol, followed by digestion with DNase and further purification using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. SuperScript II reverse transcriptase (Invitrogen) was used to generate complementary DNA with random hexamers according to the manufacturer’s instructions. The complementary DNA was diluted 5-fold prior to RT-PCR. One microliter was used in PCR with GoTaq polymerase and amplifications (95°C for 2 min, 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 0.5 min, followed by 72°C for 5 min) according to the manufacturer’s instructions. Fragments spanning the region between ctaE1 and trxA were generated using primers ctaE1trxAfor and ctaE1trxArev, within trxA using primers trxAfor and trxArev, within cruF using primers cruFfor and cruFrev, and between cruF and trxA using primers cruFctaC2for and cruFctaC2rev. Primers were verified via amplification of genomic DNA. RT samples were used as negative controls.
Characterization of Mutant Growth
All strains were inoculated using cultures grown under moderate light to late logarithmic phase and then cultured under continuous moderate light (40 µmol photons m−2 s−1), continuous high light (150 µmol photons m−2 s−1), 12-h moderate-light/dark square-wave cycling, or 12-h high-light/dark cycling. In these experiments, cells cultured in the dark were maintained at 30°C but were not shaken. A modified light-emitting diode (LED) photobioreactor with X-Controller (Infors) and an Algem Labscale photobioreactor were used to provide a sinusoidal light input simulating a 12-h day with sunrise at 8 am and a noon peak intensity of 180 µmol photons m−2 s−1 (Supplemental Fig. S2F). Cultures were agitated by sparging air into the flasks. Absorbance was measured at 680 and 750 nm to quantify chlorophyll and cell density. The amount of chlorophyll was measured to determine photobleaching by subtracting the 750-nm OD value from the 680-nm OD value and multiplying the total by 10.854. There was a strong correlation (r2 = 0.9854) in determining chlorophyll concentration between this method and the well-established chlorophyll quantification protocol as described previously (Porra et al., 1989; Bombelli et al., 2011; Supplemental Fig. S3). This amount was divided by the 750-nm OD value to give a level of chlorophyll per cell in arbitrary units. A Student’s paired t test was used for all comparisons, with P < 0.05 being considered statistically significant.
Oxygen Measurements
All oxygen measurements were carried out in BG-11 medium at 30°C using DW1 liquid-phase oxygen electrode chambers with Oxygraph meters (Hansatech Instruments). Oxygen consumption was measured in the dark. For oxygen evolution experiments, cool-white light with an intensity of 1,500 or 150 µmol photons m−2 s−1 (as indicated) was provided using MR16 LED lamps (Deltech). For photosynthesis measurements, samples were withdrawn at each time point from the incubation mixture for dry cell weight and oxygen evolution measurements. For photoinhibition experiments, lincomycin was added to a final concentration of 200 μg mL−1 and subjected to either constant light or a pulsing cycle of 5 min on/5 min off. All measurements were standardized to dry cell weight. To measure dry cell weight, 20 mL of culture was removed, washed once with distilled water, filtered, and dried prior to measurement.
Glycogen Measurements
Assays of intracellular glycogen were performed as described by Osanai et al. (2005). One milliliter of culture was suspended in 3.5% (v/v) sulfuric acid and boiled for 40 min. To this was added 1 mL of o-toluidine (Sigma), and the reaction mixture was boiled for 10 min. Glc produced by acid hydrolysis was quantified by measuring A630 and comparing with Glc standards.
Oxidative Damage Measurements
Cells were washed twice with phosphate-buffered saline, resuspended in phosphate-buffered saline, and DCHF-DA was added to a final concentration of 5 µm (Rastogi et al., 2010). Samples were incubated at 30°C in the dark for 30 min, and fluorescence was quantified using a DMG FLUOstar optima+ fluorometer using an excitation wavelength of 485 nm and an emission wavelength of 520 nm. Fluorescence from cell samples without DCHF-DA was subtracted from cell + DCHF-DA measurements and standardized to dry cell weight. As a positive control, MV was added to a final concentration of 100 µm, and cells were either exposed to 30 min of illumination (40 µmol photons m−2 s−1) or incubated in the dark prior to quantification.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Disruption of ctaC1D1E1, ctaCII, and cydAB in Synechocystis.
Supplemental Figure S2. Analysis of transcripts in regions downstream of ctaE1 and ctaCII.
Supplemental Figure S3. Correlation between the A680 − A750 value and amounts of chlorophyll measured following methanol extraction.
Supplemental Figure S4. Growth of terminal oxidase mutants under different light conditions.
Supplemental Figure S5. Validation of DCHF-DA as a measure of oxidative damage in Synechocystis.
Supplemental Table S1. Number of COX (ctacC1), ARTO (ctacII), Cyd (cydA), and PTOX terminal oxidases present in the 60 cyanobacteria with sequenced genomes.
Supplemental Table S2. Nucleotide changes between the published Synechocystis genome and the strain used in this study.
Supplemental Table S3. Respiratory rates are greatly reduced in strains deficient in thylakoid-localized terminal oxidases.
Supplemental Table S4. Sequences of primers used in this study.
Acknowledgments
We thank Adrian Barbrook, Paolo Bombelli, and Alistair McCormick (University of Cambridge) and David Parker, Andrew Murphy, and Karina Almeida Lenero (Shell Global Solutions) for useful discussion as well as Nick Musgrove and Daniel Bruecher of Infors for use of the modified LED photobioreactor. We acknowledge Algenuity (www.algenuity.com) for the generous loan of an Algem Labscale photobioreactor. We thank Shilo Dickens and Robert Bradley (University of Cambridge) for MiSeq genome sequencing and analysis.
Glossary
- cyt b6f
cytochrome b6f complex
- RT
reverse transcription
- OD
optical density
- ROS
reactive oxygen species
- DCHF-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DCF
dichlorofluorescein
- MV
methyl viologen
- RFU
relative fluorescence units
- LED
light-emitting diode
- DCW
dry cell weight
References
- Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikström M. (2000) The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nat Struct Biol 7: 910–917 [DOI] [PubMed] [Google Scholar]
- Agarwal R, Matros A, Melzer M, Mock HP, Sainis JK. (2010) Heterogeneity in thylakoid membrane proteome of Synechocystis 6803. J Proteomics 73: 976–991 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. J Mol Biol 215: 403–410 [DOI] [PubMed] [Google Scholar]
- Bailey S, Melis A, Mackey KR, Cardol P, Finazzi G, van Dijken G, Berg GM, Arrigo K, Shrager J, Grossman A. (2008) Alternative photosynthetic electron flow to oxygen in marine Synechococcus. Biochim Biophys Acta 1777: 269–276 [DOI] [PubMed] [Google Scholar]
- Ben-Porath J, Zehr JP. (1994) Detection and characterization of cyanobacterial nifH genes. Appl Environ Microbiol 60: 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman-Frank I, Lundgren P, Chen YB, Küpper H, Kolber Z, Bergman B, Falkowski P. (2001) Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294: 1534–1537 [DOI] [PubMed] [Google Scholar]
- Berry S, Schneider D, Vermaas WFJ, Rögner M. (2002) Electron transport routes in whole cells of Synechocystis sp. strain PCC 6803: the role of the cytochrome bd-type oxidase. Biochemistry 41: 3422–3429 [DOI] [PubMed] [Google Scholar]
- Bombelli P, Bradley RW, Scott AM, Philips AJ, McCormick AJ, Cruz SM, Anderson A, Yunus K, Bendall DS, Cameron PJ, et al. (2011) Quantitative analysis of the factors limiting solar power transduction by Synechocystis sp. PCC 6803 in biological photovoltaic devices. Energy and Environmental Science 4: 4690–4698 [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz RW. (1988) Culturing methods for cyanobacteria. Methods Enzymol 167: 68–93 [Google Scholar]
- Cooley JW, Vermaas WFJ. (2001) Succinate dehydrogenase and other respiratory pathways in thylakoid membranes of Synechocystis sp. strain PCC 6803: capacity comparisons and physiological function. J Bacteriol 183: 4251–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow JP. (1997) Dichlorodihydrofluorescein and dihydrorhodamine 123 are sensitive indicators of peroxynitrite in vitro: implications for intracellular measurement of reactive nitrogen and oxygen species. Nitric Oxide 1: 145–157 [DOI] [PubMed] [Google Scholar]
- Ding WX, Shen HM, Zhu HG, Ong CN. (1998) Studies on oxidative damage induced by cyanobacteria extract in primary cultured rat hepatocytes. Environ Res 78: 12–18 [DOI] [PubMed] [Google Scholar]
- Fry IV, Huflejt M, Erber WW, Peschek GA, Packer L. (1986) The role of respiration during adaptation of the freshwater cyanobacterium Synechococcus 6311 to salinity. Arch Biochem Biophys 244: 686–691 [DOI] [PubMed] [Google Scholar]
- Gutthann F, Egert M, Marques A, Appel J. (2007) Inhibition of respiration and nitrate assimilation enhances photohydrogen evolution under low oxygen concentrations in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1767: 161–169 [DOI] [PubMed] [Google Scholar]
- Hagemann M. (2011) Molecular biology of cyanobacterial salt acclimation. FEMS Microbiol Rev 35: 87–123 [DOI] [PubMed] [Google Scholar]
- Hart SE, Schlarb-Ridley BG, Bendall DS, Howe CJ. (2005) Terminal oxidases of cyanobacteria. Biochem Soc Trans 33: 832–835 [DOI] [PubMed] [Google Scholar]
- He YY, Häder DP. (2002) Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci 1: 729–736 [DOI] [PubMed] [Google Scholar]
- Houille-Vernes L, Rappaport F, Wollman FA, Alric J, Johnson X. (2011) Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc Natl Acad Sci USA 108: 20820–20825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt CA, Udall PK, Vermaas WFJ. (1999) Type 2 NADH dehydrogenases in the cyanobacterium Synechocystis sp. strain PCC 6803 are involved in regulation rather than respiration. J Bacteriol 181: 3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitt CA, Vermaas WFJ. (1998) Quinol and cytochrome oxidases in the cyanobacterium Synechocystis sp. PCC 6803. Biochemistry 37: 17944–17951 [DOI] [PubMed] [Google Scholar]
- Huang F, Fulda S, Hagemann M, Norling B. (2006) Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics 6: 910–920 [DOI] [PubMed] [Google Scholar]
- Huang F, Parmryd I, Nilsson F, Persson AL, Pakrasi HB, Andersson B, Norling B. (2002) Proteomics of Synechocystis sp. strain PCC 6803: identification of plasma membrane proteins. Mol Cell Proteomics 1: 956–966 [DOI] [PubMed] [Google Scholar]
- Huang TC, Chow TJ. (1986) New type of N2-fixing unicellular cyanobacterium (blue-green alga). FEMS Microbiol Lett 36: 109–110 [Google Scholar]
- Jeanjean R, Matthijs HCP, Onana B, Havaux M, Joset F. (1993) Exposure of the cyanobacterium Synechocystis PCC6803 to salt stress induces concerted changes in respiration and photosynthesis. Plant Cell Physiol 34: 1073–1079 [Google Scholar]
- Jeanjean R, Onana B, Peschek GA, Joset F. (1990) Mutants of the cyanobacterium Synechocystis PCC6803 impaired in respiration and unable to tolerate high salt concentrations. FEMS Microbiol Lett 68: 125–129 [Google Scholar]
- Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, et al. (1996) Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions (supplement). DNA Res 3: 185–209 [DOI] [PubMed] [Google Scholar]
- Lindberg P, Park S, Melis A. (2010) Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab Eng 12: 70–79 [DOI] [PubMed] [Google Scholar]
- Liu LN, Bryan SJ, Huang F, Yu J, Nixon PJ, Rich PR, Mullineaux CW. (2012) Control of electron transport routes through redox-regulated redistribution of respiratory complexes. Proc Natl Acad Sci USA 109: 11431–11436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijs HCP, Lubberding HJ (1988) Dark respiration in cyanobacteria. In LJ Rogers, JR Gallon, eds, Biochemistry of the Algae and Cyanobacteria. Clarendon Press, Oxford, pp 131–145
- McDonald AE, Ivanov AG, Bode R, Maxwell DP, Rodermel SR, Hüner NP. (2011) Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX). Biochim Biophys Acta 1807: 954–967 [DOI] [PubMed] [Google Scholar]
- Mi HL, Endo T, Ogawa T, Asada K. (1995) Thylakoid membrane-bound, NADPH-specific pyridine-nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp PCC-6803. Plant Cell Physiol 36: 661–668 [Google Scholar]
- Nomura CT, Persson S, Shen G, Inoue-Sakamoto K, Bryant DA. (2006) Characterization of two cytochrome oxidase operons in the marine cyanobacterium Synechococcus sp. PCC 7002: inactivation of ctaDI affects the PS I:PS II ratio. Photosynth Res 87: 215–228 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Pakrasi HB, Ogawa T. (2000) Two types of functionally distinct NAD(P)H dehydrogenases in Synechocystis sp. strain PCC6803. J Biol Chem 275: 31630–31634 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Sonoda M, Shibata M, Ogawa T. (2001) Localization of NAD(P)H dehydrogenase in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol 183: 4938–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai T, Kanesaki Y, Nakano T, Takahashi H, Asayama M, Shirai M, Kanehisa M, Suzuki I, Murata N, Tanaka K. (2005) Positive regulation of sugar catabolic pathways in the cyanobacterium Synechocystis sp. PCC 6803 by the group 2 sigma factor sigE. J Biol Chem 280: 30653–30659 [DOI] [PubMed] [Google Scholar]
- Peschek GA, Obinger C, Fromwald S, Bergman B. (1994) Correlation between immunogold labels and activities of the cytochrome c oxidase (Aa(3) type) in membranes of salt-stressed cyanobacteria. FEMS Microbiol Lett 124: 431–437 [Google Scholar]
- Peschek GA, Obinger C, Paumann M. (2004) The respiratory chain of blue-green algae (cyanobacteria). Physiol Plant 120: 358–369 [DOI] [PubMed] [Google Scholar]
- Pils D, Schmetterer G. (2001) Characterization of three bioenergetically active respiratory terminal oxidases in the cyanobacterium Synechocystis sp. strain PCC 6803. FEMS Microbiol Lett 203: 217–222 [DOI] [PubMed] [Google Scholar]
- Pisareva T, Shumskaya M, Maddalo G, Ilag L, Norling B. (2007) Proteomics of Synechocystis sp. PCC 6803: identification of novel integral plasma membrane proteins. FEBS J 274: 791–804 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rastogi RP, Singh SP, Häder DP, Sinha RP. (2010) Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397: 603–607 [DOI] [PubMed] [Google Scholar]
- Ried JL, Collmer A. (1987) An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57: 239–246 [DOI] [PubMed] [Google Scholar]
- Rocap G, Distel DL, Waterbury JB, Chisholm SW. (2002) Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol 68: 1180–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso D, Bode R, Li W, Krol M, Saccon D, Wang S, Schillaci LA, Rodermel SR, Maxwell DP, Hüner NP. (2009) Photosynthetic redox imbalance governs leaf sectoring in the Arabidopsis thaliana variegation mutants immutans, spotty, var1, and var2. Plant Cell 21: 3473–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze M, Forberich B, Rexroth S, Dyczmons NG, Roegner M, Appel J. (2009) Localization of cytochrome b(6)f complexes implies an incomplete respiratory chain in cytoplasmic membranes of the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta 1787: 1479–1485 [DOI] [PubMed]
- Smith D, Howe CJ. (1993) The distribution of photosystem-I and photosystem-II polypeptides between the cytoplasmic and thylakoid membranes of cyanobacteria. FEMS Microbiol Lett 110: 341–347 [Google Scholar]
- Srivastava R, Pisareva T, Norling B. (2005) Proteomic studies of the thylakoid membrane of Synechocystis sp. PCC 6803. Proteomics 5: 4905–4916 [DOI] [PubMed] [Google Scholar]
- Staal M, Meysman FJ, Stal LJ. (2003) Temperature excludes N2-fixing heterocystous cyanobacteria in the tropical oceans. Nature 425: 504–507 [DOI] [PubMed] [Google Scholar]
- Stal LJ, Moezelaar R. (1997) Fermentation in cyanobacteria. FEMS Microbiol Rev 21: 179–211 [Google Scholar]
- Stepien P, Johnson GN. (2009) Contrasting responses of photosynthesis to salt stress in the glycophyte Arabidopsis and the halophyte Thellungiella: role of the plastid terminal oxidase as an alternative electron sink. Plant Physiol 149: 1154–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb P, Josse EM, Gallouet E, Baptist F, Kuntz M, Cornic G. (2005) Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ 28: 1123–1135 [Google Scholar]
- Suzuki E, Ohkawa H, Moriya K, Matsubara T, Nagaike Y, Iwasaki I, Fujiwara S, Tsuzuki M, Nakamura Y. (2010) Carbohydrate metabolism in mutants of the cyanobacterium Synechococcus elongatus PCC 7942 defective in glycogen synthesis. Appl Environ Microbiol 76: 3153–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DJ, Avenson TJ, Thomas JB, Herbert SK. (1998) A cyanobacterium lacking iron superoxide dismutase is sensitized to oxidative stress induced with methyl viologen but is not sensitized to oxidative stress induced with norflurazon. Plant Physiol 116: 1593–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaas WF. (2001) Photosynthesis and Respiration in Cyanobacteria: Encyclopedia of Life Sciences. Macmillan Publishers Ltd., Basingstoke, UK
- Williams JGK. (1988) Construction of specific mutations in photosystem-II photosynthetic reaction center by genetic-engineering methods in Synechocystis 6803. Methods Enzymol 167: 766–778 [Google Scholar]
- Xu H, Vavilin D, Funk C, Vermaas W. (2004) Multiple deletions of small Cab-like proteins in the cyanobacterium Synechocystis sp. PCC 6803: consequences for pigment biosynthesis and accumulation. J Biol Chem 279: 27971–27979 [DOI] [PubMed] [Google Scholar]
- Zehr JP, Bench SR, Carter BJ, Hewson I, Niazi F, Shi T, Tripp HJ, Affourtit JP. (2008) Globally distributed uncultivated oceanic N2-fixing cyanobacteria lack oxygenic photosystem II. Science 322: 1110–1112 [DOI] [PubMed] [Google Scholar]
- Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, Aro EM. (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16: 3326–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Eisenhut M, Brandt AM, Carmel D, Silén HM, Vass I, Allahverdiyeva Y, Salminen TA, Aro EM. (2012) Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24: 1952–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwirglmaier K, Jardillier L, Ostrowski M, Mazard S, Garczarek L, Vaulot D, Not F, Massana R, Ulloa O, Scanlan DJ. (2008) Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ Microbiol 10: 147–161 [DOI] [PubMed] [Google Scholar]