Premature loss of leaves, flowers, and fruit can reduce crop yield; manipulating the plant hormone auxin in abscission zone cells alters the timing of organ shedding.
Abstract
A number of novel strategies were employed to examine the role of indoleacetic acid (IAA) in regulating floral organ abscission in Arabidopsis (Arabidopsis thaliana). Analysis of auxin influx facilitator expression in β-glucuronidase reporter plants revealed that AUXIN RESISTANT1, LIKE AUX1, and LAX3 were specifically up-regulated at the site of floral organ shedding. Flowers from mutants where individual family members were down-regulated exhibited a reduction in the force necessary to bring about petal separation; however, the effect was not additive in double or quadruple mutants. Using the promoter of a polygalacturonase (At2g41850), active primarily in cells undergoing separation, to drive expression of the bacterial genes iaaL and iaaM, we have shown that it is possible to manipulate auxin activity specifically within the floral organ abscission zone (AZ). Analysis of petal breakstrength reveals that if IAA AZ levels are reduced, shedding takes place prematurely, while if they are enhanced, organ loss is delayed. The At2g41850 promoter was also used to transactivate the gain-of-function AXR3-1 gene in order to disrupt auxin signaling specifically within the floral organ AZ cells. Flowers from transactivated lines failed to shed their sepals, petals, and anthers during pod expansion and maturity, and these organs frequently remained attached to the plant even after silique desiccation and dehiscence had taken place. These observations support a key role for IAA in the regulation of abscission in planta and reveal, to our knowledge for the first time, a requirement for a functional IAA signaling pathway in AZ cells for organ shedding to take place.
The shedding of plant organs plays a key role during the life cycle of a plant (Roberts et al., 2002). It can limit the spread of systemic invasion by pathogens, provide a mechanism to remove damaged or inefficiently functioning tissues, remove competition for pollinators from fertilized flowers, and contribute to seed dispersal in dry and fleshy fruits (Leslie et al., 2007). The timing of flower and fruit abscission is a process of substantial interest to the horticultural and agricultural industries, as it can affect both the quantity and quality of yield. Indeed, the formation of an abscission zone (AZ) was one of the first traits to be manipulated during the advent of agricultural practices (Doebley, 2004). Considerable research interest, therefore, has been dedicated to identifying the endogenous and environmental factors that trigger the process and regulate the rate at which it proceeds.
Research by Jackson and Osborne (1970) showed that ethylene was a natural regulator of abscission and that exposure to the gas hastened the shedding of leaves, flowers, and fruit. Prior to this discovery, it had been reported that the attachment of orchid (Dendrobium spp.) pollinia, known to be rich in auxin, to excised coleus tissue dramatically slowed abscission (Laibach, 1951) and that application of indoleacetic acid (IAA) to the distal end of bean (Phaseolus vulgaris) leaf explants delayed cell separation (Jacobs, 1962). These observations led to the hypothesis that the timing of shedding was regulated by the auxin-ethylene balance within the AZ tissues, with a high flux of IAA into these cells, from the subtending organ, preventing abscission from taking place (Taylor and Whitelaw, 2001). The generation of plant material unresponsive to endogenous hormones has allowed this hypothesis to be tested, and while the shedding of floral organs is delayed in the ethylene-insensitive Arabidopsis (Arabidopsis thaliana) mutants ethylene resistant1-1 (etr1-1) and ethylene insensitive2, abscission does take place (Patterson and Bleecker, 2004). This observation suggests that while ethylene may play an important role in dictating the timing of organ shedding, the action of the gas is not critical for abscission to take place, and it has been proposed that the level of IAA within the AZ tissues might play a more crucial role (Roberts and González-Carranza, 2007).
The characterization of the auxin/indoleacetic acid (Aux/IAA) and Auxin Response Factor (ARF) family of genes, which regulate many plant responses to auxin (Ellis et al., 2005), has provided a strategy to explore the role of IAA during abscission. For an auxin response to take place in plants, the ARF proteins bind to the auxin response elements present in the promoter of a gene, which are regulated by auxin and either suppress or activate the expression of these genes (Ulmasov et al., 1997). Ellis et al. (2005) demonstrated a change in organ abscission in Arabidopsis material containing transfer DNA (T-DNA) insertions in the ARF1 and ARF2 genes. Those authors demonstrated delayed floral abscission in the arf2-8 single mutant and the arf1-4 arf2-8 double mutants, where the floral organs were shed at flower positions 10 and 11, respectively, compared with shedding by flower position 6 or 7 in the wild types. Position 1 is identified where petals of the flower are first visible by eye, and later positions are as described by Patterson (2001). However, the arf1 single mutant typically shed its floral organs at the same time as the wild-type plants, which led to the conclusion that ARF1 enhanced the effect of a mutation in ARF2. The authors further demonstrated tissue-specific expression of both ARF1 and ARF2 in the AZ of Arabidopsis flowers using GUS reporter lines. Transcript profiling analysis in tomato (Solanum lycopersicum) has revealed that flower removal, a treatment that hastens pedicel abscission, leads to a rapid decline in the expression of a spectrum of Aux/IAA genes. Intriguingly, this reduction in expression is not regulated by ethylene (Meir et al., 2010). These observations add weight to the hypothesis that IAA may act within the AZ layer to regulate the timing of organ shedding. However, direct evidence based on manipulating auxin levels within the AZ cells has not yet been forthcoming.
For abscission to occur, one of the most important steps is cell wall degradation, which is brought about by the action of several hydrolytic enzymes including polygalacturonases (PGs) and cellulases (Roberts et al., 2002; Rose et al., 2003). González-Carranza et al. (2002) identified an abscission-related gene encoding a PG from canola (Brassica napus) and used this sequence information to identify its ortholog (At2g41850) in Arabidopsis. Temporal and spatial studies to monitor the activity of the promoter of this gene, by fusion to GUS or GFP reporters, demonstrated expression specifically in the floral AZ cells (González-Carranza et al., 2002). A T-DNA insertion line of this gene exhibited delayed floral organ shedding, indicating that the PG played a role in cell separation (González-Carranza et al., 2007). The QUARTET2 gene has also been shown to encode a PG that is specifically expressed in the AZ of floral organs of Arabidopsis (Ogawa et al., 2009).
In this paper, we have investigated the expression of auxin influx facilitators (Kramer and Bennett, 2006) during flower development and have analyzed the consequence of down-regulating their expression on the time course of organ shedding. We also describe the exploitation of the At2g41850 promoter as a tool to manipulate auxin levels and signaling, specifically within the AZ cells of floral organs of Arabidopsis. Our data demonstrate that by altering the concentration of IAA, it is possible to attenuate the timing of shedding and that disruption of IAA signaling within the AZ cells prevents abscission from taking place.
RESULTS
Expression of IAA Influx Facilitators during Flower Development
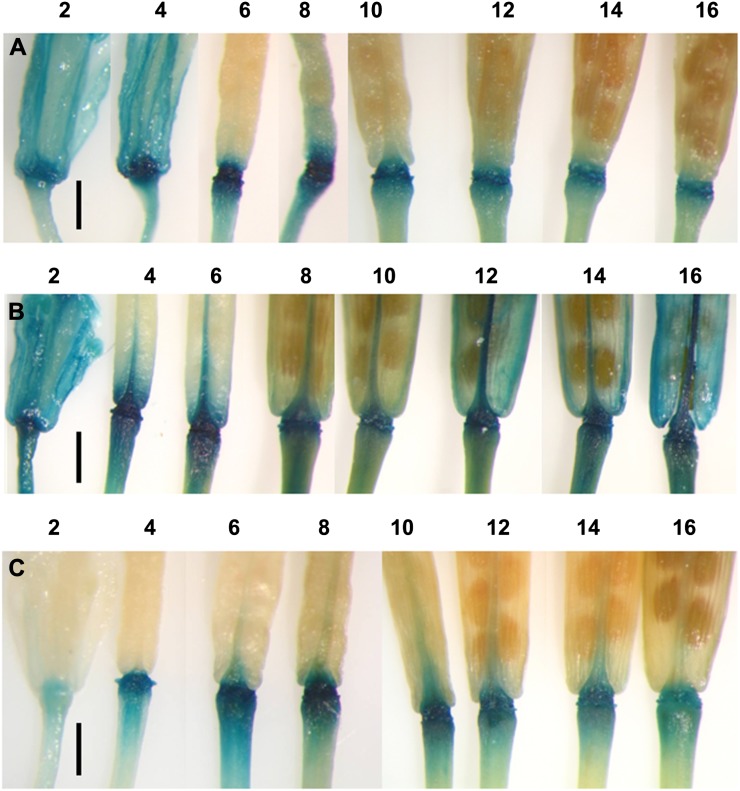
The expression of the IAA influx carriers AUXIN RESISTANT1 (AUX1), LIKE AUX1 (LAX1), LAX2, and LAX3 was analyzed, throughout flower and silique development, in homozygous transgenic plants where the promoters of the individual genes had been fused to the reporter GUS (Parry et al., 2001). A study of expression from flower position 2 to the stage where siliques were fully mature (position 16; for the definition of floral stages, see Patterson [2001]) revealed intense GUS expression in ProAUX1::GUS, ProLAX1::GUS, and ProLAX3::GUS plants at the site of floral organ shedding (Fig. 1). Expression in all three genotypes was evident by position 4 and was discretely localized in ProAUX1::GUS and ProLAX3::GUS plants. In ProLAX1::GUS plants, expression spread from the AZ into the pedicel and valve margin tissues. Expression of the reporter could only be seen at a low level in ProLAX2::GUS material and was not focused in any specific cell type (data not shown).
Figure 1.
Temporal and spatial expression of GUS in Arabidopsis floral AZs and developing siliques from ProAUX1::GUS (A), ProLAX1::GUS (B), and ProLAX3::GUS (C) plants. The time course of expression is shown from floral development positions 2 to 16, where position 1 is the first flower where petals are visible to the eye. Bars = 1 mm.
Impact of Silencing IAA Influx Facilitators on Floral Organ Abscission
The impact of down-regulating the expression of members of the IAA influx facilitator family on the timing of floral organ abscission was examined using the aux1 mutant and homozygous lines containing a T-DNA insertion (Swarup et al., 2008) into either LAX1 (lax1) or LAX3 (lax3). Shedding in the double mutant aux1 lax3 (Swarup et al., 2008) and the quadruple mutant aux1 lax1 lax2 lax3 (Swarup et al., 2008) was also investigated. Abscission was monitored in flowers at positions 2, 3, and 4 using a petal breakstrength assay specifically designed for the purpose (González-Carranza et al., 2012).
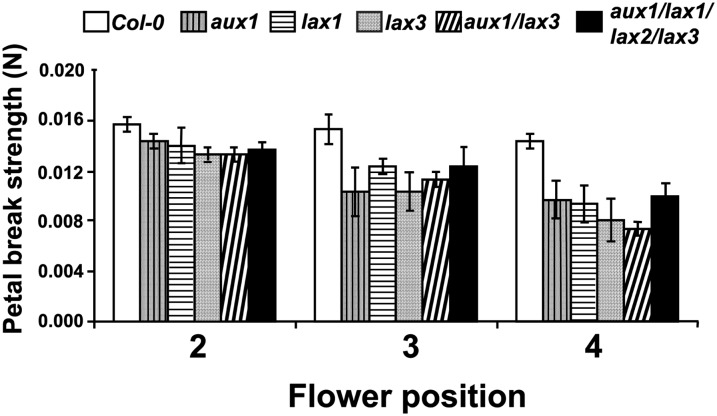
The data in Figure 2 demonstrate that the force necessary to separate petals at flower positions 3 and 4 in the ecotype Columbia-0 (Col-0) wild type was significantly greater than that required to carry out the same process in the aux1, lax1, and lax3 mutant lines. Interestingly, no synergistic breakstrength effect was observed in either the double or the quadruple mutant compared with the single mutants (Fig. 2). Floral organ abscission in glasshouse-grown plants routinely took place earlier in the mutant lines (position 5 or 6) than in the Col-0 wild-type plants (position 7 or 8; Fig. 2; Supplemental Fig. S1).
Figure 2.
Breakstrength analysis of petals from Col-0, aux1, lax1, lax3, aux1 lax3, and aux1 lax1 lax2 lax3 plants. Breakstrength was determined at flower positions 2, 3, and 4 (n = 3).
Expression of At2g41850 during Floral Organ Abscission
In order to manipulate IAA at the site of organ shedding, it was necessary to exploit the use of a promoter that was active specifically within the AZ cells. Reporter gene studies had previously shown that expression of the PG At2g41850 was restricted to the base of sepal, petal, and anther filament tissues at the time of abscission (González-Carranza et al., 2002). To identify the timing of At2g41850 expression during flower development, real-time PCR was carried out on RNA extracted from the AZ tissues from Col-0 wild-type flowers at positions 4 to 9 on the inflorescence. Figure 3 shows that while At2g41850 transcript could be detected throughout this developmental period, a peak of expression was apparent at flower positions 6 and 7 when normalized using the housekeeping gene ACT2 (At3g18780).
Figure 3.
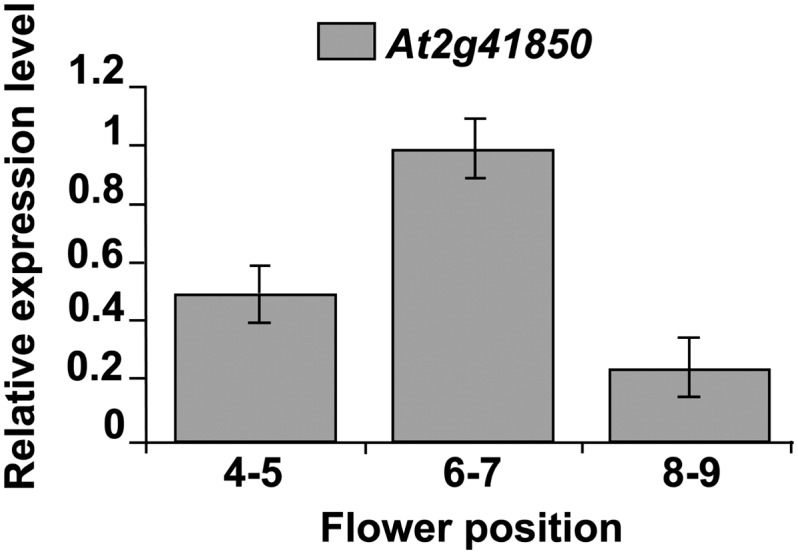
QPCR expression of At2g41850 at flower positions 4 to 5, 6 to 7, and 8 to 9.
Manipulation of IAA Concentrations within the Floral Organ AZ Cells
The approach utilized to manipulate the concentration of IAA within the AZ cells was to fuse the bacterial gene iaaM (Trp-2-monooxygenase) or iaaL (IAA-Lys synthetase) downstream of the At2g41850 promoter. iaaM converts Trp to indoleacetamide, and up-regulation can promote auxin production (Klee et al., 1987). iaaL can reduce auxin levels in cells by conjugating free IAA to IAA-Lys (Klee and Estelle, 1991). It had previously been shown that a 1,476-bp fragment was sufficient to drive abscission-specific expression (González-Carranza et al., 2002). Homozygous transgenic ProAt2g41850::iaaM and ProAt2g41850::iaaL plants were generated, and expression of the transgenes was confirmed by reverse transcription (RT)-PCR (Supplemental Figs. S3 and S4).
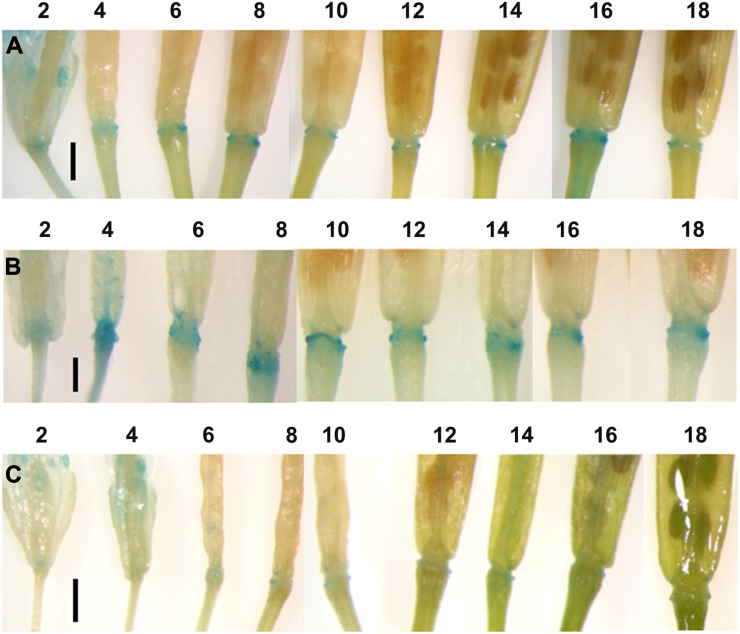
Plants containing the synthetic IAA reporter gene DR5::GUS were crossed with ProAt2g41850::iaaM or ProAt2g41850::iaaL lines to report the levels of auxin activity within the AZ tissues. A spatial and temporal analysis of reporter activity was undertaken in the DR5::GUS parent line and compared with what was observed in the F1 plants of the crosses. GUS expression could be detected, within the AZ, in the parental control from flower position 2 to position 18 (Fig. 4A). The intensity of the GUS staining was more intense, especially during developmental stages 4 to 10, in the ProAt2g41850::iaaM plants (Fig. 4B), while little GUS staining could be detected in the ProAt2g41850::iaaL plants (Fig. 4C).
Figure 4.
Temporal and spatial expression of DR5::GUS in Arabidopsis floral AZs and developing siliques from Col-0 (A), ProAt2g41850::iaaM (B), and ProAt2g41850::iaaL (C) lines. The time course of expression is shown from floral development positions 2 to 18, where position 1 is the first flower where petals are visible to the eye. Bars = 1 mm.
Floral Organ Abscission in ProAt2g41850:iaaM and ProAt2g41850:iaaL Plants
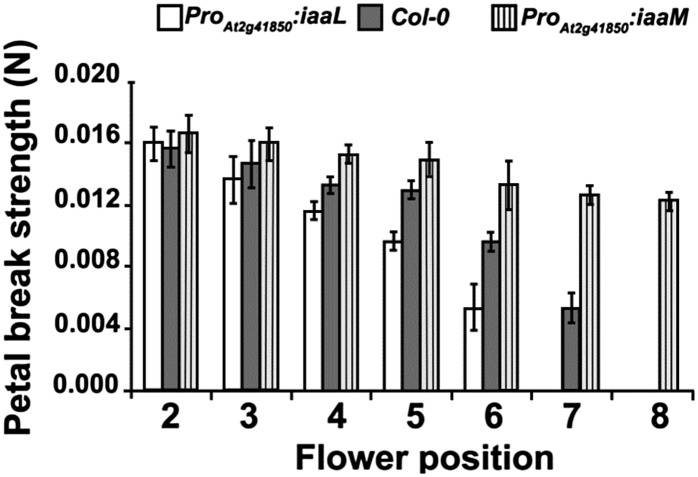
Petal breakstrength was determined in flowers from positions 2 to 8 in Col-0, ProAt2g41850::iaaM, and ProAt2g41850::iaaL plants (Fig. 5). No difference in breakstrength was apparent at the earliest position; however, by position 4, the force required to remove petals from ProAt2g41850::iaaL flowers was significantly less, while that from ProAt2g41850::iaaM plants was significantly greater, than from wild-type material. This difference was even more marked by position 6, and only flowers from ProAt2g41850::iaaM plants still retained their petals by position 8. Floral organs from ProAt2g41850::iaaL were routinely shed at flower position 5 or 6, while those on ProAt2g41850::iaaM plants were frequently retained until position 15 or 16 under glasshouse conditions. Sepal yellowing was also delayed in ProAt2g41850::iaaM plants (Supplemental Fig. S5). No other distinguishing phenotypic characteristics in the aerial tissues were observed between Col-0, ProAt2g41850::iaaL, and ProAt2g41850::iaaM plants.
Figure 5.
Breakstrength analysis of petals from Col-0, ProAt2g41850::iaaM, and ProAt2g41850::iaaL plants. Breakstrength was determined at flower positions 2 to 8.
Consequences of Targeting the Expression of AXR3-1 in Floral Organ AZ Cells
The axr3-1 mutant of Arabidopsis is a gain-of-function semidominant mutation arising from the production of an IAA17/AXR3 protein with enhanced stability that disrupts auxin signaling (Rouse et al., 1998). By expressing the AXR3-1 protein specifically in AZ cells, the contribution of auxin signaling in the regulation of abscission could be investigated. The AXR3-1 gene was transactivated specifically in floral organ AZ cells of Arabidopsis first by generating a homozygous ProAt2g41850::GAL4 VP16 driver line and then crossing this to a ProUAS::axr3-1 (Swarup et al., 2005) line. F1 plants were identified based on PCR amplification of both the ProAt2g41850::GAL4 and ProUAS::arx3-1 constructs (Supplemental Figs. S6 and S7). In ProAt2g41850>>AXR3-1 plants, the shedding of petals, sepals, and anther filaments was delayed substantially beyond position 5 or 6, where wild-type plants normally shed their floral parts, and frequently these organs were retained until the time of silique desiccation and dehiscence (Fig. 6). In addition, floral organs in ProAt2g41850>>AXR3-1 plants showed visual signs of senescence earlier than wild-type plants, and by position 10, they showed signs of desiccation.
Figure 6.

Flower and silique development in At2g41850Pro>>AXR3-1 plants. The time course of floral development is shown from positions 2 to 16 and at silique dehiscence, where position 1 is the first flower where petals are visible to the eye. Floral organ abscission in wild-type plants normally takes place in the glasshouse at position 5 or 6. Bar = 1 mm. [See online article for color version of this figure.]
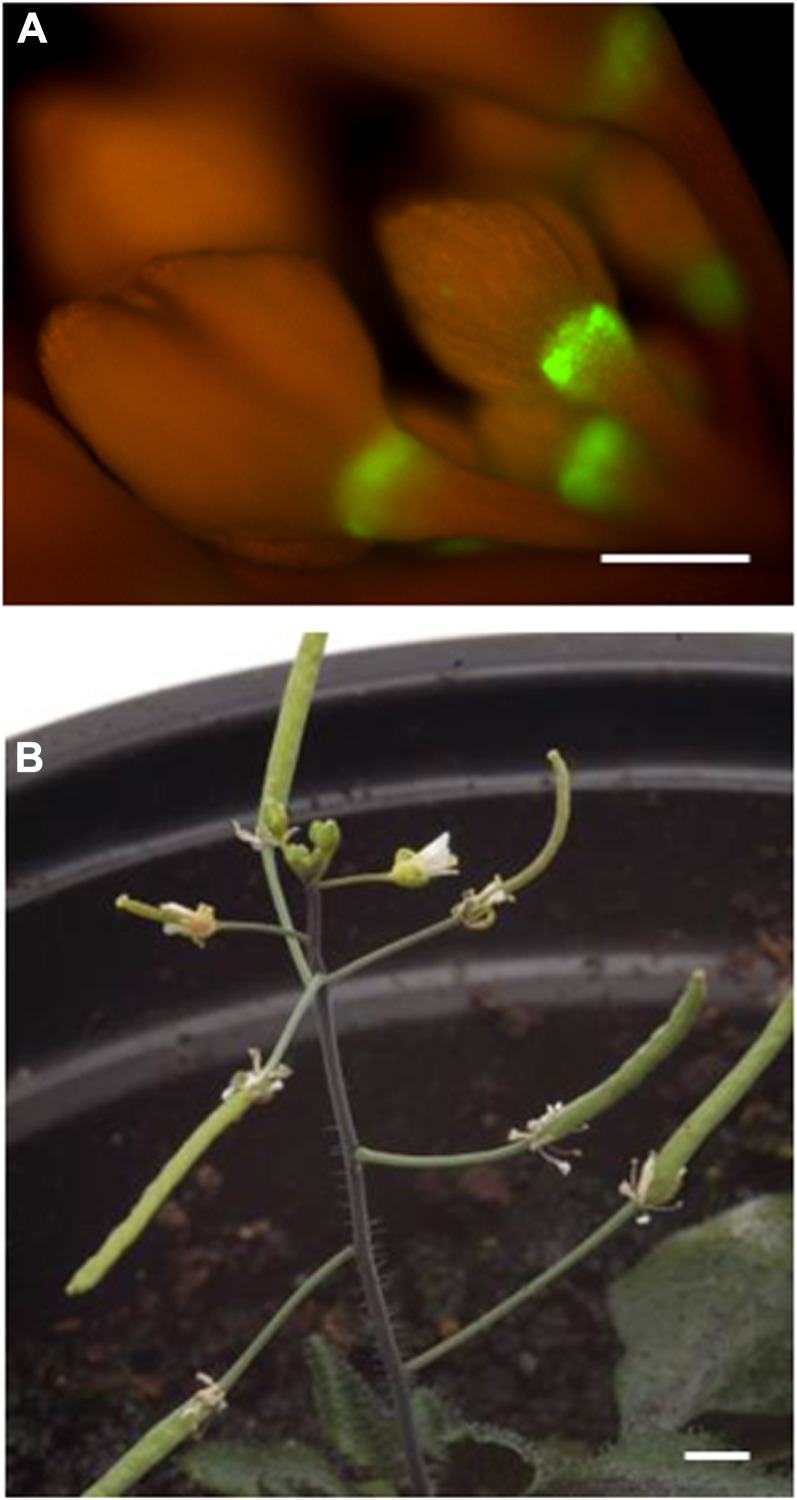
As the proposed role of the protein encoded by At2g41850 is to promote cell wall dissolution, it is expressed toward the end of the shedding process. To explore the consequences of silencing the AZ cells to auxin at an early stage in abscission, a GAL4-GFP-tagged enhancer trap line (M0223), from the Haseloff collection (Haseloff et al., 1997), was identified that exhibits expression in the AZ region of flower buds (Fig. 7A). This M0223 driver line was crossed with the ProUAS::Axr3-1 line to generate the line ProM0233>>AXR3. Floral organ abscission in the progeny of this cross was abolished (Fig. 7B).
Figure 7.
A, GFP expression in M0233 GFP plants. Bar = 1 mm. B, M0233Pro>>AXR3 plant showing retention of floral organs throughout inflorescence development. Bar = 1 mm. [See online article for color version of this figure.]
DISCUSSION
It has been well documented that the timing of organ shedding can be influenced by manipulating the flux of auxin through the AZ tissues (Taylor and Whitelaw, 2001). Studies have shown, using the classical bean leaf explant system, that if a supply of auxin is delivered to the distal tissue, abscission at the junction between the pulvinus and the petiole is delayed (Rubinstein and Leopold, 1963; Sexton and Roberts, 1982). In intact plants, removal of the IAA source by deblading the leaf lamina or by application of auxin transport inhibitors distal to the AZ tissue accelerates shedding (Taylor and Whitelaw, 2001). Indeed, one of the initial effects of exposure to ethylene is to impede the polar transport of IAA into the AZ cells (Beyer, 1975), and it has been proposed that the auxin/ethylene balance ultimately dictates the triggering of abscission and the rate at which it proceeds. However, much of the evidence of an involvement of auxin in the regulation of abscission is correlative and is based on manipulating hormone concentrations by tissue excision and auxin application. In this study, endogenous IAA activity and signaling, to our knowledge for the first time, have been specifically manipulated within the cells that constitute the AZ. The data reveal that auxin not only regulates the timing of organ shedding in planta but that there is also an absolute requirement for IAA signaling to be maintained for abscission to take place.
Auxin Transport and Abscission
In this study, the expression of the auxin influx facilitators AUX1, LAX1, LAX2, and LAX3 was analyzed in GUS reporter lines (Swarup et al., 2008; Ugartechea-Chirino et al., 2010) during the time course of flower and silique development. The expression of AUX1, LAX1, and LAX3 was strongly localized to the sites of floral organ abscission, with GUS accumulation in this area preceding sepal, petal, or anther filament shedding. The intensity and degree of localization varied, with AUX1 and LAX3 expression being tightly restricted to the AZ, while the activity of the LAX1 promoter was spread from the AZ into the pedicel and valve margin tissues. The expression of LAX2 was at a low level throughout floral and silique material. The differing spatial and temporal expression patterns of the AUX1/LAX family members illustrate their likely diversity of function. While auxin influx facilitators are not critical for IAA to be delivered into a cell (Kramer and Bennett, 2006), their localization of expression at the sites where organ shedding takes place suggests that loading of the hormone into the AZ cells may be important for regulating the timing of abscission and the rate at which it proceeds. Certainly, an elevated level of IAA activity is associated with these regions of the flower, as revealed by ProDR5::GUS control material (Fig. 4A). This hypothesis is supported by our observations that shedding in the glasshouse consistently took place earlier (position 5 or 6) in the aux1 mutant and the lax1 and lax3 loss-of-function T-DNA insertion lines than in the Col-0 wild type (position 7 or 8). A detailed breakstrength analysis of material from the different genotypes confirmed that the force required to remove petals from flowers at positions 3 and 4 was significantly lower in the mutants compared with the wild-type material. The aux1, lax1, and lax3 mutants exhibited a similar breakstrength at these positions, and one interpretation of these data is that the capacity of these three carriers to deliver IAA into the AZ layer is approximately equivalent. Silencing of the IAA influx facilitators would not eliminate polar transport, as some auxin will move passively into cells (Swarup and Péret, 2012). However, as the petal breakstrength of the aux1 lax3 double mutant and the aux1 lax1 lax2 lax3 quadruple mutant at positions 3 and 4 is comparable to the reduction in the single mutants, an alternative explanation is that once the level of IAA in the AZ layer falls below a critical threshold, the process of shedding is triggered and proceeds at a specific rate. In order to distinguish between these hypotheses, further work would be necessary to explore the relationship between the auxin status of the AZ cells and the timing of abscission, and this might include expression analysis of members of the PIN and P-glycoprotein family of auxin efflux carriers (Blakeslee et al., 2005) and the consequences of silencing their expression on the cell separation process.
If IAA is a key player in the regulation of shedding, it would be predicted that tissues distal to the site of shedding might be a source of auxin. Studies carried out by Aloni et al. (2006) on the role of auxin in flower development have revealed, using a DR5::GUS reporter line, that there is a substantial quantity of free IAA available in the anthers prior to dehiscence. In Arabidopsis flowers, we have observed that anther filaments are consistently the last organs to be shed, and it is possible that free auxin may be transported basipetally from the anther locules toward the AZ cells, blocking their activation until pollination has taken place. Work by Meir et al. (2006) has demonstrated that IAA transport inhibitors, like naphthylphthalamic acid, enhance the process of leaf abscission in Mirabilis jalapa by blocking auxin uptake into the AZ cells and enhancing their sensitivity to ethylene. In addition to this, it is possible that the flux of auxin through the abscission layer might attenuate the intracellular transport of hydrolytic enzymes such as PG, which play a key role in wall dissolution (Degan et al., 2001).
Consequences of Manipulating IAA Concentrations Specifically within the AZ Cells
The strategy that we adopted in this study was to explore the consequences of manipulating IAA concentrations, specifically within the AZ cells, on the time course of organ shedding. To achieve this goal, we utilized a promoter of the At2g41850 gene that had previously been shown to be active specifically at the site where shedding takes place (González-Carranza et al., 2002) and that regulated the expression of a gene playing a role in cell separation (González-Carranza et al., 2007). Quantitative PCR (QPCR) showed that the expression of At2g41850 was highest at flower position 6 or 7, which coincides with the time of shedding of sepals, petals, and anther filaments in wild-type plants. The promoter of At2g41850 was fused to the open reading frame of the iaaL gene, and the construct was used to generate transgenic plants. The protein encoded by this bacterial gene converts free auxin into IAA-Lys, rendering it unavailable for use by the plant. A reduction in IAA activity compared with the wild type, specifically within the AZ cells, was confirmed in ProAt2g41850::iaaL plants by crossing with a ProDR5::GUS line that reports on auxin signaling. In wild-type plants, GUS accumulation could be observed at the site of organ shedding throughout flower and silique development, indicating that IAA levels are higher in the AZ than in adjacent tissues. Although GUS accumulation is discretely sustained in the AZ from positions 2 to 18, it may not be appropriate to conclude that elevated IAA signaling is maintained throughout this developmental time period, as it is well documented that the GUS protein is highly stable (Kavita and Burma, 2008). A more definitive spatial and temporal analysis of IAA signaling within AZ cells during flower development might be obtained using a GFP reporter (Brunoud et al., 2012) or by monitoring the expression of AUX/IAA genes (Abebie et al., 2008). What is evident is that GUS staining is only just detectable in the AZ region of ProAt2g41850:iaaL plants, confirming that in this region IAA activity has been considerably reduced.
In order to elevate levels of IAA in the AZ cells, the promoter of At2g41850 was fused to the open reading frame of the iaaM gene. iaaM converts Trp to indoleacetamide, and although there is no evidence that this compound is a standard intermediate in the biosynthesis of auxin, it has been shown in plants such as petunia (Petunia hybrida) that high IAA production can be induced by overexpression of the iaaM gene (Klee et al., 1987). Confirmation that IAA activity was enhanced in the ProAt2g41850::iaaM plants was obtained using the ProDR5:GUS reporter material, which revealed a more intense accumulation of GUS stain discretely within the AZ cells. This was particularly strong at positions 4 to 8, which coincide with the stages where At2g41850 is most highly expressed.
Floral organs from ProAt2g41850::iaaL plants maintained in the glasshouse were shed on average by flower position 5 or 6, while those from wild-type plants were regularly retained until position 8. The timing of abscission seen in the ProAt2g41850::iaaL material was comparable with that observed in the lines where expression of the IAA influx facilitators had been silenced or down-regulated. In contrast, sepals, petals, and anther filaments from ProAt2g41850::iaaM plants were routinely retained up to position 16. These observations, from glasshouse-grown ProAt2g41850::iaaL and ProAt2g41850::iaaM plants, were validated by petal breakstrength analyses. Interestingly, ProAt2g41850:iaaL flowers exhibit both an earlier position at which a reduction in force required to separate petals can be detected and an elevated rate at which the decline in breakstrength proceeds, suggesting that IAA is important for both the timing of abscission and the rate at which cell wall dissolution takes place. Only a small reduction in petal breakstrength was observed in ProAt2g41850::iaaM plants up to position 8, when petals no longer remained attached in wild-type plants, confirming that if elevated IAA levels are maintained within the AZ tissues, then shedding is strongly inhibited. The fact that floral organs do eventually abscise suggests that IAA may not be able to protect the AZ cells from other abscission-promoting stimuli indefinitely. The PG gene used to drive iaaM is only expressed at the later stages of abscission, and a promoter that is similarly discrete but is active at an earlier developmental phase in flower development and is more sustained might be even more effective, when fused to iaaL or iaaM, at manipulating the timing of organ shedding. For instance, it would be interesting to drive these bacterial genes using the M0223 promoter that was used in our transactivation experiments.
Meir et al. (2006) demonstrated that the petiole AZ in M. jalapa became sensitive to the abscission-inducing effects of ethylene when the leaf blades were decapitated to stop/reduce the supply of auxin to the AZ. Those authors further demonstrated that application of the ethylene action inhibitor 1-methylcyclopropene can delay abscission even after deblading, providing evidence for the existence of a fine balance of auxin-ethylene in the AZ.
The gene iaaM, when expressed specifically in the AZ under the control of the At2g41850 promoter, delayed shedding of floral organs until flower position 15 or 16, which is late compared with the wild type. Sepal yellowing was also slowed in these plants. These observations were similar to the delayed shedding of floral organs shown by Patterson and Bleecker (2004), where the entire plants were made insensitive to ethylene using the mutated version of the etr1-1 gene. Those authors concluded that by making the plant insensitive to ethylene, the auxin-ethylene balance was manipulated in favor of auxin, which delays the actions of the cell-separating enzymes. In the iaaM lines, the auxin-ethylene balance is manipulated in the same way but by synthesizing auxin in the AZ, without directly manipulating ethylene biosynthesis and or response.
Role of Aux/IAA Proteins during Abscission
The AXR3 gene belongs to the Aux/IAA family and plays an important role in the auxin-regulated degradation of the Aux/IAA proteins, which is an essential part of the auxin response (Leyser, 2002). The Aux/IAA proteins act as transcriptional regulators by producing a variety of dimers, which leads to the formation of an unusual sequence-specific DNA-binding domain. These domains bind to DNA directly and regulate transcription (Leyser, 2002). Woodward and Bartel (2005) demonstrated that the Aux/IAA transcripts accumulate following auxin exposure, and the encoded proteins apparently serve to dampen auxin signaling.
One of the mutants isolated as the gain-of-function allele axr3-1 of the AXR3 (At1g04250) gene stabilizes the Aux/IAA proteins and confers an auxin resistance phenotype (Rouse et al., 1998; Tian and Reed, 1999; Nagpal et al., 2000; Overvoorde et al., 2005). Generally, the Aux/IAA proteins have a half-life of 5 to 12 min. In wild-type plants, the half-life of AXR3 (IAA17) is 80 min (Abel et al., 1994), but the mutated axr3-1 protein has a half-life of approximately 550 min (Ouellet et al., 2001), causing an altered auxin response.
In order to manipulate auxin responses during abscission, transactivation of the gain-of-function mutation of the AXR3 gene (axr3-1) in the floral AZ was undertaken. In ProAt2g41850>>AXR3 plants, floral organs started to senesce from flower position 4 onward, and by position 10, they were completely desiccated; however, there was no abscission. Desiccation of the AZ cells might ultimately preclude cell separation from taking place. As the gene At2g41850 was most highly expressed at position 6 or 7, we decided to construct a ProM0233>>AXR3 line, which expresses even before anthesis in the floral AZ. Similar results were obtained in the case of M0223-driven axr3-1 transactivation compared with At2g41850-driven axr3-1 expression, indicating that a disruption of auxin signaling can abolish abscission.
Transcriptomic analysis indicates that stabilization of the AXR3-1/IAA17-1 protein acts to repress auxin signaling (Overvoorde et al., 2005), and our observation that this leads to an impairment of floral organ shedding is intriguing. Perhaps the simplest explanation for this is that ethylene-mediated abscission is compromised in the absence of auxin signaling. Certainly, it is well documented that the biosynthesis of these two hormones is closely entwined and that they can influence each other’s response pathways (Stepanova et al., 2007). Another possibility is that the “ethylene-independent” pathway (Patterson and Bleecker, 2004) that has been proposed to contribute to the abscission process exhibits cross talk with the auxin signaling process. Certainly, there is evidence that axr3-1 plants exhibit attenuated expression of type A ARRs (Overvoorde et al., 2005), and these genes are known to play a key role in the cytokinin signal transduction pathway (Brandstatter and Kieber, 1998).
A third potential explanation is that while elevated concentrations of IAA can “protect” AZ cells from abscission-inducing stimuli, a low level of auxin signaling is a prerequisite for the events associated with cell separation to take place. In support of this hypothesis, a spectrum of the genes that have been reported to be associated with abscission, including cell wall-loosening enzymes such as PGs, are up-regulated by auxin treatment (González-Carranza et al., 2007, 2012; Hruz et al., 2008). Abscission and lateral root emergence require targeted wall dissolution to take place, and many of the same genes, both at a regulatory level (Stenvik et al., 2008) and a functional level (González-Carranza et al., 2007), contribute to both processes. A model to account for how cell wall remodeling is regulated in each tier of cells in the root has been developed, and in this scheme, IAA plays a central role in coordinating the events (Swarup et al., 2008). Although we know that a high flux of auxin has the capacity to delay organ shedding, it is possible that a discrete discontinuity in auxin concentrations between AZ cells and their nonseparating neighbors could act as a trigger for the process to take place. The observations recorded in this paper that auxin facilitators are expressed specifically at the site of abscission and that levels of IAA signaling are higher in AZ cells provide some evidence to support this proposal. Further work using, for instance, more dynamic sensors of auxin signaling (Brunoud et al., 2012) will help us to determine whether this hypothesis is correct.
In conclusion, we have employed a number of novel strategies to dissect the role of IAA in regulating floral organ abscission in Arabidopsis. By studying mutants that are compromised in their ability to regulate auxin uptake into cells and transgenic lines where IAA concentrations have been manipulated specifically within the AZ, we have determined that both the timing and the rate of petal shedding can be attenuated. Furthermore, using a transactivation approach to suppress auxin responses specifically with the AZ cells, we have revealed, to our knowledge for the first time, that a functional IAA signaling pathway is necessary for abscission to take place. These observations may contribute to the formulation of new strategies that can be employed to manipulate organ shedding in crop plants.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants (Col-0) were either grown in a growth chamber under 18 h of daylight at 20°C ± 2°C and 6 h of night at 16°C ± 2°C or in a glasshouse under at least 16 h of light using supplementary lighting (76 W m−2) when necessary; the compost used to grow all plants in this study was Levington Professional M3 (Scotts). The aux1 mutant (Marchant and Bennett, 1998), lax1, lax2, and lax3 loss-of-function T-DNA insertion lines, aux1 lax3 double mutant, aux1 lax1 lax2 lax3 quadruple mutant (Swarup et al., 2008; Ugartechea-Chirino et al., 2010), and ProAUX1:: GUS, ProLAX1::GUS, ProLAX2::GUS, and ProLAX3::GUS lines (Parry et al., 2001), along with the ProUAS::axr3-1 line (Swarup et al., 2005) and the ProDR5::GUS (Ulmasov et al., 1997) auxin reporter line, were provided by Prof. Malcolm Bennett’s group (Division of Plant and Crop Sciences, University of Nottingham). The GAL4 enhancer trap line M0223 (NASC N9336) from the Haseloff collection (Haseloff et al., 1997) was ordered from the Nottingham Arabidopsis Stock Centre.
Plasmid Construction and Plant Transformation
Bacterial Escherichia coli strains (DH5α) containing the genes iaaL and iaaM (Østergaard et al., 2007; Sorefan et al., 2009) in the pCR2.1 vector (Invitrogen) along with ProAt2g41850::GUS (González-Carranza et al., 2002) and GAL4-VP16 in the binary vector pMOG-402 (Swarup et al., 2005) were grown at 37°C overnight in Luria-Bertani medium supplemented with 50 µg mL−1 kanamycin in a controlled-environment rotary incubator shaker (New Brunswick Scientific). The next morning, cultures were subjected to plasmid DNA extraction using the Gen Elute Plasmid Miniprep Kit (Sigma-Aldrich) following the manufacturer’s protocol and were quantified using the Nanodrop ND1000 Spectrophotometer V3.7 (Thermo Fisher Scientific). The DNA from plasmids was then used as a template in different PCRs to amplify fragments from the genes iaaL, iaaM, and GAV4-VP16; the primers used were IAAL-For/IAAL-Rev (1,234 bp), IAAM-For/IAAM-Rev (2,314 bp), and GAL4-VP16-For/GAL4-VP16-Rev (833 bp), respectively. All primer sequences used for the PCR performed in this work can be found in Supplemental Table S1.
The PCR conditions used to amplify the fragments were as follows: 94°C for 3 min for denaturation; 94°C for 30 s, 55°C for 1 min, and 72°C for 2 min for 35 cycles; and 72°C for 7 min as a final extension. This was followed by 4°C hold using the AB GeneAmp PCR 9700 thermal cycler in a 50-µL reaction (1× PCR buffer, 1.5 mm MgCl2, and 0.2 mm deoxyribonucleotide triphosphates [Pharmacia], 2.5 units of platinum Taq DNA polymerase [Invitrogen], and 1 μL of 10 μm gene-specific primers). The PCR-amplified fragments were then reamplified to introduce restriction sites as follows, iaaL (BamHI and SacI), iaaM (XbaI and SacI), and GAL4 VP16 (XbaI and SacI), and subcloned downstream of the 1,476-bp At2g41850 promoter into the pMOG-402 binary vector. The PCR conditions and reactions to reamplify these segments was the same as for fragments without restriction sites except that the melting temperature used was 60°C. The integrity of the clones was verified by PCR using the primer PGAZAT ScrFr with the corresponding reverse primer for each segment. PCR conditions and reactions were performed as mentioned earlier. Once the At2g41850 promoter-driven constructs for iaaL, iaaM, and GAL4-VP16 in the pMog402 vector were generated, plasmids were extracted from bacterial strains (DH5α) using the Gen Elute Plasmid Miniprep Kit (Sigma-Aldrich) following the manufacturer’s instructions. The plasmids were then electroporated into Agrobacterium tumefaciens C58 strain and grown to an optical density at 600 nm of 0.5 to 0.8. Arabidopsis Col-0 plants were transformed using the floral dip method as described by Clough and Bent (1998).
After transformation, the plants were kept in the greenhouse under at least a 16-h photoperiod, and seeds were collected at maturity. Transgenic selection was carried out using plates containing 4.33 g L−1 Murashige and Skoog basal salt mixture (Sigma; Murashige and Skoog, 1962), pH 5.9, 0.8% (v/v) agar, and supplemented with 40 mg L−1 kanamycin; surviving plants were transferred to Levington Professional M3 compost and grown under growth room conditions as described previously.
RNA Extraction
Total RNA was extracted from the AZ of flowers at positions 4 to 9 (in this study, flower position 1 is considered the flower with the first visible petals in the inflorescence) from the control plants (Col-0) along with ProAt2g41850::iaaM and ProAt2g41850::iaaL lines using the Qiagen RNeasy Plant RNA Extraction Kit following the manufacturer’s instructions.
RT-PCR Analysis of Transgenes iaaL and iaaM in Floral AZs
First-strand complementary DNA was synthesized from total RNA extracted from floral AZ of Col-0, ProAt2g41850::iaaM, and ProAt2g41850::iaaL lines as per the manufacturer’s instructions supplied with the Invitrogen RT Kit. PCR was carried out to analyze the expression of the transgenes iaaL and IaaM using the primer combinations At2g41850Pro and gene-specific reverse primers IAAL-Rev and IAAM-Rev, respectively. PCR conditions were as described above in “Plasmid Construction and Plant Transformation.”
Genetic Crosses and Genotypic Characterization
The auxin reporter ProDR5::GUS line was crossed with the Columbia wild-type ecotype and the generated lines ProAt2g41850::iaaL and ProAt2g41850::iaaM to study levels of auxin in the floral AZs. DNA was extracted from F1 plants using the Sigma ExtractnAmp DNA Kit following the manufacturer’s instructions, and crosses were verified by PCR analyses of the transgenes iaaL and iaaM as described above in “Plasmid Construction and Plant Transformation”; the presence of the DR5::GUS was confirmed with GUS assays.
Crosses between ProAt2g41850::GAL4-VP16 and ProUAS::axr3-1, M0223 and ProUAS::axr3-1, and ProM0233::AXR3 and ProAt2g41850::GAL4-VP16 were generated to produce transactivating lines. DNA was extracted from the F1 plants, and two separated PCRs were carried out using At2g41850-For and GAL4-VP16-Rev primers (883 bp) and UAS-For and AXR3-1-Rev primers (784 bp) to confirm the crosses. PCR conditions were described above in “Plasmid Construction and Plant Transformation.” ProAt2g41850::AXR3 and ProM0223::AXR3 crosses were segregated to obtain homozygous lines, and the rest of the lines analyzed were F1 populations.
QPCR
QPCR was used to quantify At2g41850 expression in the AZ cells at different flower stages (4–5, 6–7, and 8–9) in the Col-0 plants. The fluorescent dye SYBR Green I (provided in the Brilliant SYBR Green QPCR Master Mix Kit [Agilent Technologies]) was used to detect and quantify gene transcripts. QPCR was performed using the Stratagene MX3005P QPCR machine, which includes a QPCR machine and the software (MXPRO QPCR Software) for designing the PCR program and analyzing data (Agilent Technologies). All solutions except primers and templates were provided in the Brilliant SYBR Green QPCR Master Mix Kit.
The experimental reaction comprised 12.5 µL of 2× master mix (2× Brilliant SYBR Green QPCR Master Mix), 0.5 µL of At2g41850-For and At2g41850-Rev (10 μm), 0.375 µL of reference dye (2 µm), and 0.5 µL of complementary DNA- and nuclease-free water to make a final volume of 25 µL. Due to the sensitivity of the QPCR, all samples were in triplicate, and 40 PCR cycles were run (95°C for 30 s, 57°C for 30 s, and 72°C for 40 s) followed by a dissociation step to detect any nonspecific amplification or primer-dimer formation during amplification. ACT2-For and ACT2-Rev primers were used to amplify the housekeeping gene ACT2 (At3g18780) to normalize the relative expression of At2g41850. All samples were run in triplicate, and relative expression was determined using the comparative threshold cycle analysis method (Livak and Schmittgen, 2001).
GUS Assay
GUS expression analysis from AZ flowers of F1 crosses between ProDR5::GUS and ProAt2g41850::iaaL, ProDR5::GUS and ProAt2g41850::iaaM, and IAA influx and efflux carrier reporter lines ProAUX1::GUS, ProLAX1::GUS, ProLAX2::GUS, and ProLAX3::GUS was undertaken by collecting various floral positions (2–16) as described by Patterson (2001); the GUS was visualized using the protocol of Willemsen et al. (1998). Tissue was cleared with 70% (v/v) ethanol at room temperature. Flowers were then examined for GUS expression, and tissue was viewed and photographed using a Zeiss Semi SV6 Stereomicroscope and a Canon digital camera (Power Shot A620).
Breakstrength Analysis
The breakstrength of petals from Arabidopsis flowers from different lines and from different floral stages was measured as described by González-Carranza et al. (2012). The raw data obtained from the breakstrength analysis of Col-0 control, ProAt2g41850::iaaL, ProAt2g41850::iaaM, aux1 mutant, lax1 and lax3 loss-of-function insertion lines, aux1 lax3 double mutant, and aux1 lax1 lax2 lax3 quadruple mutant were imported into Microsoft Excel. Mean and sd values were calculated using the Excel program, and a bar graph depicting the relative breakstrength at different floral positions was generated.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Natural shedding at various flower positions in the Col-0 wild type and AUX1 and LAX1 knockouts.
Supplemental Figure S2. Natural shedding at various different flower positions in the LAX3 knockout, AUX1-LAX3 double knockout, and AUX1-LAX1-LAX2-LAX3 quadruple knockout lines.
Supplemental Figure S3. Genomic PCR of positive primary transformants containing PGAZAT::iaaL and PGAZAT::iaaM transgenes.
Supplemental Figure S4. RT-PCR results confirming the expression of PGAZAT::iaaL and PGAZAT::iaaM in the floral AZ.
Supplemental Figure S5. Floral organ shedding in Col-0 wild-type control, PGAZAT::iaaL, and PGAZAT::iaaM plants.
Supplemental Figure S6. Genomic PCR of five independent positive primary transformants containing PGAZAT::GAL4-VP16 transgenes.
Supplemental Figure S7. RT-PCR results confirming the expression of PGAZAT::GAL4-VP16 in the floral AZ.
Supplemental Table S1. Primer sets employed in this research.
Acknowledgments
We thank Malcolm Bennett and Ranjan Swarup for providing us with the aux1 mutant line, the lax1, lax2, and lax3 insertion lines, the aux1 lax3 double mutant and aux1 lax1 lax2 lax3 quadruple mutant, the ProAUX1:: GUS, ProLAX1::GUS, ProLAX2::GUS, and ProLAX3::GUS lines, and the ProUAS::axr3-1 and ProDR5::GUS lines. We thank Lars Østergaard for providing us with the iaaL and iaaM constructs.
Glossary
- AZ
abscission zone
- IAA
indoleacetic acid
- Aux/IAA
auxin/indoleacetic acid
- PG
polygalacturonase
- Col-0
Columbia-0
- RT
reverse transcription
- QPCR
quantitative PCR
- T-DNA
transfer DNA
References
- Abebie B, Lers A, Philosoph-Hadas S, Goren R, Riov J, Meir S. (2008) Differential effects of NAA and 2,4-D in reducing floret abscission in cestrum (Cestrum elegans Schlecht) cut flowers are associated with their differential activation of Aux/IAA homologous genes. Ann Bot (Lond) 101: 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Oeller PW, Theologis A. (1994) Early auxin-induced genes encode short-lived nuclear proteins. Proc Natl Acad Sci USA 91: 326–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni R, Aloni E, Langhans M, Ullrich CI. (2006) Role of auxin in regulating Arabidopsis flower development. Planta 223: 315–328 [DOI] [PubMed] [Google Scholar]
- Beyer EM. (1975) Abscission: the initial effect of ethylene is in the leaf blade. Plant Physiol 55: 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS. (2005) Auxin transport. Curr Opin Plant Biol 8: 494–500 [DOI] [PubMed] [Google Scholar]
- Brandstatter I, Kieber JJ. (1998) Two genes with similarity to bacterial response regulators are rapidly and specifically induced by cytokinin in Arabidopsis. Plant Cell 10: 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoud G, Wells DM, Oliva M, Larrieu A, Mirabet V, Burrow AH, Beeckman T, Kepinski S, Traas J, Bennett MJ, et al. (2012) A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482: 103–106 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Degan FD, Child R, Svendsen I, Ulvskov P. (2001) The cleavable N-terminal domain of plant endopolygalacturonases from clade B may be involved in a regulated secretion mechanism. J Biol Chem 276: 35297–35304 [DOI] [PubMed] [Google Scholar]
- Doebley J. (2004) The genetics of maize evolution. Annu Rev Genet 38: 37–59 [DOI] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW. (2005) AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132: 4563–4574 [DOI] [PubMed] [Google Scholar]
- González-Carranza ZH, Elliott KA, Roberts JA. (2007) Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J Exp Bot 58: 3719–3730 [DOI] [PubMed] [Google Scholar]
- González-Carranza ZH, Shahid AA, Zhang L, Liu Y, Ninsuwan U, Roberts JA. (2012) A novel approach to dissect the abscission process in Arabidopsis. Plant Physiol 160: 1342–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Carranza ZH, Whitelaw CA, Swarup R, Roberts JA. (2002) Temporal and spatial expression of a polygalacturonase during leaf and flower abscission in oilseed rape and Arabidopsis. Plant Physiol 128: 534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S. (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P. (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv Bioinformatics 2008: 420747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Osborne DJ. (1970) Ethylene, the natural regulator of leaf abscission. Nature 225: 1019–1022 [DOI] [PubMed] [Google Scholar]
- Jacobs WP. (1962) Longevity of plant organs: internal factors controlling abscission. Annu Rev Plant Physiol 13: 403–436 [Google Scholar]
- Kavita P, Burma PK. (2008) A comparative analysis of green fluorescent protein and β-glucuronidase protein-encoding genes as a reporter system for studying the temporal expression profiles of promoters. J Biosci 33: 337–343 [DOI] [PubMed] [Google Scholar]
- Klee HJ, Estelle M. (1991) Molecular genetic approaches to plant hormone biology. Annu Rev Plant Physiol 42: 529–551 [Google Scholar]
- Klee HJ, Horsch RB, Nichee MA, Hein MB, Hoffmann NL. (1987) The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev 1: 85–96 [Google Scholar]
- Kramer EM, Bennett MJ. (2006) Auxin transport: a field in flux. Trends Plant Sci 11: 382–386 [DOI] [PubMed] [Google Scholar]
- Laibach F. (1951) Wuchstoffversuche mit lebenden Orchideenpollinen. Ber Dtsch Bot Ges 51: 336–340 [Google Scholar]
- Leslie ME, Lewis MW, Liljegren SJ. (2007) Organ abscission. Annual Plant Reviews 25: 106–136 [Google Scholar]
- Leyser O. (2002) Molecular genetics of auxin signaling. Annu Rev Plant Biol 53: 377–398 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Marchant A, Bennett MJ. (1998) The Arabidopsis AUX1 gene: a model system to study mRNA processing in plants. Plant Mol Biol 36: 463–471 [DOI] [PubMed] [Google Scholar]
- Meir S, Hunter DA, Chen J-C, Halaly V, Reid MS. (2006) Molecular changes occurring during acquisition of abscission competence following auxin depletion in Mirabilis jalapa. Plant Physiol 141: 1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir S, Philosoph-Hadas S, Sundaresan S, Selvaraj KSV, Burd S, Ophir R, Kochanek B, Reid MS, Jiang C-Z, Lers A. (2010) Microarray analysis of the abscission-related transcriptome in the tomato flower abscission zone in response to auxin depletion. Plant Physiol 154: 1929–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 18: 100–127 [Google Scholar]
- Nagpal P, Walker LM, Young JC, Sonawala A, Timpte C, Estelle M, Reed JW. (2000) AXR2 encodes a member of the Aux/IAA protein family. Plant Physiol 123: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Kay P, Wilson S, Swain SM. (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21: 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østergaard L, Borkhardt B, Ulvskov P. (2007) Dehiscence. Annual Plant Reviews 25: 137–163 [Google Scholar]
- Ouellet F, Overvoorde PJ, Theologis A. (2001) IAA17/AXR3: biochemical insight into an auxin mutant phenotype. Plant Cell 13: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. (2005) Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17: 3282–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Marchant A, May S, Swarup R, Swarup K, James N, Graham N, Allen T, Martucci T, Yemm A, et al. (2001) Quick on the uptake: characterization of a family of plant auxin influx carriers. J Plant Growth Regul 20: 217–225 [Google Scholar]
- Patterson SE. (2001) Cutting loose: abscission and dehiscence in Arabidopsis. Plant Physiol 126: 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson SE, Bleecker AB. (2004) Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol 134: 194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Elliott KA, González-Carranza ZH. (2002) Abscission, dehiscence, and other cell separation processes. Annu Rev Plant Biol 53: 131–158 [DOI] [PubMed] [Google Scholar]
- Roberts JA, González-Carranza ZH (2007) Abscission. In Encyclopedia of Life Sciences [Google Scholar]
- Rose JKC, Catala C, González-Carranza ZH, Roberts JA. (2003) Cell wall disassembly. Annual Plant Reviews 8: 264–324 [Google Scholar]
- Rouse D, Mackay P, Stirnberg P, Estelle M, Leyser O. (1998) Changes in auxin response from mutations in an AUX/IAA gene. Science 279: 1371–1373 [DOI] [PubMed] [Google Scholar]
- Rubinstein B, Leopold AC. (1963) Analysis of the auxin control of bean leaf abscission. Plant Physiol 38: 262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton R, Roberts JA. (1982) Cell biology of abscission. Annu Rev Plant Physiol 33: 133–162 [Google Scholar]
- Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, Offringa R, Friml J, Yanofsky MF, Østergaard L. (2009) A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature 459: 583–586 [DOI] [PubMed] [Google Scholar]
- Stenvik GE, Tandstad NM, Guo Y, Shi C-L, Kristiansen W, Holmgren A, Clark SE, Aalen RB, Butenko MA. (2008) The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 20: 1805–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HMO, Haseloff J, Beemster GTS, Bhalerao R, Bennett MJ. (2005) Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat Cell Biol 7: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Swarup R, Péret B. (2012) AUX/LAX family of auxin influx carriers: an overview. Front Plant Sci 3: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Whitelaw CA. (2001) Signals in abscission. New Phytol 151: 323–339 [Google Scholar]
- Tian Q, Reed JW. (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126: 711–721 [DOI] [PubMed] [Google Scholar]
- Ugartechea-Chirino Y, Swarup R, Swarup K, Péret B, Whitworth M, Bennett M, Bougourd S. (2010) The AUX1 LAX family of auxin influx carriers is required for the establishment of embryonic root cell organization in Arabidopsis thaliana. Ann Bot (Lond) 105: 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. (1997) ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868 [DOI] [PubMed] [Google Scholar]
- Willemsen V, Wolkenfelt H, de Vrieze G, Weisbeek P, Scheres B. (1998) The HOBBIT gene is required for formation of the root meristem in the Arabidopsis embryo. Development 125: 521–531 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]