Abstract
Spinal subdural abscesses (SSA) are very rare disease. The etiologies of SSA are hematogenous spread, iatrogenic contamination, and local extension. Elevated WBC counts, ESR, and C-reactive protein are usually found in laboratory tests. But they are not sensitive indicators of SSA, especially chronic abscesses patient tend to have a less specific characteristic. We report the case of a healthy man with chronic subdural abscess referred to our hospital as an intradural–extramedullary (IDEM) tumor. The patient presented with voiding difficulty and pain in the back and left leg. In a contrast MRI scan, a rim-enhanced mass-like lesion was seen at the L5/S1 level. But adjacent ill-defined epidural fat enhancement that are unusual imaging manifestation for IDEM tumors was seen. He had no fever and normal WBC, ESR, and CRP. In addition, the patient had no previous infection history or other disease, but he did have an epidural block for back pain at another hospital 2 years previously. So, we repeated the MRI with a high-resolution 3-T scanner. The newly taken MR images in our hospital revealed a clear enlargement of lesion size compared to the previous MRI taken 1 week before in other hospital. We suspected a chronic spinal subdural abscess with recent aggravation and immediately performed surgical evacuation. In the surgical field, tensed dura was observed and pus was identified after opening the abscess capsule. Because chronic spinal subdural abscesses are difficult to diagnose, we could differentiate with IDEM tumor exactly and an exact history taking, contrast MRI are required.
Keywords: Spinal subdural abscess, Chronic spinal subdural abscess, Intradural–extramedullary tumor, Spinal cord tumor
Introduction
With the development of treatment technologies in recent years, the mortality rate of spinal cord abscesses has decreased. However, spinal cord abscesses are still rare and difficult to treat. Although most spinal abscesses are extradural, they can also be intradural–extramedullary (IDEM) or intramedullary [5, 9]. Abscess in the intradural–extramedullary space are usually called spinal subdural abscesses. The Spinal subdural abscesses (SSA) were first described by Sitting in 1927, and only 69 cases of spinal subdural abscess have been reported in the literature since then [2, 12, 13]. The SSA involves hematogenous spread, iatrogenic contamination, and direct spread [2, 7, 12, 13]. Except for two previously reported cases, patients usually have obvious etiology, recent history of infection or procedure, or an anatomical defect [10, 12]. We present the case of a healthy man with a SSA without fever, leukocytosis, or meningeal irritation. The patient presented no infection signs except a history of epidural block 2 years previous.
Case report
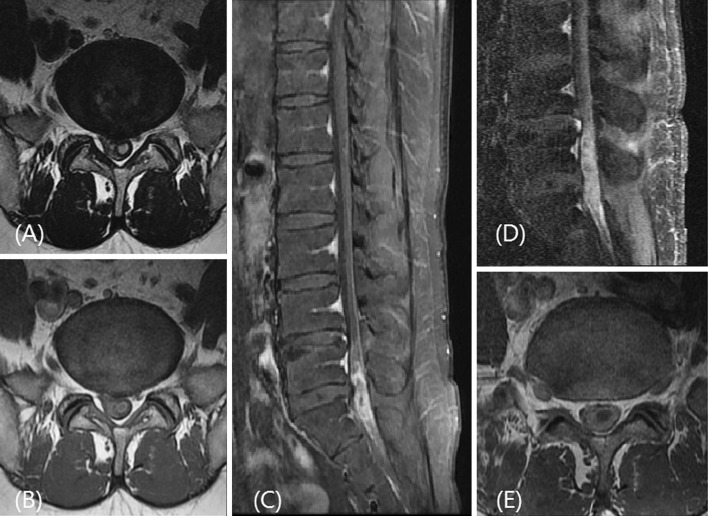
A 43-year-old man was referred to our hospital from other hospital for an intradural–extramedullary tumor. The patient complained of lower back, left buttock and posterior thigh pain for 2 years with back and leg pain aggravation starting 1 month before. He also complained a newly developed voiding difficulty. He had no other disease or past surgical history except a history of epidural block 2 years previous. On neurologic examination, no focal neurologic deficits were identified besides voiding difficulty, constipation, and erectile dysfunction. He had no fever, chill, or signs of meningeal irritation. Laboratory tests were normal (ESR 7 mm/h; normal: < 15 mm/h, CRP 3.6 mg/L; normal: 0.1–6.0 mg/L, WBC count 9,080/ml3; normal: 4,000 –10,800/ml3). One week before, the patient had undergone contrast enhanced magnetic resonance (MR) imaging at other hospital. MR images showed a mass-like lesion at the lumbosacral junction level. The signal intensity of the lesion was high on T2-weighted images and slightly lower on T1-weighted images (Fig. 1a, b). In gadolinium contrast enhanced fat-suppressed T1-weighted images, the lesion showed rim enhancement while demonstrating diffuse enhancement of adjacent epidural fat (Fig. 1c). The other hospital had diagnosed an IDEM tumor using these imaging features. Radiologists in our hospital repeated the MRI with a high-resolution 3-T scanner (Fig. 1d, e) because ill-defined epidural fat enhancement is an unusual imaging manifestation for IDEM tumors and is usually associated with arachnoiditis or meningitis. The newly taken MR images in our hospital revealed a clear enlargement of lesion size compared to the previous MRI taken 1 week before in other hospital. Although his laboratory tests and body temperature were in normal range, we focused more on abscess than tumor. Because lesion was accompanied with adjacent arachnoiditis in MRI scan and rapid growing in IDEM tumor is very rare. In addition, despite epidural block was performed 2 years ago, epidural block could lead to spinal cord infection as chronic. According to previous literatures, if abscess was presented as chronic, it could be presented with less specific symptom and sign [1, 11]. Considering the patient’s history and MRI findings, we suspected that the lesion might be an acute aggravation of chronic subdural inflammatory change. However, the possibility of IDEM tumor could not be completely ruled out using imaging.
Fig. 1.
Initial T2-weighted MR images (a), T1-weighted MR image (b), Fat suppressed contrast enhanced T1 weighted MR image c taken at other hospital. 1 weak later, We repeated contrast enhanced MRI image at our hospital (d, e)
Surgery was performed the next day. After a L5 laminectomy, the epidural space was clear and tensed dura was found. After durotomy, a soft mass with a thick capsule resembling a spinal cord tumor was identified. Whitish cheese-like pus was identified after opening the abscess capsule (Fig. 2a). Granulation tissue was also seen around the abscess. We suggest the granulation tissue was due to chronic inflammation and that the infection had been asymptomatically present for a long period of time. According to Bartels et al. [2], granulation tissue has not previously been found in a patient with acute spinal subdural abscess. The abscess was removed and copious irrigation was performed (Fig. 2b). A microbiological examination of the pus revealed Methicillin sensitivity Staphylococcus aureus (MSSA). After abscess removal, an appropriate antibiotic (Nafcillin® 2 g IV every 6 h) was injected for 4 weeks. Postoperatively his symptoms, voiding difficulty, and pain improved slightly. And postvoiding residual urine volume was decreased. Because we were undergoing surgical evacuation urgently, we could not preoperative electromyography. But postoperative electromyography reveals sacral radiculopathy and caudal equine injury. We did follow-up for 4 month in outpatient clinicand the patient symptom has not changed.
Fig. 2.
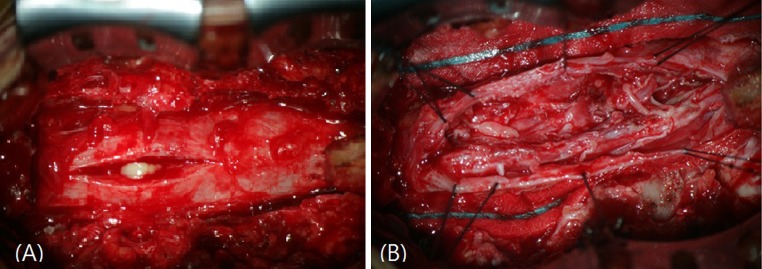
In operation, after durotomy, pus was revealed within dura (a). We put in stay sutures and removed pus. After abscess removal, granulation tissue and adhesion were found (b)
Discussion
Spinal subdural abscess is rare. Although previous literatures were well described, they are not well distinguished spinal subdural abscesses from subdural empyema. Abscesses have a capsule with normal structure that separates pus, while empyema involves the accumulation of pus in a preexisting cavity. Although abscesses and empyema are not the same, they are known to have similar etiology, pathogen, symptoms, progression, and treatment.
In the previous literatures, almost SSA has obvious etiology [10, 12]. The etiology of spinal subdural abscess was known to be hematogenous spread, iatrogenic contamination (lumbar puncture, discogram, previous surgical history), and local extension [3, 7, 8, 10]. Predisposing factors are anatomical abnormalities of the spinal cord, congenital dermal sinus, acquired immunodeficiency syndrome, DM, drug abuse, and repetitive meningitis [2, 9, 12].
The lumbar spine is the most common region for spinal subdural abscesses, and the most common pathogen is Staphyloccous aureus [2–4, 7, 12, 13]. The symptoms of SSA are fever, back pain, paralysis, and spinal symptoms such as weakness and voiding dysfunction. According to the report of Bartels et al. [2], Fever was the initial symptom in 56.6 % of 44 patients. The duration of symptoms from onset until patient death or therapeutic intervention ranged from 1 day to approximately 1 year. Among 39 cases, 6 were chronic SSA (>8 weeks) [2]. In this report, symptoms of chronic abscess were not described. In some reported cases, chronic abscesses tend to have less specific symptomatology [1, 11]. Leukocyte count, ESR, and C-reactive protein are not sensitive indicators of spinal infection, but are usually found to be elevated [12, 13]. In our case, leukocyte count, ESR, CRP, and body temperature were normal.
Myelography has previously been used, but recently contrast-enhanced MRI scans are the best modality for diagnosis [1, 4, 7, 9]. In MRI scans, increased signal intensity in T2-weighted images, decreased the signal intensity in T1-weighted images, and rim enhancement in contrast enhanced T1-weighted images strongly suggests an abscess [4, 10]. Differential diagnosis from an IDEM tumor such as schwannoma or neuroma and an epidural or subdural hematoma is needed [10]. In addition, spinal inflammatory myofibroblastic tumor (IMT) should be considered in these MRI finding though very rare [14]. Inflammatory myofibroblastic tumor called “inflammatory pseudotumor” or “plasma cell granuloma” could occur anywhere and was reported only in nine case of intradural–extramedullary tumor. IMT was histologically benign and showed a fibrous tissue mass with a mixed population of lymphocyte, plasma cells and macrophages.
Fat suppression sequence may help by subtracting the high signals of epidural fat and bone marrow [7, 10]. Although not yet proven in the spinal cord, diffusion-weighted images (DWI) can be a useful adjunct in differentiating abscesses from tumors [6]. In several studies, nearly all pyogenic abscesses showed marked hyperintense signals on DWI and corresponding reduced calculated apparent diffusion coefficients (ADCs), indicating restricted water diffusion. Restricted diffusion indicates the absence of water movement, which is usually due to the high viscosity of inflammatory cells in pus [6].
When a spinal subdural abscess is diagnosed, prompt surgical evacuation and appropriate antibiotic therapy are necessary. To determine the appropriate antibiotics, pus should be tested in aerobic and anaerobic cultures for antibiotics susceptibility.
Peculiarity of this case is that the patient has no obvious evidence of SSA and was misdiagnosed to IDEM tumor, but chronic abscess could be presented atypically, and hence, we should differentiate SSA and IDEM tumor in MRI scan.
Conclusion
In the previous literatures, only one case had similar clinical and laboratory course [11]. That case has previous kyphosis surgery history and intercostal nerve block history. Our case also has history of previous epidural block, but the patient received the procedure 2 years ago just one time. In this case, it would have been difficult to suspect pyogenic spinal cord infection without the contrast-enhanced MRI scan and previous epidural block history. Because chronic SSAs are difficult to diagnose, we could differentiate with IDEM tumor exactly and an exact history taking, contrast MRI are required.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Akhaddar A, Boulahroud O, Boucetta M. Chronic spinal cord abscess in an elderly patient. Surg Infect (Larchmt) 2011;12:333–334. doi: 10.1089/sur.2010.064. [DOI] [PubMed] [Google Scholar]
- 2.Bartels RH, de Jong TR, Grotenhuis JA. Spinal subdural abscess. Case report J Neurosurg. 1992;76:307–311. doi: 10.3171/jns.1992.76.2.0307. [DOI] [PubMed] [Google Scholar]
- 3.Chen MH, Huang JS. Cervical subdural empyema following acupuncture. J Clin Neurosci. 2004;11:909–911. doi: 10.1016/j.jocn.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Diehn FE. Imaging of spine infection. Radiol Clin North Am. 2012;50:777–798. doi: 10.1016/j.rcl.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Foley J. Intramedullary abscess of the spinal cord. Lancet. 1949;2:193–195. doi: 10.1016/S0140-6736(49)91194-0. [DOI] [PubMed] [Google Scholar]
- 6.Hood B, Wolfe SQ, Trivedi RA, Rajadhyaksha C, Green B. Intramedullary abscess of the cervical spinal cord in an otherwise healthy man. World Neurosurg. 2011;76(361):e315–e369. doi: 10.1016/j.wneu.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Levy ML, Wieder BH, Schneider J, Zee CS, Weiss MH. Subdural empyema of the cervical spine: clinicopathological correlates and magnetic resonance imaging. Report of three cases. J Neurosurg. 1994;81:160. [PubMed] [Google Scholar]
- 8.Lownie SP, Ferguson GG. Spinal subdural empyema complicating cervical discography. Spine (Phila Pa 1976) 1989;14:1415–1417. doi: 10.1097/00007632-198912000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Nadkarni T, Shah A, Kansal R, Goel A. An intradural–extramedullary gas-forming spinal abscess in a patient with diabetes mellitus. J Clin Neurosci. 2010;17:263–265. doi: 10.1016/j.jocn.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Sorar M, Er U, Seckin H, Ozturk MH, Bavbek M. Spinal subdural abscess: a rare cause of low back pain. J Clin Neurosci. 2008;15:292–294. doi: 10.1016/j.jocn.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Thome C, Krauss JK, Zevgaridis D, Schmiedek P. Pyogenic abscess of the filum terminale Case report . J Neurosurg. 2001;95:100–104. doi: 10.3171/spi.2001.95.1.0100. [DOI] [PubMed] [Google Scholar]
- 12.Velissaris D, Aretha D, Fligou F, Filos KS. Spinal Subdural Staphylococcus Aureus Abscess: case report and review of the literature. World J Emerg Surg. 2009;4:31. doi: 10.1186/1749-7922-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vural M, Arslantas A, Adapinar B, et al. Spinal subdural Staphylococcus aureus abscess: case report and review of the literature. Acta Neurol Scand. 2005;112:343–346. doi: 10.1111/j.1600-0404.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- 14.Zemmoura I, Hamlat A, Morandi X. Intradural extramedullary spinal inflammatory myofibroblastic tumor: case report and literature review. Eur Spine J. 2011;20(Suppl 2):S330–S335. doi: 10.1007/s00586-011-1783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]