Abstract
Background
Spinal melanocytoma is one of the most infrequent space-occupying lesions of the central nervous system. To the best of our knowledge, this is the first report of primary bifocal intradural melanocytoma of heterogenous pathological grade to date.
Case description
We report the case of a 43-year old patient with primary bifocal melanocytoma, clinically and radiologically resembling benign schwannoma. The patient presented with myeloradiculopathy of the left C3 dermatome. Magnetic resonance imaging of the upper spine revealed two space-occupying lesions with paraspinal extension, initially diagnosed as neurofibroma. Definitive histopathological classification of both lesions was melanocytoma. Both tumours were only partially removed due to adherence to surrounding structures. The patient underwent stereotactic external beam irradiation (EBR). Follow-up at 1 year after surgery revealed no recurrence and the patient remained free of symptoms. The clinical, radiological and pathological features of this rare tumour entity are presented and the available literature is reviewed.
Conclusions
Intradural melanocytoma, although exceedingly rare, requires a thorough work-up to exclude malignant melanoma. With only two previous reports of multifocal melanocytoma published in the literature, standard therapy has not yet been established and complete surgical removal remains the modality of choice. Patients should be closely monitored to detect local recurrence or malignant degeneration. EBR may be considered in cases where total excision is not achievable and reduces risk of local recurrences.
Keywords: Melanocytoma, Melanoma, Cervical spine, Tumour
Introduction
Pigmented tumours are among the most infrequent space occupying lesions of the central nervous system (CNS). These neoplasms are mostly found in the posterior fossa [17], although other locations are recognized. One-third occurs in the superior spinal cord [6]. Melanocytomas (MC) are slow-growing tumours that cause symptoms like myeloradiculopathy by compression of adjacent structures [12]. Malignant progression with infiltrative growth and even metastatic seeding is not uncommon [8, 19, 22]. We report the case of a patient with bifocal MC, clinically and radiologically resembling benign schwannoma. To the best of our knowledge, this is the first report detailing two MC of different pathological grades in a single patient, and the third report of multifocal MC.
Case report
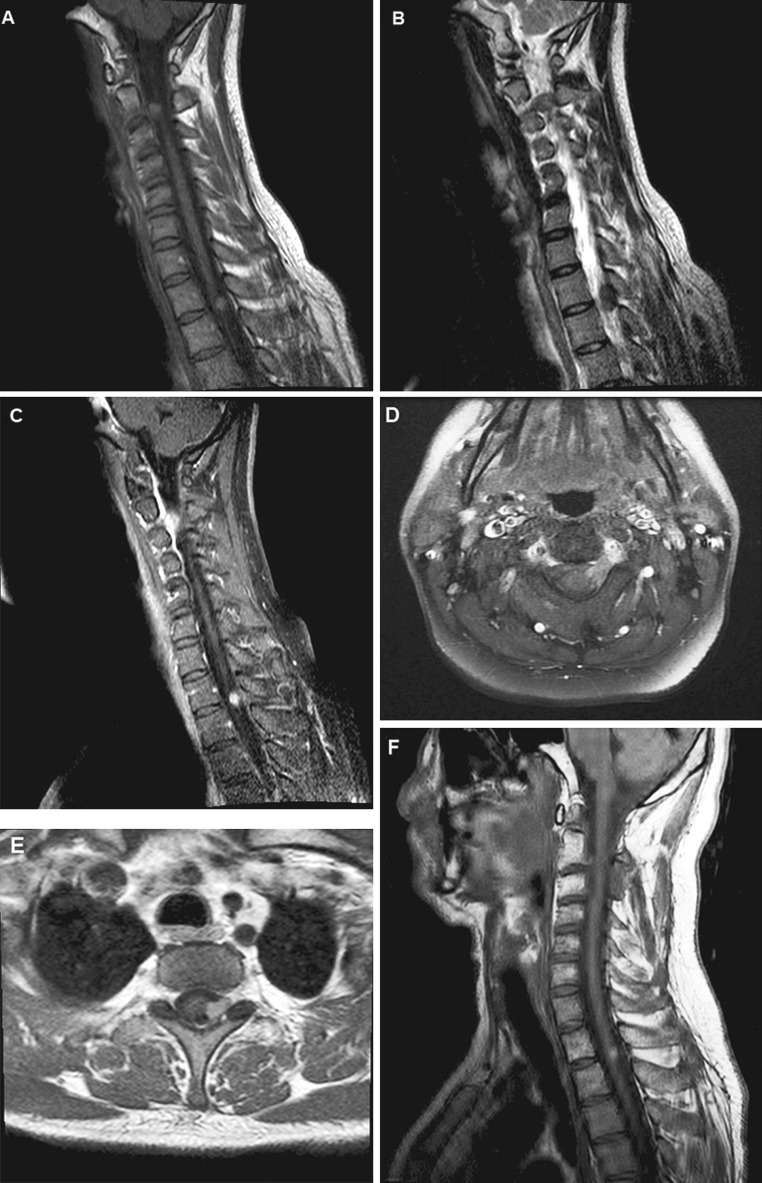
A 43-year-old non-smoker with no significant past medical history presented to a regional medical center in December 2010, with a 4-month history of left neck and shoulder pain and occasional paraesthesias of the left hand. He also complained of constant, left-sided cephalalgia radiating to the jaw. Spinal magnetic resonance imaging (MRI) revealed two space-occupying lesions in segments C2-3 and Th1-2, respectively (Fig. 1). Both tumours, which extended from the neural foramen into the dural cavity, demonstrated homogenous contrast-enhancement (Fig. 1c–e). The lesions were hyperintense on T1 (Fig. 1a) and hypointense on T2-weighted sequences (Fig. 1b), consistent with benign schwannoma.
Fig. 1.
Magnetic resonance imaging. Sagittal MRI of the cervical/upper thoracic spine reveals two intradural, extra-axial lesions, extending through the neuroforamen of C2-C3 (a, b, d) and T1-T2 (a, b, e). Both enhance homogenously after gadolinium administration (d, e), the cervical lesion shows diffuse leptomeningeal enhancement (c). No progression is seen 12 months after initial surgery and external beam irradiation (f)
T1–T2 decompression was performed, exposing a darkly pigmented tumour, extending from the neural foramen into the intradural compartment. The tumour was subtotally removed and classified as a melanotic schwannoma. The patient was then referred to our university hospital due to uncertain tumour grade and persistent symptoms.
Physical examination was significant for hypaesthesia in the left C3 dermatome, but was otherwise unremarkable. Dermatological and ophthalmological work-up failed to show evidence of cutaneous melanoma or Carney’s syndrome. Left C2–C3 hemilaminectomy with open exploration of the neural foramen was performed. Intraoperatively, a bulky, paraspinal tumour was found, extending from the epidural space of the C2-neural foramen into the dural sac, dura and nerve rootlets (Fig. 2). Diffuse leptomeningeal hyperpigmentation and dural thickening were present. The tumour was adherent to adjacent structures and incorporated multiple nerve rootlets. The lack of a clear cleavage plane as well as profound bleeding rendered GTR unachievable and the tumour was debulked to the greatest possible extent. Frozen section examination of the obtained tissue samples identified a melanotic, blue-cell tumour. The operation was aborted at this time to reduce the risk of neurological deficits and due to uncertainty regarding the tumour entity as well as suspected meningeal seeding.
Fig. 2.
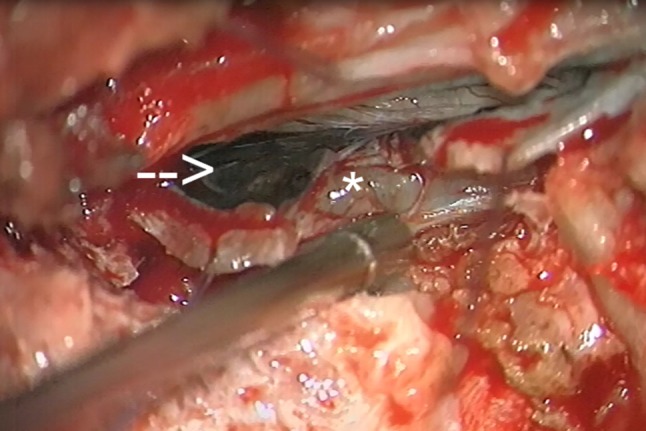
Intraoperative photography. Intraoperative photographs demonstrating the cervical lesion in the C2-3 segment as an intradural mass (arrow) with extradural extension (asterisk). Note the diffuse meningeal hyperpigmentation overlying the tumour
The patient experienced an uncomplicated postoperative recovery with significant pain reduction. To exclude metastatic seeding, cranial MRI and whole-body positron-emission computed tomography (CT) were performed, revealing no other lesions. The patient subsequently underwent stereotactic external beam irradiation (EBR) to both tumour locations. The target volumes consisted of residual tumour as visualized in MRI and planning CT (cervical volume 1.79 cm3/thoracic volume 1.49 cm3). The cervical target volume was extended to include areas of meningeal gadolinium enhancement. A total dose of 14 Gray was applied to each location. Follow-up MRI 18 months after EBR demonstrated no evidence of tumour progression (Fig. 1f). Neurological examination was normal and the patient has remained free of symptoms.
Histopathology
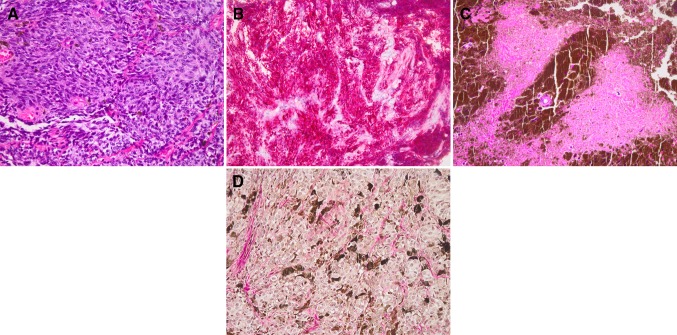
The biopsy specimen from the cervical lesion consisted of small tumour fragments composed of variably pigmented, monomorphic spindle cells, organized into tight nests (Fig. 3a). The nuclei were small and oval-shaped with occasional grooves. Mitotic figures and necrosis were absent, with a very low proliferation index (Mib1 1 %). S100-protein staining was positive in both nuclei and cytoplasm. Immunohistochemical preparations demonstrated diffuse cytoplasmic labelling with Pan-melanoma, a highly sensitive melanoma cocktail (HMB-45, Melan-A and tyrosinase antibodies, Fig. 3b).
Fig. 3.
Histopathology. Haematoxylin–eosin (HE) staining of the cervical lesion displays variably pigmented, tightly packed tumour cells in a nested growth pattern. Cells possess oval to bean-shaped nuclei, with occasional grooves (a). Pan-melanoma immuno-histochemistry preparation (b) demonstrates diffuse cytoplasmatic labeling. HE-stained sections from the thoracic biopsy reveal monomorphic tumor cells surrounded by densely pigmented cells and extracellular melanin. Note the small focus of necrosis (c). Gomori reticulin stain fails to demonstrate reticulin fibers surrounding individual tumor cells (d)
Samples from the thoracic tumour site displayed a nested growth pattern and were composed of tightly packed, spindle cells (Fig. 3c). Cells were variably pigmented and had oval to bean-shaped large nuclei with grooves. Occasionally, prominent nucleoli were seen. Similar to the previous specimen, the tumour cells immunolabeled with S100-protein and Pan-melanoma. The Mib1-index was low with a rate 3 %. Mitotic figures were not identified, but necrotic foci were seen (Fig. 3c). The lack of reticulin fibres between tumour cells excluded the initial diagnosis of pigmented Schwannoma (Fig. 3d). Due to the presence of necrosis the thoracic tumor was considered to represent an intermediate grade rather than low-grade MC as seen in the cervical tumour.
Both tumour samples were subsequently analysed for BRAF mutations, a proto-oncogene frequently found in melanoma [10]. Analysis was negative in both samples; however, the thoracic sample demonstrated beta-actin amplification in the t(12;22)-locus.
Discussion
Intradural MC is an exceedingly rare variant within the spectrum of melanocytic tumours of the CNS. Developing from leptomeningeal melanocytes, MC are considered to represent the benign variant in a continuum of pigmented CNS tumours, followed by intermediate grade MC, and malignant melanoma (MM) [1]. Cervical spinal cord involvement in MC is rare [17] and the majority of MC are found in the posterior fossa; however, other localizations are recognized [6, 7, 13, 16, 18]. Spinal MC are most often reported in the extramedullary space, with only 23 intradural lesions reported in the literature. Most cases are found in women in their fourth decade of life and are discovered after a long-standing history of progressive myeloradiculopathy [17].
It should be emphasized that distinguishing MC from melanoma can be especially challenging when sampling of the tumour is limited [23]. For the most part, low-grade MCs lack mitotic activity, cytologic atypia, necrosis and neural parenchymal invasion [12]. The intermediate grade category was introduced for melanocytic tumours with bland cytologic features that manifest signs of malignancy [3]. The initial diagnosis of melanotic schwannoma was excluded by the lack of reticulin fibers surrounding individual tumour cells [2, 15]. MC feature variable degrees of melanisation, ranging from gray to charcoal-black tumours [8]. Immunohistochemical staining is positive for HMB-45, Melan-A and S100, consistent with a melanocytic origin. To differentiate this entity from other pigmented lesions such as melanotic meningioma and schwannoma, EMA and reticulin may be used, respectively. B-Raf mutation analysis may be helpful in identifying a proto-oncogenic driver mutation, often found in MM [10].
Intraoperatively, both tumours could not be dissected from the surrounding structures, raising suspicion of infiltrative growth. On microscopy, the thoracic biopsy revealed evidence of malignancy, especially necrosis (Fig. 3c). Accordingly, some authors consider infiltration and necrosis as de facto evidence of an intermediate grade MC [1, 5]. Since the cervical tumour lacked distinct anaplastic features, it was classified as benign, low-grade MC. Samples from the thoracic lesion revealed necrosis and a higher MIB-1. Co-existence of these features prompted the classification of this tumour as an intermediate-grade MC [1, 3, 19].
It remains controversial whether multifocal MC are due to meningeal seeding [7] or form a distinct variant as proposed by Ali et al. [1]. Primarymultifocal MC are believed to be uniform in pathology [1]; however this was not the case in our patient. There is growing evidence in the literature that benign low-grade MC may degenerate into more aggressive entities [1], challenging the initial definition by Brat et al. [3]. It is therefore likely that our case resembles a primary multifocal MC of heterogeneous pathological grade, potentially in a very early stage of malignant transformation [19].
Diffuse leptomeningeal hyper-pigmentation and contrast-enhancement seen in the cervical tumour (Figs. 1c, 2a) may reflect melanocytic invasion, commonly seen in neurocutaneous melanosis [1]. This diagnosis was ruled out in our case due to absence of hydrocephalus and cutaneous lesions [12]. Leptomeningeal seeding is a tentative explanation of a bifocal manifestation, but only occurs late in the course of MC following malignant transformation [7, 21]. Given the short time course from onset of symptoms to histopathological diagnosis and lack of anaplastic features in the cervical lesion, metastatic seeding seems unlikely. A high-grade, seeding MC would be expected cranial to a metastasis. However, the intermediate-grade tumour was located in the thoracic spine, virtually excluding drop metastasis, favouring a primary multifocal origin.
At present, there is no established consensus regarding the radiological features of MC. In general, T1 iso- to hyperintensity and iso- to hypointensity on T2-weighted sequences are reported with homogeneous enhancement following gadolinium administration [2, 9, 18]. However, imaging characteristics are not specific due to varying degrees of melanisation and necrosis, which may affect the MRI signal [13]. Differential considerations include various spinal neoplasms like meningioma, schwannoma or melanoma [2, 12, 15, 23].
Neurosurgical management of MC ideally consists of gross total resection (GTR), which is associated with increased long-term survival and significantly lower recurrence rates [11, 12, 24]. However, GTR is limited when surrounding structures are infiltrated. In those cases, high-dose radiotherapy or radiosurgery are the therapeutic modalities of choice [4, 14, 20]. All patients require close follow-up with serial MRI and clinical examination as local recurrence rates are as high as 50 % [23].
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or findings specified in this paper.
Abbreviations
- CNS
Central nervous system
- MC
Melanocytoma
- MRI
Magnetic resonance imaging
- EBR
External beam irradiation
- GTR
Gross total resection
- CT
Computed tomography
- HE
Haematoxylin–eosin
References
- 1.Ali Y, Rahme R, Moussa R, Abadjian G, Menassa-Moussa L, Samaha E. Multifocal meningeal melanocytoma: a new pathological entity or the result of leptomeningeal seeding? J Neurosurg. 2009;111(3):488–491. doi: 10.3171/2009.3.JNS081096. [DOI] [PubMed] [Google Scholar]
- 2.Azarpira N, Torabineghad S, Sepidbakht S, Rakei M, Bagheri MH. Cytologic findings in pigmented melanotic schwannoma: a case report. Acta Cytol. 2009;53(1):113–115. doi: 10.1159/000325096. [DOI] [PubMed] [Google Scholar]
- 3.Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol. 1999;23(7):745–754. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Classen J, Hehr T, Paulus W, Plate K, Bamberg M. Suprasellar melanocytoma: a case of primary radiotherapy and review of the literature. J Neurooncol. 2002;58(1):39–46. doi: 10.1023/A:1015872207398. [DOI] [PubMed] [Google Scholar]
- 5.El-Khashab M, Koral K, Bowers DC, Johnson-Welch S, Swift D, Nejat F. Intermediate grade meningeal melanocytoma of cervical spine. Child’s Nerv Syst. 2009;25:407–410. doi: 10.1007/s00381-008-0782-6. [DOI] [PubMed] [Google Scholar]
- 6.Eskandari R, Schmidt MH. Intramedullary spinal melanocytoma. Rare Tumors. 2010;2(2):e24. doi: 10.4081/rt.2010.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franken SPG, Setz-Pels W, Smink-Bol M, et al. Unusual case of bifocal leptomeningeal melanocytoma in the posterior fossa with seeding in the spinal canal. Br J Radiol. 2009;82(981):e182–e188. doi: 10.1259/bjr/30756805. [DOI] [PubMed] [Google Scholar]
- 8.Gempt J, Buchmann N, Grams AE, et al. Black brain: transformation of a melanocytoma with diffuse melanocytosis into a primary cerebral melanoma. J Neurooncol. 2010;102:323–328. doi: 10.1007/s11060-010-0311-9. [DOI] [PubMed] [Google Scholar]
- 9.Goyal A, Sinha S, Singh AK, Tatke M, Kansal A. Lumbar spinal meningeal melanocytoma of the l3 nerve root with paraspinal extension: a case report. Spine. 2003;28(7):E140–E142. doi: 10.1097/01.BRS.0000051879.20360.8A. [DOI] [PubMed] [Google Scholar]
- 10.Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn EM, Nakaji P, Coons SW, Dickman CA. Surgical treatment for intramedullary spinal cord melanocytomas. JNS Spine. 2008;9:48–54. doi: 10.3171/SPI/2008/9/7/048. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal S, Tungria A, Srivastava A, Vij M, Jaiswal A, Behari S. Primary melanocytic tumors of the central nervous system: a neuroradiological and clinicopathological study of five cases and brief review of literature. Neurol India. 2011;59:413. doi: 10.4103/0028-3886.82758. [DOI] [PubMed] [Google Scholar]
- 13.Karikari IO, Powers CJ, Bagley CA, Cummings TJ, Radhakrishnan S, Friedman AH. Primary intramedullary melanocytoma of the spinal cord. Neurosurgery. 2009;64:E777–E778. doi: 10.1227/01.NEU.0000341516.22126.AA. [DOI] [PubMed] [Google Scholar]
- 14.Kurita H, Segawa H, Shin M, et al. Radiosurgery of meningeal melanocytoma. J Neurooncol. 2000;46(1):57–61. doi: 10.1023/A:1006335616839. [DOI] [PubMed] [Google Scholar]
- 15.Liubinas SV, Maartens N, Drummond KJ. Primary melanocytic neoplasms of the central nervous system. J Clin Neurosci. 2010;17(10):1227–1232. doi: 10.1016/j.jocn.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Merciadri P, Secci F, Sbaffi P, Zona G. Multifocal meningeal melanocytoma of the conus medullaris. Acta Neurochir. 2011;153(11):2283–2285. doi: 10.1007/s00701-011-1143-x. [DOI] [PubMed] [Google Scholar]
- 17.Muthappan M, Muthu T, Hussain Z, Lamont D, Balakrishnan V. Cervical intramedullary melanocytoma: a case report and review of literature. J Clin Neurosci. 2012;19(10):1450–1453. doi: 10.1016/j.jocn.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Painter TJ, Chaljub G, Sethi R, Singh H, Gelman B. Intracranial and intraspinal meningeal melanocytosis. AJNR Am J Neuroradiol. 2000;21(7):1349–1353. [PMC free article] [PubMed] [Google Scholar]
- 19.Perrini P, Caniglia M, Pieroni M, Castagna M, Parenti GF. Malignant transformation of intramedullary melanocytoma. Neurosurgery. 2010;67:E867–E869. doi: 10.1227/01.NEU.0000372919.96651.34. [DOI] [PubMed] [Google Scholar]
- 20.Rades D, Schild SE. Dose–response relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: a review of the literature. J Neurooncol. 2005;77:311–314. doi: 10.1007/s11060-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 21.Roser F, Nakamura M, Brandis A, Hans V, Vorkapic P, Samii M. Transition from meningeal melanocytoma to primary cerebral melanoma. JNS. 2004;101(3):528–531. doi: 10.3171/jns.2004.101.3.0528. [DOI] [PubMed] [Google Scholar]
- 22.Scheithauer BW, Erdogan S, Rodriguez FJ, et al. Malignant peripheral nerve sheath tumors of cranial nerves and intracranial contents. Am J Surg Pathol. 2009;33:325–338. doi: 10.1097/PAS.0b013e31818d6470. [DOI] [PubMed] [Google Scholar]
- 23.Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: radiologic-pathologic correlation. Radiographics. 2009;29(5):1503–1524. doi: 10.1148/rg.295095109. [DOI] [PubMed] [Google Scholar]
- 24.Turhan T, Oner K, Yurtseven T, Akalin T, Ovul I (2004) Spinal meningeal melanocytoma. Report of two cases and review of the literature. J Neurosurg 100(3 Suppl Spine):287–290 [PubMed]