Abstract
Purpose
In this article, we review the English literature of calcified pseudomeningoceles in the lumbar region.
Methods
A systematic review using the Medline Database using the varied nomenclature for pseudomeningoceles, as well as reviewing the reference lists of relevant article found.
Results
We discuss the different pathological theories on formation of a pseudomeningocele, the formation of a calcified wall and the optimal management for this entity. To date, 17 cases have been described, of which 13 are reviewed here. Calcification of pseudomeningocele is a rare entity and in the lumbar spine this occurs postsurgically. The only predisposing factor is prior surgery to the lumbar spine. Computer tomography, magnetic resonance imaging (MRI) and MRI myelography in combination are the preoperative investigations of choice. The radiological work-up can be preoperatively diagnostic and is important in the surgical planning.
Conclusions
The treatment is surgicel removal and the decision to treat is based on patient symptoms and correlating these with imaging. There is an average reported follow-up of 1.7 years postoperatively for these patients and the reported outcome after surgery is good.
Keywords: Calcification, Postsurgical, Pseudomeningocele, Lumbar spine
Introduction
Calcification of a pseudomeningocele was first described by Verbiest in 1951 [16]. The first article on the subject was published in 1963 [13]. We found 17 cases described to date by means of a comprehensive computer-based literature search in the PubMed database using the varied nomenclature for calcified pseudomeningoceles as well as reviewing the reference lists of relevant articles [1, 3, 5–7, 9–20]. Four of these cases are not reviewed in this article, two of which are in the cervical region, one of which is in Japanese language and one case only known through oral presentation.
The incidence of pseudomeningocele after spinal surgery is reported to be between 0.068 and 2 % [8]. For complicated cases, the incidence is higher and has been reported to be as high as 43 % in surgery for tethered cord [4]. Some authors speculate that both pseudomeningocele and the calcification of such an entity may go undiagnosed for the lack of symptoms [10, 14, 17, 18].
We aim to determine the optimal management of ossified pseudomeningoceles from the evidence of outcome as well as review the pathological theories on the subject.
Case
A 48-year-old lady presented with progressing symptoms of neurogenic claudication over 1 year. Her leg pain developed after walking a few yards, making it very disabling for this previously self-sufficient woman. The strength of the legs was MRC scale 4 bilaterally and she walked with aid of a walking stick. She also had urge incontinence. She had undergone a 4th lumbar vertebra (L4) laminectomy and excision of a benign lesion arising from the right 4th lumbar nerve root 18 years previously. The surgery was uneventful and brought about good symptom relief. She had also been diagnosed with non-Hodgkin’s lymphoma after a neck node biopsy 22 years prior, following which she underwent splenectomy and radiotherapy. She has been in remission since completion of treatment. Correlating the CT Scan, MRI and MR myelogram findings, the final diagnosis was neurogenic claudication secondary to an ossified lumbar pseudomeningocele and diagnosis was confirmed during surgery. Follow-up at 6 months and 1 year after surgery showed good symptomatic relief.
Pathology
Regarding the formation of spinal pseudomeningocele there have been several opinions and reflecting this is the inconsistent nomenclature such as meningeal pseudocyst, extradural cysts, arachnoid cysts, lumbar cysts, spurious meningocele, acquired meningocele, iatrogenic meningocele, arachnoid diverticulum, false cyst and pseudomeningocele [4, 10, 17]. Traditionally pseudomeningoceles are divided into two groups namely congenital (non-traumatic) and traumatic pseudomeningocele [13].
Traumatic pseudomeningocele are believed to form as a result of blunt trauma, inadvertent trauma or by planned dural opening for treatment purpose, excessive traction on nerve roots, infection and intramural haemorrhage [3, 10, 13]. As proposed by Shifrin et al., a tear in the dural layer may or may not involve the underlying arachnoid layer. When a dural tear is present without tear of the arachnoid it can herniate forming a meningocele with arachnoid lining, whereas if both layers are torn, the presence of cerebrospinal fluid (CSF) can form into a pseudomeningocele [17]. Histological findings differ, but if the two theories on cyst formation by Shifrin et al. are accepted, findings of pseudomeningoceles either with or without a true meningeal lining can be explained. In this review, only five cases have been reported with histological findings [10, 14, 17–19]. None of which demonstrated arachnoidal lining histologically. One case is, however, reported as formed by arachnoid herniation, due to the histological findings of three layers. The inner layer being smooth sparse connective tissue simulating the arachnoid and this finding was correlated with intraoperative findings of an intact arachnoid layer at the time of the first operation [19]. This should then be labelled a meningocele, not a pseudomeningocele.
To form a pseudomeningocele one can argue there must also be other factors that contribute, as a tear in both dural and arachnoid layers happens with a much higher frequency than that of pseudomeningoceles [10]. No article has yet eluded the reason for this, but there have been several hypotheses. One proposal is that under normal circumstances the resorptive capacity of the surrounding soft tissue usually prevents a pseudomeningocele from forming [10, 17]. It has been suggested that as a contributing factor the communication between the pseudomeningocele and the subdural space may act as a one-way flap valve, either by scar tissue or nerve elements, directing CSF towards the pseudomeningocele [10]. Other contributing factors in maintaining the amount of liquid to form pseudomeningoceles may be osmosis, the hydrostatic pressure of standing, the pulsatile nature of spinal fluid dynamics, dead space after surgery, nutritional deficits, steroids, infection and radiation [4, 10, 18]. The collection of CSF is eventually walled off presumably by a connective tissue reaction and protein precipitation developing into a non-absorbing membrane [16, 17].
The formation of a calcified wall of the pseudomeningocele is also a topic of discussion. Rosenblum and DeRow suggested that length of time is of importance, with the statement “extradural cyst was present long enough for the wall to ossify and calcify” [13]. The time from surgery to discovery of an ossified pseudomeningocele is on average 10.3 years, with a range from 6 months to 22 years (Table 1). One theory holds that calcification occurs by metaplasia of surrounding soft tissue to cartilage, which then undergoes ossification [18]. Some authors propose that presence of blood might be crucial, leading to an inflammatory response and eventually calcification [10, 17]. An indirect support to this theory is that some authors analyzed the cystic content of the pseudomeningocele and reported positive Nonne–Froin test and Pandy test [18, 19]. These tests are usually done to investigate the presence of elevated globulin and albumin in CSF, but in the reported cases the pseudomeningocele fluid was analyzed, the authors implying that this is representative of the CSF as a connection with the subarachnoid space was confirmed for both. Although there might be several causes to these tests being positive, one of them is previous haemorrhage [2]. Dysregulation of soluble factors released from the dura mater, and avulsion of a piece of periosteum into the adjacent soft tissue are other suggested causes to calcification, although these last two proposals are only mentioned in regards to ossified pseudomeningoceles in the cervical spine [12]. In the case presented by Saito et al. the ossified lesion was not communicating with the subdural space, but walled off completely. This is explained by the possibility of local adhesive lesions developing from the arachnoid membrane leading to a gradual reduction in spinal fluid efflux and eventually closure of the aperture [14].
Table 1.
A summary presentation of the reviewed cases
| Case | Sex/age | Prior surgery | Preoperative symptoms | Years after surgery | Described treatment of pseudomeningocele | Outcome | Follow-up |
|---|---|---|---|---|---|---|---|
| Rosenblum and DeRow [13] | F/49 | Herniated disc | Intermittent foot drop and pain, exacerbated | 22 years | Removal of the cystic area | N/A | N/A |
| Nash et al. [10] | F/46 | Bilateral exploration of L3,L4;L4,L5; L5,S1 interspaces | Recurrence of LBP | 6 months | Removal of dural sac after longitudinal incision thereafter dural reapproximation | Improved | N/A |
| Carollo et al. [3] | F/38 | Herniated disc | Recurrence of bilateral pain at S1-S5 | 1 year 10 months | Radical removal of calcified extradural cyst. A small fistula was accurately closed | Free of pain | 10 months |
| Schumacher et al. [15] | M/43 | Herniated disc | Recurrent pain at L4 right side | 4 years 1 month | Total resection of pseudomeningocele and closure of fistula | Free of symptoms | N/A |
| Shifrin et al. [17] | F/60 | LBP surgery | Progressive LBP and bilateral leg pain. Reduced walking to 20 m distance due to paresthesia and weakness | 8 years | Complete laminectomies, partial facetectomies and root decompression from L2 to sacrum. Cyst removal and closure of fistula by a purse-string suture and sealed with a free fat graft sutured over the repair. A large fat graft was placed on the dura and securely closing of overlying muscles | Able to walk unlimited distances with minimal discomfort | 1 year |
| Tsuji et al. [19] | M/60 | Herniated disc | Reappearance of pain in lower back and right leg | 6 years 6 months | Pseudocyst removal, recurrent extruded disc material compressing the right S1 nerve removed followed by autologous free fat grafting | Occasional slight pain in left leg | 6 years |
| Shimazaki et al. [18] | M/68 | Spinal canal stenosis | Recurrence of LBP and intermittent claudication. Back pain when in supine position | 10 years 8 months | Dorsomedial incision extending from L3 to S1 with removal of pseudomeningocele. To enter the cyst air drill was used. Artificial dura applied to defect | Free of symptoms | 1 year |
| Lee et al. [9] | M/69 | Laminectomy and discectomy for leg pain | Increasing back and left leg pain over 10 months | 11 years | The pseudomeningocele was entered with a rongeur and removed. A partial laminectomy from L5 to L3 and at L5/S1 an extruded disc was removed. Dural defect was sutured interrupted | Free of symptoms | 11 months |
| Saito et al. [14] | M/45 | Herniated disc | Gradually worsening pain in left leg, eventually difficulties standing and walking | 19 years | Removal of ossified cyst entered with a chisel. Detached from dura without any dural defect noted. A medial facetectomy was also performed and nerve roots confirmed intact | Free of pain | 1 year |
| Ishaque et al. [7] | M/70 | Degenerative Spondylolisthesis | Sudden severe back and leg pain and leg weakness. Progression to bilateral L5 weakness and bilateral sciatica | 5 years | Removal of pseudomeningocele with micro dissection technique around the nerve roots. L4 and L5 nerve roots decompressed completely. A dural patch of fat, Tisseel and surgicel was used to close the dural defect. Lumbar fixation was also performed | Immediate pain reduction | N/A |
| Ishaque et al. [7] | M/45 | LBP and Sciatica | Episode of sciatica | 19 years | The communication between dura and the pseudomeningocele was repaired with fat, Tisseel and Surgicel. A lumbar CSF drain was placed for 5 days | N/A | N/A |
| Al-Edrus et al. [1] | F/48 | Removal of benign lumbar nerve root lesion | Progressive neurogenic claudication over 1 year. Minimal weakness and urge incontinence | 18 years | Careful dissection of the pseudomeningocele after decompressing it with a puncture. The dural defect was plugged with fat and tissue glue applied (personal communication) | Significant improvement | N/A (1 year) |
| Youssef et al. [20] | F/41 | Herniated disc | Diffuse back pain | 11 years | No surgery performed | Diffuse back pain unchanged | 23 years |
Diagnosis
To correctly diagnose these patients preoperatively, both CT and MRI are important as recently described by Al-Edrus et al. [1]. If both modalities are used, one is less likely to misinterpret this very rare entity. The MRI would define the cystic collection and it is relation to the surrounding neural structures as well as degree of neural compression if present. The correlation of the MRI findings to the clinical picture is paramount in deciding the need for surgical decompression of such cases. The MRI myelography is a rather good technique to illustrate the exact location of communication between the thecal sac and the pseudomeningocele (Figs. 1, 2). The CT scans aid in confirming the calcification of the wall of the pseudomeningocele as well as the integrity of the vertebra, particularly the pedicle (Fig. 3). The expansion of the pseudomeningocele can cause erosion of the vertebral structure [4]. If such compromise in integrity of the spine is demonstrated, the need for instrumentation and fusion may need to be considered to prevent progressive deformity of the lumbar spine.
Fig. 1.
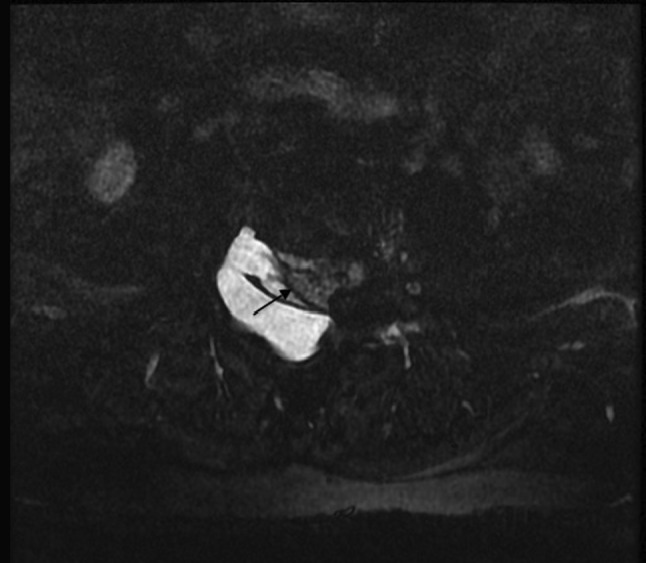
MR myelogram in axial plane showing the intact thecal sac indicated by the arrow and the pseudomeningocele
Fig. 2.
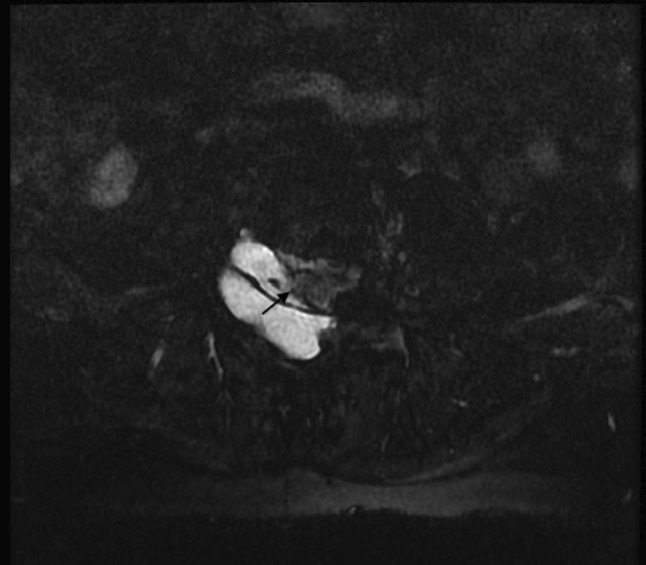
MR myelogram in axial plane showing the focal deficit in the thecal sac. The arrow indicates the site of communication between the subarachnoid space and the pseudomeningocele
Fig. 3.
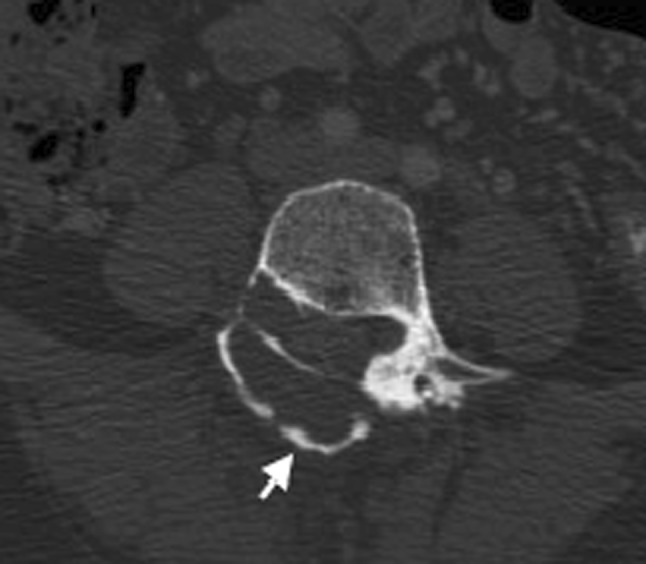
CT in axial plane in bone window with the calcified wall of the pseudomeningocele indicated by the arrow and a thinned out right pedicle
Treatment
Of the 13 cases reviewed in this article only one case was not operated due to the lack of symptoms from the ossified pseudocyst [20]. All other cases were operated (92 %) with removal of pseudomeningocele and closure of the abnormal communication. The described treatments of the pseudomeningoceles are reproduced in Table 1. The descriptions of the surgical removal are varied and somewhat sparse, but it appears that the preferred method for surgical removal of a calcified pseudomeningocele is to primarily decompress the calcified wall to inspect the cavity. In the case of any herniating nerve roots these are observed at an early stage during surgery and inadvertent injury to nerve structures is minimized. This is followed by careful dissection of the pseudomeningocele off the thecal sac so as not to create any additional punctuation to the dura (Figs. 4, 5). The choice to close the abnormal CSF communication if present ranges from using autologue free fat grafts, direct suturing and/or artificial dura. In addition, surgicel and tissue glue has been used. One of the cases reported also added a lumbar CSF drain for 5 days after the removal of the pseudomeningocele and closure of the dural defect [7].
Fig. 4.
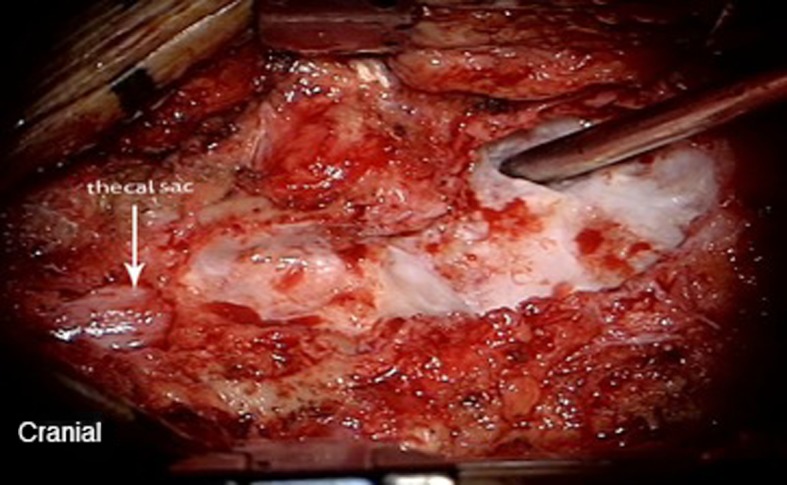
Intraoperative image of the anterior wall of the calcified pseudomeningocoele after the posterior wall has been decapped
Fig. 5.
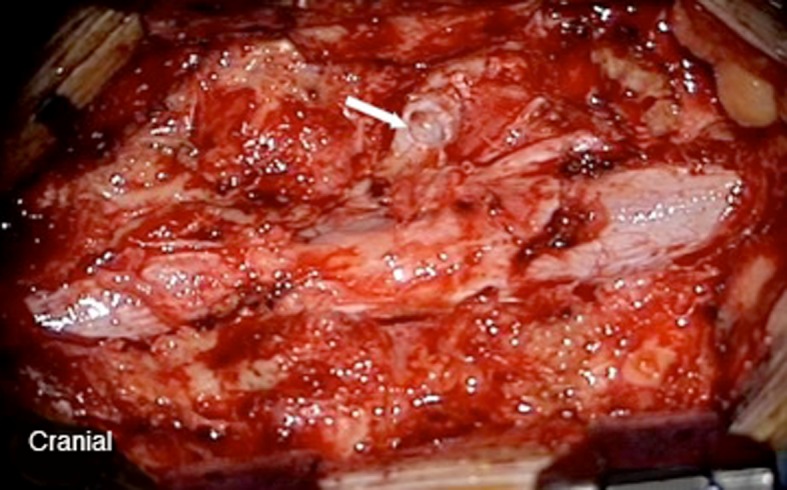
Intraoperative image of the connection between the calcified pseudomeningocele and the thecal sac (white arrow) after the wall of the pseudomeningocele is removed. It is in close proximity to the sleeve of the exiting nerve root
Discussion
The reported male:female ratio is 1.16:1 and the mean age at the point of diagnosing the calcified lumbar pseudomeningocele is 52.5 years, ranging from 38 to 70 years (Table 1). There are no cases reported located in the thoracic part of the spine, but two in the cervical region. These two differ substantially from the lumbar spine in regards to etiology, age onset, development and treatment. All cases reviewed here have had prior surgery to the lumbar spine. Detection of calcified lumbar pseudomeningocele happens at an average of 10.5 years after prior surgery, ranging from 6 months to 22 years. In the case where calcification was found after only 6 months, it was merely focal areas of calcification. This underlines that the calcification takes time to form.
Clinically the symptoms range from low back pain, sciatica to incontinence and myelopathy. There are reports of both sudden onset of symptoms and more progressive onset. There is too little information as to draw conclusions on predisposing factors to formation of calcified pseudomeningocele; however, most of the patients had surgery for lumbar disc disease (Table 1). There was one case of degenerative spondylolisthesis and benign lumbar nerve root tumour each. No information on previous corticosteroid injections is provided, although such injections are known for inducing calcification [8]. Very few of the reviewed articles mention prior medical history of their patients. The fact that all patients had prior surgery is perhaps the most important predisposing factor and not the condition itself that resulted in surgery.
The decision to surgically decompress the calcified lumbar pseudomeningocele is based on symptoms. 11 patients (85 %) had radiculopathy as the main symptom, with some having low back pain concurrently. One case was successfully managed conservatively and had only diffuse low back pain. However, Nash et al. described surgical decompression of a patient with low back pain without radiculopathy with good symptom relief. We believe the clear indication for surgical decompression in a patient with calcified lumbar pseudomeningocele is radiculopathy that correlates with the location of compression on imaging. Based on the review, we can infer that if one chooses to operate, a complete resection of the pseudomeningocele and meticulous closure of the dural defect should be made, and there seems to be no need for CSF diversion after surgery as this is also associated with certain risks [4]. As the dural defect at the ostium in most instances is calcified, the ostium is plugged with fat and sealed with tissue glue. However, if the calcified portion is removed and soft dural lining is visible then suture repair is possible.
The average reported follow-up after surgery is 1.7 years ranging from 10 months to 6 years, all with good surgical outcome. However, only seven articles include follow-up (including the addition of our case), so one must take this lack of reports on long-term outcome into account for these patients. The single patient who was not operated remained stable with regard to symptoms for 23 years [20]. It could, therefore, be postulated that a calcified pseudomeningocele on its own probably does not seem to evolve to a larger cyst over time and cause neural compression. However, if the initial pseudomeningocele has already been in close proximity with neural structures, as ossification and calcification of the wall sets in, it can cause compression of the neural elements.
Conclusion
The incidence of calcified pseudomeningocele of the lumbar spine is rare. Only 15 cases have been reported to date although it is unlikely this represent the true frequency. It occurs after lumbar spinal surgery, mainly after disc surgery. The exact reason for the formation of calcification of the wall remains unclear. It is detected at an average of 10.3 years after surgery. The symptoms related to calcified pseudomeningocele is mainly low back pain and radiculopathy. The investigation of choice prior to surgery is MRI spine, CT spine and MRI myelography in combination. The indication to surgically decompress is primarily radiculopathy. The surgery advocated would be to decap the calcified pseudomeningocele to ensure there are no nerve roots within it, decompress the remaining wall from the surrounding thecal sac and nerve roots, identify the ostium and plug it with fat and seal with tissue glue. In the instance of unossified ostium it can be closed with sutures. Postoperative external lumbar drain is not routinely required. Good symptom relief has been recorded for all the cases that have been operated. For long-term outcome on surgically treated calcified pseudomeningocele there is a lack of reports. The reported average follow-up is 1.7 years postoperatively and there is good surgical outcome.
Acknowledgments
We would like to record our appreciation to Mrs Suneet D Sidhu for editing the grammar in the text.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Al-Edrus SA, Mukari SA, Ganesan D, Ramli N. Ossified Lumbar pseudomeningocele: imaging findings. Spine J. 2011;11:796–797. doi: 10.1016/j.spinee.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Bullock FN, Fleischhacker HH. Interpretation of the pandy test. Acta Psychiatr Scand. 1951;26:149–153. doi: 10.1111/j.1600-0447.1951.tb09665.x. [DOI] [PubMed] [Google Scholar]
- 3.Carollo C, Rigobello L, Carteri A, Marin G. Case report: postsurgical calcified pseudocyst of the lumbar spine. J Comput Assist Tomogr. 1982;6:627–629. doi: 10.1097/00004728-198206000-00034. [DOI] [PubMed] [Google Scholar]
- 4.Couture D, Branch DL., Jr Spinal pseudomeningoceles and cerebrospinal fluid fistulas. Neurosurg Focus. 2003;15(6):E6. doi: 10.3171/foc.2003.15.6.6. [DOI] [PubMed] [Google Scholar]
- 5.Goel A, Desai KI, Nadkarni TD, Muzumdar DP. An unusual post-traumatic occipitocervical pseudomeningocele: case report. Surg Neurol. 2001;56:62–65. doi: 10.1016/S0090-3019(01)00503-1. [DOI] [PubMed] [Google Scholar]
- 6.Handa T, Tsuji H, Handa O, et al. A rare case of postoperative ossified extradural cyst (in Japanese) Rinsho Seikeigeka. 1985;20:173–178. [Google Scholar]
- 7.Ishaque MA, Crockard HA, Stevens JM. Ossified pseudomeningocoele following laminectomy: case reports and review of the literature. Eur Spine J. 1997;6:430–432. doi: 10.1007/BF01834074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin YJ, Chung SB, Kim KJ, Kim HJ. Dystrophic calcification in the epidural and extraforaminal space caused by repetive triamcinolone acetonide injections. J Korean Neurosurg Soc. 2011;50:134–138. doi: 10.3340/jkns.2011.50.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee KS, Hardy IM., II Postlaminectomy lumbar pseudomeningocele: report of four cases. Neurosurgery. 1992;30:111–114. doi: 10.1227/00006123-199201000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Nash CL, Kaufman B, Frankel VH. Postsurgical meningeal pseudocyst of the lumbar spine. Clin Orthop. 1971;75:167–178. doi: 10.1097/00003086-197103000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds MR, Blackburn SL, Smyth MD. Ossified pseudomeningocele following Chiari decompression surgery in a patient with Kleeblattschädel deformety Case report. J Neurosurg Pediatr. 2008;2:203–206. doi: 10.3171/PED/2008/2/9/203. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds MR. Ossified pseudomeningocele (Response in Letters to the Editor) J Neurosurg Pediatr. 2009;3:79. doi: 10.3171/2008.10.PEDS08264. [DOI] [PubMed] [Google Scholar]
- 13.Rosenblum DJ, DeRow JR. Spinal Extradural Cysts with report of an ossified spinal extradural cyst. Amer J Roentgen. 1963;90:1227–1230. [PubMed] [Google Scholar]
- 14.Saito H, Kawakami N. Postsurgical ossified extradural cyst of the lumbar spine: a case report. Spine. 1996;21:386–388. doi: 10.1097/00007632-199602010-00027. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher HW, Wassmann H, Podlinski C. Brief Communication: pseudomeningocele of the lumbar spine. Surg Neurol. 1988;29:77–78. doi: 10.1016/0090-3019(88)90127-9. [DOI] [PubMed] [Google Scholar]
- 16.Shahinfar AH, Schechter MM. Traumatic extradural cysts of the spine. Amer J Roentgenol Radium Ther Nucl Med. 1966;98:713–719. doi: 10.2214/ajr.98.3.713. [DOI] [PubMed] [Google Scholar]
- 17.Shifrin LZ, Frish E, Benarie J. Post surgical lumbar calcified extradural cyst: report of case. Spine. 1990;15:229–231. doi: 10.1097/00007632-199003000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Shimazaki K, Nisihida H, Harada Y, Hirohata K. Late recurrence of spinal stenosis and claudication after laminectomy due to an ossified extradural pseudocyst. Spine. 1991;16:221–224. [PubMed] [Google Scholar]
- 19.Tsuji H, Handa N, Handa O, Tajima G, Mori K. Post laminectomy ossified extradural pseudocyst: case report. J Neurosurg. 1990;73:785–787. doi: 10.3171/jns.1990.73.5.0785. [DOI] [PubMed] [Google Scholar]
- 20.Youssef F, Markovic D, López H, Spuler A, Kiwit J. Completely ossified pseudomeningocele, a rare complication after spinal surgery. Cent Eur Neurosurg. 2009;70:211–213. doi: 10.1055/s-0029-1224160. [DOI] [PubMed] [Google Scholar]


