Abstract
Purpose
Pseudomyogenic hemangioendothelioma is a soft tissue tumor found in young adults, predominantly males. The tumor has been reported in various locations in the body, including the head, neck, chest wall, abdominal wall, genital region, and extremities. Until now, there has been no indication of occurrence in the spine.
Methods
A 25-year-old male presented with spinal cord compression, due to an extradural tumor involving the third and fourth thoracic vertebrae with extension into the right pleural cavity.
Results
Histopathologic examination revealed a pseudomyogenic hemangioendothelioma, also described as epithelioid sarcoma-like hemangioendothelioma, or fibroma-like variant of epithelioid sarcoma.
Conclusion
We describe the first occurrence of pseudomyogenic hemangioendothelioma in the thoracic spine. According to previous reports based on other locations, the tumor has an indolent clinical course with a small risk of metastasis, therefore complete macroscopic excision is the treatment of choice. Local recurrence may occur even with complete surgical resection, requiring close follow-up; adjuvant therapy is warranted.
Keywords: Pseudomyogenic hemangioendothelioma, Epithelioid sarcoma-like hemangioendothelioma, Fibroma-like variant of epithelioid sarcoma, Thoracic spine tumor
Introduction
Pseudomyogenic hemangioendothelioma is a rare soft tissue tumor—distinct from epithelioid hemangioendothelioma [1]—originally described as a “fibroma-like variant of epithelioid sarcoma” [2]; it has been recently concluded that the “epithelioid sarcoma-like hemangioendothelioma” is essentially the same pathological entity [3]. Pseudomyogenic hemangioendothelioma occurs predominantly in males between 20 and 50 years of age. Up to 78 % of tumors arise in the extremities, often affecting multiple tissue planes; dermis and subcutaneous tissue are most commonly involved, whereas intraosseous occurrence is rare, comprising not more than 20 % of the cases. Despite its ominous multifocal presentation, the tumor seems to have a relatively favorable long-term prognosis [4]. We present a case of pseudomyogenic hemangioendothelioma occurring in a new and entirely unusual localization, the thoracic spine.
Case report
History and examination
A 25-year-old male presented with a history of progressive bilateral lower extremity weakness during the last month. His past medical history and review of systems were unremarkable. He also complained of right-sided position- and activity-dependent chest pain. On neurological examination he had M 0/5 weakness of all muscle groups in the lower extremities, hyperactive deep tendon reflexes, bilateral Babinski reflexes and increased muscle tone, representing spasticity. Sensory examination revealed pinprick sensory loss below the fourth thoracic dermatome, with preservation of deep pressure sensation.
Imaging studies
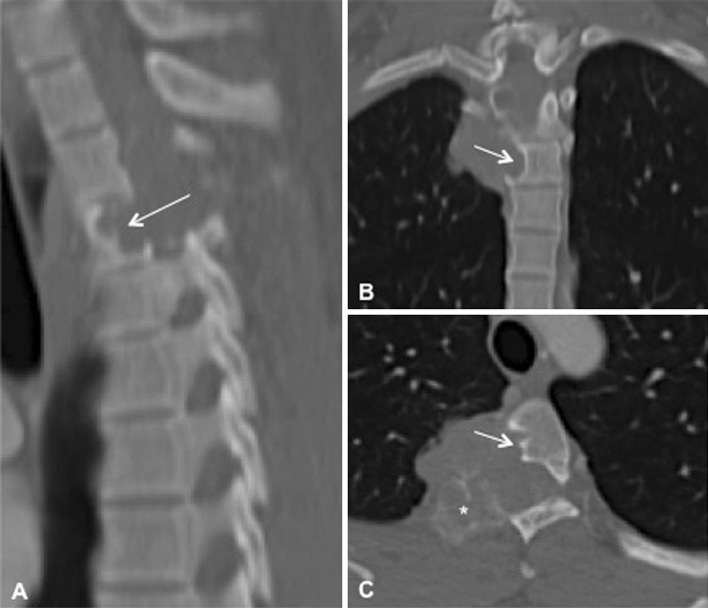
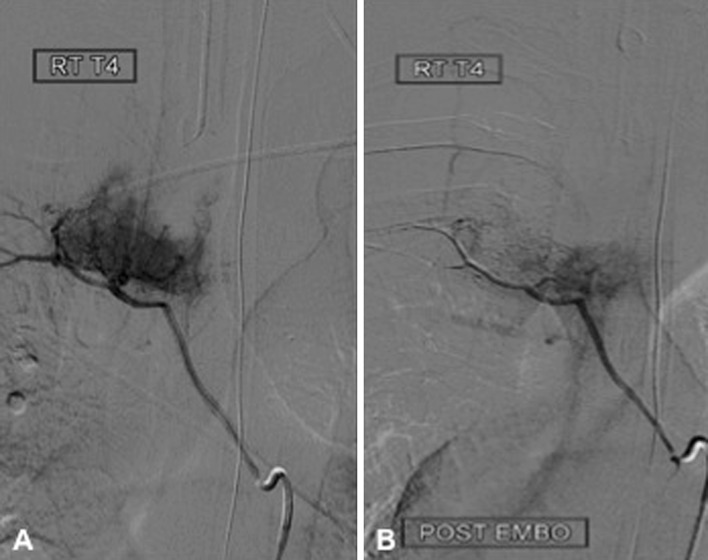
Magnetic resonance imaging (MRI) of the thoracic spine showed a large (4.5 × 3.5 × 3 cm3) mass involving the right costovertebral region of the fourth thoracic vertebra. The tumor extended into the right thoracic cavity and involved the right pedicle, transverse process, and rib. The lesion was multilobulated, isointense on T1-weighed images (T1WI), and heterogeneously hyperintense on T2WI. Following contrast administration it showed consistent enhancement (Fig. 1). The computed tomography (CT) of the involved region revealed two different morphologic patterns of tumor behavior with respect to bone. The tumor caused scalloping of the vertebral body without bone destruction, whereas it involved the pedicle, lamina, and transverse process, expanding these structures from within (Fig. 2). Preoperative angiography through the fourth thoracic segmental artery revealed an intensive tumor blush, which could be significantly reduced following embolization with polyvinyl alcohol (Fig. 3).
Fig. 1.
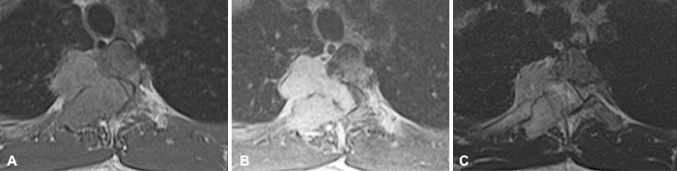
Magnetic resonance imaging (MRI) of the fourth thoracic vertebral segment. a T1-weighed image (T1WI) illustrating the isointense mass extending into the right thoracic cavity, pedicle, transverse process, and rib. b Following contrast administration the mass enhances homogenously. c On T2WI the tumor is heterogeneously hyperintense
Fig. 2.
Computed tomography of the fourth thoracic vertebral segment on sagittal (a), coronal (b), and axial (c) images. There are two noticeable patterns of tumor behavior with respect to bone: bone scalloping of the vertebral body (arrow), and bone expansion of the lamina, and transverse process (asterisk)
Fig. 3.
Preoperative angiography through the fourth thoracic segmental artery revealed an intensive tumor blush (a), which was significantly reduced following embolization with polyvinyl alcohol (b)
Surgery
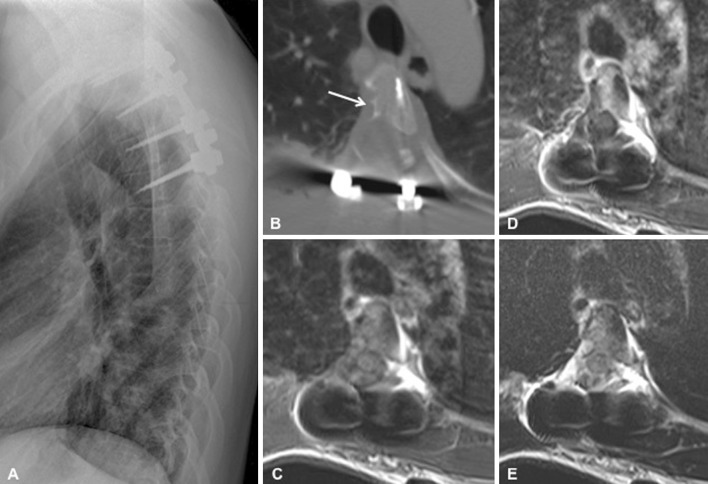
Gross total resection of the tumor was performed through a posterior approach. Macroscopically the tumor was solid, well circumscribed, and yellowish as it extended into the pleura. It became more fragile, bloody, infiltrating, and dark gray as it extended into the pedicle and transverse process. Its consistency was solid in the part causing scalloping of the vertebral body, where the tumor was easily peeling off from the corticalized surface of the vertebral body. Through right-sided costotransversectomy complete macroscopic resection of the tumor could be achieved (Fig. 4).
Fig. 4.
Postoperative imaging. Lateral view thoracic spine radiography with instrumentation at levels T2–T5 (a). Axial computed tomography image of the T4 vertebra shows the right-sided costotransversectomy, resection of the right pedicle and posterior elements, and the corticalized scalloping of the vertebral body (arrow) (b). Magnetic resonance imaging (MRI) demonstrates complete resection of the tumor without residual contrast enhancement, and re-expansion of the thecal sac and spinal cord on T1-weighed axial image (T1WI) (c), following contrast administration (d), and on T2WI image (e)
Surgical pathology
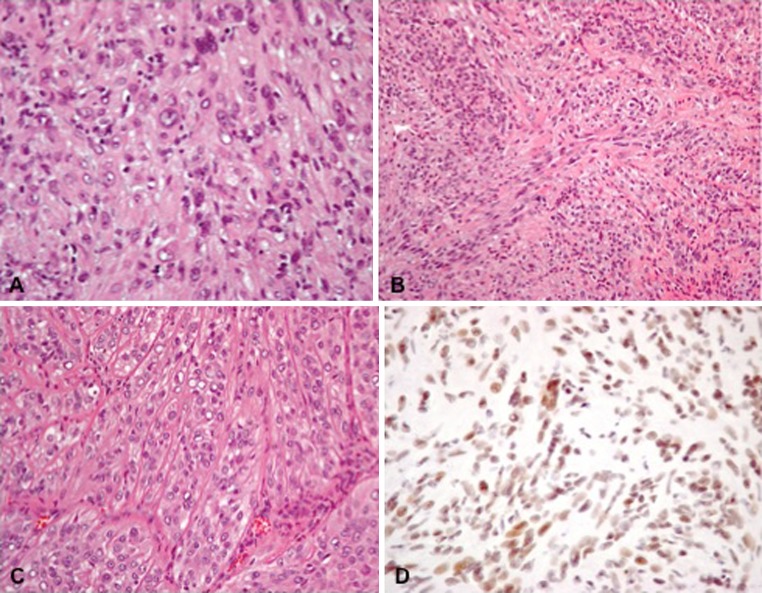
The tumor consisted of densely distributed pleomorphic cells, ranging in configuration from small spindled elements to large plump epithelioid variants with prominent pink cytoplasm. Scattered cells with vacuolated cytoplasm were also present. Prominent pleomorphic nuclei contained optically empty centers with peripheral marginalization of the chromatin, in addition to conspicuous nucleoli. Multinucleated cells were also present. Mitotic figures were relatively inconspicuous, in keeping with the low proliferation index (Ki-67). Occasional foci of cellular necrosis were also present. The large epithelioid cells resembled rhabdoid elements. The cells were oriented in a poorly defined, intersecting fascicular pattern, while the amorphic stroma assumed a bluish tinge or cast. Large blood vessels were visible within the stroma. These vessels branched into progressively smaller vascular elements, ultimately forming a network of small capillaries between the individual cells. Stains for keratin (AE1/3 and CAM5.2) were strongly positive, whereas CD31 immunohistochemistry (platelet endothelial cell adhesion molecule—PECAM-1) was focally positive. The negative S100 staining excluded the diagnosis of malignant peripheral nerve sheath tumor. The tumor was desmin-negative and integrase interactor 1 (INI-1)-positive. The hematoxylin–eosin characteristics and immunohistochemistry staining profile of the tumor were conclusive for the diagnosis of pseudomyogenic hemangioendothelioma (Fig. 5).
Fig. 5.
Histopathological microphotographs of the tumor. a Epithelioid cells with vacuolated nuclei and prominent nucleoli (hematoxylin eosin, original magnification ×400). b Spindle cell component of the tumor with poorly defined fascicular pattern (hematoxylin eosin, original magnification ×200). c Prominent capillaries in the epithelioid part of the tumor (hematoxylin eosin, original magnification ×200). d immunohistochemistry for integrase interactor 1 protein (INI-1, original magnification ×400)
Discussion
Pseudomyogenic hemangioendothelioma is a soft tissue tumor, named and extensively described by Hornick and Fletcher in 2011 [4]. It is generally accepted that this tumor is identical to the “fibroma-like variant of epithelioid sarcoma”, which was described in five patients by Mirra et al. [2]. Although the relationship of pseudomyogenic hemangioendothelioma to “epithelioid sarcoma-like hemangioendothelioma” is subject to significant controversy and academic debate, they seem to be co-identical [3]. For those reasons, our literature search as to past-published cases included all three terms: pseudomyogenic hemangioendothelioma, fibroma-like variant of epithelioid sarcoma, and epithelioid sarcoma-like hemangioendothelioma (Table 1).
Table 1.
Reported cases of pseudomyogenic hemangioendothelioma (epithelioid sarcoma-like hemangioendothelioma, fibroma-like variant of epithelioid sarcoma)
| References | No. of cases | Locations |
|---|---|---|
| Mirra et al. [2] | 5 | Heel, arm, thigh, toe, and foot |
| Billings et al. [7] | 7 | Thigh, knee, scalp, calf, chest, and forearm |
| Tokyol et al. [8] | 1 | Forearm |
| Watabe et al. [9] | 2 | Index finger and forearm |
| Hornick et al. [4] | 50 | Thigh, forearm, calf, foot, hand, chest, abdominal wall, upper arm, shoulder, back, scrotum, nose, penis, scalp, and finger |
| Cai et al. [10] | 3 | Neck, ilium, and shoulder |
| Present case | 1 | Thoracic spine |
Pseudomyogenic hemangioendothelioma affects predominantly young males. The tumor is multifocal, involving different tissue planes and, although, it shares some histopathologic features with epithelioid sarcoma, it has a different, spindle cell morphology with common positivity for CD31, lack of CD34 reactivity, and intact INI-1 immunoreactivity [4, 5].
The molecular genetics of pseudomyogenic hemangioendothelioma is largely unknown with only one published study in the literature [6]. In a G-banding analysis of cultured metaphase tumor cells, Trombetta et al. showed a balanced translocation between chromosomes 7 and 19 [t(7;19) (q22;q13)] as the sole clonal change. Fluorescence in situ hybridization disclosed that the breakpoints were located within bacterial artificial chromosome clones RP4-747G18 in band 7q22.1, and CTB-171A8 in band 19q13.31-q13.32. The breakpoint region on chromosome 7 contained six genes (SERPINE1, APIS1, VGF, MOGAT3, PLOD3, and ZNHIT1), and the breakpoint on chromosome 19 contained four known genes (CEACAM19, CEACAM16, BCL3, and CBLC). Although the analysis of transcriptional directions of the genes allowed considering putative chimeric transcripts, the pathogenetic significance of these changes remains to be determined. Due to limited access to fresh tumor material, the authors also performed interphase fluorescence in situ hybridization, which showed unbalanced translocation der (7) t(7;19) in one out of nine analyzed cases. These results suggest that the translocation t(7;19) is of pathogenetic importance, however, only in a subset of tumors. The majority of pseudomyogenic hemangioendotheliomas seem to develop through other mechanisms [6].
The tumor has been described in various locations of the body, such as the head, chest wall, abdominal wall, genital region, and extremities [2, 4, 7–10]. No case of pseudomyogenic hemangioendothelioma has been reported in the spine (Table 1). In our patient the tumor involved several tissue planes including the thoracic vertebra, paravertebral muscles and the parietal pleura. During surgery, it was well-demarcated in its extension into the pleural cavity causing only scalloping, but no invasion of the vertebral body. Conversely, the tumor was more aggressive in its dorsal extension, infiltrating the vertebral pedicle, lamina, and transverse process.
In conclusion, we describe the first occurrence of pseudomyogenic hemangioendothelioma in the thoracic spine. Based on previous reports in other locations, the tumor has a more indolent clinical course with a small risk of metastasis [4]. Therefore, complete macroscopic excision is the treatment of choice. Local recurrence must be considered, even with complete, gross surgical resection; close follow-up and adjuvant therapy is warranted.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Ma J, Wang L, Mo W, Yang X, Xiao J. Epithelioid hemangioendothelioma of the spine: clinical characters with middle and long-term follow up under surgical treatments. Eur Spine J. 2011;20(8):1371–1376. doi: 10.1007/s00586-011-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mirra JM, Kessler S, Bhuta S, Eckardt J. The fibroma-like variant of epithelioid sarcoma. A fibrohistiocytic/myoid cell lesion often confused with benign and malignant spindle cell tumors. Cancer. 1992;69(6):1382–1395. doi: 10.1002/1097-0142(19920315)69:6<1382::AID-CNCR2820690614>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma (pseudomyogenic hemangioendothelioma) Am J Surg Pathol. 2011;35(7):1088. doi: 10.1097/PAS.0b013e31821caf1c. [DOI] [PubMed] [Google Scholar]
- 4.Hornick JL, Fletcher CD. Pseudomyogenic hemangioendothelioma: a distinctive, often multicentric tumor with indolent behavior. Am J Surg Pathol. 2011;35(2):190–201. doi: 10.1097/PAS.0b013e3181ff0901. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Fanburg-Smith JC, Virolainen M, Shmookler BM, Fetsch JF. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30(8):934–942. doi: 10.1016/S0046-8177(99)90247-2. [DOI] [PubMed] [Google Scholar]
- 6.Trombetta D, Magnusson L, Vult von Steyern F, Hornick JL, Fletcher CD, Mertens F. Translocation t (7;19)(q22;q13)-a recurrent chromosome aberration in pseudomyogenic hemangioendothelioma? Cancer Genet. 2011;204:211–215. doi: 10.1016/j.cancergen.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48–57. doi: 10.1097/00000478-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Tokyol C, Uzum N, Kuru I, Uluoglu O. Epithelioid sarcoma-like hemangioendothelioma: a case report. Tumori. 2005;91(5):436–439. doi: 10.1177/030089160509100512. [DOI] [PubMed] [Google Scholar]
- 9.Watabe A, Okuyama R, Hashimoto A, Hosaka M, Hatori M, Kariya Y, et al. Epithelioid sarcoma-like hemangioendothelioma: a case report. Acta Derm Venereol. 2009;89(2):208–209. doi: 10.2340/00015555-0599. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Peng F, Li L, Cheng Y, Wang J. Epithelioid sarcoma-like hemangioendothelioma: a clinicopathologic and immunohistochemical study of 3 cases. Zhonghua Bing Li Xue Za Zhi. 2011;40(1):27–31. [PubMed] [Google Scholar]