Abstract
Purpose
Beside mechanical complications, the majority of adverse events after total disc arthroplasty (TDA) are related to the surgical approach. Septic complications are very uncommon and only one previous case has been published. The objective of this article is to describe the clinical circumstances, treatment, and outcomes of septic complication after TDA at L4–L5, involving an uncommon pathogen (Mycoplasma hominis).
Methods
A 38-year-old woman underwent a MobiDisc® TDA at L4–L5 level for discogenic pain. One month postoperatively, she complained of acute low back and abdominal pain associated with fever (39 °C). C-reactive protein level was elevated (197 mg/L; normal <5 mg/L) and the white blood cell count was normal (7 × 109/L; normal 4–10 × 109/L). A computerized tomography (CT) showed a left psoas-based retroperitoneal abscess. Treatment consisted of open debridement, drainage and empirical antibiotic therapy. Intraoperative cultures yielded M.hominis after 7 days incubation. Antibiotic therapy was adapted and discontinued after 2 months. The patient had failed to mention earlier that she had been suffering from abnormal vaginal discharge for some time and was using an intrauterine contraceptive device.
Results
At 1.5-year follow-up, review confirmed healing of the infection with biological normalization without residual collection, radiolucent lines or osteolysis around the prosthesis at radiographs, CT and MRI.
Conclusions
Mycoplasma hominis can be involved as an extragenital pathogen in musculoskeletal infections. Because its culture and identification are difficult, special media and real-time PCR are required in case of postoperative deep wound infection after anterior lumbar spine surgery, especially in the case of previous genitourinary infections, to decrease the delay in diagnosis and treatment.
Keywords: Total disc arthroplasty, Anterior lumbar revision surgery, Infection, Complications
Introduction
Beside mechanical complications, the majority of adverse events and complications of total disc arthroplasty (TDA) are related to the anterior surgical approach of the lumbar spine [1–4]. Infection after TDA is very uncommon [1, 5] and to our knowledge this is the second report of an infection after TDA in literature [6]. As such, there are no standard, approved guidelines for the treatment of an infected TDA. However, all mobile joint replacements have some incidence of late infection [6]. Depending on the time that has elapsed between the arthroplasty and the diagnosis of infection, treatment options may include long-term antibiotic therapy, open debridement, removal of the prosthesis and either exchange arthroplasty or joint fusion [7]. Several articles describe revision strategies for accessing total disc prosthesis [8–12]. They point out that revision anterior approach of the lumbar spine has an increased potential for iatrogenic injury during exposure due to the formation of adhesions of veins, arteries, ureter, visceral organs, and peritoneum to the spine after the index surgery [11].
We present a case of acute infection after TDA with an atypical pathogen and its treatment, including open debridement without implant removal and antibiotic therapy.
Case presentation
A 38-year-old woman with DDD at L4–L5 underwent TDA with the MobiDisc® prosthesis (LDR Medical, Troyes, France) (Fig. 1) through a left retroperitoneal approach using a midline incision. Her surgical history included two bariatric surgeries: a gastric banding surgery and a sleeve gastrectomy. Surgery was uneventful, except for a peritoneal breach during exposure that was successfully repaired. Prophylactic cefuroxime was initiated intravenously during induction of anesthesia and then four times during the first 24-h period after surgery. The early postoperative course was uneventful and the patient was discharged home 7 days after surgery.
Fig. 1.
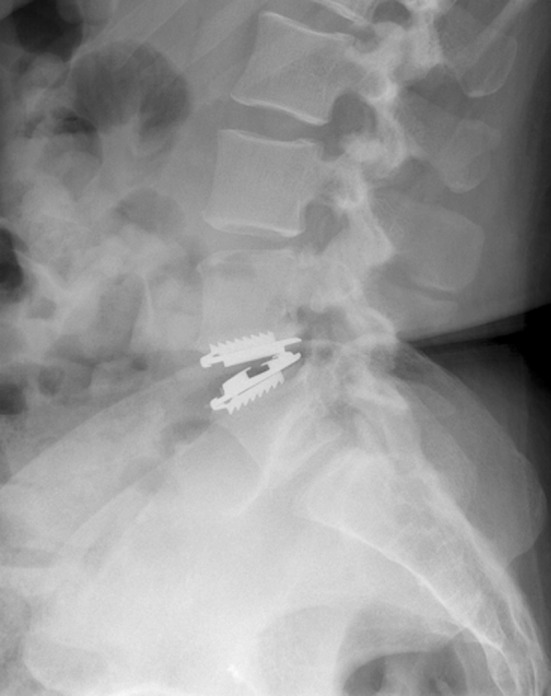
Postoperative lateral view of MobiDisc at the L4–L5 disc level
One month later, she was admitted to another facility because of acute low back and abdominal pain in the left iliac fossa. She was febrile to 39 °C with complaints of nausea. There was no wound complication. Infection parameters showed an elevated C-reactive protein (CRP) level (197 mg/L; normal <5 mg/L) and a normal white blood cell count (WBC) (7 × 109/L; normal 4–10 × 109/L). A computerized tomography (CT) showed a left psoas-based retroperitoneal abscess measuring 36 mm × 24 mm (Fig. 2). Empirical antibiotic treatment by cephotaxime was initiated intravenously, and the patient was subsequently transferred to our facility.
Fig. 2.
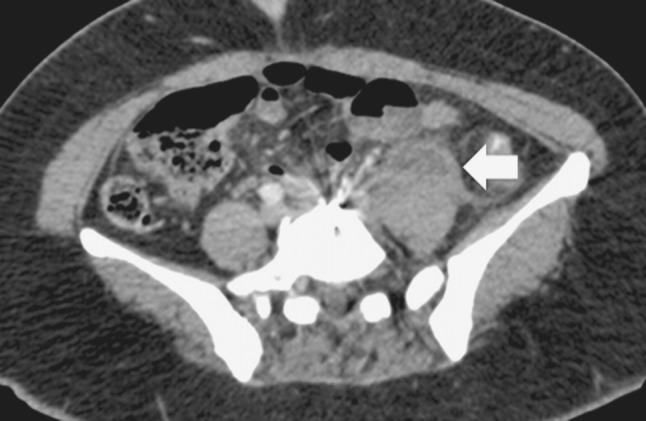
Computerized tomography showing left psoas abscess at the L4–L5 disc level (white arrow) 1 month after TDA
Interventional radiologists could not drain the abscess percutaneously. Thus, a surgical debridement was performed through a different approach (left lombotomy). Because dissection of the great vessels appeared too difficult to access the prosthesis due to inflammatory adhesions, implant was left in place. Abscess was treated by open debridement, extensive irrigation with pulsed lavage, and drainage. According to protocol, cultures were taken by fluid aspiration and from the abscess wall. Adjuvant therapy with intravenous cephotaxime and fosfomycine was initiated postoperatively.
All intraoperative cultures yielded Mycoplasma hominis after 7 days incubation. When re-interrogating the patient, we discovered that she had failed to mention that she was using an intrauterine contraceptive device and had been suffering from abnormal vaginal discharge for some time. Cephotaxime and fosfomycine were stopped, and doxycycline 100 mg was administered orally at 12-h intervals. The patient was discharged 8 days after the second surgery. The intrauterine contraceptive device was removed but culture and a real time PCR test for detection of Mycoplasma remained negative due to yeast contamination.
After 2 months, the infection parameters were normalized and the wound was healed. Antibiotics were discontinued. At 1.5-year follow-up, standard radiographs showed a well-functioning prosthesis (Fig. 3). CT showed no osteolysis around the prosthesis and no migration or subsidence of the implant (Fig. 4). MRI showed no residual collection at the L4–L5 disc level (Fig. 5).
Fig. 3.
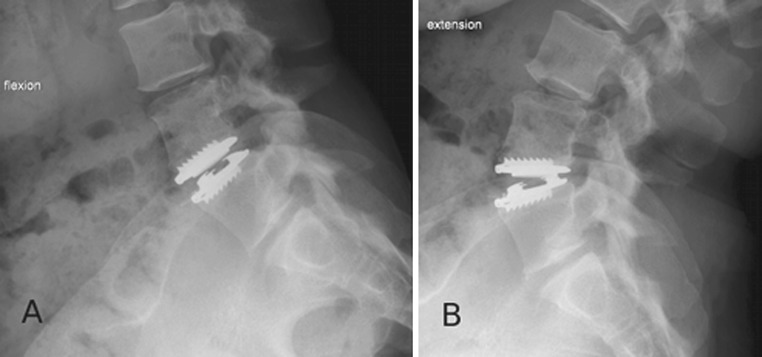
Postoperative dynamic lateral views at 1.5-year follow-up of a well-functioning TDA. a Flexion and b extension. Range of motion is 8° at 1.5-year follow-up and was 2° postoperatively
Fig. 4.
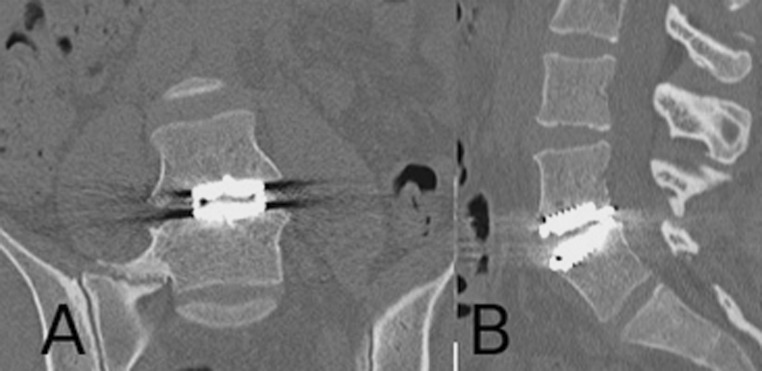
Computerized tomography 1-year after open debridement and drainage of a left psoas abscess at the L4–L5 disc level: no residual collection and no signs of periprosthetic osteolysis. a Frontal reconstruction b sagittal reconstruction
Fig. 5.
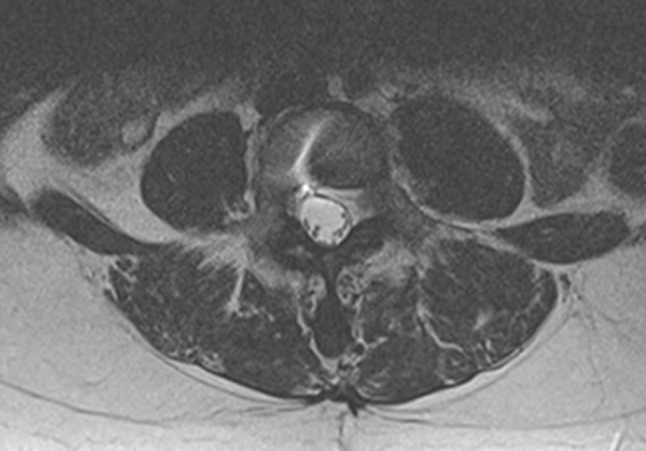
Magnetic resonance imaging at 1-year follow-up showing no residual collection
Discussion
Mycoplasma hominis is a commensal bacterium of the urogenital tract. It is usually responsible for genitourinary tract infections and gynecological surgical wounds infections [13, 14]. A review of extragenital infections involving M. hominis revealed that they fall into five categories: septicemia, joint infections, central nervous system infections, respiratory tract infections, and wound infections (particularly after surgery) [15]. Most of the patients were found to be debilitated or immunocompromised before the infection developed [16]. Three cases of M. hominis infection were reported in literature after scoliosis surgery [13, 15]. Two cases of retroperitoneal abscess due to M. hominis have been reported, one in a polytraumatized man, and the other one postpartum [17, 18].
It is difficult to know the physiopathology of this bacterial infection. In our case, two possible causes of contamination can be considered. First, the intrauterine device endometritis could be a source of colonization of the peritoneum. During surgical approach, the peritoneum has been lacerated, causing contamination of the operative field. Second, another possible cause could be systemic spread through the bloodstream, which is the most frequent mode of contamination for M. hominis extragenital infections. In any case, local hematoma harboring bacteria at the end of the procedure served as an excellent culture medium for the pathogen.
In the presented case, intraoperative local cultures were positive for M. hominis, but blood culture remained negative. The diagnosis of a Mycoplasma infection was delayed due to the fastidious nature of mycoplasmas, not seen on gram-stained smears. On the usual culture media, M. hominis grows poorly and slowly. In addition, culture plates are usually not kept long enough and are not scrutinized with sufficient attention to detect the tiny colonies of M. hominis [19]. Real-time PCR on deep cultures provides a rapid alternative with a higher sensitivity and specificity than culture for its detection [20]. However, in our case, M. hominis was not the first pathogen to be considered in postoperative deep wound infection after anterior lumbar spine surgery, especially since the patient had failed to inform us about her abnormal vaginal discharge. The incidence of such infections may be underestimated. Indeed, extragenital infections due to M. hominis have often been discovered fortuitously by growth of the organism on blood agar or in routine blood cultures, or because treatment failure with antimicrobial agents ineffective against mycoplasma aroused physician suspicion [16].
There are lack of data regarding management of an infected disc arthroplasty since this pathology is uncommon. One case of Streptococcus intermedius infection after a two-level TDA has been reported [6], treated by percutaneous abscess drainage and suppressive antibiotics. Despite favorable evolution, the patient wished to have his implants removed and converted to a fusion.
There is a theoretical similarity in the treatment of infection after TDA and infection after other joint prosthesis. However, the environment of the intervertebral disc is not necessarily comparable because of the absence of synovial membrane, which is a very immunologically active structure. The treatment of postoperative infections in anterior spine surgery is unclear in the literature, with some authors advocating interbody implant preservation and others interbody implant removal [21]. The implants should probably be left in place unless definite signs of osteomyelitis of the vertebral bodies are present, or if implant loosening is documented. Conservative management is all the more advisable since dissection of the great vessels to access the prosthesis can be too difficult because of inflammatory adhesions. Our case demonstrates that infection can be treated successfully with aggressive irrigation and debridement of the abscess, antibiotic therapy and removal of the causal factor (intrauterine device).
We report a case of deep lumbar TDA infection due to M. hominis. This pathogen can be incriminated in infection after spine surgery, especially if intraoperative peritoneal breach occurred during the anterior approach. To prevent secondary infections due to M. hominis, careful preoperative patient history is essential. In patients with abnormal vaginal discharge, it is important to first exclude endometritis, which should postpone anterior approach of the lumbar spine to perform either TDA or interbody fusion. Implant removal does not seem to be required to heal the infection, but open debridement, drainage and suitable antibiotic therapy are mandatory. Since M. hominis isolation is difficult, specific culture media and/or real-time PCR may be required, especially in case of pathology in the gynecological history and/or intraoperative peritoneal breach.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Mayer HM. Total lumbar disc replacement. J Bone Joint Surg Br. 2005;87:1029–1037. doi: 10.1302/0301-620X.87B8.16151. [DOI] [PubMed] [Google Scholar]
- 2.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults. A review of 1223 procedures. Spine (Phila Pa 1976) 1995;20:1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wood KB, Schwender JD. Lumbar intervertebral cages: limitations and complications. Oper Tech Orthop. 2000;10:320–324. doi: 10.1016/S1048-6666(00)80032-4. [DOI] [Google Scholar]
- 4.Sasso RC, Best NM, Mummaneni PV, Reilly TM, Hussain SM. Analysis of operative complications in a series of 471 anterior lumbar interbody fusion procedures. Spine (Phila Pa 1976) 2005;30:670–674. doi: 10.1097/01.brs.0000155423.18218.75. [DOI] [PubMed] [Google Scholar]
- 5.Guyer RD, Ohnmeiss DD. Intervertebral disc prostheses. Spine (Phila Pa 1976) 2003;28:S15–S23. doi: 10.1097/01.BRS.0000076843.59883.E1. [DOI] [PubMed] [Google Scholar]
- 6.Spivak JM, Petrizzo AM. Revision of a lumbar disc arthroplasty following late infection. Eur Spine J. 2010;19:677–681. doi: 10.1007/s00586-009-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 8.Bertagnoli R, Zigler J, Karg A, Voigt S. Complications and strategies for revision surgery in total disc replacement. Orthop Clin North Am. 2005;36:389–395. doi: 10.1016/j.ocl.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Leary SP, Regan JJ, Lanman TH, Wagner WH. Revision and explantation strategies involving the CHARITE lumbar artificial disc replacement. Spine (Phila Pa 1976) 2007;32:1001–1011. doi: 10.1097/01.brs.0000260794.73938.93. [DOI] [PubMed] [Google Scholar]
- 10.McAfee PC, Geisler FH, Saiedy SS, Moore SV, Regan JJ, Guyer RD, Blumenthal SL, Fedder IL, Tortolani PJ, Cunningham B. Revisability of the CHARITE artificial disc replacement: analysis of 688 patients enrolled in the U.S. IDE study of the CHARITE artificial disc. Spine (Phila Pa 1976) 2006;31:1217–1226. doi: 10.1097/01.brs.0000217689.08487.a8. [DOI] [PubMed] [Google Scholar]
- 11.Patel AA, Brodke DS, Pimenta L, Bono CM, Hilibrand AS, Harrop JS, Riew KD, Youssef JA, Vaccaro AR. Revision strategies in lumbar total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1276–1283. doi: 10.1097/BRS.0b013e3181714a1d. [DOI] [PubMed] [Google Scholar]
- 12.Wagner WH, Regan JJ, Leary SP, Lanman TH, Johnson JP, Rao RK, Cossman DV. Access strategies for revision or explantation of the Charite lumbar artificial disc replacement. J Vasc Surg. 2006;44:1266–1272. doi: 10.1016/j.jvs.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 13.Krijnen MR, Hekker T, Algra J, Wuisman PI, Van Royen BJ. Mycoplasma hominis deep wound infection after neuromuscular scoliosis surgery: the use of real-time polymerase chain reaction (PCR) Eur Spine J. 2006;15(Suppl 5):599–603. doi: 10.1007/s00586-005-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers PO, Khabiri E, Greub G, Kalangos A. Mycoplasma hominis mediastinitis after acute aortic dissection repair. Interact Cardiovasc Thorac Surg. 2010;11:857–858. doi: 10.1510/icvts.2010.244608. [DOI] [PubMed] [Google Scholar]
- 15.Madoff S, Hooper DC. Nongenitourinary infections caused by Mycoplasma hominis in adults. Rev Infect Dis. 1988;10:602–613. doi: 10.1093/clinids/10.3.602. [DOI] [PubMed] [Google Scholar]
- 16.Taylor-Robinson D. Infections due to species of Mycoplasma and Ureaplasma: an update. Clin Infect Dis. 1996;23:671–682. doi: 10.1093/clinids/23.4.671. [DOI] [PubMed] [Google Scholar]
- 17.Brunner S, Frey-Rindova P, Altwegg M, Zbinden R. Retroperitoneal abscess and bacteremia due to Mycoplasma hominis in a polytraumatized man. Infection. 2000;28:46–48. doi: 10.1007/s150100050011. [DOI] [PubMed] [Google Scholar]
- 18.Barbera J, Gasser I, Almirante B, Pigrau C. Postpartum retroperitoneal abscess due to Mycoplasma hominis. Clin Infect Dis. 1995;21:698–699. doi: 10.1093/clinids/21.3.698. [DOI] [PubMed] [Google Scholar]
- 19.Fenollar F, Casalta JP, Lepidi H, Piquet P, Raoult D. Mycoplasma infections of aneurysms or vascular grafts. Clin Infect Dis. 1999;28:694–695. doi: 10.1086/517225. [DOI] [PubMed] [Google Scholar]
- 20.Baczynska A, Svenstrup HF, Fedder J, Birkelund S, Christiansen G. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 2004;4:35. doi: 10.1186/1471-2180-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappou IP, Papadopoulos EC, Sama AA, Girardi FP, Cammisa FP. Postoperative infections in interbody fusion for degenerative spinal disease. Clin Orthop Relat Res. 2006;444:120–128. doi: 10.1097/01.blo.0000203446.06028.b5. [DOI] [PubMed] [Google Scholar]


