Abstract
Purpose
Hemangiopericytoma (HPC) is a rare tumor of the central nervous system. Primary spinal occurrence of this tumor is extremely uncommon and cases involving the intramedullary spinal cord are even more rare. The purpose of this study was to explore the clinical features, surgical strategies, outcome and pathology in a consecutive series of patients treated at a single institution.
Methods
The authors performed a retrospective review of the clinicopathological characteristics of four patients with a pathological diagnosis of spinal HPC.
Results
Four cases with intradural as well as intra/extra-medullary components were identified. Gross total resection with no recurrence at the operative site was achieved in the majority of patients with a spinal HPC. One patient had significant recurrence and eventually, succumbed to the disease.
Conclusion
Increased awareness of these tumors’ capability to occur intradurally and intramedullarly can help surgeons accurately diagnose and choose an effective plan of care. Gross total resection of hemangiopericytomas is the mainstay of treatment and should be pursued if feasible. Histopathology is essential to the diagnosis.
Keywords: Hemangiopericytoma, Intradural, Thoracic, Extramedullary
Introduction
Originally characterized as the angioblastic form of meningiomas, Stout and Murray [1] coined the term hemangiopericytoma (HPC) in 1942 to describe a vascular neoplasm thought to be derived from capillary pericytes. Although most frequently arising from the meninges, the WHO originally classified these neoplasms as “mesenchymal non-meningothelial” tumors (grade II and III) in 1993 [2]. The updated WHO classification of Tumors of Soft Tissue and Bone, categorized HPCs as fibroblastic with no evidence of pericytic differentiation [2]. This suggests that HPCs are a continuum with solitary fibrous tumors (SFT) due to their similar characteristics, and are often only separated by histopathology [3]. Most HPCs occur outside the central nervous system. However, when within the CNS, the tumors are more commonly intracranial. Cases of spinal HPCs are extremely rare and are most often intradural. There have been only 19 reported cases of intradural tumors, most being extramedullary [4–14]. Only two prior case reports have been documented to have intramedullary involvement [6, 10]. We present four cases of a HPC with intradural-extramedullary components that had invaded the spinal cord, along with a review of the prior intradural tumor reports.
Case series
Case 1
History and examination
A 27-year-old female with no past medical history presented with progressive worsening of right lower extremity numbness followed by minimal loss of strength in the left lower extremity over a 2-month course. Neurological examination revealed full strength of the right lower limb, while her left lower extremity had diffuse 4+/5 strength in the proximal muscle groups and 3/5 in her left tibialis anterior and extensor hallucis longus. Both lower extremities displayed 3+ hyperreflexia, with a 3-beat clonus of the left foot, and 1-beat clonus of the right. Radiographic findings are presented in Table 1 (Fig. 1a, b).
Table 1.
Radiographic findings for the cases presented
| Case | Spinal level | MRI findings |
|---|---|---|
| 1 | T7–8 | The tumor displayed hyperintense signaling on T1. It appeared concentric with homogenous enhancement with evidence of severe compression of the spinal cord (Fig. 1a, b) |
| 2 | Occiput—C3 | A intradural-extramedullary lesion extending from the occiput down to C3 in the ventrolateral position with central stenosis and cord compression was seen (Fig. 2a, b). MRI of the brain and thoracic spine were both within normal limits |
| 3 | T10–12 | MRI of thoracic and lumbar spine demonstrated multiple lesions most notable at the right T9–12 with severe spinal cord compression (Fig. 3a, b). |
| 4 | T9–10 | MRI of the thoracic spine illustrated a posterior T9–10 epidural mass with both intradural and extramedullary components as well as right-sided neural foramen extension. The mass was significantly compressing the cord and appeared vascular (Fig. 4a, b) |
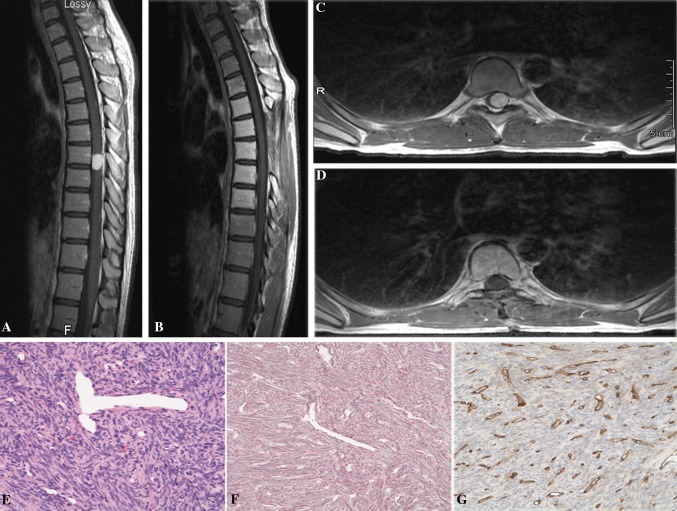
Fig. 1.
a, b Preoperative T1 sagittal and axial MRI with contrast showed a enhancing lesion at the T7–8 levels with severe compression of the spinal cord. c, d Postoperative T1 sagittal and axial MRI with contrast demonstrating resection of the tumor. e, f, g Hematoxylin and eosin (H&E) stained section showed moderately cellular proliferation of short nuclei, associated with prominent “staghorn” vasculature (e), embedded in a rich network of reticulin fibers (f), but showing only a weak staining for CD34 (g). Original magnification ×200
Operation
The patient underwent a midline T6–9 laminectomy with ultrasound localization of the mass. The tumor was visualized beneath the arachnoid, shifted to the left side. After the arachnoid was incised, a firm red mass was found to be emanating, intramedullary, from the spinal cord. There was no clear border between the tumor and the spinal cord, suggesting that the tumor was both intramedullary and extramedullary. The extramedullary portion of the tumor was defined and debulked with ultrasonic aspiration allowing the intramedullary portion of the mass to be better evaluated. Gross total resection of the tumor was obtained by dissection of the tumor from within the spinal cord. The arachnoidal adhesions surrounding the spinal cord were mobilized, and the spinal cord was gently returned to a midline position. The dura was closed without complications.
Postoperative course
The postoperative course was uneventful. MRI revealed complete resection (Fig. 1c, d). The patient’s postoperative neurological exam remained stable with resolution of her hyperreflexia. With therapy, the patient continued further improvement in her balance and left lower extremity weakness. She remains tumor-free at 3 years follow-up.
Pathology
Hematoxylin-eosin–stained sections revealed a spindle cell proliferation with a fascicular growth pattern and staghorn type vessels (Fig. 1e). Mitotic count approached 2 per 10 high-power fields. Reticulin stain highlighted a pericellular pattern (Fig. 1f). Immunohistochemical studies showed the tumor cells to be diffusely positive for CD34 and negative for S100 (Fig. 1g). Features were characteristic of a hemangiopericytoma, WHO grade II.
Case 2
History and examination
The patient was a 56-year-old male who presented with several months complaint of neck discomfort that had not been responding to anti-inflammatories. His pain radiated into his head and both shoulders. On neurological exam, motor and sensory examinations were intact throughout the upper and lower extremities, with normal reflexes. Cerebellar, Romberg, and gait examinations were normal. Radiographic findings are presented in Table 1 (Fig. 2a, b).
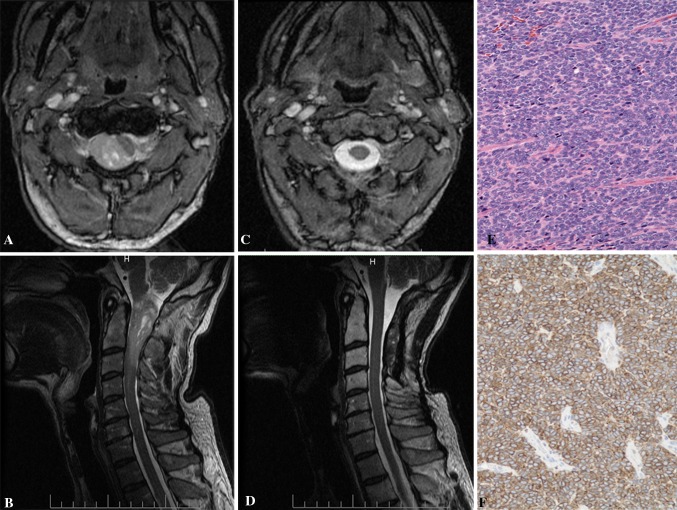
Fig. 2.
a, b Preoperative T1 sagittal and axial MRI with contrast showed a enhancing lesion at the C1–3 levels with severe compression of the spinal cord. c, d Postoperative T1 sagittal and axial MRI with contrast demonstrating resection of the tumor. e, f H&E stained sections disclosed tightly packed oval nuclei forming fascicles (e). Scattered tumor cells were positive for CD34 (f). Original magnification ×200
Operation
The patient underwent a suboccipital craniectomy accompanied with a C1–3 complete laminectomy. After midline dural opening under microscope visualization, a red and solid appearing tumor surrounded with clear arachnoid was noted occupying the right dorsolateral gutter—pushing the spinal cord to the left. The tumor was mobilized and removed in multiple large fragments and further debulked with the use of a Cavitron, as several nerves were coursing through and near the tumor. The tumor was found to be densely adherent to the spinal cord at the C1 and 2 levels; however, a gross total excision of visible tumor was achieved. The spinal cord was brought back to midline, meticulous hemostasis was achieved, and closure was obtained without any complications.
Postoperative course
The patient had an uneventful postoperative course. MRI of the operative site revealed complete resection of the tumor (Fig. 2c, d). His 6-month postoperative thoracic MRI revealed tumor recurrence at the T2–3 level. The patient underwent successful radiosurgery of the thoracic area at an outside facility. Three years later, he is asymptomatic and doing well.
Pathology
Macroscopic examination of the tissue revealed grossly hemorrhagic bulky mass. Microscopic sections disclose a densely cellular proliferation of monotonous cells with enlarged nuclei displaying speckled chromatin and showing numerous (up to 30 per 10 high-power fields) mitotic figures (Fig. 2e). No necrosis was seen, but there were foci of hemorrhage. The tumor showed only minimal reticulin deposition, stained positive with antibodies to CD34 (Fig. 2f) and Bcl2, but negative for smooth muscle actin, epithelial membrane antigen, Synaptophysin and glial fibrillary acidic protein. The Ki-67 proliferative index was 12 %.
Case 3
History and examination
A 49-year-old male with past medical history of metastatic malignant hemangiopericytoma presented emergently with left lower extremity weakness and bilateral radicular pain. Four years prior to the current admission he had undergone a T8–10 resection of paravertebral mass at outside hospital. Histologically the tumor was proved to be a hemangiopericytoma. After an initial period of well-controlled disease, the patient developed increasing pain in his bilateral lower extremities, he had become wheelchair-bound. He was without any bowel or bladder incontinence issues. On neurological exam, he displayed full strength in his right lower extremity with 4/5 diffuse strength in his left lower extremity. There was no hyperreflexia or clonus, and his sensation was intact across all dermatomes. Radiographic findings are presented in Table 1 (Fig. 3a, b).
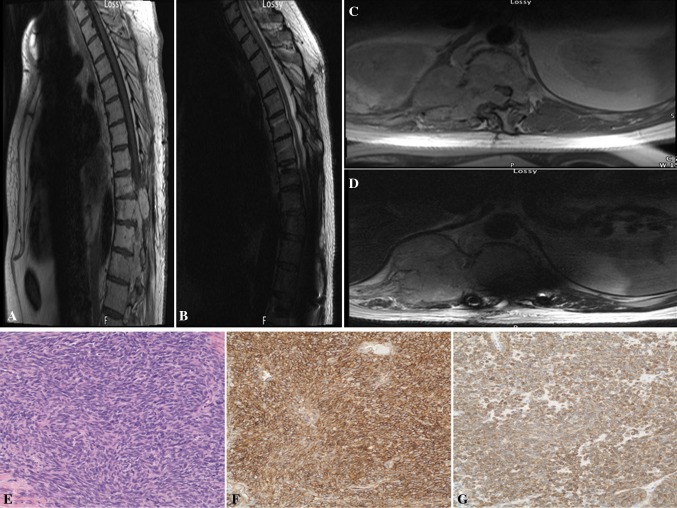
Fig. 3.
a, b Preoperative T1 sagittal and axial MRI with contrast showed a enhancing lesion at the T9–12 levels with severe compression of the spinal cord. c, d Postoperative T1 sagittal and axial MRI with contrast demonstrating recurrent tumor at T10–12 with complete obliteration of the spinal cord (d). e, f, g H&E stained sections revealed intersecting fascicles of tightly juxtaposed elongate tumor cells, associated with collagen bands (e). The tumor cells showed strong diffuse staining for CD34 and Bcl-2 (f, g, respectively). Original magnification ×200
Operation
Due to the vascular appearance of the tumor on CT and MRI, the patient was taken for preoperative embolization of the tumor via the right T8 and left T10 intrathoracic arteries. The following day, the patient was taken to the operating room where he underwent complete wide T9–12 laminectomies. Through a midline dural approach, the tumor was immediately identified by its reddish color and areas of hematoma and necrotic tissue. Significant adhesion and scar tissue were mobilized proximally, and the extramedullary tumor was debulked from T10–12. The spine was stabilized from T6–L3 with pedicle screw instrumentation.
Postoperative course
Four months following a stable postoperative course, the patient was readmitted emergently with gradual onset urinary incontinence and bilateral lower extremity incomplete paraparesis. MRI with gadolinium demonstrated a large intracanal recurrence of the tumor from T10–12, and complete obliteration of the spinal cord (Fig. 3c, d). The patient was emergently taken to the operative room for a revision tumor debulking and decompression. His motor strength remained unchanged postoperatively. He developed multiple metastatic tumors to non-spine areas including the iliac wing, left femur and right rib/chest wall. Despite palliative radiation therapy, he was eventually transferred to hospice care and expired a year from his second spinal decompression.
Pathology
Gross evaluation revealed a 3.5 × 3.0 × 2.0 cm fragment of firm gray-tan tissue. Microscopic sections disclosed metastatic sarcoma composed of intersecting fascicles of rather bland and mitotically inactive elongate nuclei with inconspicuous nucleoli associated with minimal amounts of pink cytoplasm (Fig. 3e). The tumor contained thick bands of collagen and only incipient foci of necrosis. Blood vessels showed “staghorn” appearance. The tumor was positive for CD34 and Bcl-2 (Fig. 3f, g, respectively), deposited pericellular reticulin fibers and showed 10 % Ki-67 proliferative index. The tumor was designated as a metastatic hemangiopericytoma.
Case 4
History and examination
A 57-year-old male with a past medical history of prostate cancer, presented with a 6-month period of worsening lower back pain. He had noticed a band of numbness across his abdominal area for years. With Valsalva, he experienced worsening of his pain and electrical shock sensations in his legs. On neurological exam, he displayed full strength in his bilateral lower extremities without hyperreflexia or clonus. His sensation was intact in all dermatomes. Radiographic findings are presented in Table 1 (Fig. 4a, b).
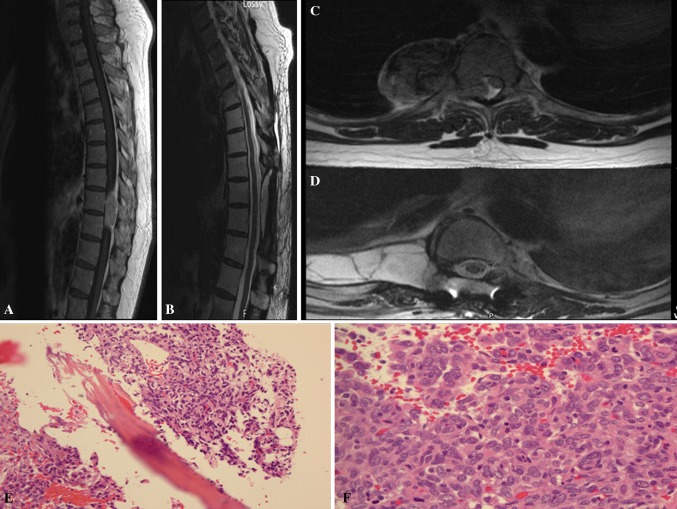
Fig. 4.
a, b Preoperative T1 sagittal and axial MRI with contrast showed a enhancing lesion at the T9–10 levels with severe compression of the spinal cord. c, d Postoperative T1 sagittal and axial MRI with contrast demonstrating resection of the tumor. e, f There is focal bony involvement by tumor (e, H&E, 20×) and increased mitosis f, 40×)
Operation
The patient was taken to interventional radiology and underwent successful embolization of the nutrient tumor vasculature via the T9 spinal artery. He was subsequently taken to the operating room for T9–10 wide laminectomy and micro-dissection of the intradural, extramedullary, as well as extradural component of the tumor. Tumor erosion of the 9th and 10th ribs was noted and the ribs were detached at the costovertebral joint. A large, gray tumor, noted to be attached to the T9 nerve root, was separated from the pleura and resected. The patient was stabilized from T7–11 with pedicle screw instrumentation.
Postoperative course
The patient had a transient right lower extremity weakness, which improved with rehabilitation. He also displayed a loss of sensation around the right T8–9 dermatomes. His postoperative MRI revealed a gross total resection without any further abnormalities (Fig. 4c, d). He underwent a course of postoperative radiation therapy (IMRT of 5580 cGy over 48 elapsed days) to his resection cavity. Currently, he is doing well and has remained tumor-free for over 3 years.
Pathology
The tumor is hypercellular consisting of spindle cells forming sheets with focal whorling pattern, mimicking a meningioma. The tumor cells show mild to moderate cytologic atypia with scattered mitosis (2–3/10 HPF) and focally involve the adipose tissue and bone (Fig. 4e). There is a focal area showing 3–4 mitoses/10HPF. In addition, there are findings consistent with pre-embolization-related changes. Multifocal remote hemorrhage and focal myxoid change are also noted. The classic HPC-like vascular pattern is apparent (Fig. 4f). Reticulin stain shows a predominantly pericellular pattern. Immunohistochemical studies show tumor cells to be positive for vimentin and CD34, and negative for EMA, AE1/AE3, and S-100.
Discussion
Hemangiopericytomas are aggressive, locally recurring malignant tumors that metastasize disproportionate to their incidence. They are still described by many to arise from pericytes, which are modified smooth muscle cells typically found around postcapillary venules [2]. A group at the WHO, however, is proposing that HPCs show no pericytic differentiation and are more fibroblastic in nature, placing them along the continuum of the cellular form of SFTs [3]. The histopathology of our first case was consistent with a WHO grade II and had the typical “staghorn” type vessels with focal mild to moderate cellularity. Intradural HPCs are rarely encountered primarily arising as extradural tumors. The first intradural case was reported in 1961 by Kruse [11]. Since then, 23 cases have been reported, including our four present cases [4–14]. The occurrence of HPCs has been reported throughout the cervical, thoracic and lumbar spine, but the cervicothoracic region appears to have the greatest prevalence (Table 2). Betchen et al. [5] reported that extradural tumors are associated with more pain than intradural tumors. In a review of the literature, 5 out of the 19 patients had pain complaints involving intradural lesions [4, 8, 9].
Table 2.
Cases of hemangiopericytoma of the spine described in present and previous reports
| Authors (references) | Case | Age (years)/sex | Location | Clinical presentation | Intramedullary? | Treatment | Recurrence | Follow-up | Survival (at last F/U) |
|---|---|---|---|---|---|---|---|---|---|
| Kruse [11] | 1 | 53/M | Cervical | L-sided weakness, hyperreflexia and paresthesia | No | GTR + RT | At 3 years | 4 years | Alive |
| Pitlyk et al. [12] | 2 | 60/M | Cervical | R-sided weakness and paresthesia | No | GTR | NR | None | NR |
| 3 | 49/F | Cervical | L-sided weakness and paresthesia | No | GTR | No | 10 years | Alive | |
| 4 | 39/M | Thoracic | Paraplegia | No | GTR | At 9, 17, and 18 years | 18 years | Alive | |
| Ciapetta et al. [7] | 5 | 48/M | Cervical | LUE paresthesia | No | GTR | At 6 years | 7 years | Alive |
| Dufour et al. [8] | 6 | 45/M | Cervical | Paraparesis | No | GTR | No | 2 years | Alive |
| 7 | 18/F | Dorsal | Dorsalgia | No | STR + RT | No | 4.6 years | Alive | |
| 8 | 43/F | Dorsal | Intercostal neuralgia | No | GTR | No | 4.1 years | Alive | |
| 9 | 38/M | Dorsal | Intercostal neuralgia | No | GTR | No | 12.6 years | Alive | |
| Someya et al. [13] | 10 | 68/F | Cervical | Foot drop and paresthesia | No | GTR + RT | No | 30 months | Alive |
| Betchen et al. [5] | 11 | 31/M | Lumbar | B/l LE paresthesia, bladder dysf | No | GTR | No | 6 months | Alive |
| Zhao and Zhao [14] | 12 | NR | Cervical | No extremity involvement | No | STR + RT | At 2 years | Mean = 4.7 years | Alive |
| 13 | NR | Cervical | No extremity involvement | No | GTR | Unknown time | Mean = 4.7 years | Alive | |
| 14 | NR | Thoracic | No extremity involvement | No | GTR | No | Mean = 4.7 years | Alive | |
| 15 | NR | Thoracic | No extremity involvement | No | GTR | No | Mean = 4.7 years | Alive | |
| Kashiwazaki et al. [10] | 16 | 31/M | Subpial | L-sided hyperreflexia and numbness | Yes | GTR | No | 3 years | Alive |
| Fitzpatrick et al. [9] | 17 | 54/M | Lumbar | L-paraspinal, buttock, and anterolateral thigh pain | No | NR | NR | NR | NR |
| Chou et al. [6] | 18 | 80/M | Thoracic | B/l paresthesias, weakness of LE and bladder dysf | Yes | GTR | No | 3 years | Alive |
| Ackerman et al. [4] | 19 | 58/M | Thoracic | Back pain that radiated down b/l thighs ant and post | NR | GTR | NR | NR | NR |
M male, F female, R right, L left, B/l bilateral, LE lower extremity, UE upper extremity, GTR gross total resection, STR subtotal resection, RT radiation treatment, NR not reported
Radiographic evaluation
Most radiographic findings are non-specific and share similar imaging characteristics with other tumors, whether intracranial or intradural. Computed tomography (CT) imaging typically shows a hyperintense dural-based lesion that homogeneously enhances strongly with contrast [15]. MRI findings on T1- and T2-weighted imaging are usually of an isodense lesion that enhances heterogeneously with contrast [16]. However, our first case was slightly hyperintense on T1- and T2-weighted imaging and enhanced homogenously with contrast. These findings further support the non-specificity of radiographic findings. Flow voids are a frequent finding with HPC, more so than with meningiomas. In addition, intradural tumors are often well-circumscribed lesions that enhance markedly with contrast, making the diagnosis difficult [4–6, 9, 10, 14]. They are often misdiagnosed as a meningioma, schwannoma, neurofibroma, and less frequently as ependymoma, sarcoidosis, SFT, and lymphoma [16]. Angiography may be considered and has been recommended to aid in diagnosis and preoperative planning of HPCs, but these characteristics are also non-specific and superior ways of controlling intraoperative bleeding are now recommended [17].
Surgical treatment
Aggressive gross total resection (GTR) has been advocated by most as reports have found greater recurrence-free interval (RFI) and overall survival (OS) in patients [5, 17–21]. Schiariti et al. [20] reported that patients with GTR had both longer mean OS (235 vs. 175 months, respectively; p = 0.47) and mean RFI (117 vs. 53 months, respectively; p = 0.0045). Soyuer et al. [21] reported the 5-year local control rates for patients treated with GTR and subtotal resection were 84 and 38 %, respectively (p = 0.0034). Of the reported follow-up data in this review, only 2 out of the 19 did not receive GTR. Of note, the earliest recurrence occurred in one of the two patients who did not undergo GTR even after receiving radiotherapy. Of the cases with follow-up data regarding survival (12) and recurrence (14), the 2-year survival rate was 100 % (12/12) and the recurrence rate from 6 months to 18 years was 5/14 (35 %). The tumors with intramedullary involvement had a recurrence rate of 0 % and overall survival rate of 100 % at 3 years. In our series, three of the four patients with gross tumor resection have done well during the 3 years of follow-up, with one patient who had recurrence without significant tumor removal unfortunately passing away.
Adjuvant treatment
Radiotherapy has been studied to a greater extent with intracranial tumors and while inconclusive overall, has shown a trend of increased local control and overall survival [17–21]. In the present literature review, 5 of 19 patients received radiotherapy; no conclusion could be drawn regarding its effectiveness since doses were not reported in each case. Guthrie et al. [18] reported that 9 of 17 irradiated tumors recurred on average 75 months after surgery, while 13 of 15 non-irradiated tumors recurred on average 34 months after surgery (p < 0.05). Irradiated patients survived 30 months longer than non-irradiated. The 5-, 10-, and 15-year recurrence rates for irradiated tumors were 38, 64, and 80 %, respectively. The effective radiation dose is quoted by most studies to be >50 Gy [17–19, 21–24]. In a recent systematic review of 563 intracranial HPCs cases (277 had follow-up data), Rutkowski et al. [25] reported that patients receiving >50 Gy of radiation had worse survival outcomes (median survival 4 vs. 18.6 years, p < 0.01). This study notes the possibility of selection biases in tumor quality and more aggressive tumors receiving higher doses. In our patient series, three had radiation therapy, however, our first patient did not undergo radiation due to complete tumor resection, isolated low-grade tumor, and young age. While tumor control is obtained more often with radiotherapy than surgery alone, many studies fail to see a significant gain in overall survival.
Prognosis and recurrence
The rarity of HPCs makes it difficult to gather conclusive information pertaining to the most effective plan of treatment. Even with local control rates of 76 % at 5 years in one study, metastasis still occurred in 29 % of patients [24]. Stereotactic radiosurgery, although few in numbers and limited follow-up, is a treatment that shows comparable control rates with local aggressive, recurrent, or metastatic disease without the morbidity associated with external beam radiation [17, 24, 26]. Chemotherapy is another treatment modality that has largely been ineffective but new studies using antiangiogenic drugs may yield better results [15, 27, 28]. The prognosis of the current reported case remains guarded due to the history of this tumor recurring as late as 26 years [5]. In this review of the literature, patients had recurrences ranging from 2 to 18 years later. This information underlies the importance of close follow-up over a prolonged period of time. Betchen et al. [5] reported extradural tumors recurred earlier than intradural tumors at 2.6 versus 6 years, respectively. It appears that intradural tumors carry a better prognosis than extradural spinal tumors, however, longer follow-up studies are needed in conjunction with larger numbers of patients to make any significant conclusions.
Current recommendations
The current recommendation for spinal hemangiopericytomas is en bloc resection along with the dura when feasible. Tumors where GTR or en bloc resection is not possible should be debulked after preoperative embolization if HPC is expected or recurrent. Stabilization of the spine is recommended based on location and extent of bone involvement. There has been no consistent benefit of radiotherapy regarding overall survival or recurrence for spinal tumors, irrespective of resection type, and we currently do not recommended it following GTR. Radiotherapy is recommended for cases involving subtotal resection, higher-grade tumors, and recurrences where repeat surgery would have significant morbidity. However, no therapy has shown to be effective in preventing metastasis. The course of treatment should be customized for each individual patient and long-term follow-up should be employed with more aggressive surveillance in the first 5 years after treatment whether dealing with primary or recurrent disease.
Conclusion
Hemangiopericytomas are extremely rare tumors. Diagnosis is difficult due both to its infrequency and to the non-specific findings on both advanced imaging and physical exam. Our case series highlights the exceedingly rare extension of HPC into the intradural and intramedullary tissue, and the essential role of histopathology in the diagnosis. Surgically, gross total resection is the mainstay of treatment and should be pursued with plans for long-term follow-up care. Radiation therapy and non-surgical interventions have both been shown to have inconclusive outcomes. Ultimately, increased awareness that these tumors can occur intradurally and intramedullarly will aid a surgeon in diagnosis, minimizing local recurrence and maximizing overall patient survival.
Conflict of interest
None of the authors has any potential conflict of interest.
Footnotes
Drs. A. Shirzadi and D. Drazin contributed equally to this work.
Contributor Information
Doniel Drazin, Email: doniel.drazin@cshs.org.
J. Patrick Johnson, Phone: +1-310-4237900, Email: Spineexperts@aol.com
References
- 1.Stout AP, Murray MR. Hemanigopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. 1942;116:26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giannini C, Rushing EJ, Hainfellner JA. Haemangiopericytoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO classification of tumours of the central nervous system. 4. Lyon: International Agency for Research on Cancer (IARC); 2007. pp. 178–180. [Google Scholar]
- 3.Sundaram C, Shantveer UG, Megha US, Rekha SJ, Panigrahi KM, Purohit AK, Rammurti S. A clinicopathological and immunohistochemical study of the central nervous system hemangiopericytomas. J Clin Neurosci. 2010;17:469–472. doi: 10.1016/j.jocn.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman PD, Khaldi A, Shea JF. Intradural hemangiopericytoma of the thoracic spine: a case report. Spine J. 2011;11(7):e9–e14. doi: 10.1016/j.spinee.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 5.Betchen S, Schwartz A, Black C, Post K. Intradural hemangiopericytoma of the lumbar spine: case report. Neurosurgery. 2002;50:654–657. doi: 10.1097/00006123-200203000-00045. [DOI] [PubMed] [Google Scholar]
- 6.Chou CW, Hsu SPC, Lin SC, Chen MH, Shih YH, Lee LS, Lin CF. Primary intradural hemangiopericytoma with intramedullary invasion. J Chin Med Assoc. 2009;72:536–541. doi: 10.1016/S1726-4901(09)70424-1. [DOI] [PubMed] [Google Scholar]
- 7.Ciappetta P, Celli P, Palma L, Mariottini A. Intraspinal hemangiopericytomas. Report of two cases and review of the literature. Spine. 1985;10:27–31. doi: 10.1097/00007632-198501000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Dufour H, Metellus P, Fuentes S, Murracciole X, Regis J, Figarella-Branger D, Grisoli F. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of the postoperative external radiotherapy. Neurosurgery. 2001;48:756–763. doi: 10.1097/00006123-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick D, Mahajan J, Lewkowitz M, Black K, Setton A, Woldenberg R. Intradural hemangiopericytoma of the lumbar spine: a rare entity. AJNR. 2009;30:152–154. doi: 10.3174/ajnr.A1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kashiwazaki D, Hida K, Yano S, Seki T, Iwasaki Y. Subpial hemangiopericytoma with marked extramedullary growth: case report. Neurosurgery. 2007;61:1336–1337. doi: 10.1227/01.neu.0000306116.93291.94. [DOI] [PubMed] [Google Scholar]
- 11.Kruse F., Jr Hemangiopericytoma of the meninges (angioblastic meningioma of Cushing and Eisenhardt). Clinico-pathologic aspects and follow-up studies in 8 cases. Neurology. 1961;11:771–777. doi: 10.1212/WNL.11.9.771. [DOI] [PubMed] [Google Scholar]
- 12.Pitlyk PJ, Dockery MB, Miller RH. Hemangiopericytoma of the spinal cord: report of three cases. Neurology. 1965;15:649–653. doi: 10.1212/WNL.15.7.649. [DOI] [PubMed] [Google Scholar]
- 13.Someya M, Sakata K, Oouchi A, Nagakura H, Satoh M, Hareyama M. Four cases of meningeal hemangiopericytoma treated with surgery and radiotherapy. Jpn J Clin Oncol. 2001;31:548–552. doi: 10.1093/jjco/hye116. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Zhao JZ. Clinical and pathological characteristics of primary intraspinal hemangiopericytoma and choice of treatment. Chin Med J (Engl) 2007;120:115–119. [PubMed] [Google Scholar]
- 15.Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery. 2008;63:720–727. doi: 10.1227/01.NEU.0000325494.69836.51. [DOI] [PubMed] [Google Scholar]
- 16.Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for treatment of recurrent intracranial hemangiopericytoma. Neurosurgery. 2002;51:905–911. doi: 10.1097/00006123-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ecker RD, Marsh WR, Pollock BE, et al. Hemangiopericytoma in the central nervous system: treatment, pathological features, and long-term follow up in 38 patients. J Neurosurg. 2003;98:1182–1187. doi: 10.3171/jns.2003.98.6.1182. [DOI] [PubMed] [Google Scholar]
- 18.Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25:514–522. doi: 10.1227/00006123-198910000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Jung HW, Kim YS, Kim CJ, Hwang SK, Paek SH, Kim DG, Kwun BD. Meningeal hemangiopericytomas: long-term outcome and biological behavior. Surg Neurol. 2003;59(1):47–54. doi: 10.1016/S0090-3019(02)00917-5. [DOI] [PubMed] [Google Scholar]
- 20.Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. J Neurosurg. 2011;114:747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]
- 21.Soyuer S, Chang EL, Selek U, et al. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100:1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 22.Combs SE, Thilmann C, Debus J, Schulz-Ertner D. Precision radiotherapy for hemangiopericytomas of the central nervous system. Cancer. 2005;104:2457–2465. doi: 10.1002/cncr.21448. [DOI] [PubMed] [Google Scholar]
- 23.Schirmer CM, Heilman CB. Hemangiopericytomas of the skull base. Neurosurg Focus. 2011;30(5):E10. doi: 10.3171/2011.2.FOCUS119. [DOI] [PubMed] [Google Scholar]
- 24.Sibtain NA, Butt S, Connor SEJ. Imaging features of central nervous system haemangiopericytomas. Eur Radiol. 2007;17:1685–1693. doi: 10.1007/s00330-006-0471-3. [DOI] [PubMed] [Google Scholar]
- 25.Rutkowski MJ, Sughrue M, Kane AJ, Aranda D, Mills SA, Barani IJ, Parsa AT. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010;113:333–339. doi: 10.3171/2010.3.JNS091882. [DOI] [PubMed] [Google Scholar]
- 26.Veeravagu A, Jiang B, Patil CG, Lee M, Scoltys SG, Gibbs IC, Chang SD. CyberKnife stereotactic radiosurgery for recurrent, metastatic, and residual hemangiopericytomas. J Hem Onc. 2011;4:26. doi: 10.1186/1756-8722-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bramwell VH, Anderson D, Charette ML. Doxorubicin-based chemotherapy for the treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003;3:CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park MS, Patel SR, Ludwig JA, Trent JC, Conrad CA, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer. 2011;117(21):4939–4947. doi: 10.1002/cncr.26098. [DOI] [PMC free article] [PubMed] [Google Scholar]