Abstract
Objective
Oil-based contrast media such as Pantopaque have not used for imaging for several decades, but because these contrast media have an extremely low clearance rate, the remnant contrast media or residual sequelae of these materials may be encountered in the clinical field.
Clinical presentation
A 63-year-old woman presented to our hospital complaining of increasing lower back pain and lower extremity paresthesia with incontinence for 2 years. A plain X-ray film revealed single droplet-like mass at the lower thoracic T9–T10. A magnetic resonance image (MRI) study revealed a dorsally placed extramedullary intradural lesion, compressing the thoracic cord and minimally displacing it anteriorly. Spinal stenosis was also noted at the L4–5 level.
Intervention
The patient was performed for two consecutive surgeries. Total laminectomy was performed at T9–T10 to remove mass. A 0.5 × 0.5 × 4 cm yellowish intradural extramedullary cystic mass was removed without any leakage of cystic contents. Partial hemi-laminectomy and foraminotomy was then done at L4–5 levels for radiculopathy symptom relief. The fluid from the cyst was composed mainly of iodide.
Conclusion
Intraspinal masses showing metal-like density in X-ray or computed tomography but in MRI showing only lipoma or cystic lesions, not metallic characteristics, the differential diagnosis should include iophendylate (Pantopaque) cyst. Oil-based contrast medium is believed to have the potential to make a syrinx formation via arachnoiditis, which can lead to severe neurologic deteriorations, so even if the patients do not represent with an acute neurologic deficit, surgical total removal of remnant material without leaking should be considered.
Keywords: Arachnoiditis, Arachnoid cysts, Contrast media, Iophendylate, Pantopaque
Introduction
Before the introduction of computed tomography (CT) scanning, magnetic resonance imaging (MRI), or other water-soluble contrast medium, iophendylate (Pantopaque or Myodil), an oil-based contrast media, was widely used through the 1980s for myelograms and other contrast dependent studies. However, this oil-based contrast media was known to have an extremely low clearance rate from cerebrospinal fluid, which has led to concerns about the development of arachnoiditis.
Through the 1980s, many cases were reported of complications and sequelae following the use of Pantopaque. In the 1990s, oil-based contrast media, like Pantopaque, were replaced with water-soluble contrast media. With this change, the incidence of sequelae or complications of oil-based contrast media largely disappeared.
However, occasional residual evidence and effects of Pantopaque used in the past are still encountered clinically. Adverse effects of oil-based dye, both acute and chronic, continue to be reported.
We will introduce our case of a Pantopaque cyst in the intradural and extramedullary space mimicking lipoma and will review the articles dealing with various symptoms and clinical and radiologic figures of Pantopaque cyst.
Clinical presentation
History
A 63-year-old woman presented to our hospital complaining of increasing low back pain and lower extremity paresthesia with incontinence for 2 years with aggravation during the previous 2 months. She also complained of claudication. Her medical history was significant for symptoms 30 years earlier which were treated with a laminectomy at L4–5 and L5–S1 level. For relief of recent symptoms including back pain and claudication of low extremity, she had received nerve blocking in the lumbar area several times during the previous 2 months. In the recent medical history, she had no examinations using contrast agents. MRI at admission to our hospital revealed an intra-thecal extramedullary mass posterior to T9–T10.
Physical examination
The findings of a motor examination consisted of normal tone in both lower extremities. Motor power was rated 5/5 throughout all muscle groups in the upper extremities. Lower extremity strength was symmetric and rated 5/5 throughout. Anal sphincter tone was normal. A sensory examination revealed diffuse paresthesia at the dermatome level L5–S1 over both lower extremities, with greater severity on the right side. Sensation in the upper extremity and trunk was intact bilaterally. Performance on straight leg raising test showed limitation at 70° on both lower extremities. Reflexes were normal and symmetric in the upper and lower extremities. Pathologic Babinski’s sign and ankle clonuses were not seen. There was no evidence of upper motor neuron signs. A 5-cm scar from the previous operation was noted at the patient’s low back area.
Radiologic imaging (Fig. 1)
Fig. 1.

a Pre-operative radiologic findings. a Lateral X-ray of thoracic spine. b Sagittal T2 weighted image of magnetic resonance image (MRI). c Pre-operative computed tomography (CT) image at T9/10 level. d Axial T2 weighted image of lumbar 4/5 disc level
A plain X-ray film revealed a single droplet-like mass at the T10 spinal canal level. An MRI study of the thoracolumbar region revealed a dorsally placed extramedullary intradural lesion at T10, compressing the thoracic cord. The abnormal areas were non-enhancing, high signal on both T1 and T2 weighted images. Axial MRI scans at the same levels revealed an ovoid and slightly right-displaced, well-defined mass. This mass was compressing the thoracic spinal cord, which was minimally displaced anteriorly. There was no syrinx or intramedullary abnormal signal. In the CT study of the same region, there was also a high-density mass noted at the same location.
Axial and sagittal MRI scans at the L4/5 levels revealed spinal stenosis with mild disc protrusion and foraminal stenosis of both sides. There was no evidence of severe arachnoiditis such as clumping of nerve roots at any spinal level.
Operation
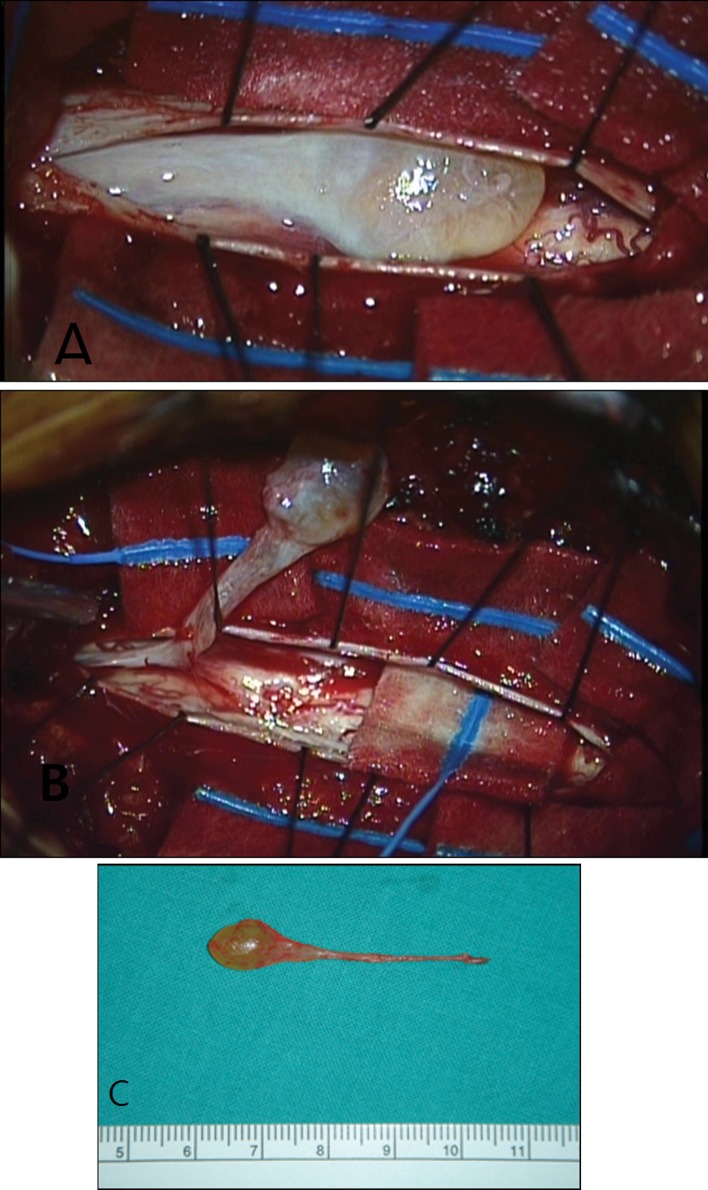
The patient was scheduled for two consecutive surgeries. Total T9–T10 laminectomy was performed. Localization of the radio-opaque mass was performed using C-arm image intensifier. A 5-cm linear incision was made on the intact dura at the midline. After multiple tenting sutures, a 0.5 × 0.5 × 4 cm yellowish intradural extramedullary cystic mass was revealed. The ventral portion of the cyst was adhered to arachnoids of the thoracic spinal cord. Under microscopic magnification, the arachnoids, which was thickened and adherent to the spinal cord, were carefully dissected. Fine arachnoid dissection was performed and the cystic mass was totally removed en-bloc (Fig. 2). We also could figure out this mass with C-arm image intensifier. The mass was seen in the C-arm image intensifier as a “drop with tail”. The cystic wall was not torn with dissection, so cystic fluid was not leaked. There was no damage to the thoracic spinal cord and no definite vascular or anatomical abnormal findings (Fig. 3). Only minimal arachnoid thickening was noted around the cystic mass. The remnant of thickened arachnoid was dissected off the posterior aspect of the cord and was removed. Partial hemi-laminectomy and foraminotomy was done at L4/5 levels for radiculopathy symptom relief. Finally, a 27-gauge needle was inserted into the cyst and 1.7 cc yellowish clear fluid was aspirated.
Fig. 2.
a Yellowish cystic mass was noted in the intradural extramedullary space. b Under microscopic magnification, fine arachnoid dissection was performed and the cystic mass was totally removed en-bloc manner. c The cystic mass was 0.5 × 0.5 × 4 cm
Fig. 3.
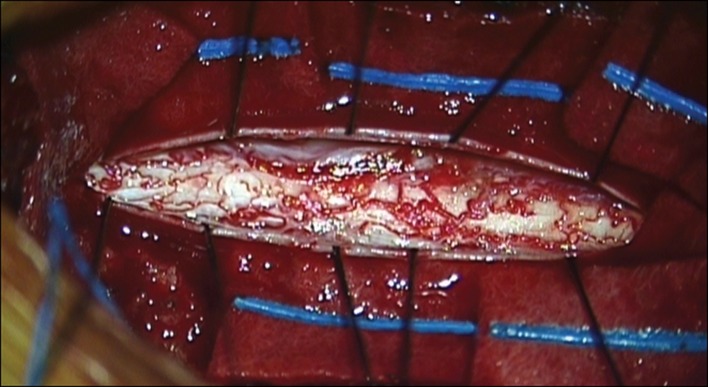
During and after the dissection, no definite vascular or anatomical abnormal findings of the spinal cord were noted. Only minimal arachnoid thickening was noted around the cystic mass. The remnant of thickened arachnoid was dissected off of the posterior aspect of the cord and was removed
Pathological findings
Specimens were submitted for pathological evaluation. The cyst wall revealed no epithelial lining. Tissue from the arachnoid wall was consistent with a benign arachnoid cyst. The fluid from the cyst was sent to the advanced analysis center of Korea Institute of Science and Technology. The result was the fluid comprised mainly of iodide.
Postoperative course
In the postoperative X-ray, the radio-opaque mass was removed totally and there were no operative or postoperative complications. The patient reported about 50 % improved sensation in both lower extremities, compared to the preoperative state. There was no sign of cerebrospinal fluid leak and both wounds were clean. Two months after the operation, bilateral lower extremity radiculopathy and claudication, which the patient had complained, were improved. Paresthesia on both extremities was not seen on examination. No other neurologic deficits were noted.
Discussion
When we viewed the radiologic results for this patient, we were initially unsure of the correct diagnosis. Our first impression was intradural extramedullary lipoma, but this diagnosis did not explain the high-density figure in the simple X-ray or CT. The presence of a metallic foreign body was also considered, but MRI findings showed no metallic artifact, and MRI signal intensity did not indicate the metal. Hemorrhage, calcified neoplasms, such as dermoid cyst or hemangiomas, also did not fit our radiologic findings. After searching online and reviewing the literature, we were satisfied we had reached a proper diagnosis for these radiologic findings.
Literature review
Before the end of the 1980s, oil-based contrast media were used widely, and many articles were published about these contrasts. Beginning in the 1990s, oil-based contrast media were rarely used [1, 2], and consequently, fewer articles about the media were published.
The authors of this paper wanted to evaluate the long-term effects of the use of oil-based contrast media to the spinal cord and spinal nerve, so we reviewed only the articles published after 1990.
The terms “Pantopaque”, “Myodil”, and “Iophendylate” were used as search terms in the published English language literature. We searched several electronic databases. Included in our analysis were articles published after 1990, treating adult patients of either gender with chronic back, neck, and/or extremity pain, who were treated or diagnosed with oil based contrast media. The literature revealed that oil-based contrast media were used for many other organs, but we included only articles that dealt with spinal lesions.
To the best of our knowledge, we identified a total of nine articles. These nine articles are summarized in Tables 1, 2, and 3, showing radiologic findings (Table 1), symptoms and neurologic findings (Table 2), and treatments (Table 3).
Table 1.
Radiologic findings of reviewed articles
| Author | Year | Location | Radiologic finding |
|---|---|---|---|
| Gopalakrishnan et al. [6] | 2010 | T6–T10 | Multiple cerebrospinal fluid (CSF) loculations anterior to the spinal cord at T6–T10 levels displacing the cord posteriorly Multiple hyper-intensities within the cord and cord atrophy Small intramedullary CSF intensity longitudinal cleft was also seen suggestive of syrinx |
| Kubota et al. [3] | 2008 | T10–T11 | Multiple droplet-like enhancements in the lower thoracic spinal canal Syrinx at the T10–T11 vertebrae and signal flow voids, suggesting venous congestion around that lesion |
| Hwang et al. [4] | 2008 | T6–T8 | Some mild contrast enhancement and several loculated cystic areas that contributed to the appearance of a dilated spinal cord Intradural intramedullary lesion |
| Krishnamoorthy et al. [9] | 2006 | Scattered T5–T11 | Dorsal to the spinal cord, focal linear hyperintense intramedullary signals, distorted cord contour |
| Navani et al. [7] | 2006 | L5–S1 | Fat containing fluid collection was 1.5–2 cm and yielded a volume of 1–2 mL A fluid–fluid level with CSF signal superiorly and with a fat signal on T1-weighted and T2-weighted images |
| Gnanalingham et al. [2] | 2006 | T6–T8, T10–T12 |
An intramedullary lesion extending from T6 to T8 and an anteriorly placed extramedullary, intradural lesion at T10–T12, compressing the thoracic cord Non-enhancing, low signal on T1 weighted images and high signal on T2 weighted images, in keeping with a thoracic syrinx and an arachnoid cyst, respectively Clumping of the nerve roots, suggestive of arachnoidal adhesions Myodil droplets were not seen |
| Shah et al. [10] | 2001 | Scattered T2–T10 | Multiple areas of extra-medullary fat signal intensity (hyperintense on T1- and hypointense on T2 weighted images) areas in the thecal sac, mainly at levels T2/T3, T7/T8 and T9/T10 The spinal cord was adherent to the posterior aspect of the thecal sac at levels T7/T8 and T9/T10. Thinned cord: T7–T10 An ill-defined hyperintensity in the cord (T2 weighted images): T7–T11 A long segment of CSF signal intensity lesion causing an impression on the anterior aspect of the cord |
| Fitzpatrick et al. [8] | 1999 | T10 | Single round, smooth margined intradural extramedullary cyst |
| Tabor et al. [5] | 1996 | T10–T11 | High signal on both pre- and post-contrast scans to the right of the cord at T10–T11 with cord compression A spinal angiogram demonstrated that no tumor blush was seen Syringomyelic cavity extending from C7 to T10 |
Table 2.
Symptoms and neurologic findings of reviewed articles
| Author | Year | Location | Symptoms and neurologic finding |
|---|---|---|---|
| Gopalakrishnan et al. [6] | 2010 | T6–T10 | Progressive weakness of both lower limbs: for 3 years Severe spasticity involving both lower limbs with mild asymmetric weakness Exaggerated deep tendon reflexes in both lower limbs with bilateral ankle clonus Sensory level to pinprick and light touch starting at T8 Urinary urgency: for 6 months |
| Kubota et al. [3] | 2008 | T10–T11 | Hypesthesia of right leg, distally dominant paraparesis. Hypesthesia at the lateral surface of his right lower leg extending to the sole of his foot The joint position sense and the vibratory sense were impaired, and the patellar tendon reflexes and Achilles reflexes were absent bilaterally |
| Hwang et al. [4] | 2008 | T6–T8 | Gait difficulty, urinary incontinence, rectal incontinence, lower extremity muscle spasms, pain radiating in the T6 dermatome bilaterally, a mid-thoracic sensory level A sensory level to pinprick and light touch starting at T-4 and was hyper-reflexive in her lower extremities Several beats of clonus in her feet and had bilaterally up going plantar reflexes. Stiff and spastic gait and decreased rectal tone |
| Krishnamoorthy et al. [9] | 2006 | Scattered T5–T11 | Very slowly progressive weakness of both lower limbs of 6 years duration Low extremity motor grade 3. Deep tendon reflexes were exaggerated |
| Navani et al. [7] | 2006 | L5–S1 | Chronic low-back pain, radiation along the posterior and lateral aspects of her thighs and legs down to the ankles bilaterally Decreased range of motion of the lumbar spine to 30° during forward flexion and exacerbation of her low back |
| Gnanalingham et al. [2] | 2006 | T6–T8, T10–T12 |
Chronic thoraco-lumbar back pain Progressive bilateral lower limb weakness and spasm Abnormal sensation on the soles of the feet 3-Month history of urinary and fecal urgency Spastic paraparesis, with an up going plantar response on the right Bilateral weakness of hip flexion (motor grade 4) Difficulty walking independently Reduced proprioception at the toes bilaterally |
| Shah et al. [10] | 2001 | Scattered T2–T10 | Chronic backache, mainly in the dorsal and lumbar regions, associated with episodic shooting pain (in the lower limbs, trunk and chest, without any provocation or posture change) Power of grade 3–5 in both the lower limbs Reduced muscle mass particularly in the left lower limb Increased tone and positive Babinski’s sign bilaterally Ankle, knee and abdominal reflexes were exaggerated A few patchy areas of sensory loss—over the trunk and lower limbs |
| Fitzpatrick et al. [8] | 1999 | T10 | Back pain and decreased sensory Lt trunk Radiculopathy T10 |
| Tabor et al. [5] | 1996 | T10–T11 | Significantly increased tone in both lower extremities Performance on heel-to-shin testing: slow and difficult to assess because of severe spasticity Diffusely decreased sensation to both light touch and pinprick over both lower extremities, the deficit being most dense below the knees |
Table 3.
Treatments of reviewed articles
| Author | Year | Location | Treatment |
|---|---|---|---|
| Gopalakrishnan et al. [6] | 2010 | T6–T10 | Surgical decompression with arachnoid cyst removal |
| Kubota et al. [3] | 2008 | T10–T11 | Surgical decompression with cyst removal Syrinx–subarachnoid and subarachnoid–subarachnoid shunt |
| Hwang et al. [4] | 2008 | T6–T8 | Surgical decompression with cyst removal and resection of the mass (Intraoperatively, the spinal cord was displaced anteriorly by a large, calcified mass adherent to the arachnoid and dura. The calcifications were so large and dense that they had to be removed using a Kerrison) |
| Krishnamoorthy et al. [9] | 2006 | Scattered T5–T11 | Conservative treatment |
| Navani et al. [7] | 2006 | L5–S1 | Conservative treatment—epidural steroid injection |
| Gnanalingham et al. [2] | 2006 | T6–T8, T10–T12 |
Surgical decompression Syringo- and cysto-subarachnoid shunts |
| Shah et al. [10] | 2001 | Scattered T2–T10 | Surgical decompression with cyst removal (the cyst was found to be of the communicating type and extended from the vertebral levels T7 to T11. The cyst was resected and the cord was freed from the adhesions) |
| Fitzpatrick et al. [8] | 1999 | T10 | Surgical decompression |
| Tabor et al. [5] | 1996 | T10–T11 | Surgical decompression with cyst removal |
Radiologic findings
In the nine cases we reviewed, the most common radiologic feature of MRI was region of high signal intensity (SI) on T1 weighted image and iso- to high SI on T2 weighted image, without enhancement with contrast at thoracic spine. There were many variations between cases and they are summarized in Table 1. In some cases, multiple enhancements were noted [3–5]. Secondary anatomical changes, such as syrinx [2, 5, 6] or arachnoid cysts or fluid–fluid level forming cysts [6, 7], and clumping of the nerve roots due to arachnoiditis were also reported. Calcified masses were also noted and regarded as results of inflammation [4].
Except for one case, which was located at the lumbosacral junction [7], all the cases developed at the thoracic spine level, where lesions could be noted both intramedullary and extramedullary. The number of lesion also varied; in some articles only one lesion was noted, but in other articles multiple lesions were scattered in the spinal cord.
In our case, located at the T9–T10 level, the lesion was high signal intensity at both T1-weighted image and T2-weighted image showing a single intradural extramedullary mass with round and smooth margins with a tail. Both the round cystic portion and the tail portion were radio-opaque in X-ray and connected intra-lumenally.
Symptoms and neurologic findings
Except for two of the nine cases [7, 8], motor weakness was noted. Upper motor neuron signs such as increased deep tendon reflexes, urinary incontinence, fecal urgency, positive Babinski’s sign were also noted. Severe spasticity of both the lower extremities or reduced muscle mass was noted in some cases. Back pain, sensory change, hypesthesia, or sensory loss is common in some articles.
Four of the nine articles we reviewed showed a distinguishing pattern of progressive motor weakness [2, 5, 9, 10]. However, in our case, the patient had only back pain and L5 dermatome hypesthesia, with no prominent motor weakness. It is not commonly believed that radio-opaque masses at the T9/10 level cause these symptoms, so there was some debate among our team about the efficacy of operating on the T9/10 mass, since the patient’s spinal stenosis and foraminal stenosis with disc extrusion at L4/5 might have been the cause of the symptoms (Table 2).
Treatment
Of the nine cases we reviewed, seven were treated surgically and two were treated conservatively (Table 3; [7, 9]). Mass removal and cystic wall removal was the main surgical procedure, and in cases where a syrinx had a compressive effect on the cord, syrinx–subarachnoid or subarachnoid–subarachnoid shunt or cysto-subarachnoid shunting was also performed. Adhesiolysis, or removal of thickened arachnoid membrane, was also performed in some cases. Of the two conservative cases, one case was treated with an epidural steroid injection procedure [7].
In our case, we completed two different surgical approaches. The first was made at T9/10 for cyst removal. The second was made at L4/5 with partial hemi-laminectomy and foraminotomy for L4/5 spinal stenosis and foraminal stenosis. In pre-operative MRIs, we noted that the radio-opaque cyst had compressed the thoracic spinal cord, so the thoracic spinal cord was slightly displaced anteriorly. Despite this, the patient did not show any abnormal neurologic, motor system, or upper motor neuron symptoms indicating cord compression. This is consistent with other radio-opaque Pantopaque cysts, which do not typically cause significant symptoms or neurologic deficits. In these cases, there may be controversy about performing an operation on a patient, who has no, or only mild symptoms.
Controversy over indication for operation
Pantopaque is well known for its ability to create both clinical and subclinical arachnoid reactions. Pantopaque has been known to be cleared by a process in which the dye is encysted in the subarachnoid space in approximately 6 days and absorbed at a rate of 0.5–3 cc per year [7]. This extremely slow clearance rate can be another explanation for syrinx formation that is another major feature of Pantopaque cysts. The syringomyelic cavity may have been related to the focal arachnoid scarring, which interferes with normal cerebrospinal fluid dynamics in the subarachnoid space [5].
However, in some cases, like ours, this process is halted by a well-encapsulated cyst wall, which seems to halt problems such as progressive neurologic deterioration. In these circumstances, clinicians may hesitate to provide aggressive surgical removal of these “silent Pantopaque cyst region.”
Some studies suggest that degenerative disc disease, or minor spine trauma, interaction with blood and exaggerated effect with incomplete removal of contrast medium could have a synergistic role in arachnoiditis [5, 11, 12]. This means that even though well sealed Pantopaque cysts may not cause any problems for several decades, common conditions may trigger progression of sealed region. Because there is not a particular surgical treatment option for spinal arachnoiditis, preventive procedure for Pantopaque cysts could be important before leaking contents or rupture of cyst wall cause rapidly accelerating neurologic deficits.
Although Pantopaque has fallen out of favor, residual sequelae of its long-term use may continue to present themselves infrequently in clinically interesting and surgically challenging ways. So even if there are no definite present neurologic deficits, Pantopaque cysts should be removed because of their potential to make a long-term arachnoiditis. In our case, the patient’s Pantopaque cyst was discovered accidently. Even though that Pantopaque cyst was not causing any neurologic deficits, we believe we made the best choice to surgically remove it, without leakage of the cyst contents. Because of this surgery, we may have prevented long-lasting arachnoiditis and its complications such as syringomyelia.
Conclusion
Oil-based contrast media have not been used for several decades, so many current clinicians have no clinical information or experience with these materials. However, because these contrast media have extremely low clearance rates, the remnant contrast media or residual sequelae of these materials could be encountered in present-day clinical field. Intraspinal masses showing metal density in X-ray or computed tomography, but not showing a metallic characteristic in MRI and showing only lipoma or cystic lesion in MRI should include the differential diagnosis of Pantopaque cyst. Even though patients with these cysts do not typically experience acute neurologic deficits, total surgical removal of remnant material, without causing leaks, should be considered. Because oil-based contrast media is believed to have the potential to make a syrinx formation via arachnoiditis, without removal they can lead to severe neurologic deteriorations.
Conflict of interest
None of the authors has any potential conflict of interest.
References
- 1.Guyer DW, Wiltse LL, Eskay ML, Guyer BH. The long-range prognosis of arachnoiditis. Spine. 1989;14:1332–1341. doi: 10.1097/00007632-198912000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Gnanalingham KK, Joshi SM, Sabin I. Thoracic arachnoiditis, arachnoid cyst and syrinx formation secondary to myelography with Myodil, 30 years previously. Eur Spine J. 2006;15(Suppl 5):661–663. doi: 10.1007/s00586-006-0204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubota M, Shin M, Taniguchi M, Terao T, Nakauchi J, Takahashi H. Syringomyelia caused by intrathecal remnants of oil-based contrast medium. J Neurosurg Spine. 2008;8:169–173. doi: 10.3171/SPI/2008/8/2/169. [DOI] [PubMed] [Google Scholar]
- 4.Hwang SW, Bhadelia RA, Wu J. Thoracic spinal iophendylate-induced arachnoiditis mimicking an intramedullary spinal cord neoplasm: case report. J Neurosurg Spine. 2008;8:292–294. doi: 10.3171/SPI/2008/8/3/292. [DOI] [PubMed] [Google Scholar]
- 5.Tabor EN, Batzdorf U. Thoracic spinal Pantopaque cyst and associated syrinx resulting in progressive spastic paraparesis: case report. Neurosurgery. 1996;39:1040–1042. doi: 10.1097/00006123-199611000-00033. [DOI] [PubMed] [Google Scholar]
- 6.Gopalakrishnan CV, Mishra A, Thomas B. Iophendylate myelography induced thoracic arachnoiditis, arachnoid cyst and syrinx, four decades later. Br J Neurosurg. 2010;24:711–713. doi: 10.3109/02688697.2010.522746. [DOI] [PubMed] [Google Scholar]
- 7.Navani A, Dominguez CL, Hald JK, Fishman SM. An injection from the past: fluoroscopic evidence of remote injections of radiopaque substances. Reg Anesth Pain Med. 2006;31:82–85. doi: 10.1016/j.rapm.2005.07.193. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick MO, Goyal K, Johnston RA. Thoracic radiculopathy caused by a myodil cyst. Br J Neurosurg. 2000;14:351–353. doi: 10.1080/026886900417379. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamoorthy T, Thomas B. Unknown case. Spine. 2006;31:1633–1634. doi: 10.1097/01.brs.0000220220.87011.52. [DOI] [PubMed] [Google Scholar]
- 10.Shah J, Patkar D, Parmar H, Prasad S, Varma R. Arachnoiditis associated with arachnoid cyst formation and cord tethering following myelography: magnetic resonance features. Australas Radiol. 2001;45:236–239. doi: 10.1046/j.1440-1673.2001.00911.x. [DOI] [PubMed] [Google Scholar]
- 11.Barsoum AH, Cannillo KL. Thoracic constrictive arachnoiditis after Pantopaque myelography: report of two cases. Neurosurgery. 1980;6:314–316. doi: 10.1227/00006123-198003000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hill CA, Hunter JV, Moseley IF, Kendall BE. Does myodil introduced for ventriculography lead to symptomatic lumbar arachnoiditis? Br J Radiol. 1992;65:1105–1107. doi: 10.1259/0007-1285-65-780-1105. [DOI] [PubMed] [Google Scholar]