Abstract
A homoserine auxotroph strain of Corynebacterium glutamicum accumulates storage compound trehalose with lysine when limited by growth. Industrially lysine is produced from C. glutamicum through aspartate biosynthetic pathway, where enzymatic activity of aspartate kinase is allosterically controlled by the concerted feedback inhibition of threonine plus lysine. Ample threonine in the medium supports growth and inhibits lysine production (phenotype-I) and its complete absence leads to inhibition of growth in addition to accumulating lysine and trehalose (phenotype-II). In this work, we demonstrate that as threonine concentration becomes limiting, metabolic state of the cell shifts from maximizing growth (phenotype-I) to maximizing trehalose phenotype (phenotype-II) in a highly sensitive manner (with a Hill coefficient of 4). Trehalose formation was linked to lysine production through stoichiometry of the network. The study demonstrated that the net flux of the population was a linear combination of the two optimal phenotypic states, requiring only two experimental measurements to evaluate the flux distribution. The property of linear combination of two extreme phenotypes was robust for various medium conditions including varying batch time, initial glucose concentrations and medium osmolality.
Electronic supplementary material
The online version of this article (doi:10.1007/s11693-013-9107-5) contains supplementary material, which is available to authorized users.
Keywords: Corynebacterium glutamicum, Elementary modes, Optimal phenotypic state, Lysine, Osmotic stress
Introduction
Quantification of metabolic network provides a methodology to obtain insights into the key phenomena of metabolism by characterizing a phenotypic state of an organism. Several methods, such as flux balance analysis (FBA) (Edwards et al. 2002; Ibarra et al. 2002), elementary mode analysis (EMA) (Klamt and Stelling 2003; Papin et al. 2004; Schilling et al. 2000; Schuster and Hilgetag 1994), extreme pathway analysis, in tandem with experimental data are used for their quantification. These methods are being integrated with other omics-data such as transcriptional expression to provide predictive knowledge about the phenotype (Covert and Palsson 2002; Haverkorn van Rijsewijk et al. 2011). Quantification of metabolic network has in the past, helped in determining the controlling steps and key regulatory nodes in the metabolism (Stephanopoulos and Vallino 1991; Vallino and Stephanopoulos 1993, 1994a, b; Kromer et al. 2006). Such an analysis requires perturbations in the network, measuring the accumulation rates of metabolites, determining the fluxes in the metabolic network and then comparing the same with the control. The information regarding the regulation of metabolism helps in engineering the network towards a desired phenotype for applications. Although several studies have been reported to identify the bottlenecks in a metabolic network (Stephanopoulos and Vallino 1991; Vallino and Stephanopoulos 1994a, b), few studies characterize the phenotypic response given a well-characterized regulation in a network. Such a study will provide insights into the relevance of regulation towards a specific phenotypic behavior. A well characterized regulation of lysine synthesis exists in Cornybacterium glutamicum and is a prototype for metabolic network analysis (Vallino and Stephanopoulos 1993).
Cornybacterium glutamicum is a widely used organism in biotechnology industry for the large scale fermentative production of l-amino acids, such as, lysine and glutamate. Lysine is produced through the aspartate biosynthetic pathway. l-Aspartate is derived from oxaloacetic acid (OAA) through transamination process. l-Aspartate is phosphorylated by aspartate kinase, an enzyme which is tightly regulated through feedback control, and is further reduced to yield l-aspartate semialdehyde. This intermediate is at an important branch point where one path leads to l-threonine, l-methionine and l-isoleucine synthesis and the other to lysine (Eggeling 1994). Shiio and Miyajima (1969) found that the enzymatic activity of aspartate kinase was allosterically controlled by the concerted feedback inhibition of threonine plus lysine (Shiio and Miyajima 1969). However, the activity of aspartate kinase is not significantly affected by the presence of either lysine or threonine separately (Eikmanns et al. 1993). In lysine over producer strain of C. glutamicum, the enzyme homoserine dehydrogenase involved in the conversion of aspartic semialdehyde into l-homoserine is inactive. Therefore, this strain cannot synthesize threonine resulting in the release of feedback inhibition exerted by threonine on aspartate kinase. As a result aspartic semialdehyde produced is utilized in the over production of lysine (Fig. 1) (Kromer et al. 2004; Tryfona and Bustard 2005). Thus threonine plays a pivotal role in the regulation of lysine formation and growth of the organism.
Fig. 1.
Lysine biosynthesis in homoserine auxotroph of C. glutamicum. Mutation causing inactivation of homoserine dehydrogenase (marked by X) resulted in inhibition of homoserine biosynthesis. This in turn resulted in release of feedback inhibition by threonine and lysine on aspartate kinase
Studies on lysine synthesis by C. glutamicum have mainly focused on determining metabolic flux distribution of the network as well as at various branch points during the growth and overproduction of lysine (Vallino and Stephanopoulos 1993; Vallino and Stephanopoulos 1994a, b) and on the influence of environmental conditions, such as, salt stress (Rajvanshi and Venkatesh 2011; Benjamin et al. 2010; Guillouet and Engasser 1995a, b Skjerdal et al. 1995, 1996; Kempf and Bremer 1998; Morbach and Kramer 2003; Varela et al. 2003; Heermann and Jung 2004; Varela et al. 2004), nutritional conditions with use of variety of sugars and organic acids as carbon sources (Wittmann et al. 2004; Dominguez et al. 1998; Cocaign and Lindley 1995; Gerstmeir et al. 2003), on the physiological behavior and in vivo flux distribution of C. glutamicum. Various methodologies, such as, 13C flux analysis (Wittmann et al. 2004; Wendisch et al. 2002), flux balance analysis (Takac et al. 1998), metabolite balancing technique (Varela et al. 2003; Vallino and Stephanopoulos 1993, 1994a, b) and metabolic pathway analysis (elementary mode analysis (EMA) and extreme pathway analysis) (Rajvanshi and Venkatesh 2011; Radhakrishnan et al. 2010; Gayen and Venkatesh 2006; Gayen et al. 2007; Chen et al. 2009; Kromer et al. 2006) have been used for flux determination. EMA is a promising mathematical tool to represent the structure of a metabolic network and has been used to evaluate steady state flux distribution for different nutritional and environmental conditions (Schuster et al. 1999; Edwards and Palsson 2000; Stelling et al. 2002; Klamt and Stelling 2003; Papin et al. 2004; Poolman et al. 2004; Gayen and Venkatesh 2006; Schwartz and Kanehisa 2006; Radhakrishnan et al. 2010; Rajvanshi and Venkatesh 2011). In addition to analyzing metabolic network and in identifying target genes for manipulations, elementary modes also give insights about the redundancy and robustness in the network. It shows all the optimal and suboptimal solutions available for the network under consideration, gives insights about the maximum possible theoretical yields and bounds on nutrient uptake rates for production of individual metabolites under the constraint of the stoichiometry of the reactions of metabolic network (Radhakrishnan et al. 2010; Llaneras and Picó 2008).
In the current work, having known that threonine controls both the growth and lysine formation, we address the question as to how is this regulation translated to the phenotypic response at the metabolic level. To study the effect of the threonine concentration on the inhibition of lysine synthesis and growth rate, elementary mode analysis was used to determine the fluxes under threonine limiting conditions. We demonstrate the following through the analysis (1) existence of two distinct optimal phenotypes, one maximizing growth rate under threonine rich condition and the other maximizing trehalose synthesis under threonine limiting condition (2) the flux distribution for the growth of C. glutamicum is found to be a linear combination of these two optimal phenotypic states (3) the flux distribution could be evaluated using only two accumulation rates using the constraint of the linear combination for various growth conditions including osmotic stress (4) the switching between the two phenotypes is purely brought about by the regulation of threonine on the lysine synthesis. The analysis demonstrated a robust control by threonine to bring about linear combination of the two optimal phenotypes characterizing growth and storage under various growth conditions.
Materials and methods
Experimental protocol
Organism and materials
Corynebacterium glutamicum (CECT 79) was obtained from the Spanish Type Culture Collection (CECT), Valencia, Spain and was used in all the experiments.
Fermentation protocol
The strain was cultivated and maintained as reported previously (Vallino and Stephanopoulos 1993). The protocol for obtaining seed culturing, preculturing and fermentation are documented in detail in literature (Vallino and Stephanopoulos 1993). Experiments were performed to determine the effect of threonine concentration on cells metabolic response under extreme conditions, namely, in absence and excess of threonine. For this study, cells were grown under two extreme conditions (a) a medium in which threonine (0.73 g/l) was not limiting related to the concentration of carbon (15 g/l) representing excess threonine condition (b) threonine was not added in the medium. Phenotypic response to these two extreme conditions will be referred to as phenotype-I (for excess threonine condition) and phenotype-II (for threonine starvation condition). Various fermentation studies were carried out using different initial glucose concentration in the media ranging from 15, 25, 50 and 100 g/l. It has to be noted that the initial concentrations of the other constituents were kept constant and only glucose concentration was altered. Also, the media was designed to limit the concentration of threonine which constrained the cellular growth in case of fermentations with 50 and 100 g/l. Similarly osmotic stress studies were conducted with 100 g/l initial glucose concentration and 25 and 40 g/l NaCl in the medium to invoke stress. Laboratory bioreactor with working volume of 1.5 l (BIOSTAT B plus, Sartorius, Goettingen, Germany) was used for all the fermentations. Airflow rate was maintained at one liter per minute per liter of the reactor volume. The stirrer speed of the fermentor was monitored at 1,000 rpm and pH was controlled at 7.0 by 2.5 N NaOH.
Online measurements
Online measurements were performed through data acquisition software supplied by Sartorius (Goettingen, Germany). Temperature, airflow rate, dissolved oxygen and pH were monitored during the experiment. Off gas (oxygen uptake rate and carbon dioxide evolution rate) was also monitored by an off gas analyzer (Emerson process management, Germany).
Off line measurements
Samples were drawn in regular intervals during the course of fermentation for analysis of dry cell weight, glucose, trehalose, lysine and ammonium sulfate. Dry cell weight was estimated using a spectrophotometer (V-540, Jasco, Tokyo, Japan) with the absorbance at 600 nm. Glucose and trehalose were estimated using RI detector in HPLC (Hitachi, Merck, KgaA, Darmstndt, Germany) with a HP-Aminex-87-H column (Biorad, Inc., Hercules, CA) at 65° C, with a mobile phase of 5 mM sulfuric acid; flow rate was kept at 0.6 ml/min. The concentration of lysine was measured by HPTLC method as reported by Pachuski et al. (2002). Ammonia concentration was measured spectrophotometrically using Nessler’s reagent at 410 nm.
Network model
The metabolic network of C. glutamicum included the glucose phosphotransferase system, reactions of glycolytic pathway, reactions of TCA cycle and PPP as the core metabolism. Also, anaplerotic reaction connecting pyruvate to OAA was included as it plays an important role for lysine synthesis. The ammonia assimilation was through GS/GOGAT system. Further, the oxidative phosphorylation accounted for NADH recycling with ATP synthesis, while the biomass formation was included as a stoichiometric reaction involving the internal metabolites (Gayen and Venkatesh 2006; Stelling et al. 2002; Schuster et al. 1999; Klamt and Stelling 2003; Schwartz and Kanehisa 2006; Papin et al. 2004; Poolman et al. 2004; Vallino 1991). The reaction network employed for the current analysis was taken from the literature (Vallino and Stephanopoulos 1993). However, we made use of pyruvate carboxylase as an anaplerotic reaction instead of phosphoenol pyruvate carboxylase since it is demonstrated that the leading anaplerotic reaction is from pyruvate node (de Graaf 2000; Schuster et al. 2007).
Computation of the elementary modes
Elementary modes were generated using the public domain python based software named ‘ScrumPy’ (http://www.gnu.org/licenses/gpl.html), which was developed by M. G. Poolman and D. A. Fell (from Oxford Brooks University, UK). Elementary modes represent the smallest possible independent sub-networks which exhibit identical structure as that of the metabolic network. Thus, a metabolic network can be represented by a set of elementary modes which connect the extracellular metabolites through various independent feasible routes (Klamt and Stelling 2003). A total of 542 elementary modes were observed by incorporating all the possible extracellular metabolites of C. glutamicum. Experimental evidence suggested that the accumulation of lactate, acetate, pyruvate, alanine and valine were negligible during the period of fermentation studied. Therefore, these metabolites were not included in the analysis. This modification resulted in only eighteen elementary modes (see Supplementary Table S1) by considering a total number of 39 reactions and 38 metabolites.
Determination of fluxes of the elementary modes through linear optimization
The fluxes of the elementary modes were evaluated using linear optimization technique under the constraints of the stoichiometries of the elementary modes. For details of the methodology, refer (Gayen and Venkatesh 2006). Mathematically, the linear optimization formulation can be represented as:
 |
1 |
where, Mi is the accumulation rate of a specific metabolite (such as biomass), M is vector representing the accumulation rates of the external metabolites and S is the matrix, indicating the stoichiometry of the elementary modes. The elements of E{e1, e2, e3, …en} represents the vector of unknown fluxes of the elementary modes. The minimum number of experimental accumulation rates needed for the closure of molar balance was four, resulting in a unique flux distribution for the network (Gayen and Venkatesh 2006). Analysis of flux distribution of two extreme phenotypes (phenotype-I and phenotype-II) was performed using this method.
Determination of fluxes of metabolic network through linear combination
Determination of net flux distribution needs only two extracellular metabolite measurements when the system is constrained with the condition of linear combination of the two optimal phenotypic states viz. optimal growth (phenotype-I) and trehalose production (phenotype-II) (Fig. 2). Biomass formation is associated with phenotype-I. Therefore, biomass formation rate can be used to obtain the fraction of total glucose consumed for phenotype-I (G1) and the glucose uptake rate for phenotype-II can be evaluated by subtracting G1 from the total measured glucose uptake rate GT (see Fig. 5). By multiplying coefficients of metabolites with G1 and G2, accumulation rates of various metabolites can be estimated. All the data sets with different initial concentration of glucose and osmotic stress conditions were analyzed using this method.
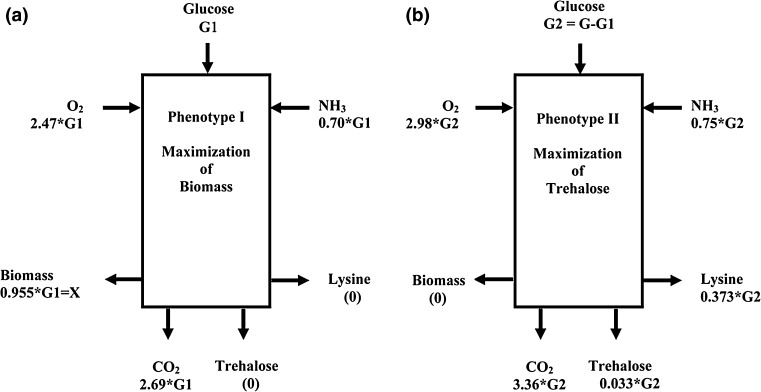
Fig. 2.
Flux distribution maps of the two phenotypes. a Flux distribution for phenotype-I (maximization of biomass) obtained under high threonine condition, b Flux distribution for phenotype-II (maximization of trehalose) obtained under threonine starvation condition. Uptake rate of glucose and production rates of lysine, trehalose and biomass were used as constraints. The fluxes were expressed in mM/h and normalized with respect to glucose uptake rate
Fig. 5.
Evaluation of fluxes assuming linear combination of the two optimal phenotypes. a Maximization of biomass (phenotype-I) and b Maximization of trehalose (phenotype-II). Stoichiometric coefficients of various extracellular metabolites for the two phenotypes were obtained from Fig. 2. The analysis utilizes measurement of any two accumulation rates. The above figure demonstrates the evaluation method for glucose uptake rate (G) and biomass formation rate (X). G1 and G2 = (G − G1) are the fractions of the total glucose taken up by phenotype-I and phenotype-II, respectively. It can be observed that synthesis of lysine and trehalose is zero in phenotype-I while biomass production is zero in phenotype-II. Using the value of X, the fluxes of other variables can be evaluated for phenotype-I including G1. Further, G2 = (G − G1) can be used to determine the fluxes for phenotype-II
Results
Corynebacterium glutamicum was grown under two extreme conditions (1) a medium in which threonine was not limiting till the end of fermentation and (2) a medium completely lacking threonine. In the first case a high growth rate of 0.45 h−1 was observed with neither accumulation of lysine nor trehalose (see Supplementary Fig.S1a), while in the second case, accumulation of lysine and trehalose was observed from the beginning with no growth beyond 3 h (see Supplementary Fig. S1b). The uptake rates obtained from these experiments were used to determine fluxes in the central metabolism of C. glutamicum using elementary modes (see “Materials and methods” for details) for the two cases. Four accumulation rates obtained through experiments were used to obtain a unique flux distribution to represent the two extreme phenotypes, as four accumulation rates of the extra-cellular metabolites are required for analysis of network for unique closure of molar balance. Figure 2 shows the normalized flux distribution for the 2 phenotypic states. For the case with growth on high threonine levels (phenotype-I), the biomass yield was 95.5 relative to 100 mM/h of glucose uptake without any accumulation of trehalose or lysine. While in the case of growth on zero threonine concentration (phenotype-II), the flux towards biomass was zero with accumulation rate of 37.3 and 3.3 mM/h for lysine and trehalose, respectively. It is clear from the figure, that there is a higher flux towards pentose phosphate pathway to satisfy elevated NADPH requirement for growth in case of phenotype-I as compared to that observed for phenotype-II. Further, as has been reported in previous studies, the anaplerotic reaction of pyruvate carboxylase was high in the lysine producing phenotype-II as compared to phenotype-I. The normalized ammonia uptake rates were comparable for the two cases (70.3 and 74.7 mM/h for phenotype-I and phenotype-II, respectively). The oxygen uptake rate for phenotype-II was 1.2 times that observed for phenotype-I and similarly the CO2 formation rate for phenotype-II was 1.5 times that of phenotype-I. The elementary mode analysis also demonstrated that phenotype-I represented optimal growth while phenotype-II represented optimal trehalose formation (Supplementary Fig. S2). It was interesting to note that the optimal growth in phenotype-I was represented by four operational modes each leading to the formation of biomass and CO2. Phenotype-II was similarly represented by eight elementary modes leading to the formation of trehalose and lysine. The stoichiometry of the fifteenth to eighteenth elementary modes yielded entire trehalose accumulated by the cells. These modes are associated with lysine formation. Thus, the constraint of the stoichiometry of the metabolic network in C. glutamicum linked the formation of lysine and trehalose (Fig. 3). The above mentioned two phenotypes can be represented as net stoichiometric reactions, described below:
 |
2 |
 |
3 |
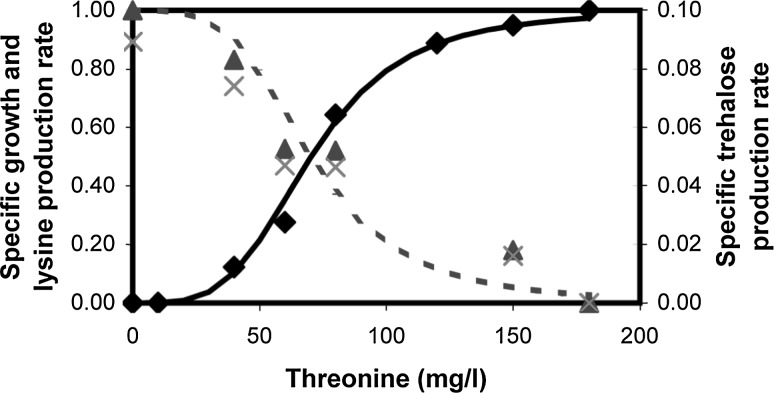
The above study demonstrated the existence of two optimal phenotypes due to the two conditions namely, non-limiting threonine and limiting carbon for phenotype-I and absence of threonine for phenotype-II. Experiments were performed by varying threonine concentration in a medium containing 8 g/l of glucose to achieve transition between the two optimal phenotypes. Figure 4 shows the specific growth rate and specific accumulation rate of lysine and trehalose at various threonine concentrations. The specific growth rate was fitted with a Hill equation in relation to threonine, yielding a Hill coefficient of 4 and an Half saturation constant of 70 mg/l. The accumulation rates of lysine and trehalose were inhibited by threonine with the same values of Hill coefficient and half saturation constant. This demonstrated that the regulation of growth and lysine, trehalose formation by threonine was reciprocal and sensitive. The above study suggested that the cells demonstrate the two optimal phenotypes at the extremes of threonine regulation and combination of the two in the intermediate concentrations. Further, since the regulation of growth and trehalose formation is reciprocal in nature, one can hypothesize that the flux distribution may be a convex linear combination of the two optimal phenotypic states in response to threonine concentration.
Fig. 3.
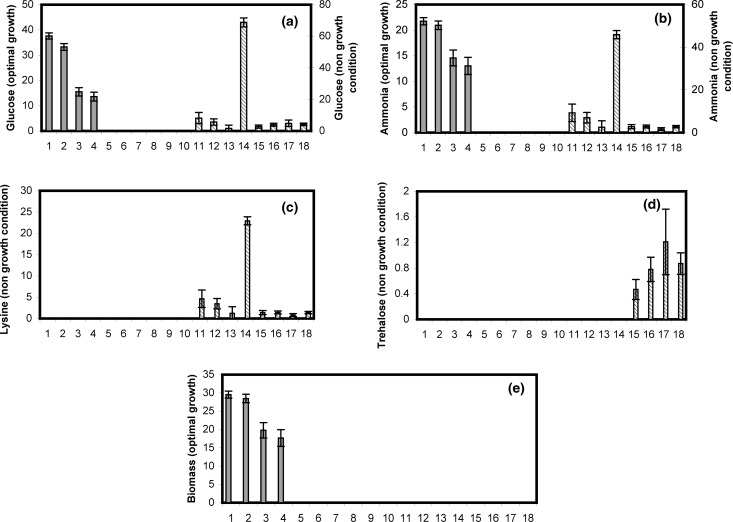
Quantification of fluxes through the elementary modes. Quantification of the fluxes of elementary modes gives insights regarding the modes active under the two phenotypic states discussed and contribution of total accumulation rates of various metabolites through the individual elementary modes. 14 elementary modes were obtained for central metabolism of C. glutamicum (Table S1). The modes demonstrate the conversion of the substrates [glucose (a), oxygen and ammonia (b)], to the products [lysine (c), trehalose (d) and biomass (e)]. Solid bars show modes active under the condition of biomass maximization (phenotype-I) and striped bars show modes active under trehalose maximizing condition (phenotype-II). The fluxes (in mM/h) were computed by linear programming using maximization of ammonia, CO2 and O2 as an objective function and average is shown as histogram with standard deviation. Uptake rate of glucose and production rates of lysine, trehalose and biomass were used as decision variables. Under the condition where cells were in phenotypic-I state, only first four modes were active leading to production of biomass (e) and under phenotypic-II state modes leading to lysine and trehalose production (11–18) were active (c and d)
Fig. 4.
Profiles of specific growth rate (Filled diamond), specific accumulation rate of lysine (Filled triangle) and trehalose (x). Profiles obtained at varying threonine concentrations and 8 g/l initial glucose concentration. Solid line shows specific growth rate fitted with a Hill equation in relation to threonine and dotted line shows lysine and trehalose accumulation rate fitted with Hill equation. Hill coefficients and half saturation constants of both the profiles were 4 and 70 mg/l, respectively
To verify the hypothesis of linear combination of the 2 optimal phenotypic states, several experiments were performed with varying initial glucose concentration (ranging from 25 to 100 g/l) with a fixed threonine concentration. The flux distribution at various time points was determined by assessing a linear combination of the two optimal phenotypes (see Fig. 5). In such an analysis only two accumulation rates are needed to evaluate the flux distribution due to the additional constraint of the linear combination. Note that overall stoichiometric description of the two phenotypes (Eqs. 2 and 3) clearly highlight the requirement of only two measurements to determine the accumulation rates of all the other metabolites. Figure 6 shows the comparison of experimental data with the estimate of accumulation rates of various metabolites using the hypothesis of linear combination of the two optimal phenotypes and using the accumulation rates of glucose and biomass. Note that in this case, the accumulation rates of all the other metabolites were independently predicted using the stoichiometry of the two optimal extreme phenotypic states, representing optimal growth (phenotype-I) and optimal trehalose formation (phenotype-II). It is clear from the figure that the hypothesis is valid as one could estimate the accumulation rates of all metabolites within a maximum error of 12 %. Similar accurate estimates could be obtained using any other two measurements (see Supplementary Fig. S3 for predictions based on accumulation rates of ammonia and biomass). Figure 6 also includes data from experiments performed using 100 g/l of glucose in a medium containing salt of 25 and 40 g/l, to study the effect of osmotic stress. It was interesting to note that the linear combination of the optimal phenotypes was valid even for growth under osmotic stress. The analysis was also used to determine the fractional contribution of the individual phenotypes on the overall flux distribution. Figure 6g and h show the fractional contribution of the two phenotypes in case of growth on 100 g/l of glucose in absence and presence of osmotic shock, respectively. It is clear from the figure that the contribution from the growth phenotype-I is higher during initial batch times and crosses over to phenotype-II during the fermentation.
Fig. 6.
Comparison of the predicted accumulation rates of external metabolites obtained through linear combination of flux distribution for the two phenotypes using biomass and glucose accumulation rates for flux quantification, with experimental accumulation rates. Figures show comparison of (a) NH3 consumption rates (b) Lysine production rates (c) Biomass production rates (d) Trehalose production rates (e) O2 consumption rates and (f) CO2 evolution rates. Symbols indicate data from various experiments: experiment with 100 g/l initial glucose ‘open diamond’, 50 g/l initial glucose ‘open triangle’, 25 g/l initial glucose ‘filled diamond’, 100 g/l initial glucose with 25 g/l NaCl ‘asterisk’, 100 g/l initial glucose with 40 g/l NaCl ‘filled circle’, dynamic switching of phenotypic states controlled by threonine ‘open circle’, data reported by Vallino (Vallino 1991) ‘filled triangle’. g and h show the estimated fraction associated with glucose consumption rate for the two phenotypes during the course of fermentation with normal (100 g/l initial glucose concentration) and osmotic stress conditions (100 g/l glucose and 40 g/l NaCl), respectively
The flux distribution obtained using the hypothesis of linear combination was compared with that obtained through linear optimization. Studies reported in the literature indicated that larger numbers of measurements are required to obtain the flux distribution (Vallino and Stephanopoulos 1993; Varela et al. 2003, 2004). To evaluate flux distribution of the network using linear optimization four accumulation rates and the stoichiometry of the elementary modes were used (see Fig. 7) while in the above method using linear combination of optimal phenotypes, only two measurements were used to predict the accumulation rates of the other metabolites. It is clear from the comparison that predictions obtained using two rate measurements along with linear combination of optimal phenotypes can yield flux distribution equivalent to that obtained from linear optimization strategies. This demonstrates the validity of the linear combination. Figure 7 b and c also shows a similar comparison for growth under osmotic stress reiterating the validity of the hypothesis even under stress. Dynamic shift experiments were also performed to check if the phenotypes alter dynamically the flux distribution to changing external conditions (see Supplementary Fig. S4). When cells exposed to high threonine were allowed to exhaust the threonine reserves shifting the cells from phenotype-I to phenotype-II and on further addition of high threonine levels, the cells shifted back to phenotype-I in about 30 min. This confirmed that the two extreme phenotypes are robust and are also reversible depending on the availability of threonine.
Fig. 7.
Comparative flux distribution profiles. Comparison of flux distribution using EMA (through specification of four constraints namely, accumulation rates of glucose, lysine, trehalose and biomass) (values shown in parentheses) with flux distribution obtained assuming linear combination of two optimal phenotypic states (as described in Fig. 5). Comparison of flux distribution at (a) t = 12 h for experimental condition with 100 g/l initial glucose concentration (b) t = 16 h for osmotic stress condition with 25 g/l NaCl and 100 g/l initial glucose concentration (c) t = 22 h for osmotic stress condition with 40 g/l NaCl and 100 g/l initial glucose concentration. For all the three experimental conditions, glucose concentration was kept at 100 g/l. All flux values are expressed in mM/h
Discussion
Homoserine auxotrophic strain of C. glutamicum, accumulates lysine because strain is deficient in homoserine dehydrogenase activity leading to inhibition of threonine synthesis. Since no threonine is synthesized, therefore, the concerted feedback inhibition on aspartate kinase is relieved and hence lysine is overproduced (Tryfona and Bustard 2005). The existence of a rigid node at PEP further controls the extent of overflow metabolism, where in PEP carboxylase is tightly regulated by its activator acetyl-CoA regulating in the flow of carbon towards TCA for energy and towards OAA for lysine formation (Vallino 1991). By eliminating the threonine synthesis in a wild type strain, the growth of the organism is dependent on the extracellular levels of threonine which also decides the extent of overflow metabolism. The dependency of growth and lysine formation rates on threonine was experimentally demonstrated to be highly ultra sensitive (with ηH = 4). The sensitivity and threshold of half saturation constant for the activation of growth and inhibition of lysine formation were found to be equal indicating a reciprocal relationship between the two. Further, the formation of storage compound, trehalose was stoichiometrically linked to the lysine formation. Thus, the accumulation of lysine was directly connected to the flux towards lysine and trehalose on eliminating threonine from the medium resulting in reduced growth.
The sensitive regulation of growth and accumulation of lysine by threonine yielded two extreme phenotypes, which were experimentally evaluated. In medium with excess threonine, the accumulation of lysine was absent with maximum growth indicating an optimal growth phenotype. In contrast, in a medium lacking threonine, the accumulation of lysine was maximum representing an optimal storage phenotype with accumulation of trehalose and lysine. A stoichiometric reaction scheme was obtained for the two optimal phenotypes quantifying the stoichiometry of biomass formation from phenotype-I and lysine/trehalose formation from phenotype-II (see Eqs. 2 and 3). It was interesting to note that each of the phenotype had fluxes through distinct elementary modes (see Fig. 3). The overall stoichiometric reaction scheme for the two phenotypes can be related to these distinct elementary modes. The elementary modes (1–4) representing optimal growth condition characterize stoichiometric reaction scheme of Eq. (2), while modes (11–18) represent optimal storage condition characterize stoichiometric reaction scheme of Eq. (3). It was interesting to note that the ammonia uptake for both the extreme phenotypes was similar while the glucose uptake was reduced by 1.5 fold in the second phenotype as compared to the optimal growth phenotype. Thus, the accumulation of lysine accounted for the carbon and nitrogen balance by accumulating trehalose and the amino acid, lysine. Further, in a medium with intermediate threonine levels the metabolic state was a linear combination of the two extreme phenotypes, thus regulating the accumulation of lysine to balance the excess carbon and nitrogen uptake over that required for growth. The regulation of the accumulation of lysine was highly robust as the assumption of linear combination of the two extreme phenotypes could predict the metabolic flux distribution at various batch times for growth on different glucose/nitrogen concentrations. Further, the same linearity of extreme phenotypes was valid for flux distribution for growth under osmotic stress. Since there stoichiometry of the extreme phenotypes was fixed through the unique elementary modes representing their metabolism, only two measurements were sufficient to characterize the extent of overflow metabolism. It was also interesting to note that the elementary modes representing the second extreme phenotype characterizing storage compound stoichiometrically linked lysine and trehalose accumulation. This stoichiometric linkage resulted in the accumulation of trehalose through the regulation of lysine formation, thus balancing, both carbon and nitrogen under lysine accumulation. The above studies indicated that lysine and trehalose formation are linked through stoichiometry as indicated by the elementary modes. It is well known that lysine synthesis is tightly regulated through threonine and the rigid node at PEP purely through enzymatic inhibition/activation (Vallino 1991). However, the role of regulation of trehalose in phenotype-II is unclear. Genome analysis of C. glutamicum has revealed that it possesses five genes encoding the enzymes of three different trehalose biosynthesis pathways (OtsAB, TreYZ and TreS) (Wolf et al. 2003). In vivo, TreS-mediated trehalose synthesis occurs when maltose is used as the carbon source (Wolf et al. 2003). Gene knockout leading to pathway inactivation experiments have shown that OtsAB and TreYZ pathways play a dominant role in trehalose synthesis as deletion of both the pathways or all the three pathways (OtsAB, TreYZ and TreS) leads to impaired growth and inability to synthesize trehalose (Wolf et al. 2003). Mutants lacking either otsAB or treYZ genes are capable of synthesizing trehalose to meet the requirement of the cell while growing on glucose, indicating that TreS pathway is not crucial. However, reduction in trehalose production was close to 50 % when either of the two pathways was deleted (Tzvetkov et al. 2003). The stoichiometric linkage of lysine and trehalose may be due to transcriptional regulation of the trehalose biosynthesis pathways, or due to activation/inhibition of enzyme activity or a consequence of the stoichiometric distribution around glucose 6-phosphate node (Wolf et al. 2003).
It is well known that threonine imparts enzyme inhibition on aspartate kinase regulation, the flux from aspartate towards amino acid synthesis including lysine, isoleucine, methionine and threonine (Tryfona and Bustard 2005). Formation of aspartate, itself is tightly regulated through a rigid PEP node; where in the formation of OAA from PEP through PEP carboxylase, necessary for aspartate formation, is also controlled (Vallino 1991). The net result of these enzymatic regulations is the highly sensitive control of growth and lysine accumulation by threonine. We have demonstrated that the regulation by threonine also results in a linear combination of the extreme optimal phenotype during transition from high to low concentrations of threonine. Further, the experimental validation of the predictions assuming linear combination for various media used demonstrated that the regulation by threonine is indeed robust. Invoking the constraint of linear combination on predictions, flux balance required measurement of only two accumulation rates, while higher number of measurements was required in previous studies to characterize the metabolic state (Vallino and Stephanopoulos 1993; Varela et al. 2003, 2004). Thus, the linear combination of the extreme phenotypes under threonine limitation was due to the sensitive regulation caused by enzyme inhibition. In future, it will be interesting to see if the phenomenon of linear combination of optimal phenotype will be valid under other stresses such as that imposed through changes in pH and temperature.
Electronic supplementary material
Acknowledgments
KVV acknowledges financial support for research from Department of Science and Technology, India. Authors are thankful to Dr. M. G. Poolman and Prof. D. A. Fell (Oxford Brooks University) for providing the “ScrumPy” software. Authors are also thankful to Prof. Sharad Bhartiya and Dr P. K. Vinod for their useful suggestions.
References
- Benjamin F, Christian T, Christian R, Jörn K, Ansgar P, Dirk Andreas W. Adaptation of Corynebacterium glutamicum to salt-stress conditions. Proteomics. 2010;10(3):445–457. doi: 10.1002/pmic.200900482. [DOI] [PubMed] [Google Scholar]
- Chen N, Du J, Liu H, Xu QY. Elementary mode analysis and metabolic flux analysis of l-glutamate biosynthesis by Corynebacterium glutamicum. Ann Microbiol. 2009;59(2):317–322. doi: 10.1007/BF03178334. [DOI] [Google Scholar]
- Cocaign BM, Lindley ND. Pyruvate overflow and carbon flux within the central metabolic pathways of Corynebacterium glutamicum during growth on lactate. Amsterdam: Elsevier; 1995. [Google Scholar]
- Covert MW, Palsson BO. Transcriptional regulation in constraints-based metabolic models of Escherichia coli. J Biol Chem. 2002;277(31):28058–28064. doi: 10.1074/jbc.M201691200. [DOI] [PubMed] [Google Scholar]
- de Graaf AA (2000) Metabolic flux analysis of Corynebacterium glutamicum. In: Schügerl KB, Bellgardt KH (ed) Bioreaction engineering, modelling and control. Springer, New york, pp 506–555
- Dominguez H, Rollin C, Guyonvarch A, Guerquin-Kern J-L, Cocaign-Bousquet M, Lindley ND. Carbon-flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur J Biochem. 1998;254(1):96–102. doi: 10.1046/j.1432-1327.1998.2540096.x. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Palsson BO. The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities. Proc Natl Acad Sci U S A. 2000;97(10):5528–5533. doi: 10.1073/pnas.97.10.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JS, Covert M, Palsson B. Metabolic modelling of microbes: the flux-balance approach. Environ Microbiol. 2002;4(3):133–140. doi: 10.1046/j.1462-2920.2002.00282.x. [DOI] [PubMed] [Google Scholar]
- Eggeling L. Biology of l-lysine overproduction by Corynebacterium glutamicum. Amino Acids. 1994;6(3):261–272. doi: 10.1007/BF00813746. [DOI] [PubMed] [Google Scholar]
- Eikmanns BJ, Eggeling L, Sahm H. Molecular aspects of lysine, threonine, and isoleucine biosynthesis in Corynebacterium glutamicum. Antonie Van Leeuwenhoek. 1993;64(2):145–163. doi: 10.1007/BF00873024. [DOI] [PubMed] [Google Scholar]
- Gayen K, Venkatesh KV. Analysis of optimal phenotypic space using elementary modes as applied to Corynebacterium glutamicum. BMC Bioinform. 2006;7:445. doi: 10.1186/1471-2105-7-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayen K, Gupta M, Venkatesh KV (2007) Elementary mode analysis to study the preculturing effect on the metabolic state of Lactobacillus rhamnosus during growth on mixed substrates. In Silico Biology 7(2):123–139 [PubMed]
- Gerstmeir R, Wendisch VF, Schnicke S, Ruan H, Farwick M, Reinscheid D, Eikmanns BJ. Acetate metabolism and its regulation in Corynebacterium glutamicum. J Biotechnol. 2003;104(1–3):99–122. doi: 10.1016/S0168-1656(03)00167-6. [DOI] [PubMed] [Google Scholar]
- Guillouet S, Engasser JM. Growth of Corynebacterium glutamicum in glucose-limited continuous cultures under high osmotic pressure. Influence of growth rate on the intracellular accumulation of proline, glutamate and trehalose. Appl Microbiol Biotechnol. 1995;44(3):496–500. doi: 10.1007/BF00169950. [DOI] [Google Scholar]
- Guillouet S, Engasser JM. Sodium and proline accumulation in Corynebacterium glutamicum as a response to an osmotic saline upshock. Appl Microbiol Biotechnol. 1995;43(2):315–320. doi: 10.1007/BF00172831. [DOI] [Google Scholar]
- Haverkorn van Rijsewijk BRB, Nanchen A, Nallet S, Kleijn RJ, Sauer U (2011) Large-scale 13C-flux analysis reveals distinct transcriptional control of respiratory and fermentative metabolism in Escherichia coli. Mol Syst Biol 7, Article number 477. doi:10.1038/msb.2011.9 [DOI] [PMC free article] [PubMed]
- Heermann R, Jung K. Structural features and mechanisms for sensing high osmolarity in microorganisms. Curr Opin Microbiol. 2004;7(2):168–174. doi: 10.1016/j.mib.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ibarra RU, Edwards JS, Palsson BO. Escherichia coli K-12 undergoes adaptive evolution to achieve in silico predicted optimal growth. Nature. 2002;420(6912):186–189. doi: 10.1038/nature01149. [DOI] [PubMed] [Google Scholar]
- Kempf B, Bremer E. Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments. Arch Microbiol. 1998;170(5):319–330. doi: 10.1007/s002030050649. [DOI] [PubMed] [Google Scholar]
- Klamt S, Stelling J. Two approaches for metabolic pathway analysis? Trends Biotechnol. 2003;21(2):64–69. doi: 10.1016/S0167-7799(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Kromer JO, Sorgenfrei O, Klopprogge K, Heinzle E, Wittmann C. In-depth profiling of lysine-producing Corynebacterium glutamicum by combined analysis of the transcriptome, metabolome, and fluxome. J Bacteriol. 2004;186(6):1769–1784. doi: 10.1128/JB.186.6.1769-1784.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer JO, Wittmann C, Schroder H, Heinzle E. Metabolic pathway analysis for rational design of l-methionine production by Escherichia coli and Corynebacterium glutamicum. Metab Eng. 2006;8(4):353–369. doi: 10.1016/j.ymben.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Llaneras F, Picó J. Stoichiometric modelling of cell metabolism. J Biosci Bioeng. 2008;105(1):1–11. doi: 10.1263/jbb.105.1. [DOI] [PubMed] [Google Scholar]
- Morbach S, Kramer R. Impact of transport processes in the osmotic response of Corynebacterium glutamicum. J Biotechnol. 2003;104(1–3):69–75. doi: 10.1016/S0168-1656(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Pachuski J, Fried B, Sherma J. HPTLC analysis of amino acids in Biomphalaria glabrata infected with Schistosoma mansoni. J Liq Chromatogr Rel Technol. 2002;25(13–15):2345–2349. [Google Scholar]
- Papin JA, Stelling J, Price ND, Klamt S, Schuster S, Palsson BO. Comparison of network-based pathway analysis methods. Trends Biotechnol. 2004;22(8):400–405. doi: 10.1016/j.tibtech.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Poolman MG, Venkatesh KV, Pidcock MK, Fell DA. A method for the determination of flux in elementary modes, and its application to Lactobacillus rhamnosus. Biotechnol Bioeng. 2004;88(5):601–612. doi: 10.1002/bit.20273. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan D, Rajvanshi M, Venkatesh K. Phenotypic characterization of Corynebacterium glutamicum using elementary modes towards synthesis of amino acids. Syst Synth Biol. 2010;4(4):281–291. doi: 10.1007/s11693-011-9073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajvanshi M, Venkatesh K. Phenotypic characterization of Corynebacterium glutamicum under osmotic stress conditions using elementary mode analysis. J Ind Microbiol Biotechnol. 2011;38(9):1345–1357. doi: 10.1007/s10295-010-0918-z. [DOI] [PubMed] [Google Scholar]
- Schilling CH, Letscher D, Palsson BO. Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective. J Theor Biol. 2000;203(3):229–248. doi: 10.1006/jtbi.2000.1073. [DOI] [PubMed] [Google Scholar]
- Schuster S, Hilgetag C. On elementary flux modes in biochemical reaction systems at steady state. J Biol Syst. 1994;2(2):165–182. doi: 10.1142/S0218339094000131. [DOI] [Google Scholar]
- Schuster S, Dandekar T, Fell DA. Detection of elementary flux modes in biochemical networks: a promising tool for pathway analysis and metabolic engineering. Trends Biotechnol. 1999;17(2):53–60. doi: 10.1016/S0167-7799(98)01290-6. [DOI] [PubMed] [Google Scholar]
- Schuster S, Kamp A, Pachkov M (2007) Understanding the roadmap of metabolism by pathway analysis. In: Metabolomics. Method Mol Biol. Humana Press, Iotowa, pp 199–226 [DOI] [PubMed]
- Schwartz J-M, Kanehisa M. Quantitative elementary mode analysis of metabolic pathways: the example of yeast glycolysis. BMC Bioinform. 2006;7(1):186. doi: 10.1186/1471-2105-7-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiio I, Miyajima R. Concerted inhibition and its reversal by end products of aspartate kinase in Brevibacterium flavum. J Biochem. 1969;65(6):849–859. doi: 10.1093/oxfordjournals.jbchem.a129089. [DOI] [PubMed] [Google Scholar]
- Skjerdal OT, Sletta H, Flenstad SG, Josefsen KD, Levine DW, Ellingsen TE. Changes in cell volume, growth and respiration rate in response to hyperosmotic stress of NaCl, sucrose and glutamic acid in Brevibacterium lactofermentum and Corynebacterium glutamicum. Appl Microbiol Biotechnol. 1995;43(6):1099–1106. doi: 10.1007/BF00166932. [DOI] [Google Scholar]
- Skjerdal OT, Sletta H, Flenstad SG, Josefsen KD, Levine DW, Ellingsen TE. Changes in intracellular composition in response to hyperosmotic stress of NaCl, sucrose or glutamic acid in Brevibacterium lactofermentum and Corynebacterium glutamicum. Appl Microbiol Biotechnol. 1996;44(5):635–642. doi: 10.1007/BF00172497. [DOI] [Google Scholar]
- Stelling J, Klamt S, Bettenbrock K, Schuster S, Gilles ED. Metabolic network structure determines key aspects of functionality and regulation. Nature. 2002;420(6912):190–193. doi: 10.1038/nature01166. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G, Vallino JJ. Network rigidity and metabolic engineering in metabolite overproduction. Science. 1991;252(5013):1675–1681. doi: 10.1126/science.1904627. [DOI] [PubMed] [Google Scholar]
- Takac S, Calik G, Mavituna F, Dervakos G. Metabolic flux distribution for the optimized production of l-glutamate. Enzyme Microb Technol. 1998;23(5):286–300. doi: 10.1016/S0141-0229(98)00047-7. [DOI] [Google Scholar]
- Tryfona T, Bustard MT. Fermentative production of lysine by Corynebacterium glutamicum: transmembrane transport and metabolic flux analysis. Process Biochem. 2005;40(2):499–508. doi: 10.1016/j.procbio.2004.01.037. [DOI] [Google Scholar]
- Tzvetkov M, Klopprogge C, Zelder O, Liebl W. Genetic dissection of trehalose biosynthesis in Corynebacterium glutamicum: inactivation of trehalose production leads to impaired growth and an altered cell wall lipid composition. Microbiology. 2003;149(Pt 7):1659–1673. doi: 10.1099/mic.0.26205-0. [DOI] [PubMed] [Google Scholar]
- Vallino JJ (1991) Identification of branch point restrictions in microbial metabolism through metabolic flux analysis and local network perturbations. Thesis, Massachusets Institute of Technology, Boston
- Vallino JJ, Stephanopoulos G. Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Biotechnol Bioeng. 1993;41(6):633–646. doi: 10.1002/bit.260410606. [DOI] [PubMed] [Google Scholar]
- Vallino JJ, Stephanopoulos G. Carbon flux distributions at the glucose 6-phosphate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog. 1994;10(3):327–334. doi: 10.1021/bp00027a014. [DOI] [Google Scholar]
- Vallino JJ, Stephanopoulos G. Carbon flux distributions at the pyruvate branch point in Corynebacterium glutamicum during lysine overproduction. Biotechnol Prog. 1994;10(3):320–326. doi: 10.1021/bp00027a013. [DOI] [Google Scholar]
- Varela C, Agosin E, Baez M, Klapa M, Stephanopoulos G. Metabolic flux redistribution in Corynebacterium glutamicum in response to osmotic stress. Appl Microbiol Biotechnol. 2003;60(5):547–555. doi: 10.1007/s00253-002-1120-7. [DOI] [PubMed] [Google Scholar]
- Varela CA, Baez ME, Agosin E. Osmotic stress response: quantification of cell maintenance and metabolic fluxes in a lysine-overproducing strain of Corynebactetium glutamicum. Appl Environ Microbiol. 2004;70(7):4222–4229. doi: 10.1128/AEM.70.7.4222-4229.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendisch VF, de Graaf AA, Sahm H, Eikmanns BJ. Quantitative determination of metabolic fluxes during coutilization of two carbon sources: comparative analyses with Corynebacterium glutamicum during growth on acetate and/or glucose. J Bacteriol. 2002;182:3088–3096. doi: 10.1128/JB.182.11.3088-3096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C, Kiefer P, Zelder O. Metabolic fluxes in Corynebacterium glutamicum during Lysine production with sucrose as carbon source. Appl Environ Microbiol. 2004;70(12):7277–7287. doi: 10.1128/AEM.70.12.7277-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Kramer R, Morbach S. Three pathways for trehalose metabolism in Corynebacterium glutamicum ATCC13032 and their significance in response to osmotic stress. Mol Microbiol. 2003;49(4):1119–1134. doi: 10.1046/j.1365-2958.2003.03625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.