Abstract
Surgery can suppress in vivo levels of NK cell cytotoxicity (NKCC) through various mechanisms, including catecholamine-, glucocorticoid (CORT)-, and prostaglandin (PG)-mediated responses. However, PGs are synthesized locally following tissue damage, driving proinflammatory and CORT responses, while their systemic levels are often unaffected. Thus, we herein studied the role of adrenal factors in mediating in vivo effects of PGs on NKCC, using adrenalectomized and sham-operated F344 rats subjected to surgery or PGE2 administration. In vivo and ex-vivo approaches were employed, based on intravenous administration of the NK-sensitive MADB106 tumor line, and based on ex-vivo assessment of YAC-1 and MADB106 target-line lysis. Additionally, in vitro studies assessed the kinetics of the impact of epinephrine, CORT, and PGE2 on NKCC. The results indicated that suppression of NKCC by epinephrine and PGE2 are short lasting, and cannot be evident when these compounds are removed from the in vitro assay milieu, or in the context of ex-vivo assessment of NKCC. In contrast, the effects of CORT are long-lasting and are reflected in both conditions even after its removal. Marginating-pulmonary NKCC was less susceptible to suppression than circulating NKCC, when tested against the xenogeneic YAC-1 target line, but not against the syngeneic MADB106 line, which seems to involve different cytotoxicity mechanisms. Overall, these findings indicate that elevated systemic PG levels can directly suppress NKCC in vivo, but following laparotomy adrenal hormones mediate most of the effects of endogenously-released PGs. Additionally, the ex-vivo approach seems limited in reflecting the short-lasting NK-suppressive effects of catecholamines and PGs.
Keywords: prostaglandins, glucocorticoids, catecholamines, surgery, in vivo, in vitro, ex-vivo, NK activity
Introduction
Natural Killer (NK) cells play a significant role in immune protection against virally infected cells and malignant cells, and in controlling cancer metastasis (Biron and Brossay, 2001; Shakhar and Ben-Eliyahu, 1998a). Stress and surgical excision of a primary tumor are associated with immune suppression, specifically reduction in NK cell cytotoxicity (NKCC), and surgery is suggested to promote metastatic progression through immune suppression (Neeman and Ben-Eliyahu, 2012; Neeman et al., 2012; Neuhaus et al., 2000) and through direct effects of stress factors on the remaining malignant tissue (Armaiz-Pena et al., 2012).
Potential mechanisms through which stress and surgery can suppress NK activity have been studied in vitro and in vivo, and substantial evidence support the involvement of various factors, among them are catecholamines (Ben-Eliyahu et al., 2000; Shakhar and Ben-Eliyahu, 1998b), glucocorticoids (CORT) (Deguchi et al., 1998; Shakhar and Blumenfeld, 2003) and prostaglandins (PGs) (Baxevanis et al., 1993; Skibinski et al., 1992; Yakar et al., 2003), all of which are elevated during the perioperative period (Neeman and Ben-Eliyahu, 2012).
Specifically, psychological and physiological stressors activate the sympathetic nervous system (SNS) and the HPA axis, leading to the release of catecholamines and CORT, respectively. Studies have shown that β-adrenergic agonists can suppress NK cell activity in vitro (Takamoto et al., 1991; Whalen and Bankhurst, 1990) and in vivo (Shakhar and Ben-Eliyahu, 1998a), and consequently can promote cancer metastasis in animal models. Furthermore, β-adrenergic blockade was shown to prevent suppression of NK activity and promotion of cancer metastasis by stress and surgery (Ben-Eliyahu et al., 2000). CORT was shown to cause a marked suppression of NK cytotoxicity in vitro, in presumably physiological levels (Ben-Eliyahu, 1998; Gatti et al., 1986b), but in vivo studies are inconsistent as to whether endogenous physiological stress levels of CORT are sufficient to suppress NK activity (Bodner et al., 1998; Shakhar and Blumenfeld, 2003).
It is not clear whether PGs directly suppress NK activity in vivo when released endogenously. PG-E2 (PGE2) is locally synthesized following tissue damage, and was shown to suppress NK activity in vitro and in vivo, but mainly when used in pharmacological levels/doses, (Gatti et al., 1986a; Okuno et al., 1995; Yakar et al., 2003). For example, we previously reported that systemic administration of PGE2 that doubled plasma levels of this factor also suppressed in vivo levels of NK cytotoxicity (Yakar et al., 2003). However, it is critical to note that only some studies reported elevated systemic PGE2 levels after surgery, while other studies reported no such systemic increase (Baxevanis et al., 1994; Buvanendran et al., 2006; Parsson et al., 2000; Vitoratos et al., 1996; Yakar et al., 2003), questioning a direct effect of PGs on NK cytotoxicity. Additionally, although COX inhibitors were shown to reduce post surgical immune suppression (Benish et al., 2008; Faist et al., 1990; Melamed et al., 2005; Yakar et al., 2003), such treatments are also known to reduce CORT levels (Glasner et al., 2010; Shaashua et al., In press). Last, PGs were shown to directly increase adrenal release of CORT (Mohn et al., 2005). Thus, it cannot be determined whether PGE2 affects NK activity in vivo directly, or through modulating CORT levels.
To this end, this study aims at examining mechanisms through which PGE2 suppresses NK activity in vivo, specifically the potential involvement of adrenal hormones (CORT, epinephrine, and opioids) in mediating such effects. To address this question we compared adrenalectomized rats to sham operated rats, while subjecting both groups to systemic administration of PGE2 or to surgery. To study NK activity we employed in vitro, ex-vivo, and in vivo approaches using Fischer 344 rats. Potential in vivo alterations of NK activity were studied using the MADB106 tumor line, quantifying its lung tumor retention (LTR) following intravenous inoculation of this syngeneic mammary adenocarcinoma. This in vivo index is highly sensitive to NK activity. Specifically, in vivo selective depletion of NK cells increased LTR by more than a hundred-fold, and replacement of LGL/NK cells restored normal ability to prevent the development of lung metastasis (Barlozzari et al., 1985; Barlozzari et al., 1983; Ben-Eliyahu et al., 1996b; Shakhar and Ben-Eliyahu, 1998a). Thus, this index sensitively reflects in vivo alterations in NK activity. This index also predicts the number of metastases that will be evident weeks later (Shakhar and Ben-Eliyahu, 1998a; Yakar et al., 2003; Melamed et al., 2005). Importantly, we have previously shown that the administration of PGE2 (as in the current study) elevates LTR exclusively through suppressing NK activity, as this effect (but not other effects) was abrogated by selective depletion of NK cells (Yakar et al., 2003). In the current study we have also employed the in vitro 4hr cytotoxicity assay that directly indicates NK activity, whether used to study in vitro manipulations or in an ex-vivo approach. However, the ex-vivo approach assesses cytotoxicity in a context markedly distinct from the in vivo milieu, specifically in the absence of endogenous hormones. Last, we studied both circulating NK cells and a unique NK cell population that reside in the lungs' capillary (marginating pulmonary NK cells), as only the latter can effectively lyse the syngeneic MADB106 tumor cells in vitro (Melamed et al., 2005) and as marginating pulmonary NK cells seem critical in determining in vivo levels of LTR (Melamed et al., 2010) (also see Discussion).
Materials and Methods
Animals and counterbalancing
Four-month old male and female Fisher 344 (F344) rats, were housed 3–4 per cage in our vivarium with ad-libidum access to food and water on a 12:12 light:dark cycle at 22± 1°C. Animals were handled daily during the four days prior to experimentation to reduce potential procedural stress. Body weight, sex, and drug administration were counterbalanced across all experimental procedures. Housing conditions were monitored by the Institutional Animal Care and Use Committee of Tel Aviv University, which also approved all studies described herein.
Adrenalectomy
Rats were anesthetized with 2.5% isoflurane, and a 4cm midline abdominal incision was performed. Both adrenal glands were removed and inspected for being intact outside the animal, and the abdominal cavity was sutured. To facilitate the animals' recovery, CORT was administered subcutaneously immediately after the surgery (1.5mg/kg), as well as 3 and 6hr following surgery (3mg/kg). Thereafter, adrenalectomized animals received saline with low CORT levels (15mg/1L) as their drinking substance. This paradigm was reported to result in baseline levels of CORT in the serum of adrenalectomized rats while drinking along the active day period, thus simulating the natural circadian rhythm of CORT levels (Zagron and Weinstock, 2006). In order to eradicate these low levels of CORT, drinking was replaced with CORT-free saline 2hr before the initiation of the experiment. Control animals underwent sham operation, in which a 4cm midline abdominal incision was performed and sutured, without the removal of the adrenal glands.
Experimental laparotomy (surgery)
This procedure has been described elsewhere (Page et al., 1994). Briefly, rats were anesthetized with 2.5% isoflurane and a 4cm midline abdominal incision was performed. The small intestine was externalized, rubbed with a PBS-soaked gauze pad, and left hydrated for 40 minutes. Finally, the intestine was internalized and the abdomen was sutured.
Corticosterone plasma level assessment
CORT plasma levels were measured by radioimmunoassay (RIA) (ImmuChem double antibody corticosterone 125I RIA kit, MP Biomedicals, Orangeburg, NY), as per the manufacturers' instructions. The intra assay coefficient of variability was less than 5%, as is also reported by the manufacturer.
Drugs and their administration
Drug sources
all drugs and substances (i.e., mannide monooleate, mineral oil, PGE2, metaproterenol, epinephrine and corticosterone) were obtained from Sigma-Aldrich, Rehovot, Israel, except for complete medium which was purchased from Biological Industries, Kibbutz Beit Haemek, Israel.
Complete Medium (CM)
RPMI-1640 medium supplemented with 10% heatinactivated fetal calf serum (FCS), 50µg/mL of gentamicin, 2 mM of L-glutamine, 0.1 mM of nonessential amino-acids, and 1 mM of sodium pyruvate.
Slow-release emulsion
the emulsion is based on a mixture of PBS, mineral oil, and mannide monooleate (a non-ionic surface active emulsifier), in a 4:3:1 ratio, respectively. Drugs are dissolved in the PBS of the emulsion before the emulsifying agent is added, and before a rigorous vortexing that is needed for creating the emulsion. Rats were injected subcutaneous (s.c.) with 0.5ml of the emulsion. Unpublished data from our laboratory have shown that drugs carried in this emulsion, were released slowly and were effective for at least 12hr.
Prostaglandin E2
The drug was dissolved in ethanol and diluted 1:10 in PBS. For in vivo subcutaneous (s.c.) administration, PGE2 was administered s.c. (250µg/kg) in the slow-release emulsion (see above). This dosage was shown in our previous studies to impact NK activity and MADB106 lung tumor retention, causing similar effects to those of surgery (Yakar et al., 2003). Control rats were injected with vehicle. For the in vitro study, PGE2 was dissolved in ethanol and diluted in PBS, and further in complete medium, to reach a final concentration of 0.001% ethanol in the assay medium. This concentration was used in the entire study in all conditions. In previous studies we found that a 5-fold higher concentration of ethanol is needed to start impacting NK cytotoxicity in vitro (Yirmiya et al., 1992).
Corticosterone
For in vivo administration, a fine powder of the drug was dissolved in corn oil, and administered s.c. (3mg/kg). Our previous study in male rats indicated that 1hr following administration of 3mg/kg CORT, serum levels increased to approximately 700ng/ml, halved after 3hr, and completely dissipated to baseline levels (50ng/ml) by 6hr (Haim et al., 2003). For the in vitro study, the drug was dissolved in ethanol and then diluted in complete medium, reaching a final concentration of 0.001% ethanol in the assay medium.
Epinephrine
For the in vitro study, the drug was dissolved in PBS and then diluted in complete medium, and ethanol was added to reach a final concentration of 0.001% in the assay medium.
Tumor cell lines and their maintenance
MADB106
MADB106 is a selected variant cell line obtained from a pulmonary metastasis of a chemically induced mammary adenocarcinoma (MADB100) in the F344 rat (Barlozzari et al., 1985). MADB106 tumor cells metastasize only to the lungs (Barlozzari et al., 1985), and lung tumor retention (LTR), which is highly indicative of the number of metastases that would have developed weeks later, is dependent upon NK cells in this model (Barlozzari et al., 1985; Ben-Eliyahu and Page, 1992; Ben-Eliyahu et al., 1996b; Shakhar and Ben-Eliyahu, 1998a). Additionally, as the metastatic process of MADB106 is sensitive to NK activity predominantly in the first 24hr following inoculation (Barlozzari et al., 1985; Ben-Eliyahu and Page, 1992), LTR is more reflective of in vivo NK activity levels than the number of actual metastases (Shakhar and Ben-Eliyahu, 1998a). The MADB106 cell line was maintained in monolayer cultures in CM, in 100% humidity, 5% CO2 at 37°C. Cells were removed from the culture flask with trypsin solution (0.25% in PBS), and were washed with CM. This cell line was used for both in vivo assessment of lung tumor retention and in vitro examination of NK cytotoxicity.
YAC-1
YAC-1 mouse lymphoma is the standard target cell line used for the assessment of rodent in vitro NK cytotoxicity. The cell line was maintained in suspension cultures in CM in 100% humidity, 5% CO2 at 37°C.
Radiolabeling of MADB106 tumor cells and assessment of lung tumor retention
Tumor cell DNA radiolabeling for assessment of LTR is accomplished by adding 0.5µCi/ml of 125Iododeoxyuridine (125IDUR, Daniel Biotech, Rehovot, Israel) to the cell culture for 24hr. For tumor cell injection, rats are lightly anesthetized with isoflurane, and 4×105/kg MADB106 tumor cells in 2ml/kg PBS containing 0.1% bovine serum albumin (BSA) were injected into their tail vein. For assessment of LTR, animals were sacrificed 4hr after inoculation with 125IDUR-labeled tumor cells with an overdose of isoflurane, their lungs were removed, and placed in a γ-counter to assess percent radioactivity retained in this organ. LTR is calculated using the following formula: (radioactivity count of lung – background radioactivity) ×100/(radioactivity count of the total injected cell suspension – background radioactivity).
In vitro assessment of NK cytotoxicity
Harvesting and preparing circulating leukocytes and marginating-pulmonary (MP) leukocytes for assessment of NK cytotoxicity
Rats were sacrificed with an overdose of isoflurane and the peritoneal and chest cavities opened. Five to 8ml of blood (females and male respectively) was collected from the right ventricle of the heart into heparinized syringes. One ml of blood was washed once with 3ml of PBS (400g for 10 min) and twice with 3ml of complete medium, and reconstituted to its original volume. MP leukocytes were harvested by perfusing the heart with 30U/ml of heparinized PBS. PBS was injected into the right ventricle and perfusate was collected from the left ventricle. The first 3ml of perfusate, which were contaminated with blood, were discarded, and the following 25ml were collected and concentrated to 1ml. This was achieved by centrifuging the perfusate (400g for 10 min), discarding the supernatant, and suspending the pellet in 3ml of complete medium, centrifuging the perfusate again (400g for 10 min) and concentrating the perfusate into 1ml.
Assessment of NK cytotoxicity
The standard whole blood 51Cr release assay was used. This procedure assesses anti-tumor NKCC per ml of effector cells without prior purification of peripheral blood mononuclear cells or marginating-pulmonary leukocytes. Earlier studies have indicated that cytotoxicity measured using this procedure is attributable to NK cells, rather than other cell types or soluble factors, as the selective depletion of NK cells abolishes all target-cell killing (Ben-Eliyahu et al., 1996a; Ottenhof et al., 1981). The advantages of this procedure include shorter duration, less interference with the effector cells, and better representation of the original in vivo milieu of cell composition.
Six different effector to target (E:T) ratios were formed by serially diluting 150µl aliquots of the effector-cell preparation in a microtiter plate. Then, 5,000 radiolabeled target cells (MADB106 or YAC-1) in 100µl complete medium were added to each well on top of the effector-cell preparation. Radiolabeling of the target cells was conducted by incubating them for 1hr with 100 µCi 51Cr (Rotem Taassiot, Dimona, Israel) in 100µl saline, 100µl FCS, and 50µl complete medium. Following this incubation, target cells were washed 3 times (300g for 10 min) in complete medium and their concentration adjusted to 5Χ104/ml. Spontaneous and maximal releases of radioactivity were determined by substituting effector cells with complete medium or Triton-X100 (Sigma, Rehovot, Israel), respectively. Prior to and following a 4hr incubation period (100% humidity, 5% CO2 at 37°C) plates were centrifuged (400g for 10 min, at 25°C and 4°C, respectively). This creates a "buffy coat" of leukocytes and tumor cells on top of the red blood cells surface, enabling efficient effector-target interaction. Finally, 100µl samples of supernatant, were recovered for the assessment of radioactivity in a γ-counter. Specific killing was calculated as:
[(sample release * HCF - spontaneous release)/(maximal release - spontaneous release)] * 100
Hematocrit correction factor (HCF) compensates for changes in the hematocrit-supernatant volume over different E:T ratios. This correction factor is included to consider the changing volume of cell-free medium in which the released radioactive molecules are dispersed.
Flow Cytometry
Standard procedures were used to prepare cells for flow cytometric analysis (Melamed et al., 2005). NK cells in both blood and lung perfusate were identified by the FITC-conjugated anti-NKR-P1 mAb (PharMingen, San Diego) as being NKR-P1bright (CD161bright) cells. This criterion has been shown to exclusively identify more than 95% of cells that exhibit NK activity. T cells were identified using a PE-conjugated anti-CD5 mAb (eBioscience, San Diego), and NKT cells were identified as NKR-P1+CD5+ lymphocytes. Granulocytes and lymphocytes were identified based on forward and side scatters. Flow cytometry analysis was conducted using a FACScan (Becton Dickinson). To assess the absolute number of cells per µl of sample (or a specific cell subtype), 300 polystyrene microbeads (20µm, Duke Scientific, Palo Alto) per µl sample were added to each sample, and the following formula was used: (# of cells in sample/ # of microbeads in sample) × 300.
Statistical Analyses
One- or two-way analysis of variance (ANOVA) with a pre-determined significance level of 0.05 was conducted. In the in vitro NK cytotoxicity study (Exp 1), a 3 × 4 × 4 repeated measures ANOVA was conducted (incubation schedule, drug doses, and 4 highest E:T ratios as the repeated index). Similarly, when ex vivo NK cytotoxicity was assessed (Exp 2), a 2 × 3 × 4 repeated measures ANOVA was conducted (adrenalectomy, stress paradigm, and E:T ratios). Provided significant group differences were found, Fisher’s protected least significant differences (Fisher’s PLSD) contrasts were performed to compare specific pairs of groups, based on a priori hypotheses. Pearson correlations were used for assessing the associations between continuous variables (average cytotoxicity levels & number of NK cells), and the Fisher r-to-z transformation test was used to compare significant difference between correlations.
Procedures and Results
Experiment 1: The kinetics of the in vitro effects of CORT, PGE2 and epinephrine on NKCC
This study was designed to test the impact of incubating circulating leukocytes for a 4hr period with stress hormones, and to test the impact of this incubation on NKCC when this incubation occurs: (i) simultaneously with the 4hr cytotoxicity assay, or when the incubation period starts (ii) 8hr or (iii) 4hr before the assessment of NK cytotoxicity, and the hormones are washed away at the end of the incubation period (prior to NKCC assessment in the last two conditions).
Procedure
Blood was withdrawn by cardiac puncture from 10 male F344 rats, pooled and washed three times (once with 3-fold volume of PBS (400g for 10 min) and twice with 3-fold volume of complete medium). CORT, PGE2, and epinephrine were incubated with aliquots of the washed pooled blood, employing different incubation schedules (see text below) and different drug concentrations: CORT - 0 M (control), 10−9 M, 3 × 10−9 M and 3 × 10−8 M; PGE2 - 0 M (control), 10−9 M, 3 × 10−9 M and 10−8 M; and epinephrine - 0 M (control), 10−8 M, 3 × 10−8 M and 10−7 M. These concentrations were chosen as they were the lowest in vitro concentrations that affect NK cytotoxicity, and theoretically overlapping with their levels in some physiological compartments. CORT concentrations also overlap with physiological plasma levels of unbound CORT. NK cytotoxicity against YAC-1 target cells was assessed as described in the Method section, in the presence or absence of the drugs as described in detail below. The study was conducted in two runs/replicates. In the first run, only CORT and PGE2 were used, and each drug/time condition was tested in triplicates (including the incubation with the drug and the cytotoxicity assay). The second run also included epinephrine, and was conducted in the exact same manner (triplicate for each condition). Except for the schedule of drug incubation, the different blood aliquots were subjected to the exact same procedures, including the timing and number of washes and the cytotoxicity assay.
Incubation at −8 to −4hr before NK cytotoxicity assay ('−8 to −4hr' condition): blood was co-incubated with the stress hormones for 4hr, then washed and maintained in room temperature for additional 4hr, and washed again and used in the NK cytotoxicity assay procedure.
Incubation at −4 to 0hr before NK cytotoxicity assay ('−4 to 0hr' condition): blood was maintained in room temperature for 4hr, then washed, incubated with the stress hormones for 4hr, washed again, and used in the NK cytotoxicity assay procedure.
Simultaneous incubation ('simultaneous' condition): blood was maintained in room temperature for 4hr, washed and maintained in room temperature for additional 4hr. Thereafter, stress hormones were added to the blood which was immediately used in the NK cytotoxicity assay procedure.
Results
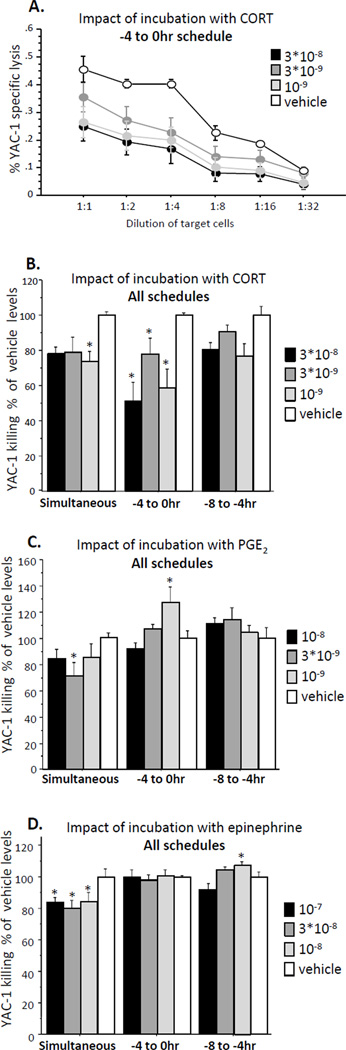
The two runs of the study yielded similar patterns of results and were combined. For each drug, a 3 × 4 × 4 repeated measures ANOVA was employed (3 time schedules, 4 drug concentrations, and 4 E:T ratios as the repeated index). A representative complete E:T cytotoxicity curve is presented for a single drug-time schedule (Fig 1A).
Fig. 1. The effects of different drugs and schedule of their application on NK cytotoxicity against YAC-1 target cells.
(A) A representative cytotoxicity curve - Incubation with CORT at the −4 to 0hr schedule. (B, C, D) Cytotoxicity presented as % of Vehicle levels at each schedule and drug. (B) Incubation with CORT reduced NK cell cytotoxicity in a similar manner in all schedules. (C) Incubation with PGE2 reduced NK cell cytotoxicity in the simultaneous schedule, elevated it in the −4 to 0hr schedule, and had no effects in the −8 to −4hr schedule. (D) Incubation with epinephrine reduced NK cell cytotoxicity in the simultaneous schedule, had no effects in the −4 to 0hr schedule, and elevated it in the −8 to −4hr schedule. * indicates a significant reduction or elevation in NK cell cytotoxicity compared to the respective control (Vehicle) group. Data presented as mean + SEM.
For clarity and ease of presentation (not for statistical comparisons), a single index of NK cytotoxicity per condition was composed by averaging killing levels of the 4 highest E:T ratios, and all results were transformed to % of control levels (0 M drug) for each time schedule and hormone (Fig. 1 B, C, D).
Incubation with CORT
A two-way repeated measures ANOVA revealed a main effect for schedule (F(2,51) = 4.222, p < 0.05) and for concentration (F(3,51) = 7.962, p < 0.05), without a significant interaction, indicating that CORT similarly suppressed NK activity along the three time points. Fisher's PLSD Post-Hoc Comparisons indicated that in the simultaneous condition, CORT in concentration of 3*10−8 and 10−9 M significantly reduced NKCC compared to the matched control. In the −4 to 0hr condition, CORT reduced NKCC in all concentrations compared to matched control (p < 0.05). No significant effects were evident in the −8 to 4hr condition, but a trend (p < 0.1) was evident in two concentrations (Fig. 1B).
Incubation with PGE2
A two-way repeated measures ANOVA revealed a main effect for schedule (F(2,51) = 13.071, p < 0.05) and a significant interaction between schedule and concentration (F(6,51) = 2.619, p < 0.05), indicating that the effects of PGE2 on NK activity were time-dependent. Fisher's PLSD Post-Hoc Comparisons indicated that in the simultaneous condition, PGE2 in a concentration of 3*10−9 M reduced NKCC compared to matched control (p < 0.05). Unlike the effects seen with CORT, a rebound effect was evident in the −4 to 0hr condition, where PGE2 in a concentration of 10−9 significantly elevated NKCC compared to the matched control. No effects were evident in the −8 to −4hr condition (Fig. 1C).
Incubation with epinephrine
had a similar effect to that seen in PGE2. A two-way repeated measures ANOVA revealed a main effect for schedule (F(2,24) = 22.754, p < 0.05) and a significant interaction between schedule and concentration (F(6,24) = 3.255, p < 0.05), indicating that the effects of epinephrine on NK activity was time-dependent. Fisher's PLSD Post-Hoc Comparisons indicated that in the simultaneous condition, epinephrine in all concentrations reduced NKCC compared to the matched control (p < 0.05). A rebound effect was evident in the −8 to −4hr condition, where epinephrine in a concentration of 10−8 significantly elevated NKCC compared to the matched control (p < 0.05). No effects were evident in other schedules (Fig. 1D).
Experiment 2: Effects of PGE2 administration and surgery on in vivo and ex-vivo indices of NK activity in adrenalectomized and sham-operated animals
Procedure
One hundred and fourteen F344 rats underwent adrenalectomy or sham operation (see Methods). Three weeks later, rats were subdivided to undergo laparotomy, or to receive PGE2 or vehicle injection (control). Immediately after, all rats were inoculated intravenously with radiolabeled MADB106 tumor cells, and 4 additional hr later sacrificed for harvesting of circulating and MP leukocytes for assessment of NK cell number and cytotoxicity, and for assessing MADB106 LTR in the excised lungs. In blood samples, CORT plasma levels were also assessed. All groups had approximately equal numbers of males and females.
Results
General
Females and males exhibited the same pattern of results, and no interaction between sex and any measure was revealed. Thus, the results are analyzed and presented irrespective of sex. In addition to presenting killing levels in the different E:T ratios, and conducting the statistical analysis on the 4 E:T ratios, for clarity of presentation in the form of the 2 × 3 design used, the 4 E:T ratios were also averaged, yielding a single index of NK cytotoxicity per sample. Of the total 114 rats, 10 unsuccessful/contaminated lung perfusate samples, and few unsuccessful blood samples, similarly scattered in the six experimental groups, were not included in the final analyses.
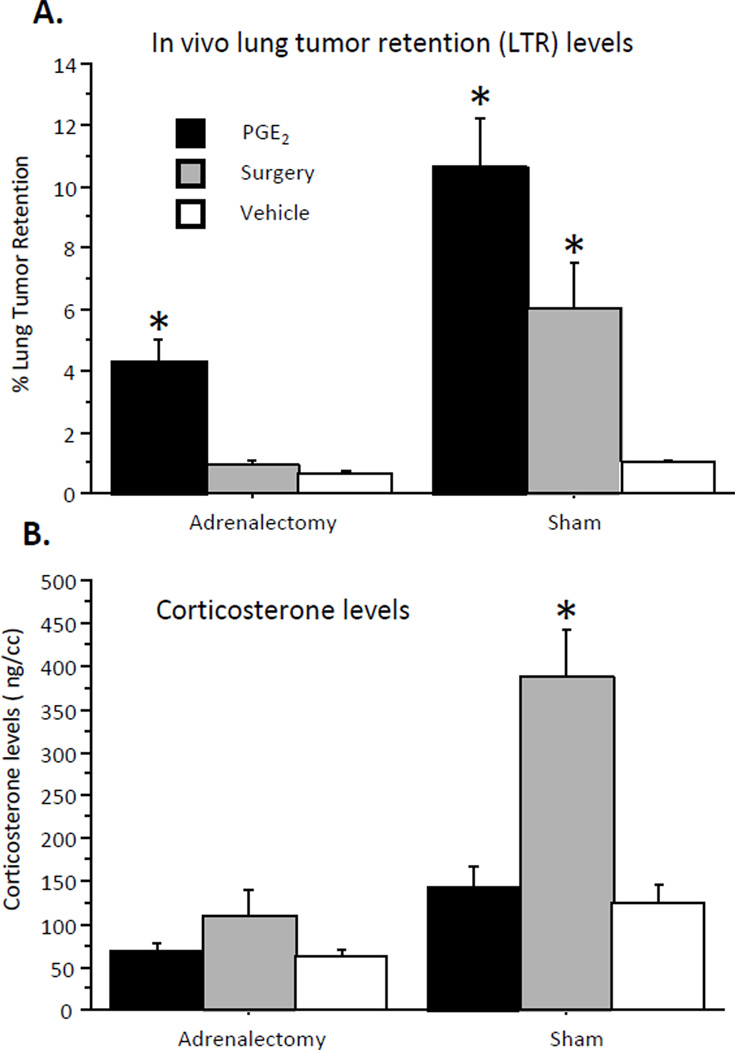
Lung tumor retention (Fig. 2A)
Fig. 2. The effects of surgery and of PGE2 administration on LTR levels (A) and on plasma corticosterone levels (B) in sham operated and in adrenalectomized animals.
(A) Surgery elevated LTR levels in sham operated but not adrenalectomized animals. PGE2 elevated LTR levels in both sham operated and adrenalectomized animals, and this effect was greater in sham operated animals. (B) Surgery elevated CORT levels in sham operated, but not in adrenalectomized animals, and PGE2 administration did not elevate CORT levels in either conditions. * indicates a significant elevation in LTR/CORT levels compared to respective control group. Data presented as mean + SEM.
A two-way ANOVA revealed a main effect for adrenalectomy (F(1,108) = 22.743, p < 0.05), and a significant interaction between adrenalectomy and stress paradigm (PGE2/surgery/vehicle) (F(2,108) = 6.136, p < 0.05), in a manner that the adrenalectomy obliterated the effects of surgery, but not of PGE2 administration. Fisher's PLSD Post-Hoc Comparisons indicated that PGE2 administration significantly increased LTR levels both in sham operated and in adrenalectomized animals; while surgery increased LTR levels only in sham operated animals (p < 0.05).
Corticosterone levels (Fig. 2B)
A two-way ANOVA revealed a main effect for adrenalectomy (F(1,107) = 46.542, p < 0.05), and a significant interaction between adrenalectomy and stress paradigm (PGE2/surgery/vehicle) (F(2,107) = 9.803, p < 0.05), in a manner that adrenalectomy abolished the effects of surgery on CORT levels. Fisher's PLSD Post-Hoc Comparisons indicated that in sham operated animals, surgery, but not PGE2 administration elevated CORT levels (p < 0.05). No effects of surgery or PGE2 were evident in adrenalectomized animals.
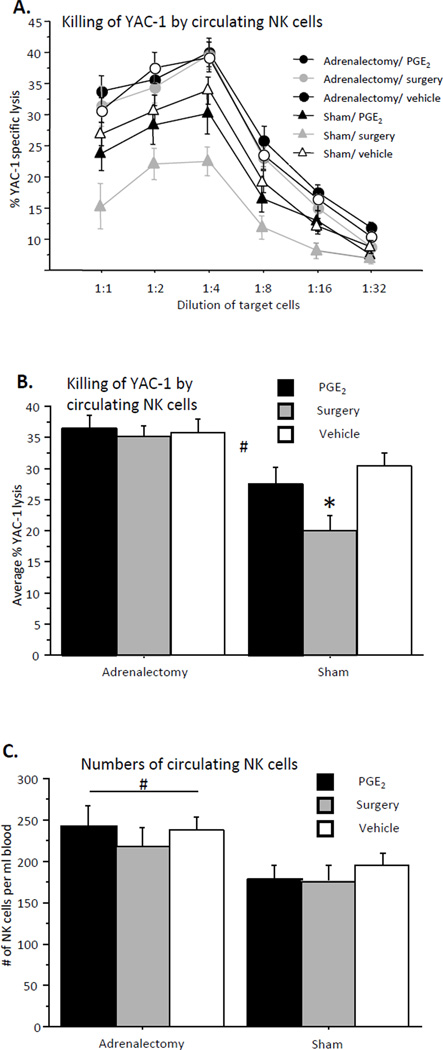
Circulating NK cell cytotoxicity against YAC-1 target cells (Fig. 3A/B)
Fig. 3. Killing of YAC-1 target cells by circulating NK cells (A – all E:T ratios; B – average of 4 highest E:T ratios).
(A, B) Surgery but not PGE2 administration reduced NK cell cytotoxicity in sham operated animals. Both treatments did not affect NK cytotoxicity in adrenalectomized animals. (C) Circulating NK numbers: Adrenalectomy elevated circulating NK cell numbers compared to sham operated animals. * indicates a significant reduction in NK cell cytotoxicity compared to respective control group. # indicates a significant elevation in NK cell numbers in adrenalectomized animals compared to sham operated animals. Data presented as mean + SEM.
A two-way repeated measures (4 highest E:T ratios) ANOVA revealed a main effect for adrenalectomy (F(1,108) = 22.932, p < 0.05). Specifically, Fisher's PLSD Post-Hoc Comparisons indicated that surgery reduced NKCC in sham operated but not in adrenalectomized animals (p < 0.05). PGE2 administration did not affect NK cytotoxicity in sham or adrenalectomized animals. This pattern of results is expected in such ex-vivo studies, as the effect of CORT on NKCC should occur in adrenal-intact, but not in adrenalectomized animals, and the potential impact of PGE2 administration should vanish given its removal from the assay milieu.
Circulating NK numbers (Fig. 3C)
A two-way ANOVA revealed a main effect for adrenalectomy (F(1,109) = 8.951, p < 0.05), with adrenalectomized animals showing higher numbers of circulating NK cells, and no significant interaction. Neither surgery nor PGE2 administration affected NK numbers in sham or in adrenalectomized animals.
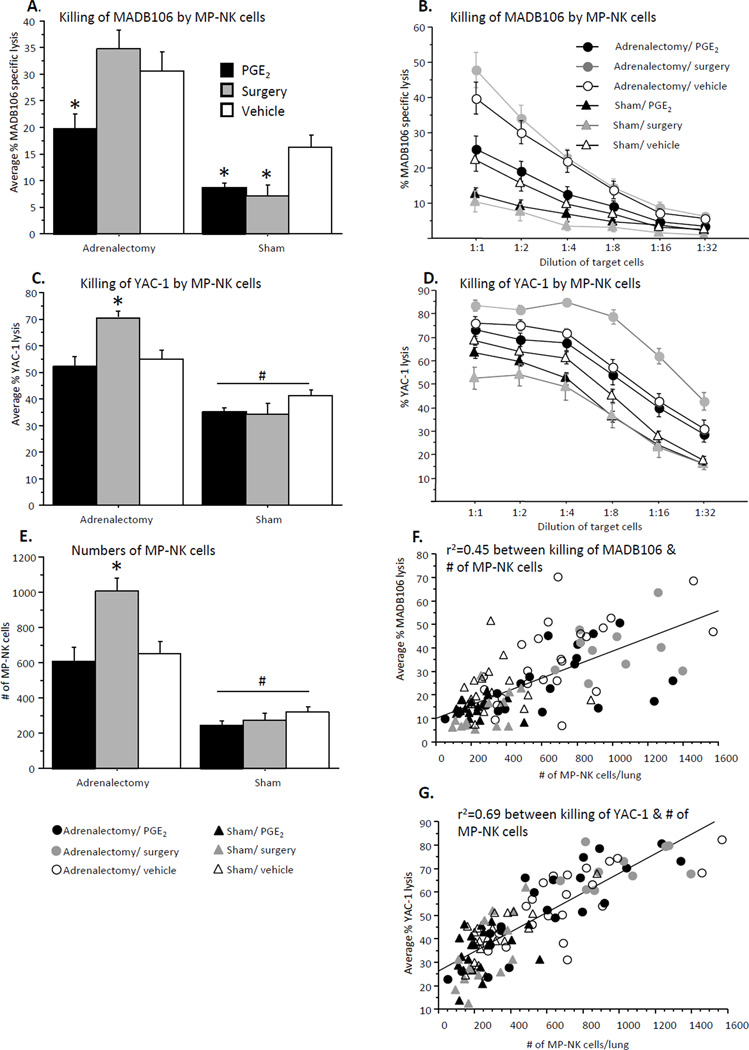
MP-NK cell cytotoxicity against MADB106 target cells (Fig. 4A/B)
Fig. 4. Numbers and cytotoxicity of MP-NK cells against syngeneic MADB106 and xenogeneic YAC-1 target cells.
(A, B) Killing of MADB106 (B – all E:T ratios; A – average of 3 highest E:T ratios): PGE2 administration reduced MP-NK cell cytotoxicity in both sham operated and adrenalectomized animals, while surgery caused such a decrease only in sham operated animals. (C, D) Killing of YAC-1 (D – all E:T ratios; C – average of 4 lowest E:T ratios): Surgery elevated MP-NK cell cytotoxicity in adrenalectomized but not sham operated animals. PGE2 administration had no effects. (E) Number of MP-NK cells per lung: Similar to C/D, surgery elevated MP-NK cell numbers in adrenalectomized, but not sham operated animals. PGE2 administration had no effects, and adrenalectomy increased MP-NK cell numbers in all groups. (F, G) A significant Pearson correlation was found between numbers of MP-NK cells per lung, and their total cytotoxicity against MADB106 target cells (r2=0.45) (F), which is significantly lower than the same correlation when cytotoxicity was tested against the YAC-1 target cells (r2=0.69) (G). * indicates a significant elevation/reduction in MP-NK cell cytotoxicity/numbers compared to respective control group. # indicates a significant overall elevation in MP-NK cell cytotoxicity/numbers in adrenalectomized animals compared to sham operated animals. Data presented as mean + SEM.
A two-way repeated measures (4 highest E:T ratios) ANOVA revealed a main effect for adrenalectomy (F(1,99) = 55.336, p < 0.05) – increased cytotoxicity in adrenalectomized animals, and a significant interaction between adrenalectomy and stress paradigm (PGE2/surgery/vehicle) (F(2,99) = 3.853, p < 0.05). Fisher's PLSD Post-Hoc Comparisons indicated significant reduction in NK cytotoxicity by both surgery and PGE2 in sham-operated animals, and a reduction only by PGE2 (but not by surgery) in adrenalectomized animals (p < 0.05). A different pattern of results was evident here in comparison to cytotoxicity against YAC-1 target cells, however, these results correspond well (inverted to) with those seen in the LTR assessment.
MP-NK cell cytotoxicity against YAC-1 target cells (Fig. 4C/D)
A two-way repeated measures (4 lowest E:T ratios) ANOVA revealed a main effect for adrenalectomy (F(1,104) = 75.532, p < 0.05) – an increased cytotoxicity in adrenalectomized animals, and a significant interaction between adrenalectomy and stress paradigm (PGE2/surgery/vehicle) (F(2,104) = 6.570, p < 0.05). Fisher's PLSD Post-Hoc Comparisons indicated that surgery elevated MP-NK cell cytotoxicity in adrenalectomized animals (but not in sham operated), and PGE2 administration showed no effect in any condition.
MP-NK cell numbers (Fig. 4E)
A two-way ANOVA revealed a main effect for adrenalectomy (F(1,106) = 85.449, p < 0.05), adrenalectomized animals showing higher numbers of MP-NK cells, and a significant interaction between adrenalectomy and stress paradigm (PGE2/surgery/vehicle) (F(2,106) = 4.977, p < 0.05). Fisher's PLSD Post-Hoc Comparisons indicated a significant elevation in MP-NK numbers by surgery in adrenalectomized animals (but not in sham-operated animals) (p < 0.05). PGE2 did not affect NK numbers in sham or adrenalectomized animals.
A significant Pearson correlation was found between MP-NK numbers and cytotoxicity against YAC-1 target cells (r = 0.83, r2 = 0.69) (Fig. 4G), which is significantly higher (p < 0.05) then the correlation between MP-NK numbers and cytotoxicity against MADB106 target cells (r = 0.67, r2 = 0.45) (Fig. 4F).
Discussion
The present study aimed to elucidate potential mechanisms through which PGE2 affects NKCC in vivo. PGE2 is known to directly decrease NK cytotoxicity in vitro (Gatti et al., 1986a; Yakar et al., 2003), through its membrane prostanoid receptors and subsequent elevated intracellular cAMP and activated protein kinase A1 (PKA1) levels (Torgersen et al., 1997a). However, since PGE2 is released in vivo locally (around the damaged tissue), and its systemic endogenous levels are often not affected (Parsson et al., 2000), it is not clear whether the in vivo effects of PGE2 depend on a mediating mechanism, such as CORT, which, in the context of surgery, is elevated by PGs (Asoh et al., 1987; Shaashua et al., In press) and by a host of other pro-inflammatory factors (DeKeyser et al., 2000; Turnbull et al., 1994). As will be elaborated below, increased systemic levels of PGs, induced herein by its administration, directly suppressed NKCC in vivo. However, following surgery, adrenal hormones were necessary to mediate these effects. In both cases, these in vivo-suppressing effects cannot be reflected by the standard ex-vivo assessment of NKCC.
Critical for the interpretation of the current findings are results from our previous studies employing the same methods of surgery and PGE2 administration in F344 rats. First, no effects of surgery on systemic PGE2 levels at various time points after surgery were found, while a robust two-fold increase of PGE2 levels were observed following its systemic administration. Second, PGE2 administration increased MADB106 LTR (as was in the current study) through suppressing NK activity, as selective depletion of NK cells abrogated this effect, but not other effects that were NK-independent (Yakar et al., 2003). Last, the MADB106 LTR-increasing effects of surgery (also evident herein) were additively reduced by a COX inhibitor and by a β-adrenergic blocker, together abolishing up to 85% of the deleterious effects of surgery (Benish et al., 2008; Melamed et al., 2005). These later findings clearly indicate the involvement of CAs and of PGs in the LTR-increasing effects of surgery.
As is the common practice, ex-vivo levels of NK activity are measured following removal of all in vivo factors from the assay's milieu. Thus, to simulate the in vivo presence of CORT, catecholamines, or PGs during stress or surgery, and their absence in the ex-vivo setting, we first tested various schedules which included utilizing and removing these compounds in vitro, and then measuring NK cytotoxicity against the standard YAC-1 target cell. CORT suppressed NK activity when employed simultaneously with the assessment of cytotoxicity, along the 4hr 51Cr release assay, as well as if removed immediately before this assessment or 4hr earlier. PGE2 and epinephrine, on the other hand, suppressed NK activity only if employed simultaneously with the assessment of cytotoxicity. When removed before this assessment, no suppression was evident, and in PGE2- and epinephrine-extreated cells an increase in cytotoxicity (rebound) was observed. These findings correspond well with the known mechanism of enduring CORT action on NK cytotoxicity, mediated through activation of DNA-dependent mechanisms and protein synthesis (Zhou et al., 1997), in contrast to the transient cytoplasmatic-mediated effects of PGE2 and epinephrine in regulating NK activity, through elevation of cAMP and activation of PKA1 (Torgersen et al., 1997b). Our herein in vitro findings also correspond with those of Hellstrand et al (Hellstrand et al., 1985), reporting in vitro suppression of human NK activity by epinephrine, which was followed by a rebound effect following its removal. Given these previous findings and our current findings, it is expected that ex-vivo studies of NK cytotoxicity, which involve the replacement of the plasma with hormone-free medium, will reflect in vivo effects of elevated CORT levels, but will not reflect the in vivo effects of elevated PGE2 or epinephrine levels, as indeed found in our current study and discussed below.
The in vivo assessment of NK activity, through studying MADB106 LTR in the current and in our previous studies, was conducted alongside exposure of animals to surgery or PGE2 (PGE2 was administered simultaneously with tumor cells in a slow release preparation). Thus, the effects of PGE2 administration or of surgery are expected to be reflected in this index, as are the effects of CORT. Importantly, in our previous study we have directly showed that the increase in LTR following PGE2 administration is mediated in vivo by suppression of NK activity (Yakar et al., 2003). In the current study, in sham operated animals, both surgery and systemic administration of PGE2 elevated LTR levels, whereas in adrenalectomized animals, only PGE2 administration elevated this index. Thus, systemic PGE2 administration can suppress NKCC in vivo without the mediating effects of CORT or other adrenal hormones. However, the effects of the surgical procedure used herein were mediated by adrenal hormones (e.g. epinephrine & CORT), rather than directly by PGs, as adrenalectomy abrogated them. Therefore, based on the literature and the above findings, it seems that PGs can suppress NK activity through two different non-exclusive mechanisms – directly by impacting NK cells, provided that PGs systemic levels are sufficiently elevated, and indirectly through inducing adrenal hormones response. We further hypothesize that CORT is the critical mediating factor of these effects of PG response during surgery, rather than other adrenal factors (epinephrine or opioids), as in the context of surgery PGE2 can elevate CORT levels (Asoh et al., 1987), and as PG inhibitors reduce post-operative CORT levels (Glasner et al., 2010; Shaashua et al., In press). The effects of PGs on CORT levels can be mediated through cells of the adrenal cortex (Mohn et al., 2005), or through elevating IL-6 levels (Page and Ben-Eliyahu, 2002). To the best of our knowledge, PGs are not known to elevate other adrenal hormones, specifically epinephrine or opioids. Nevertheless, more severe surgical procedures than the one used herein, or other traumatic tissue-damaging events, could elevate systemic levels of PGE2 (Faist et al., 1987), which could then directly suppress NK activity, as following PGE2 systemic administration.
Our ex-vivo findings correspond with our predictions based on our in vitro and in vivo experiments. As would be expected, in sham operated animals, surgery, but not PGE2 administration, decreased ex-vivo NK cytotoxicity levels. These effects are attributable to the herein evident increase in CORT levels following surgery, but not following PGE2 administration, and the transient effects of PGE2 that cannot be seen due to its removal from the assay milieu. In adrenalectomized animals, neither surgery nor PGE2 administration affected NK cytotoxicity, given the absence of CORT response, and as ex-vivo effects of PGs and catecholamines are not expected to be evident. Together, these findings clearly suggest that when studying circulating NK cytotoxicity levels, the ex-vivo effects observed following surgery in otherwise naïve rats are those that had been induced in vivo by CORT, but not those that were potentially induced in vivo by catecholamines or PGs. It is crucial to note that in vivo effects of catecholamines and PGs on NK cytotoxicity are profound and critical (Ben-Eliyahu et al., 2000; Benish et al., 2008; Glasner et al., 2010), and last as long as the levels of these hormones are elevated, even though such effects are not reflected in ex-vivo measurement. Elevated CORT levels in the context of surgery can potentially be induced through local PG release (Asoh et al., 1987), through nociception and pain, or through a systemic catecholamine and/or PG response to the surgical procedure (Glasner et al., 2010; Shaashua et al., 2012). In the current study no increase in CORT levels was observed following systemic PGE2 administration, suggesting that the mechanisms through which endogenous PGs elevate CORT in the context of surgery are not activated by systemic administration of PGE2 in the dose used herein.
Leukocytes of the MP compartment are unique in several characteristics when compared to circulating leukocytes. Their cellular composition is enriched with "proinflammatory cells" (NK cells, monocytes, granulocytes) (Melamed et al., 2010), they show a cellular profile of activated immunocytes (including high levels of intracellular IFNγ and large NK cells), and finally, MP-NK cells, but not circulating NK cells, can lyse the syngeneic MADB106 cell line in vitro (Melamed et al., 2010). When studying ex-vivo cytotoxicity of this MP population against the xenogeneic YAC-1 target cells, the herein findings indicated a remarkably high correlation between the total number of MP-NK cells and the total cytotoxicity they exhibit (r2 = ~0.7, fig. 4E). In contrast to the findings regarding circulating leukocytes, surgery did not alter cytotoxicity of the MP-leukocytes against the YAC-1 target line. These findings indicate that adrenal and non-adrenal factors, including CORT, are relatively ineffective in impacting MP cytotoxicity per NK cell in an ex-vivo approach against the YAC-1 target line.
However, when using syngeneic MADB106 cells as targets for assessing MP-NK cytotoxicity, using aliquots from the same MP-leukocyte samples used to study lysis of xenogeneic YAC-1 cells, a profoundly different pattern of effects was evident. First, although these are the same samples, the r2 between the numbers of NK cells and their cytotoxicity significantly decreased from ~0.7 (for YAC-1) to ~0.45 (for MADB106). Second, surgery and PGE2 administration significantly reduced cytotoxicity in sham operated animals, and PGE2 (but not surgery) did so also in adrenalectomized animals. None of these effects occurred when studied against the YAC-1 target line. Thus, it seems that the mechanisms used to exert MP-NK cytotoxicity against the MADB106 line are more susceptible to PGE2 and surgery-induced stress responses than the mechanisms used for lysing YAC-1 target cells. We have previously reported evidence for such a difference in lysing syngeneic versus allogeneic/xenogeneic target lines in both rats and mice (Benish et al., In press; Melamed et al., 2010). It is also worthy to note that the cytotoxicity level of MP-NK cells against MADB106 target cells were an inverted picture to MADB106 LTR, as we also reported in previous studies (Goldfarb et al., 2009).
There are several shortcomings to the study. First, the in vitro study cannot be expected to comprehensively simulate the in vivo conditions where NK cells are naturally subjected to the different effects of hormones. For example, the in vivo milieu is characterized by simultaneously ever changing levels of many hormones, which was beyond the scope of this study to simulate. Rather, we focused on separately assessing the impact of a single hormone at specific time schedules, and did not study potential in vitro interactions at different schedules and doses. Second, the approach to study NK activity in vivo relies on an indirect in vivo index that is specific to the lungs, although this approach was validated as indicating NK activity in many of our previous studies described above. Last, the study is not designed to elucidate potential NK cell interactions with other leukocytes, or to identify molecular mediating mechanisms that contribute to the observed findings.
In summary, the in vivo and the in vitro experiments, and the supporting literature, suggest that the standard assessment of ex-vivo cytotoxicity against allogeneic/xenogeneic target lines cannot be expected to reflect in vivo alterations in NK activity caused directly by catecholamines and PGs; these effects may dissipate immediately following the removal of these ligands (and potentially inducing rebound effects) although may bear long-term critical in vivo consequences (Glasner et al., 2010; Inbar et al., 2011), including reduced cancer survival rates. On the other hand, the effects of CORT are long-lasting and are reflected in the ex-vivo setting, irrespective of whether CORT is a minor or major in vivo player, or/and a mediator of other factors altering in vivo levels of NK cytotoxicity (Neeman and Ben-Eliyahu, 2012). Second, MP-NK cells show different in vivo susceptibility to PGE2 and stress hormones when their cytotoxicity is studied against standard allogeneic/xenogeneic standard cell lines (e.g., YAC-1) compared to the syngeneic cell lines (e.g., MADB106). Thus, evidence from ex-vivo NK activity studies that use standard target lines, should be cautiously interpreted regarding their implications to cancer patients bearing autologous (syngeneic) tumor cells (**). Third, elevated systemic PGE2 levels (when occurs) can suppress NK activity in vivo irrespective of adrenal hormones, but ex-vivo this suppression is evident only in MP-leukocytes against the syngeneic MADB106 line. Nevertheless, in the context of the surgical paradigm used herein, the endogenous (apparently local) PG response depends on adrenal hormones to cause NK suppression (in vivo and ex-vivo).
Acknowledgment
This work was supported by NIH/NCI grant # CA125456 grant (SBE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: All authors declare that there are no conflicts of interest.
references
- Armaiz-Pena GN, Cole SW, Lutgendorf SK, Sood AK. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asoh T, Shirasaka C, Uchida I, Tsuji H. Effects of indomethacin on endocrine responses and nitrogen loss after surgery. Ann Surg. 1987;206(6):770–776. doi: 10.1097/00000658-198712000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, Reynolds CW. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134(4):2783–2789. [PubMed] [Google Scholar]
- Barlozzari T, Reynolds CW, Herberman RB. In vivo role of natural killer cells: involvement of large granular lymphocytes in the clearance of tumor cells in anti-asialo GM1-treated rats. Journal of Immunology. 1983;131(2):1024–1027. [PubMed] [Google Scholar]
- Baxevanis CN, Papilas K, Dedoussis GV, Pavlis T, Papamichail M. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71(1):82–88. doi: 10.1006/clin.1994.1055. [DOI] [PubMed] [Google Scholar]
- Baxevanis CN, Reclos GJ, Gritzapis AD, Dedousis GV, Missitzis I, Papamichail M. Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer. 1993;72(2):491–501. doi: 10.1002/1097-0142(19930715)72:2<491::aid-cncr2820720227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S. Stress, natural killer cell activity, and tumor metastasis: The role of catecholamines and corticosteroids. In: Levi A, Grauer E, Ben-Nathan D, De-Kloet ER, editors. New Frontiers in Stress Research: Modulation in Brain Function. Amsterdam: Harwood Academic Publishers GmbH; 1998. pp. 203–215. [Google Scholar]
- Ben-Eliyahu S, Page GG. In vivo assessment of antural killer activity in rats. Prog Neuroendocrineimmunol. 1992;5:199–214. [Google Scholar]
- Ben-Eliyahu S, Page GG, Shakhar G, Taylor AN. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. Br J Cancer. 1996a;74(12):1900–1907. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Taylor AN. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996b;2(4):457–460. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–164. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benish M, Melamed R, Rosenne E, Neeman E, Sorski L, levi B, Shaashua L, Matzner P, Ben-Eliyahu S. The marginating pulmonary immune compartment in mice exhibits increased NK cytotoxicity and unique cellular characteristics. doi: 10.1007/s12026-013-8435-6. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13(4):458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- Bodner G, Ho A, Kreek MJ. Effect of endogenous cortisol levels on natural killer cell activity in healthy humans. Brain Behav Immun. 1998;12(4):285–296. doi: 10.1006/brbi.1998.0533. [DOI] [PubMed] [Google Scholar]
- Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104(3):403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- Deguchi M, Isobe Y, Matsukawa S, Yamaguchi A, Nakagawara G. Usefulness of metyrapone treatment to suppress cancer metastasis facilitated by surgical stress. Surgery. 1998;123(4):440–449. [PubMed] [Google Scholar]
- DeKeyser FG, Leker RR, Weidenfeld J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7(4):182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- Faist E, Ertel W, Cohnert T, Huber P, Inthorn D, Heberer G. Immunoprotective effects of cyclooxygenase inhibition in patients with major surgical trauma. J Trauma. 1990;30(1):8–17. doi: 10.1097/00005373-199001000-00002. discussion 17–18. [DOI] [PubMed] [Google Scholar]
- Faist E, Mewes A, Baker CC, Strasser T, Alkan SS, Rieber P, Heberer G. Prostaglandin E2 (PGE2)-dependent suppression of interleukin alpha (IL-2) production in patients with major trauma. J Trauma. 1987;27(8):837–848. doi: 10.1097/00005373-198708000-00001. [DOI] [PubMed] [Google Scholar]
- Gatti G, Cavallo R, Sartori ML, Marinone C, Angeli A. Cortisol at physiological concentrations and prostaglandin E2 are additive inhibitors of human natural killer cell activity. Immunopharmacology. 1986a;11(2):119–128. doi: 10.1016/0162-3109(86)90032-9. [DOI] [PubMed] [Google Scholar]
- Gatti G, Cavallo R, Sartori ML, Marinone C, Angeli A. Cortisol at physiological concentrations and prostaglandin E2 are additive inhibitors of human natural killer cell activity. Immunopharmacology. 1986b;11(2):119–128. doi: 10.1016/0162-3109(86)90032-9. [DOI] [PubMed] [Google Scholar]
- Glasner A, Avraham R, Rosenne E, Benish M, Zmora O, Shemer S, Meiboom H, Ben-Eliyahu S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–2457. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- Goldfarb Y, Benish M, Rosenne E, Melamed R, Levi B, Glasner A, Ben-Eliyahu S. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother. 2009;32(3):280–291. doi: 10.1097/CJI.0b013e31819a2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim S, Shakhar G, Rossene E, Taylor AN, Ben-Eliyahu S. Serum levels of sex hormones and corticosterone throughout 4- and 5-day estrous cycles in Fischer 344 rats and their simulation in ovariectomized females. J Endocrinol Invest. 2003;26(10):1013–1022. doi: 10.1007/BF03348201. [DOI] [PubMed] [Google Scholar]
- Hellstrand K, Hermodsson S, Strannegard O. Evidence for a betaadrenoceptor-mediated regulation of human natural killer cells. Journal of Immunology. 1985;134(6):4095–4099. [PubMed] [Google Scholar]
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6(4):e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Benish M, Goldfarb Y, Levi B, Ben-Eliyahu S. The marginating-pulmonary immune compartment in rats: characteristics of continuous inflammation and activated NK cells. J Immunother. 2010;33(1):16–29. doi: 10.1097/CJI.0b013e3181b0b146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Mohn CE, Fernandez-Solari J, De Laurentiis A, Prestifilippo JP, de la Cal C, Funk R, Bornstein SR, McCann SM, Rettori V. The rapid release of corticosterone from the adrenal induced by ACTH is mediated by nitric oxide acting by prostaglandin E2. Proc Natl Acad Sci U S A. 2005;102(17):6213–6218. doi: 10.1073/pnas.0502136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: New outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman E, Zmora O, Ben-Eliyhau S. A new approach to reducing postsurgical cancer recurrence: perioperative targeting of catecholamines and prostaglandins. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-12-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus SJ, Watson DI, Ellis T, Rofe AM, Jamieson GG. The effect of immune enhancement and suppression on the development of laparoscopic port site metastases. Surg Endosc. 2000;14(5):439–443. doi: 10.1007/s004640000157. [DOI] [PubMed] [Google Scholar]
- Okuno K, Jinnai H, Lee YS, Nakamura K, Hirohata T, Shigeoka H, Yasutomi M. A high level of prostaglandin E2 (PGE2) in the portal vein suppresses liver-associated immunity and promotes liver metastases. Surg Today. 1995;25(11):954–958. doi: 10.1007/BF00312380. [DOI] [PubMed] [Google Scholar]
- Ottenhof PC, Morales A, Baines MG. Quantitation of a whole blood assay for human natural killer cell activity. J Immunol Methods. 1981;42(3):305–318. doi: 10.1016/0022-1759(81)90159-9. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S. Indomethacin attenuates the immunosuppressive and tumor-promoting effects of surgery. J Pain. 2002;3(4):301–308. doi: 10.1054/jpai.2002.125184. [DOI] [PubMed] [Google Scholar]
- Page GG, Ben-Eliyahu S, Liebeskind JC. The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun. 1994;8(3):241–250. doi: 10.1006/brbi.1994.1022. [DOI] [PubMed] [Google Scholar]
- Parsson HN, Lord RS, Scott K, Zemack G. Maintaining carotid flow by shunting during carotid endarterectomy diminishes the inflammatory response mediating ischaemic brain injury. Eur J Vasc Endovasc Surg. 2000;19(2):124–130. doi: 10.1053/ejvs.1999.0954. [DOI] [PubMed] [Google Scholar]
- Shaashua L, Rosenne E, Neeman E, Sominsky L, Matzner P, Page G, Ben-Eliyahu S. In vivo suppression of plasma IL-12 levels by stress: mediation by corticosterone and prostaglandins but not catecholamines. doi: 10.1016/j.psyneuen.2013.12.001. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaashua L, Sominsky L, Levi B, Sorski L, Reznick M, Page GG, Ben-Eliyahu S. In vivo suppression of plasma IL-12 levels by acute and chronic stress paradigms: Potential mediating mechanisms and sex differences. Brain Behav Immun. 2012;26(6):996–1005. doi: 10.1016/j.bbi.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998a;160(7):3251–3258. [PubMed] [Google Scholar]
- Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. Journal of Immunology. 1998b;160(7):3251–3258. [PubMed] [Google Scholar]
- Shakhar G, Blumenfeld B. Glucocorticoid involvement in suppression of NK activity following surgery in rats. J Neuroimmunol. 2003;138(1–2):83–91. doi: 10.1016/s0165-5728(03)00118-8. [DOI] [PubMed] [Google Scholar]
- Skibinski G, Kelly RW, Harrison CM, McMillan LA, James K. Relative immunosuppressive activity of human seminal prostaglandins. J Reprod Immunol. 1992;22(2):185–195. doi: 10.1016/0165-0378(92)90015-v. [DOI] [PubMed] [Google Scholar]
- Takamoto TY, Hory Y, Koga H, Yokoyama MM. Norepinephrin inhibits human natural killer cell activity in vitro. International Journal of Neuroscience. 1991;58:127. doi: 10.3109/00207459108987189. [DOI] [PubMed] [Google Scholar]
- Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J Biol Chem. 1997a;272(9):5495–5500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicityJ. J Biol Chem. 1997b;272(9):5495–5500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Dow RC, Hopkins SJ, White A, Fink G, Rothwell NJ. Mechanisms of Activation of the Pituitary-Adrenal Axis by Tissue-Injury in the Rat. Psychoneuroendocrinology. 1994;19(2):165–178. doi: 10.1016/0306-4530(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Vitoratos N, Hassiakos D, Louridas C, Limuris G, Zourlas PA. Prostaglandin F1a and prostaglandin E2 plasma levels after transvaginal cervical cerclage. Clin Exp Obstet Gynecol. 1996;23(1):21–25. [PubMed] [Google Scholar]
- Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochemical Journal. 1990;272(2):327–331. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakar I, Melamed R, Shakhar G, Shakhar K, Rosenne E, Abudarham N, Page GG, Ben-Eliyahu S. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Ben-Eliyahu S, Gale RP, Shavit Y, Liebeskind JC, Taylor AN. Ethanol increases tumor progression in rats: possible involvement of natural killer cells. Brain Behav Immun. 1992;6(1):74–86. doi: 10.1016/0889-1591(92)90061-r. [DOI] [PubMed] [Google Scholar]
- Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175(2):323–328. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Zhou J, Olsen S, Moldovan J, Fu X, Sarkar FH, Moudgil VK, Callewaert DM. Glucocorticoid regulation of natural cytotoxicity: effects of cortisol on the phenotype and function of a cloned human natural killer cell line. Cell Immunol. 1997;178(2):108–116. doi: 10.1006/cimm.1997.1138. [DOI] [PubMed] [Google Scholar]