Abstract
Nodal peripheral T-cell lymphoma (nodal PTCL) has an unfavorable prognosis, and specific pathogenic alterations have not been fully identified. The biological and clinical relevance of the expression of CD30/T-cell receptor (TCR) genes is a topic under active investigation. One-hundred and ninety-three consecutive nodal PTCLs (89 angioimmunoblastic T-cell lymphomas (AITL) and 104 PTCL-unspecified (PTCL-not otherwise specified (NOS)) cases) were analyzed for the immunohistochemical expression of 19 molecules, involving TCR/CD30 pathways and the associations with standard prognostic indices. Mutually exclusive expression was found between CD3 and TCR-beta F1 with CD30 expression. Taking all PTCL cases together, logistic regression identified a biological score (BS) including TCR molecules (TCR-beta F1 and EZRIN) that separates two subgroups of patients with a median survival of 34.57 and 5.20 months (P<0.001). Multivariate analysis identified BS and the prognostic index for PTCL (PIT) score as independent prognostic factors. This BS maintained its significance in multivariate analysis only for the PTCL-NOS subgroup of tumors. In AITL cases, only a high level of ki67 expression was related to prognosis. A BS including molecules involved in the TCR signaling pathway proved to be an independent prognostic factor of poor outcome in a multivariate analysis, specifically in PTCL-NOS patients. Nevertheless, validation in an independent series of homogeneously treated PTCL patients is required to confirm these data.
Keywords: T-cell lymphoma, TCR-beta F1, EZRIN, JUNB, BLIMP1
Introduction
Peripheral T-cell lymphomas (PTCLs) are neoplasms derived from mature post-thymic T-cell lymphocytes, which account for ∼12% of all lymphoid neoplasms.1 They are subclassified as either predominantly nodal or extranodal on the basis of their main site of involvement. Among nodal PTCLs, angioimmunoblastic T-cell lymphoma (AITL) and PTCL-not otherwise specified (NOS) are the most frequent tumor types.1, 2 Both are aggressive diseases with a ⩽35% 5-year overall survival (OS) probability, despite aggressive chemotherapy.2
Gene-expression profiling studies have revealed that PTCL-NOS is derived from activated T cells3 with AITL harboring a gene-expression signature, suggesting derivation from follicular helper T cells.4 In spite of the progress made in their histological and immunophenotypical classification, and our understanding of their molecular biology, the molecular basis of nodal PTCLs pathogenesis and aggressiveness remains elusive. Recent findings suggest that the survival of normal and, frequently, neoplastic T cells depends upon T-cell receptor (TCR) signaling.5, 6, 7, 8 The rare translocations found in PTCL seem to confirm this observation. Thus, the chromosomal translocation t(5;9)(q33;q22) that generates the interleukin-2-inducible T-cell kinase (ITK)–spleen tyrosine kinase (SYK) fusion tyrosine kinase has been identified in a subgroup of PTCL. The inducible T-cell kinase–SYK associates constitutively with lipid rafts in T cells and triggers antigen-independent phosphorylation of TCR proximal proteins, leading to activation of downstream pathways and acute cellular outcomes that correspond to regular TCR ligation, although it is known that SYK is overexpressed in more than 90% of PTCL cases.9 In addition, inhibition of syk protein tyrosine kinase (PTK) induces apoptosis and blocks proliferation in T-cell lymphoma cell lines, highlighting its biological relevance.10
On the other hand, not all PTCLs are dependent on TCR signaling, as described by de Leval et al.4 Gene-expression arrays studies subclassify PTCLS according to gene signatures associated with the expression of CD30. The CD30-positive PTCLs overexpress many enzymes with transferase, synthase and kinase activity, and genes involved in the control of transcription, such as the Jun dimerization protein, which acts as an interleukin-2 repressor. The CD30-negative PTCLs overexpress genes involved in T-cell activation, such as the CD28 receptor, the CD69 activation antigen and molecules involved in the TCR signaling pathway.4 An inverse relationship between the levels of expression of CD30 and TCR genes was confirmed by Geissinger et al.11 However, the clinical consequences of these findings are still unexplored or controversial.4, 11, 12, 13
The TCR is a multimeric complex composed of two ligand-binding glycoproteins containing variable regions (αβ- or γδ-TCR heterodimers), which are expressed on the cell surface in association with four CD3 molecules. TCR-αβ or TCR-γδ recognizes the antigen, whereas CD3 molecules control assembly and signal transduction. Each of the CD3γ, CD3δ and CD3ɛ subunits contain one ITAM, and CD3ζ contains three ITAMs.14 Upon receptor ligation, two tyrosine residues within each ITAM are rapidly phosphorylated by a member of the src-family PTK, transforming them into high-affinity ligands for Syk PTK such as ZAP-70. The coordinated actions of Src and Syk PTK initiate a cascade of signals that ultimately leads to cell proliferation, cytokine secretion and effector functions.15 However, propagation and termination of TCR signaling needs T-cell signaling molecules (for example, PKCθ, Lck and ZAP-70) to form a cluster at the site of cell–cell contact, generating an immunological synapse. Moreover, the distal pole complex promotes T-cell activation by sequestering proteins that inhibit events at the immunological synapse. The ERM proteins, moesin, ezrin and radixin, regulate linkage between the cytoskeleton and plasma membrane, especially in actin-containing cell-surface structures, and are central regulators of distal pole complex formation. Only moesin and ezrin are expressed in T cells, the former being the most common.16, 17
Here we have examined whether the expression of different molecules related to TCR/CD3 and CD30 signaling pathways can identify different prognostic subgroups of nodal PTCLs. The expression of some selected TCR and CD30-related markers was found to be associated with prognosis. A biological score (BS) constructed from two such markers proved to be an independent prognostic factor of survival in a multivariate analysis, considering the whole cohort of patients or solely the PTCL-NOS subgroup of tumors. Validation of these data in a homogeneously treated series of PTCL could confirm the potential usefulness of these observations.
Materials and methods
Tissue samples
We analyzed a group of 193 consecutive nodal PTCLs from a larger series of cases submitted for diagnosis or second opinion to the CNIO Pathology Laboratory between 2000 and 2008. Only AITL and PTCL-NOS cases were considered in this study. Criteria for the diagnosis were based on the 2008 WHO classification.18, 19 CD30-positive cases were considered to be PTCL-NOS if none of them fulfilled the morphological criteria for classification as anaplastic large-cell lymphomas (ALCLs), or expressed either epithelial membrane antigen or anaplastic lymphoma kinase.18, 19 Moreover, specimens from patients with CD30-positive PTCL-NOS were restricted to those that lacked all B-cell antigens, including PAX5, and that expressed at least one T-cell-associated antigen or had a TCR gene rearrangement (data not shown). The project was supervised by the Ethical Committee of the Hospital Carlos III.
Tissue microarray construction
Representative areas from formalin-fixed, paraffin-embedded lymphomas were carefully selected on Hematoxylin and eosin stain sections and two 1-mm-diameter tissue cores were obtained from each specimen. All samples belonged to the initial diagnostic specimen. The tissue cores were precisely arrayed into a new paraffin block using a tissue microarray workstation (Beecher Instruments, Silver Spring, MD, USA), following methods described elsewhere.20
Immunohistochemistry
Tissue microarray sections were immunohistochemically stained by the Endvision method with a heat-induced antigen-retrieval step. Sections were immersed in boiling 10 mℳ sodium citrate at pH 6.5 for 2 min in a pressure cooker. A panel of 19 antibodies related to TCR and CD30 pathways was analyzed (Supplementary Table 1). Epithelial membrane antigen, anaplastic lymphoma kinase and PAX5 were only performed in CD30-positive cases during the diagnostic procedure of the cases to exclude diffuse large B-cell lymphoma, Hodgkin lymphomas or ALCL anaplastic lymphoma kinase-positive. Staining intensity and percentage of tumoral cells were taken into consideration for scoring markers. Three groups were established: 0, 0–10% positive cells with low-intensity staining; 1, 10–80% positive cells, irrespective of staining intensity; 2, more than 80% positive neoplastic cells with high-intensity staining (Table 1). Depending on the marker, analyzed cases included in group 1 were considered either positive or negative. A rationale for these cutoffs is included in Supplementary Table 1. Cases positive for CD3 and TCR were subdivided into cytoplasmic or membranous groups, according to their predominant pattern of expression. Reactive tonsil tissue was included as a control. The primary antibodies were omitted to provide negative controls.
Table 1. Score of the proteins analyzed in this series.
| Antibody | Total studied cases | Number/percentage of positive cases |
|---|---|---|
| CD3 | 168 | 162/96.4 |
| TCR-beta F1 | 164 | 157/95.7 |
| TCRGAMMA | 160 | 0/0 |
| CD30 | 185 | 47/25.4 |
| ZAP-70 | 174 | 147/84.5 |
| PD1 | 180 | 122/67.7 |
| EZRIN | 148 | 115/77.7 |
| MOESIN | 152 | 147/96.7 |
| CAVEOLIN 1 | 173 | 22/12.7 |
| FASCIN | 174 | 25/16.1 |
| BLIMP1 | 179 | 25/13.9 |
| JUNB | 168 | 21/12.5 |
| NF-κB-P50 | 146 | 13/8.9 |
| NF-κB-P52 | 144 | 22/15.3 |
| NF-κB-RELB | 152 | 12/7.9 |
| NF-κB-P65 | 173 | 9/5.2 |
| Ki67 | 191 | 48/25.13 |
Clinical data
Clinical data and the standard prognostic indices21, 22, 23, 24 for this series are shown in Table 2. All prognostic indices were assessed at the time of initial diagnosis. The detail of therapeutic regimens for all patients is shown in Supplementary Table 2.
Table 2. Clinical features of the series (N=193).
| Whole group | Frequency | Percentage |
|---|---|---|
| Age (years) | 193 | 99.5 |
| ⩽60 | 79 | 40.9 |
| >60 | 114 | 59.1 |
| Sex | 193 | 99.5 |
| Male | 118 | 61.1 |
| Female | 75 | 38.9 |
| ECOG | 160 | 82.5 |
| ⩽1 | 114 | 71.3 |
| >1 | 46 | 28.8 |
| LDH | 155 | 79.9 |
| Normal | 70 | 45.2 |
| High | 85 | 54.8 |
| Ann Arbor stage | 165 | 85.1 |
| I–II | 41 | 24.8 |
| III–IV | 124 | 75.2 |
| B-symptoms | 161 | 83.0 |
| Yes | 92 | 57.1 |
| No | 69 | 42.9 |
| Extranodal involvement | 165 | 85.1 |
| <2 sites | 124 | 75.2 |
| ⩾2 sites | 41 | 24.8 |
| BM involvement | 149 | 76.8 |
| Negative | 107 | 71.8 |
| Positive | 42 | 28.2 |
| Platelet cell count | 76 | 39.2 |
| ⩽150 × 109/l | 22 | 28.9 |
| >150 × 109/l | 54 | 71.1 |
| IPI | 163 | 84 |
| Low risk | 24 | 14.7 |
| Low-intermediate risk | 54 | 33.1 |
| High-intermediate risk | 53 | 32.5 |
| High risk | 32 | 19.6 |
| PIT | 150 | 77.3 |
| Low risk | 29 | 19.3 |
| Low-intermediate risk | 39 | 26.0 |
| High-intermediate risk | 44 | 29.3 |
| High risk | 38 | 25.3 |
| mPIT | 160 | 82.5 |
| Low risk | 71 | 44.4 |
| Intermediate risk | 49 | 30.6 |
| High risk | 40 | 25.0 |
| IPTCLP | 76 | 39.2 |
| Low risk | 22 | 38.9 |
| Low-intermediate risk | 33 | 43.4 |
| High-intermediate risk | 18 | 23.7 |
| High risk | 3 | 3.9 |
| First line treatment | 146 | 75.25 |
| CHOP or CHOP-like | 124 | 84.93 |
| Others | 22 | 15.06 |
| Response | 142 | 73.2 |
| CR* | 77 | 54.2 |
| PR** | 29 | 20.4 |
| P*** | 36 | 25.4 |
| Recurrence | 138 | 71.1 |
| Yes | 32 | 23.2 |
| No | 106 | 76.8 |
| State of the patient | 166 | 85.6 |
| Dead | 97 | 58.4 |
| Alive | 69 | 41.6 |
Abbreviations: BM=bone marrow; CHOP=combination chemotherapy with cyclophosphamide, doxorubicin, vincristine and prednisone; ECOG=performance status (Eastern Cooperative Oncology Group); IPI=international Prognostic Index; IPTCLP=international peripheral T-cell lymphoma project score; mPIT=modified prognostic Index for T-cell lymphoma; LDH=serum lactate dehydrogenase; PIT=prognostic Index for T-cel lymphoma. *CR, complete response; **PR, partial response; ***P, progression.
Statistical analysis
To assess associations between categorical variables, we used the X2 contingency test with Yates' correction, or Fisher's exact test, as appropriate. OS was taken as the period between the date of diagnosis and the date of death from any cause or of last contact for living patients. Disease-specific OS was calculated as the period from date of diagnosis to death from the tumor. Kaplan–Meier survival analyses were carried out for OS and lymphoma-specific survival, using the long-rank test to examine differences between groups. A multivariate Cox regression model was also derived. Estimates were considered statistically significant for two-tailed probabilities of P<0.05. All analyses were carried out with SPSS 12.0 (SPSS Inc., Chicago, IL, USA).
We also constructed a biological predictor score based on the results of the Cox functional survival curves of the significant variables. The cutoff point for subclassifying patients as being at low- or high risk of prognosis was calculated considering the optimal sensitivity and specificity of the Cox curve. The model is represented by the following equation: h(t)=h0(t)x exp(bxx+byy+….+bzz) (www.whatisseries.co.uk).
Results
Whole cohort of nodal PTCL cases
There were 89 AITL (43.52%) and 104 PTCL-NOS cases (53.88%), of which 47 expressed CD30 (24.35% of the total cohort). Seventeen AITL and 19 PTCL-NOS cases had low levels of CD30 expression, whereas one AITL and 10 PTCL-NOS cases presented high levels (more than 80% of the tumoral cells) of CD30 expression. These results are in accordance with those of previous reports.12 None of the cases expressed TCRGAMMA, PAX5, epithelial membrane antigen or anaplastic lymphoma kinase, which excludes the diagnosis of gamma-delta-T-cell lymphoma, diffuse large B-cell lymphoma, Hodgkin lymphoma and ALCL,12, 18, 19 which are tumor types with their own specific molecular pathogenesis.
Figure 1 shows some of the immunohistochemical characteristics of this series. Associations between the proteins analyzed in the whole series of nodal PTCL cases are shown in Supplementary Table 3A. Although P-values changed, correlations between different makers were alike, independently of the cutoff chosen for CD30 (data not shown). In general, taking all the data together, CD3 and TCR-beta F1 expression were directly correlated with each other and inversely correlated with the presence of CD30. We also found that TCR-beta F1-negative cases had cytoplasmic CD3 expression and vice versa. In addition, CD30-negative cases expressed both TCR-beta F1 and CD3 in the membrane, whereas CD30-positive cases expressed these two markers in the cytoplasm (data not shown).
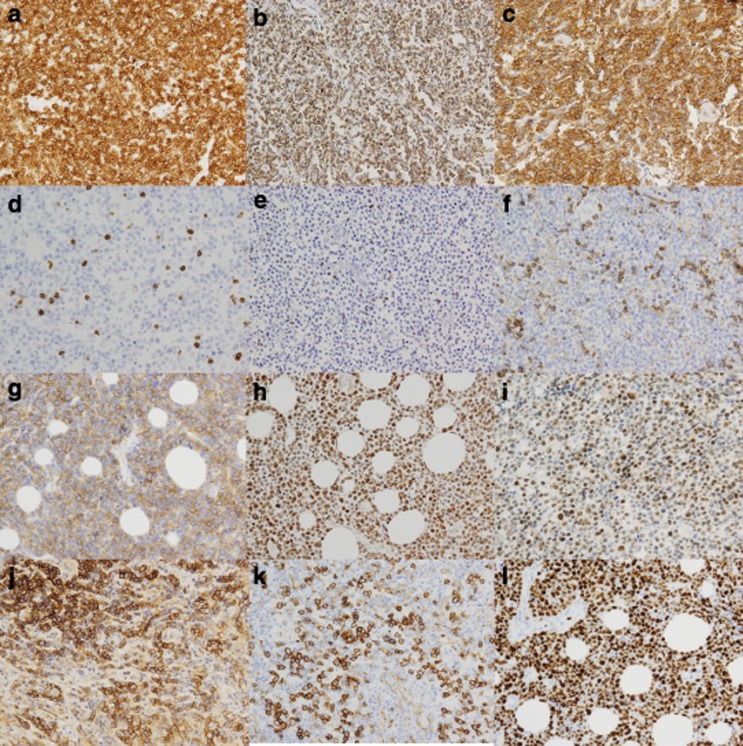
Figure 1.
Representative pictures of nodal PTCL expressing CD3 (a), TCR-beta F1 (b) and EZRIN (c). Nodal PTCL cases negative for CD3 (d), TCR-beta F1 (e) and EZRIN (f). Nodal PTCL expressing low levels of CD30 (g). Nodal PTCL cases positive for JUNB (h) and BLIMP1 (i). A representative AITL case expressing both PD1 (j) and Caveolin-1 (k). Nodal PTCL case showing a high level of KI67 expression (l).
Cases expressing CD3/TCR-beta F1 in the membrane expressed EZRIN. Moreover, ZAP-70 expression was associated with the presence of TCR-beta F1, whereas PD1 and MOESIN expression was associated with the presence of CD3. No significant association was found between EZRIN and MOESIN expression.
Conversely, CD30-positive cases tended to express FASCIN, BLIMP1 and JUNB. In addition, they showed nuclear expression of p50, p52 and RelB nuclear factor kappa B (NF-κB) subunits, whereas CD3/TCR-beta F1-positive cases more frequently expressed p65. Moreover, JUNB-positive cases showed nuclear staining of p50, p52 and RELB, whereas BLIMP1 was only significantly associated with the presence of RELB and p52.
Caveolin-1 was not associated with the expression of CD3, TCR-beta F1 or with other cytoskeletal proteins.
Cases with a high proliferation index tended to express CD30, JUNB, BLIMP1, FASCIN and Caveolin-1, and to have nuclear expression of some NF-κB subunits, whereas those with a low proliferation index expressed CD3 and TCR-beta F1 in the membrane, as well as MOESIN.
After a mean follow-up of 23.44 months (ranging from 0 to 150 months), 75 of 162 (46.30%) nodal PTCL patients had died, and 66 of 150 (44%) patients had died because of the tumor. The overall cumulative probability of survival at 5 years was 44.4% and the tumor-specific survival probability was 51.3% (Supplementary Figure 1).
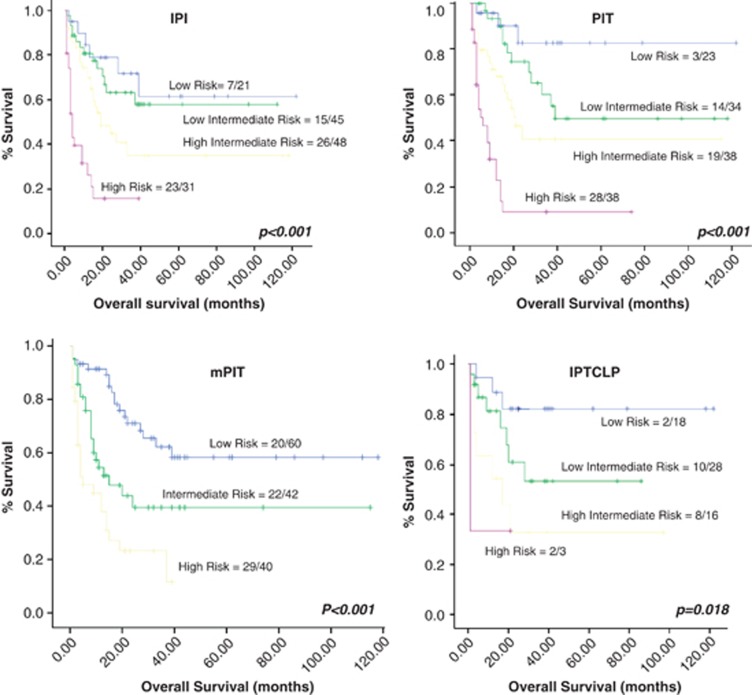
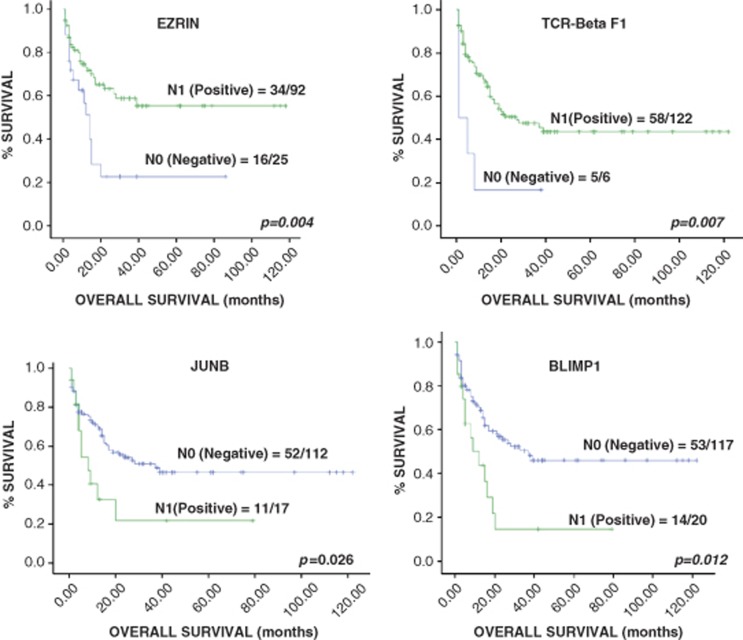
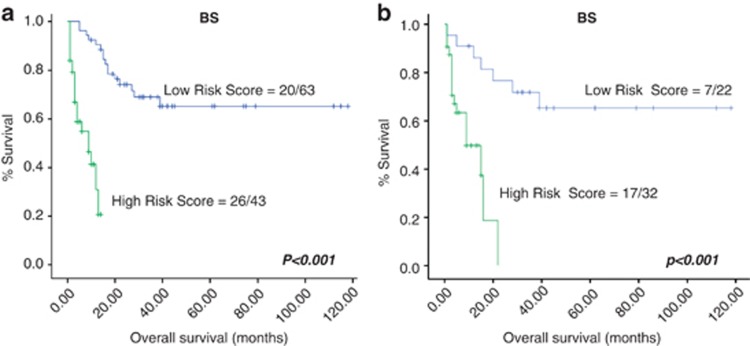
Taking all the cases together, high IPI, PIT, mPIT and IPTCLP were correlated with poor outcome in the univariate analysis (Figure 2 and Supplementary Table 4). The Cox multivariate analysis identified PIT as an independent prognostic factor of disease-specific OS (Supplementary Table 5). The expression of JUNB and BLIMP1, as well as TCR-beta F1 and EZRIN loss were significantly associated with shorter survival in the univariate analysis (Supplementary Table 4 and 6), although only TCR-beta F1 and EZRIN were identified as independent prognostic factors in the multivariate analysis (Figure 3 and Supplementary Table 5). A BS combined these two biological variables according to the equation: h(t)=h0(t)x exp((−1.054)TCR-beta F1+(−0.430) EZRIN) was constructed. This was able to identify patients who were at low and high risk of dying from their disease. The low-risk group contained 63 patients (59.4%, median survival 34.57 months; range 1–150 months) and there were 43 cases in the high-risk group (40.6%, median survival 5.20 months; range 1–14 months). The BS was highly correlated with patient outcome (P<0.001) and maintained its independent prognostic value in the Cox multivariate analysis after including PIT (Figure 4 a and Supplementary Table 5). The entire set of markers was available for 96 patients, since individual data being missing for some cases. The analysis of this smaller group of 96 cases yielded the same results (data not shown).
Figure 2.
Univariate Kaplan–Meier survival curves of significant prognostic clinical indices in the present series of nodal PTCL cases.
Figure 3.
Univariate Kaplan–Meier survival curves of significant biological markers.
Figure 4.
(a) Univariate Kaplan–Meier survival curves of the BS created on the basis of the combination of biological variables that were significant in the Cox multivariate analysis (EZRIN and TCR-beta F1) in nodal PTCL patients. (b) Univariate Kaplan–Meier survival curves of the BS created on the basis of the combination of biological variables that were significant in the Cox multivariate analysis (EZRIN and TCR-beta F1) in PTCL-NOS patients.
Analyzing data based on histological subgroups of tumors (AITL vs PTCL-NOS)
When analyzing the expression of the studied proteins according to histological diagnosis (AITL vs PTCL-NOS), we found that AITL cases were distinguished by the expression of PD1 (P<0.001) and EZRIN (P=0.010), whereas PTCL-NOS cases displayed increased expression of JUNB (P=0.010), BLIMP1 (P=0.017), RelB (P=0.056), p52 (0.017) and CD30 (0.029).
The relationship between most of the proteins analyzed here showed the same association as found in the entire set of patients (Supplementary Table 3B).
Patients groups were not significantly different in terms of age of onset of the disease (P=0.106), sex (P=0.658), performance status (P=0.205), stage (P=0.356), or low- or high-risk groups according to various clinical prognostic indices (P=0.345; 0.181; 0.417 and 0.871 for IPI, PIT, m PIT and IPTCLP, respectively). The OS of the two groups was not significantly different either (P=0.259; data not shown).
The BS was analyzed for the AITL and PTCL-NOS cases separately. Interestingly, we found that the previously defined BS was only applicable to PTCL-NOS.
In the group of AITL cases, univariate analysis showed high PIT (P=0.001), mPIT (P=0.009); data not shown) and high Ki67 (P=0.022) to be indicators of poor outcome (Supplementary Figure 3 and Supplementary Table 6). Only the PIT prognostic index maintained its prognostic value when including mPIT, KI67 and PIT in the Cox multivariate study (Supplementary Table 7).
Conversely, high scores of IPI, PIT, mPIT and IPTCLP continued to be associated with poor outcome in the univariate analysis in the PTCL-NOS tumor group (P<0.01 in all comparisons) (Supplementary Figure 2). IPI and IPTCLP both proved to be independent prognostic factors of poor outcome in the Cox multivariate analysis (P=0.043 and P=0.029, respectively) (Supplementary Table 7). In this group, the presence of BLIMP1 (P=0.025), and the absence of both TCR-beta F1 (P<0.001) and EZRIN (P=0.026) were correlated with poor survival in the univariate analysis, but only TCR-beta F1 and EZRIN maintained their significance after multivariate analysis (Supplementary Table 7 and Supplementary Figure 3). A similar BS, again combining these two variables, was created. Using this BS, all high-risk patients (32 cases) died of disease before 20 months of follow-up, whereas most low-risk patients (22 cases) were alive at that time after 20 months of following up (Figure 4b). This BS also maintained its independent prognostic value in the Cox multivariate analysis after including mPIT and IPTCLP (Supplementary Table 7).
Discussion
We have investigated the expression of TCR and NF-κB-associated molecules in a large series of PTCLs. The results of the study seem to confirm that PTCL-NOS and AITL have specific diagnostic and prognostic features that justify their separate recognition. Thus, AITL more frequently expresses follicular helper T-cell markers (TFH markers), such as PD1, whereas PTCL-NOS expresses the highest levels of JUNB, BLIMP1, RelB, p52 and CD30.
The first objective of the study was to investigate whether the expression of these molecules was hazardous, or if associations between distinct sets of molecules could contribute to a better understanding of PTCL pathogenesis. In general, nodal PTCL cases express either CD3/TCR-beta F1 or CD30. These findings are in accordance with those of previous reports, implying that there are different subgroups of tumors whose neoplastic cells appear to use diverse intracellular machinery genes to maintain cell survival and proliferation.4, 11, 12, 13 Essentially, TCR-beta F1/CD3-positive tumors resembled normal T-cell lymphocytes as they expressed ZAP-70, EZRIN, MOESIN and PD1, whereas the expression of CD30 was correlated with the presence of FASCIN, JUNB and BLIMP1. Tumoral cells expressing TCR-beta F1 also expressed CD3 in their membranes, as it has been shown that surface expression of the TCR complex requires a fully assembled set of the complex subunits.25 Absence of surface CD3 (with presence of cytoplasmic CD3 expression) and TCR expression have been described in both systemic and cutaneous ALCL cases,11, 12, 13 as well as in enteropathy-associated T-cell lymphomas,26 a subgroup of tumors characterized by CD30 expression.4, 11, 12, 13 EZRIN is a protein that serves as a scaffold facilitating efficient signal transduction on the cytoplasmic face of the plasma membrane, and is a negative regulator of apoptosis.27 Moreover, it is lost in acute leukemia cell lines that are resistant to antimicrotubule agents (vincristine).28 Caveolae are lipid microdomains or rafts with a high sphingolipid and cholesterol content that are thought to be the platforms for the TCR/CD3 signaling complex.29 Gene-expression arrays have revealed that Caveolin-1 is upregulated in the neoplastic cells of adult T-cell leukemia/lymphoma patients,30 and induces T-cell proliferation and NF-κB activation in a CD3/TCR-dependent manner.31 FASCIN is another actin-binding protein mainly involved in the organization of actin-based structures that function in cell adhesion and migration,32 but is little expressed, if at all, in T cells.33 In our series, FASCIN was correlated with the presence of CD30 and the loss of CD3, consistent with findings of previous reports.34, 35 Loss of EZRIN and gain of FASCIN have both been related to epithelial–mesenchymal transition, which is involved in invasiveness and metastasis.36, 37 Moreover, the hedgehog signaling pathway has been found to be active in epithelial–mesenchymal transition,38, 39 as well as in ALCL and some aggressive diffuse large B-cell lymphomas.40, 41
The second purpose of the study was to investigate whether the expression of these TCR and NF-κB genes could provide prognostic markers. Thus, we have identified a BS based on the loss of TCR-beta F1 and EZRIN that distinguishes two prognostic groups of nodal PTCLs. This signature was exclusively maintained in the PTCL-NOS subgroup of tumors when cases were divided according to the WHO classification criteria into PTCL-NOS and AITL. In addition, this BS was independent of classical prognostic indices after multivariate analysis.
Using these thresholds, neither CD30 nor NF-κB subunit expression was significantly associated with changes in survival probability.
In normal T cells, signaling through TCR or CD30 leads to the activation of the NF-κB pathway through a variety of intermediate molecules.42, 43, 44 However, it is not known whether this activation results in the expression of the same set of pro-survival genes.45, 46 In this series, NF-κB transcription factors were expressed in the nucleus of the neoplastic cells in both CD30-positive and CD30-negative nodal PTCLs. Although CD30-positive cases expressed nuclear p50, p52 and RelB NF-κB subunits, CD3/TCR-beta F1-positive cases expressed p65. In contrast to what has been found in gene-expression array studies,47 there was no association between the expression of any of the NF-κB subunits and prognosis. Discrepancies between these studies could be because of inherent differences in the techniques used.
BLIMP1 and JUNB are two NF-κB genes that are important in T-cell differentiation.44 BLIMP1 (also called PRDI-BF1 in humans) is encoded by the Prdm1 gene and is a critical transcription factor in peripheral T cells.48 Using an antibody that recognizes both PRDM1 isoforms, we noted that BLIMP1 expression was related to the PTCL-NOS subgroup of tumors, the activation of the alternative NF-κB pathway (RELB and p52) and to a poor outcome in the univariate analysis, consistent with results of previous studies of PTCL that used semiquantitative reverse transcriptase PCR.49, 50 Interestingly, bortezomib (a proteasome inhibitor) has been shown to downregulate PRDM1β through NF-κB inactivation, overcoming the chemotherapy resistance in HUT-78 cell lines.51 A clinical trial using bortezomib plus CHOP in advanced aggressive PTCL yielded a complete remission rate of 61.5%.51, 52 These data suggest that BLIMP1 expression in nodal PTCLs could be a surrogate marker of prognosis and a therapeutic target.
JUNB is an activator member of the AP-1 protein transcription family.53 Its overexpression and amplification are both known to be associated with neoplastic transformation54 in CD30-positive lymphomas through an NF-κB-dependent mechanism.44 In our series, JUNB was also associated with the presence of CD30, absence of CD3 and activation of the NF-κB pathway. Moreover, as in normal T lymphocytes,55 JUNB expression was correlated with BLIMP1 and associated with the PTCL-NOS subgroup of tumors in our series. Moreover, we also found JUNB expression to be a negative prognostic factor, our results being consistent with those of previous studies of mycosis fungoides.56
Taking these data together suggests that there are different means by which the classic and alternative NF-κB pathways are activated in distinct subgroups of nodal PTCLs, and that these warrant further investigation.
In the present series including all cases, KI67 expression by itself was not correlated with prognosis, but was stronger in the CD30-positive subgroup of tumors. These data are in accordance with the findings of Cuadros et al.57 and Gutiérrez-García, et al.23 and in contrast to those of Went et al.21 Surprisingly, it proved to be a predictor of poor outcome in the univariate statistical analyses for the AITL subgroup of patients. Cuadros et al.57 identified a poor prognostic signature related to proliferation using gene-expression arrays that did not differ between AITL and PTCL-NOS, although they did not study the AITL cases alone.
Tjon et al.26 suggested that the downregulation of the TCR by T-cell neoplastic cells might be a way of escaping immune-regulatory processes. Moreover, the lack of representative cell lines of most of these types of tumors and of engraftments of human tissue in immunocompromised mice, suggests that the tumoral cell depends strongly on its microenvironment.58 Here we showed that the loss of TCR-beta F1 is associated with CD30 expression, substitution of normal ezrin cytoskeletal proteins by fascin and the activation of oncogenes such as BLIMP1 and JUNB, which confer a more aggressive behavior on these tumors. In contrast with B-cell neoplasms, there is no clear proof of the existence of a normal counterpart for each type of T-cell lymphoma, and the findings described here could be interpreted as evidence of the existence of tumor-specific signatures, reflecting individual molecular events that characterize the neoplastic transformation of T cells.
In conclusion, we confirmed the mutually exclusive expression of TCR and CD30 molecules in PTCL, and identified some specific markers that may be used to predict survival independently of the clinical variables, specifically among the PTCL-NOS tumor subgroup. Nevertheless, these patients were heterogeneously treated and immunohistochemical cutoff values were manually identified. The results would require confirmation in an independent series of homogeneously treated PTCL patients.
Acknowledgments
This study was supported by grants from the Ministerio de Ciencia e Innovación, Spain (RETICC, SAF2008-03871) and the Spanish Association against Cancer (AECC). We thank the CNIO Tumour Bank, especially Laura Cereceda and Maria Jesús Artiga. We are also grateful to CI Gómez, C Gonzalez and A Gutierrez for their help with the database and the statistical analysis.
Disclaimer
The submitted material is original, has not been previously reported and is not under consideration for publication elsewhere. All the authors have reviewed the manuscript, agree with its content, and approve of its submission.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Rizvi MA, Evens AM, Tallman MS, Nelson BP, Rosen ST. T-cell non-Hodgkin lymphoma. Blood. 2006;107:1255–1264. doi: 10.1182/blood-2005-03-1306. [DOI] [PubMed] [Google Scholar]
- Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- Ballester B, Ramuz O, Gisselbrecht C, Doucet G, Loi L, Loriod B, et al. Gene expression profiling identifies molecular subgroups among nodal peripheral T-cell lymphomas. Oncogene. 2006;25:1560–1570. doi: 10.1038/sj.onc.1209178. [DOI] [PubMed] [Google Scholar]
- de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109:4952–4963. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–169. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- Serwold T, Hochedlinger K, Swindle J, Hedgpeth J, Jaenisch R, Weissman IL. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci USA. 2010;107:18939–18943. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Werneck MB, Wilson BG, Kim HJ, Kluk MJ, Thom CS, et al. TCR-dependent transformation of mature memory phenotype T cells in mice. J Clin Invest. 2011;121:3834–3845. doi: 10.1172/JCI37210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechloff K, Holch J, Ferch U, Schweneker M, Brunner K, Kremer M, et al. The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med. 2010;207:1031–1044. doi: 10.1084/jem.20092042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AL, Sun DX, Law ME, Novak AJ, Attygalle AD, Thorland EC, et al. Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas. Leukemia. 2008;22:1139–1143. doi: 10.1038/leu.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RA, Sun DX, Novak A, Dogan A, Ansell SM, Feldman AL. Inhibition of Syk protein tyrosine kinase induces apoptosis and blocks proliferation in T-cell non-Hodgkin's lymphoma cell lines. Leukemia. 2010;24:229–232. doi: 10.1038/leu.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissinger E, Sadler P, Roth S, Grieb T, Puppe B, Muller N, et al. Disturbed expression of the T-cell receptor/CD3 complex and associated signaling molecules in CD30+ T-cell lymphoproliferations. Haematologica. 2010;95:1697–1704. doi: 10.3324/haematol.2009.021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage KJ, Harris NL, Vose JM, Ullrich F, Jaffe ES, Connors JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111:5496–5504. doi: 10.1182/blood-2008-01-134270. [DOI] [PubMed] [Google Scholar]
- Bonzheim I, Geissinger E, Roth S, Zettl A, Marx A, Rosenwald A, et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004;104:3358–3360. doi: 10.1182/blood-2004-03-1037. [DOI] [PubMed] [Google Scholar]
- Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- Guy CS, Vignali DA. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol Rev. 2009;232:7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179:733–746. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer MH, Dupree RS, Zhu P, Saotome I, Schmidt RF, McClatchey AI, et al. Ezrin and moesin function together to promote T cell activation. J Immunol. 2009;182:1021–1032. doi: 10.4049/jimmunol.182.2.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary HM, Savage KJ. Update on the World Health Organization classification of peripheral T-cell lymphomas. Curr Hematol Malig Rep. 2009;4:227–235. doi: 10.1007/s11899-009-0030-5. [DOI] [PubMed] [Google Scholar]
- Swerdlow SHCE, Harris NL, Pileri SA, Stein H, Thiele J, Vardiman JW. WHO classification of Tumours and Haematopoietic and Lymphoid Tissues. IARC: Lyon, France; 2008. [Google Scholar]
- Garcia JF, Camacho FI, Morente M, Fraga M, Montalban C, Alvaro T, et al. Hodgkin and Reed-Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood. 2003;101:681–689. doi: 10.1182/blood-2002-04-1128. [DOI] [PubMed] [Google Scholar]
- Went P, Agostinelli C, Gallamini A, Piccaluga PP, Ascani S, Sabattini E, et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J Clin Oncol. 2006;24:2472–2479. doi: 10.1200/JCO.2005.03.6327. [DOI] [PubMed] [Google Scholar]
- Gallamini A, Stelitano C, Calvi R, Bellei M, Mattei D, Vitolo U, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103:2474–2479. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Garcia G, Garcia-Herrera A, Cardesa T, Martinez A, Villamor N, Ghita G, et al. Comparison of four prognostic scores in peripheral T-cell lymphoma. Ann Oncol. 2010;22:397–404. doi: 10.1093/annonc/mdq359. [DOI] [PubMed] [Google Scholar]
- Weisenburger DD, Savage KJ, Harris NL, Gascoyne RD, Jaffe ES, MacLennan KA, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- Clevers H, Alarcon B, Wileman T, Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Tjon JM, Verbeek WH, Kooy-Winkelaar YM, Nguyen BH, van der Slik AR, Thompson A, et al. Defective synthesis or association of T-cell receptor chains underlies loss of surface T-cell receptor-CD3 expression in enteropathy-associated T-cell lymphoma. Blood. 2008;112:5103–5110. doi: 10.1182/blood-2008-04-150748. [DOI] [PubMed] [Google Scholar]
- Kuo WC, Yang KT, Hsieh SL, Lai MZ. Ezrin is a negative regulator of death receptor-induced apoptosis. Oncogene. 2010;29:1374–1383. doi: 10.1038/onc.2009.417. [DOI] [PubMed] [Google Scholar]
- Verrills NM, Liem NL, Liaw TY, Hood BD, Lock RB, Kavallaris M. Proteomic analysis reveals a novel role for the actin cytoskeleton in vincristine resistant childhood leukemia--an in vivo study. Proteomics. 2006;6:1681–1694. doi: 10.1002/pmic.200500417. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H, Nishikata I, Shiraga T, Akamatsu E, Fukami T, Hidaka T, et al. Overexpression of a cell adhesion molecule, TSLC1, as a possible molecular marker for acute-type adult T-cell leukemia. Blood. 2005;105:1204–1213. doi: 10.1182/blood-2004-03-1222. [DOI] [PubMed] [Google Scholar]
- Ohnuma K, Uchiyama M, Yamochi T, Nishibashi K, Hosono O, Takahashi N, et al. Caveolin-1 triggers T-cell activation via CD26 in association with CARMA1. J Biol Chem. 2007;282:10117–10131. doi: 10.1074/jbc.M609157200. [DOI] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, Konig I, et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Pantanowitz L, Pinkus GS, Pinkus JL, Paessler ME, Roullet M, et al. Utility of fascin and JunB in distinguishing nodular lymphocyte predominant from classical lymphocyte-rich Hodgkin lymphoma. Appl Immunohistochem Mol Morphol. 2010;18:16–23. doi: 10.1097/PAI.0b013e3181a307f7. [DOI] [PubMed] [Google Scholar]
- Bakshi NA, Finn WG, Schnitzer B, Valdez R, Ross CW. Fascin expression in diffuse large B-cell lymphoma, anaplastic large cell lymphoma, and classical Hodgkin lymphoma. Arch Pathol Lab Med. 2007;131:742–747. doi: 10.5858/2007-131-742-FEIDLB. [DOI] [PubMed] [Google Scholar]
- Lan M, Kojima T, Murata M, Osanai M, Takano K, Chiba H, et al. Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Exp Cell Res. 2006;312:111–120. doi: 10.1016/j.yexcr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Isohata N, Aoyagi K, Mabuchi T, Daiko H, Fukaya M, Ohta H, et al. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int J Cancer. 2009;125:1212–1221. doi: 10.1002/ijc.24400. [DOI] [PubMed] [Google Scholar]
- Varnat F, Duquet A, Malerba M, Zbinden M, Mas C, Gervaz P, et al. Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RR, Cho-Vega JH, Davuluri Y, Ma S, Kasbidi F, Milito C, et al. Sonic hedgehog signaling pathway is activated in ALK-positive anaplastic large cell lymphoma. Cancer Res. 2009;69:2550–2558. doi: 10.1158/0008-5472.CAN-08-1808. [DOI] [PubMed] [Google Scholar]
- Singh RR, Kim JE, Davuluri Y, Drakos E, Cho-Vega JH, Amin HM, et al. Hedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferation. Leukemia. 2010;24:1025–1036. doi: 10.1038/leu.2010.35. [DOI] [PubMed] [Google Scholar]
- Schmitz ML, Krappmann D. Controlling NF-kappaB activation in T cells by costimulatory receptors. Cell Death Differ. 2006;13:834–842. doi: 10.1038/sj.cdd.4401845. [DOI] [PubMed] [Google Scholar]
- Weil R, Israel A. Deciphering the pathway from the TCR to NF-kappaB. Cell Death Differ. 2006;13:826–833. doi: 10.1038/sj.cdd.4401856. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Sasaki M, Itoh K, Higashihara M, Umezawa K, Kadin ME, et al. JunB induced by constitutive CD30-extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase signaling activates the CD30 promoter in anaplastic large cell lymphoma and Reed-Sternberg cells of Hodgkin lymphoma. Cancer Res. 2005;65:7628–7634. doi: 10.1158/0008-5472.CAN-05-0925. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- Martinez-Delgado B, Cuadros M, Honrado E, Ruiz de la Parte A, Roncador G, Alves J, et al. Differential expression of NF-kappaB pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19:2254–2263. doi: 10.1038/sj.leu.2403960. [DOI] [PubMed] [Google Scholar]
- Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, et al. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107:2354–2363. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- Zhao WL, Liu YY, Zhang QL, Wang L, Leboeuf C, Zhang YW, et al. PRDM1 is involved in chemoresistance of T-cell lymphoma and down-regulated by the proteasome inhibitor. Blood. 2008;111:3867–3871. doi: 10.1182/blood-2007-08-108654. [DOI] [PubMed] [Google Scholar]
- Lee Y, Uhm JE, Lee HY, Park MJ, Kim H, Oh SJ, et al. Clinical features and prognostic factors of patients with ‘peripheral T cell lymphoma, unspecified'. Ann Hematol. 2009;88:111–119. doi: 10.1007/s00277-008-0544-2. [DOI] [PubMed] [Google Scholar]
- Wagner EF. AP-1–Introductory remarks. Oncogene. 2001;20:2334–2335. doi: 10.1038/sj.onc.1204416. [DOI] [PubMed] [Google Scholar]
- Mao X, Orchard G, Mitchell TJ, Oyama N, Russell-Jones R, Vermeer MH, et al. A genomic and expression study of AP-1 in primary cutaneous T-cell lymphoma: evidence for dysregulated expression of JUNB and JUND in MF and SS. J Cutan Pathol. 2008;35:899–910. doi: 10.1111/j.1600-0560.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- Vasanwala FH, Kusam S, Toney LM, Dent AL. Repression of AP-1 function: a mechanism for the regulation of Blimp-1 expression and B lymphocyte differentiation by the B cell lymphoma-6 protooncogene. J Immunol. 2002;169:1922–1929. doi: 10.4049/jimmunol.169.4.1922. [DOI] [PubMed] [Google Scholar]
- Tracey L, Villuendas R, Dotor AM, Spiteri I, Ortiz P, Garcia JF, et al. Mycosis fungoides shows concurrent deregulation of multiple genes involved in the TNF signaling pathway: an expression profile study. Blood. 2003;102:1042–1050. doi: 10.1182/blood-2002-11-3574. [DOI] [PubMed] [Google Scholar]
- Cuadros M, Dave SS, Jaffe ES, Honrado E, Milne R, Alves J, et al. Identification of a proliferation signature related to survival in nodal peripheral T-cell lymphomas. J Clin Oncol. 2007;25:3321–3329. doi: 10.1200/JCO.2006.09.4474. [DOI] [PubMed] [Google Scholar]
- Waller EK, Kamel OW, Cleary ML, Majumdar AS, Schick MR, Lieberman M, et al. Growth of primary T-cell non-Hodgkin's lymphomata in SCID-hu mice: requirement for a human lymphoid microenvironment. Blood. 1991;78:2650–2665. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.