Abstract
Ezrin links the actin filaments with the cell membrane and has a functional role in the apoptotic process. It appears clear that ezrin is directly associated with Fas, leading to activation of caspase cascade and cell death. However, the exact role of ezrin in ursolic acid (UA)-induced apoptosis remains unclear. In this study, we show for the first time that UA induces apoptosis in both transformed and primary leukemia cells through dephosphorylation/downregulation of ezrin, association and polarized colocalization of Fas and ezrin, as well as formation of death-inducing signaling complex. These events are dependent on Rho-ROCK1 signaling pathway. Knockdown of ezrin enhanced cell death mediated by UA, whereas overexpression of ezrin attenuated UA-induced apoptosis. Our in vivo study also showed that UA-mediated inhibition of tumor growth of mouse leukemia xenograft model is in association with the dephosphorylation/downregulation of ezrin. Such findings suggest that the cytoskeletal protein ezrin may represent an attractive target for UA-mediated lethality in human leukemia cells.
Keywords: ursolic acid, ezrin, Rho-ROCK1 signaling, DISC formation, AML, apoptosis
Introduction
Medicinal plants are becoming an important research area for novel and bioactive molecules for drug discovery. Novel therapeutic strategies and agents are urgently needed to treat various diseases including anticancer chemotherapy. Ursolic acid (UA), an active pentacyclic triterpenoid compound, has been isolated from many kinds of medicinal plant, such as Eriobotrya japonica, Rosmarinus offıcinalis and Glechoma hederaceae.1 This compound is also the major biologically active constituent in Hedyotis diffusa,2 which is comprehensively used for antileukemic therapeutic strategies in Traditional Chinese Medicine.3 UA has recently attracted a great deal of attention since it displayed a wide range of antileukemic activities, including inhibition of cell growth, induction of cell differentiation and apoptosis.4, 5, 6 UA induces apoptosis in leukemia cells through multiple pathways including protein kinase B inactivation,4 c-Jun N-terminal kinases activation7 and Ca2+ release.8 In animal studies, UA has been shown to exhibit in vivo antileukemic activity and could be effective in the therapy of leukemia.4, 9 Preclinical data has illustrated that UA emerges as a promising anticancer agent, and it would be meaningful and challenging to develop this compound to be a novel antitumor drug. Currently UA is in human clinical trial for treating cancer.10
Ezrin is a member of the ezrin-radixin-moesin protein family, which has the ability to interact with both the plasma membrane and filamentous actin.11 The NH2-terminal region anchors ezrin in the plasma membrane, whereas the COOH-terminal domain interacts with the actin cytoskeleton. Activation of ezrin through phosphorylation on threonine 567 is dependent on the GTPase Rho and its effector Rho-associated coiled coil-containing protein kinase (ROCK).12 It has been reported that increased ezrin expression has been associated with high metastatic potential in a variety of human cancers, including pancreatic adenocarcinomas, osteosarcomas and breast carcinomas.13, 14, 15, 16 Recent study also provides the evidence that ezrin is increased in human leukemic cells and point to a new role for ezrin as signaling player in the development of leukemia.17
Fas (CD95), a transmembrane protein belonging to tumor necrosis factor receptor family, is a key player in apoptosis induction. Activated Fas recruits the adaptor molecule Fas-associated death domain protein (FADD) and the initiator to form the death-inducing signaling complex (DISC) that activates the apoptotic cascade.18 Two pathways for Fas-mediated apoptosis have been described. In Type I cells, caspase-8 is recruited to the DISC, resulting in the release of active caspase-8 in quantities suffcient to directly activate caspase-3. In Type II cells, DISC formation is strongly reduced and mitochondria may function as an amplifier, activating both caspase-8 and caspase-3, leading to cell death.19
Recently, it has been shown that the Fas linkage to actin may have an important role in Fas-mediated apoptosis.19 The Fas linkage to actin may have a role not only in conferring cell susceptibility to Fas triggering apoptosis, but also in driving the actin-dependent DISC formation and Fas internalization. It appears that ezrin can link Fas to the actin cytoskeleton, leading to Fas-induced DISC formation and apoptosis.20 The downregulation of ezrin in CEM cells abolishes their susceptibility to Fas-induced apoptosis,20 and the knockdown of ezrin or moesin in Jurkat cells decreases Fas-triggered apoptosis.21 It has also been shown that Fas-ezrin-actin linkage is involved in Fas endocytosis, and Fas-induced apoptosis was attenuated in ezrin-knock-down L12.10 cells.22 However, a more recent study argues that ezrin limits the extend of cell death triggered through Fas activation. In T cells stimulated with FasL, ezrin dissociates from Fas, resulting in activation of the DISC apoptotic cascade. Knockdown of ezrin and expression of a dominant-negative ezrin lead to an increase in Fas-mediated cell death, whereas overexpression of the full-length ezrin slightly inhibited apoptosis.23 Therefore, the exact role of ezrin in death receptor-initiated apoptosis remains unsettled.
In this study, we characterize the functional role of ezrin in UA-induced apoptosis in human leukemia cells. We found for the first time that UA induces apoptosis through dephosphorylation/downregulation of ezrin and formation of DISC, which are dependent on Rho-ROCK1 signaling pathway. Knockdown of ezrin enhanced cell death mediated by UA, whereas overexpression of ezrin attenuated UA-induced apoptosis. Our in vivo study also showed that UA-mediated inhibition of growth of mouse U937 xenograft tumors was in association with dephosphorylation/downregulation of ezrin. Moreover, evidence is presented suggesting that disrupting Rho/ROCK1/ezrin pathway has a significant functional role in UA-related lethality. Such findings suggest that treatment with UA may warrant further examination as a novel antileukemic strategy.
Materials and methods
Cell lines, primary AML cells, reagents and antibodies
Cell lines and cell culture conditions are described in Supplementary Materials and Methods. Peripheral blood samples for the in vitro studies were obtained from six patients with newly diagnosed or recurrent acute myeloid leukemia (AML) after informed consent. Approval was obtained from the Southwest Hospital (Chongqing, China) Institutional Review Board for these studies. Preparation of cells is described in Supplementary Materials and Methods.
UA was purchased from Sigma (St Louis, MO, USA). Y-27632 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Z-VAD-FMK was from EMD Biosciences (La Jolla, CA, USA). Antibodies are described in Supplementary Materials and Methods.
LC-ESI-Q-TOF MS/MS analysis and protein identification
Total cellular samples were lysed and separated by SDS–polyacrylamide gel electrophoresis. The peptides were extracted and subjected to analysis by LC-ESI-Q-TOF MS/MS (Agilent, Santa Clara, CA, USA). For details, see Supplementary Materials and Methods.
Lentiviral-mediated ezrin-overexpression cells and RNA interference
Stable overexpressing ezrin cells and ezrin-small-interfering RNA (siRNA) cells were obtained by co-transfection of lentiviral packaging plasmids into 293Ta cells. For details, see Supplementary Materials and Methods.
Immunoblotting
Immunoblotting was performed as previously described.4 For details see Supplementary Materials and Methods.
Apoptosis detection assay
Apoptotic cells were evaluated by flow cytometric analysis using fluorescein isothiocyanate-conjugated Annexin V/propidium iodide (PharMingen, San Diego, CA, USA) staining, according to the manufacturer's manual. For details see Supplementary Materials and Methods.
Death-inducing signaling complex immunoprecipitation
Cells were lysed and then incubated with anti-FasAPO-1-1 and protein G agarose for immunoprecipitation experiment. For details, see Supplementary Materials and Methods.
Immunofluorescence
The procedure is described in Supplementary Materials and Methods.
Rho activity assay
Rho activity assays were performed according to the manufacturer's instructions (Calbiochem, Merck KGaA, Darmstadt, Germany). For details, see Supplementary Materials and Methods.
Xenograft assay
NOD/SCID mice (5 weeks old) were purchased from Vital River Laboratories (VRL, Beijing, China). All animal studies were conducted according to the protocols approved by the Institutional Animal Care and Use Committee of the University. For details, see Supplementary Materials and Methods.
Statistical analysis
Tumor volumes, body weights and percentage of apoptotic cells were represented as mean±s.d. Statistical analyses were performed using the two-tailed Student's t test. P<0.05 (*) or P<0.01 (**) were considered significant.
Results
Proteomic approaches identify ezrin as candidate UA target
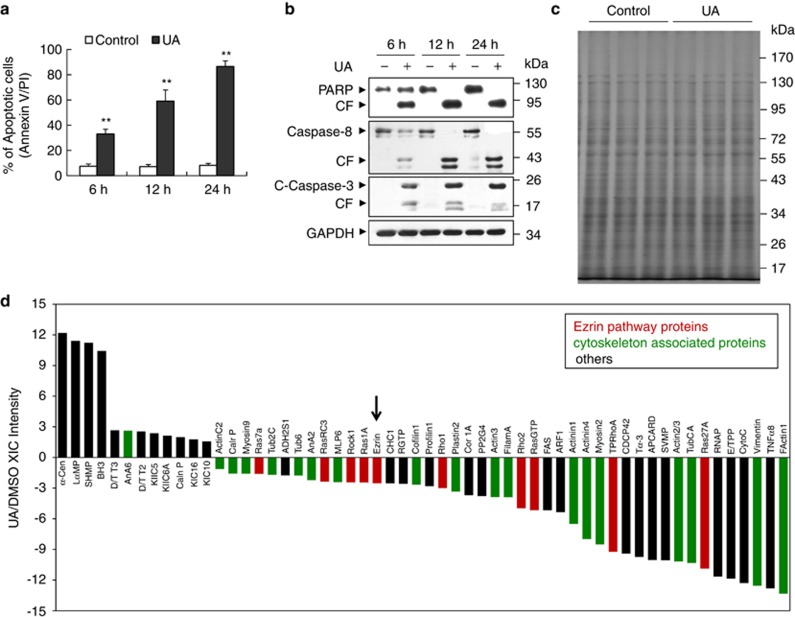
First, we examined the effects of UA on apoptosis in U937 leukemia cells. As shown in Figure 1a, exposure of cells to 20 μℳ UA resulted in a moderate increase in apoptosis as early as 6 h after drug exposure. These events became apparent after 12 h and reached near-maximal levels after 24 h of drug exposure. Western blot analysis revealed that exposure of U937 cells to 20 μℳ UA resulted in moderate increases in cleavage/activation of caspase-3 and caspase-8, as well as poly (ADP-ribose) polymerase (PARP) degradation 6 h after drug exposure. These events became apparent after 12 and 24 h of drug exposure (Figure 1b).
Figure 1.
Proteomic approaches identify ezrin as a target for UA-mediated apoptosis. (a) U937 cells were treated without or with 20 μℳ UA for 6, 12 and 24 h. Cells were stained with Annexin V/propidium iodide and apoptosis was determined by flow cytometry, as described in Materials and Methods. The values obtained represent the means±s.d. for three separate experiments. ** indicate values for cells treated with UA were significantly increased compared with control by Student's t-test; P<0.01. (b) Total protein lysates were prepared and subjected to western blot analysis using antibodies against PARP, Caspase-8 and cleaved-caspase-3 (C-Caspase-3), or glyceraldehyde-3-phosphate (GAPDH) as a loading control. Two additional studies yielded equivalent results. CF, cleavage fragment. (c) Representative SDS–polyacrylamide gel electrophoresis image of total cellular extracts from U937 cells treated without or with UA (20 μℳ, 24 h). (d) U937 cells were treated with vehicle (dimethyl sulfoxide) or UA at 20 μℳ for 24 h; LC-ESI-Q-TOF MS/MS was used to identify differentially expressed proteins. The LC-MS/MS extracted ion currents (XIC) belonging to an individual protein were summed and used to calculate differential expression ratios in UA- and dimethyl sulfoxide-treated cells. Positive ratios indicate higher expression for UA treatment; negative ratios indicate higher expression for dimethyl sulfoxide treatment. Cytoskeleton or apoptosis-related proteins changed only over twofold display in the histogram, red represents ezrin pathway proteins; green represents cytoskeleton associated proteins; black represents others.
Although various molecular targets for UA-related apoptosis have been proposed, its mechanism of action remains unclear. In order to identify further candidate targets, we employed a proteomics approach to study the regulation of apoptosis in UA treatment. We treated U937 cells with UA or vehicle (dimethyl sulfoxide) for 24 h, separated whole-cell lysate protein by 1D SDS–polyacrylamide gel electrophoresis, and then identified peptides from the gel slices by LC-ESI-Q-TOF MS/MS. Figure 1c shows the representative one-dimensional SDS–polyacrylamide gel electrophoresis images for total proteins extracted from U937 cells treated with 20 μℳ UA or vehicle for 24 h. LC-MS/MS analysis of peptides from the gel slices revealed a total of 453 proteins. Of these, 74 proteins were upregulated and 258 were downregulated, following treatment with UA. Because cytoskeletal alterations may have an important role in the initiation and regulation of apoptosis,24 we applied proteomic technology to study the regulation of actin cytoskeleton proteins in response to UA treatment. We displayed 56 cytoskeletal proteins, which were significantly altered in the histogram (Figure 1d, Supplementary Table S1); most of these proteins were downregulated. These alterations of protein expression suggest that downregulation of cytoskeletal proteins may contribute to UA-related lethality. We next further determined the role of cytoskeleton, particularly dephosphorylation/downregulation of ezrin, in the process of UA-induced apoptosis.
Dephosphorylation/downregulation of ezrin is required for the formation of DISC in a Rho- and ROCK-dependent manner
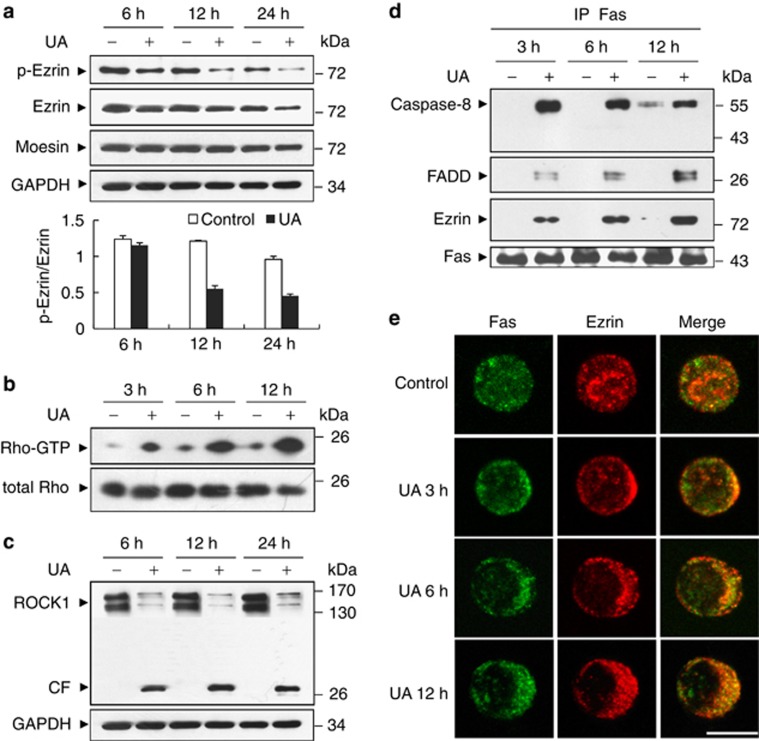
Next we studied the effect of UA on the expression of ezrin and moesin, and phosphorylation of ezrin by western blot analysis. As shown in Figure 2a, exposure of U937 cells to 20 μℳ UA for 6 h resulted in slight decrease in the expression of ezrin and phospho-ezrin. These events became apparent after 12 h and 24 h of drug exposure. In contrast, UA had no effect on the expression of moesin. As ezrin phosphorylation has been described as being potentially Rho-dependent,25 we then evaluated Rho activation by glutathione S transferase-Rho-binding domain of rhotekin pull-down assay in U937 cells treated with UA. As shown in Figure 2b, exposure of cells to UA resulted in marked increase in the amount of the GTP-Rho in a time-dependent manner, suggesting that UA markedly induces activation of Rho, which may be involved in dephosphorylation/downregulation of ezrin. As the Rho kinase ROCK1 is a major target of GTPase Rho and a key signaling molecule involved in ezrin phosphorylation,26 we next determined the effects of UA on the expression of ROCK1. Significant cleavage/activation of ROCK1 was noted after treatment of U937 cells with UA (Figure 2c). These results suggest that activation of Rho-ROCK1 signal is essential for dephosphorylation/downregulation of ezrin.
Figure 2.
Rho/Rock1-dependent ezrin dephosphorylation/downregulation regulates UA-mediated DISC formation and apoptosis in U937 cells. (a) U937 cell were treated without or with 20 μℳ UA for 6, 12 and 24 h. Total cellular extract were prepared and subjected to western blot analysis using antibodies against phospho-Ezrintyr353(p-Ezrin), Ezrin, Moesin. A densitometric analysis of the p-Ezrin and Ezrin levels were performed using the Quantity One software (Bio-Rad) and p-Ezrin/Ezrin ratio was evaluated. The values obtained represent the means±s.d. for three separate experiments. (b) Active Rho-GTP were pulled down by association with the Rho-binding domain of rhotekin at the indicated times after UA treatment, the bead/protein complexes and total Rho in lysates were detected by immunoblotting using antibody against Rho. (c) Total protein lysates were prepared and subjected to western blot analysis using antibody against ROCK1. (d) Cell lysates of control and UA-treated cells were prepared and subjected to immunoprecipitation using antibody against Fas. The associated Ezrin, Caspase-8, FADD and Fas were determined using immunoblotting. (e) U937 cells were either untreated (Control) or treated with 20 μℳ UA for 3, 6 and 12 h. Cells were collected and stained with anti-Fas antibody, followed by Alexa 488-conjugated goat anti-mouse antibody (green fluorescence for Fas); and with anti-ezrin antibody followed by Alexa 647-conjugated donkey anti-rabbit antibody (red fluorescence for ezrin). Immunofluorescence was visualized using a laser confocal scanning microscope. The merge in yellow represents colocalization between Fas and Ezrin. Scale bar represents 10 μm. Two additional studies yielded equivalent results. CF, cleavage fragment.
As Fas complexes initiate the apoptotic pathway by recruitment of the adapter molecule FADD and the initiator caspase-8 to form the DISC,19 we investigated whether association between Fas and ezrin could be involved in these events. By immunoprecipitation, we demonstrated that treatment of U937 cells with UA led to the recruitment of FADD and caspase-8 to Fas (Figure 2d). Interestingly, treatment with UA also led to the association of ezrin from Fas-activating complex. These results suggest that the association between Fas and ezrin is required for proper DISC formation and susceptible to UA-mediated apoptosis.
It was documented that polarization and colocalization of Fas and ezrin are the essential requirements for susceptibility to the Fas-triggering apoptosis.20 We then investigated the effects of UA on the polarization and colocalization of Fas and ezrin in U937 cells using immunofluorescence experiment. The results showed that Fas and ezrin were polarized and colocalized after 3 h of UA treatment. These events became apparent after 6 and 12 h of drug exposure (Figure 2e). These results support the importance of Fas/ezrin polarization and colocalization in UA-mediated apoptosis.
UA had similar effects on apoptosis in other human leukemia cell lines as well as primary human leukemia cells
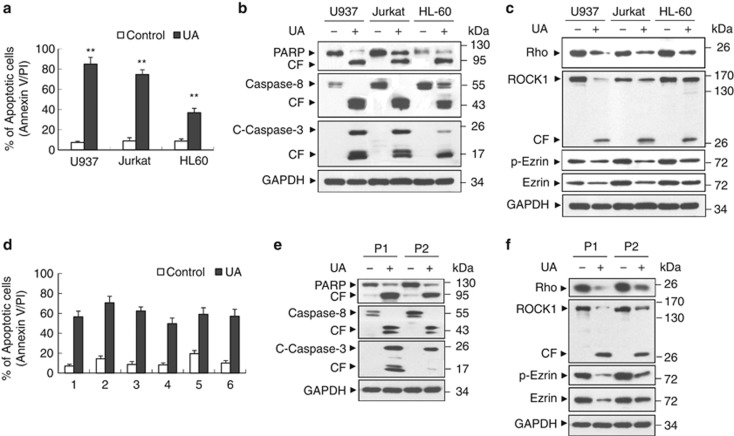
To determine whether the induction of apoptosis induced by UA was confined to U937 cells, parallel studies were performed with other human leukemia cell types, including Jurkat T-lymphoblastic and HL-60 promyelocytic leukemia cells. These cells exhibited apoptotic effects of UA similar to those observed in U937 cells, although HL-60 cells are less sensitive than U937 cells in UA-induced apoptosis (Figure 3a). Also, Jurkat and HL-60 cells exhibited comparable degrees of caspase-3 and -8 activation and PARP degradation (Figure 3b).
Figure 3.
UA had similar effects on apoptosis in other human leukemia cell lines as well as primary AML human leukemia cells. (a) U937, Jurkat and HL-60 cells were treated without or with 20 μℳ UA for 24 h. Apoptosis was evaluated by Annexin V/propidium iodide staining and flow cytometry, as described in Materials and Methods. The values obtained from Annexin V/propidium iodide assays represent the mean±s.d. for three separate experiments. ** indicate values for cells treated with UA were significantly increased compared with values obtained for control by Student's t-test; P<0.01. (b) Total cellular extracts were prepared and subjected to western blot analysis using antibodies against PARP, C-Caspase-3 and Caspase-8. (c) Western blot determined Rho/Rock1/Ezrin signaling proteins including Rho, Rock1, p-Ezrin and Ezrin. (d) Blasts from six patients with AML were isolated, after washing and counting, isolated mononuclear cells were treated without or with 20 μℳ UA for 24 h, the percentage of apoptotic cells was determined by Annexin V/propidium iodide staining and flow cytometry. (e and f) Total cellular extracts of blasts from two AML patients (P2 and P4) were prepared and subjected to western blot analysis using antibodies as indicated.
To determine whether UA-mediated dephosphorylation/downregulation of ezrin and cleavage/activation of ROCK1 were restricted to other human leukemia cell types, we then investigated the effects of UA on the expression of phospho-ezrin, ezrin, as well as ROCK1 in Jurkat and HL60 cells. As shown in Figure 3c, the ability of UA to trigger dephosphorylation/downregulation of ezrin and cleavage/activation of ROCK1 in these cells was essentially identical to effects observed in U937 cells.
Further attempts were made to determine whether UA could also trigger cell death in primary human leukemia cells isolated from patients with acute myeloid leukemia (AML), parallel experiments were done on primary mononuclear cells isolated from peripheral blood of six AML patients. Treatment of AML blasts with UA (20 μℳ) for 24 h resulted in marked increase in cell death (Figure 3d). Consistent with these results, exposure of primary leukemia cells from two AML patients to 20 μℳ UA for 24 h resulted in cleavage/activation of caspases-3 and -8, as well as PARP degradation (Figure 3e). These events were also accompanied by dephosphorylation/downregulation of ezrin and cleavage/activation of ROCK1 (Figure 3f).
UA-mediated apoptosis was associated with the caspase-independent dephosphorylation/downregulation of ezrin and cleavage/activation of ROCK1
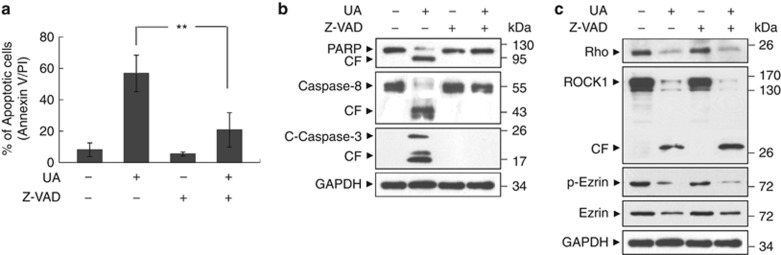
As ROCK1 is a prominent caspase-3 substrate that is cleaved and activated during apoptosis,27, 28, 29 we then investigated the effects of caspase inhibition by Z-VAD-FMK on cleavage/activation of ROCK1 and dephosphorylation/downregulation of ezrin during UA-induced apoptosis. Cotreatment of cells with the broad-spectrum caspase inhibitor Z-VAD-FMK (10 μℳ), which abrogated UA-induced apoptosis (Figure 4a), activation of caspase-3 and -8, and degradation of PARP (Figure 4b), failed to prevent cleavage/activation of ROCK1 and dephosphorylation/downregulation of ezrin mediated by UA (Figure 4c). Such findings indicate that cleavage/activation of ROCK1 and dephosphorylation/downregulation of ezrin by UA do not simply represent a secondary, caspase-dependent event. Thus, UA-induced lethality was associated with cleavage/activation of ROCK1 and dephosphorylation/downregulation of ezrin pathways.
Figure 4.
UA-mediated Rock1 activation and ezrin dephosphorylation/downregulation were not dependent on caspase activation. (a) U937 cells were pretreated with the caspase inhibitor Z-VAD-FMK (10 μℳ) for 2 h, followed by treatment with 20 μℳ UA for 12 h. Apoptosis was determined using flow cytometry. The values represent the means±s.d. for three separate experiments. ** indicate values for cells treated with UA and Z-VAD-FMK were significantly reduced compared with values obtained for UA alone by Student's t-test; P<0.01. (b) Total protein extracts were prepared and subjected to western blot assay using antibodies against PARP, Caspase-8 and C-Caspase-3. (c) Western blot determined cell signaling proteins including Rho, Rock1, p-Ezrin and Ezrin.
ROCK1 activation has an important functional role in UA-mediated dephosphorylation/downregulation of ezrin, caspase activation and apoptosis
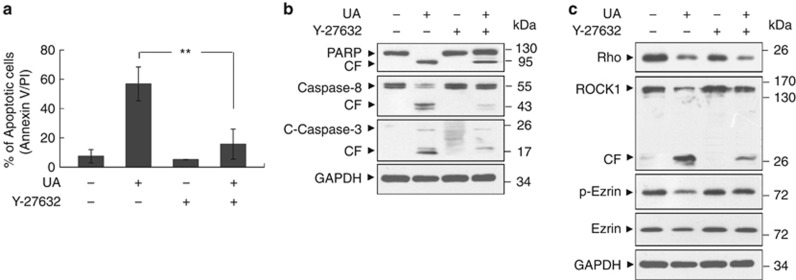
The preceding findings implied that activation of ROCK1 and dephosphorylation/downregulation of ezrin might have important roles in UA-related lethality. It has been shown that the serine/threonine kinase ROCK1 appeared as a good candidate to mediate the Rho effect on ezrin phosphorylation.26 To test this possibility, U937 cells were pretreated with a specific ROCK inhibitor, Y27632, and apoptosis was monitored. As shown in Figure 5a, coadministration of a non-toxic concentration of Y27632 (20 μℳ) with a toxic concentration of UA (20 μℳ; ∼57% for 12 h) resulted in a pronounced decrease in apoptosis (upto ∼16%). Consistently, coadministration of Y27632 also markedly attenuated UA-mediated caspases activation and PARP degradation (Figure 5b). In addition, coadministration of UA and Y27632 resulted in the virtual abrogation of ROCK1 cleavage/activation and dephosphorylation/downregulation of ezrin (Figure 5c). Together, these findings suggest that cleavage/activation of ROCK1 and dephosphorylation/downregulation of ezrin have critical roles in regulating the lethality of UA in human leukemia cells.
Figure 5.
Inhibition of Rock1 activity by Y-27632 attenuated UA-mediated apoptosis in U937 cells. (a) U937 cells were pretreated with a specific Rock1 inhibitor Y-27632 (20 μℳ) for 2 h, followed by treatment with 20 μℳ UA for 12 h. Apoptosis was evaluated by Annexin V/propidium iodide staining and flow cytometry. The values represent the means±s.d. for three separate experiments. ** indicate values for cells treated with UA and Y-27632 were significantly reduced compared with values obtained for UA alone by Student's t-test; P<0.01. (b) Total protein extracts were prepared and subjected to western blot assay using antibodies against PARP, Caspase-8 and C-Caspase-3. (c) Western blot determined cell signaling proteins including Rho, Rock1, p-Ezrin and Ezrin.
Ezrin knockdown by siRNA significantly enhances UA-mediated apoptosis and ezrin overexpression markedly attenuates UA-related apoptosis
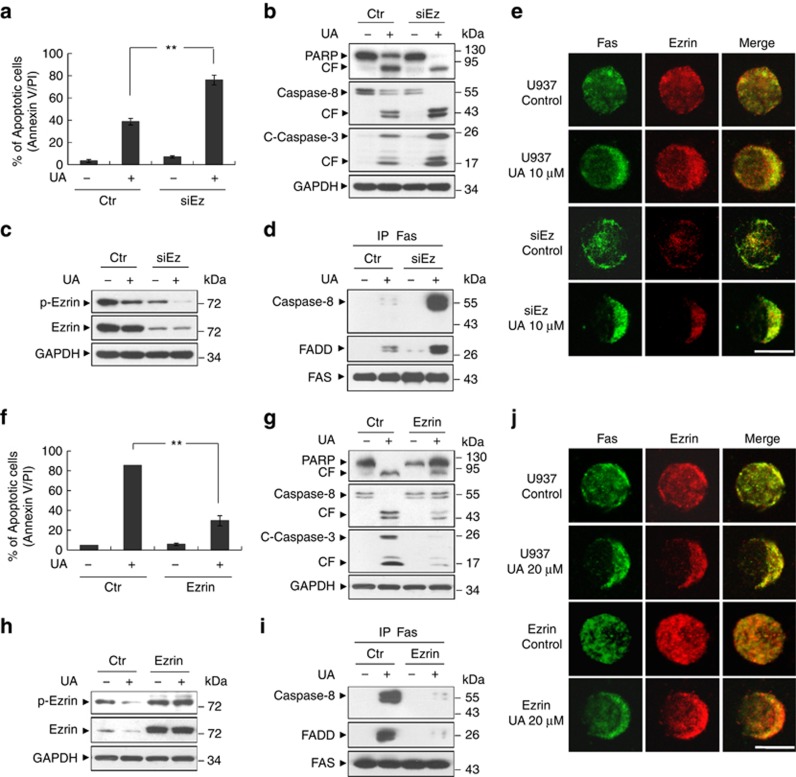
To further confirm the functional role of ezrin in UA-mediated apoptosis in human leukemia cells, U937 cells stably expressing ezrin small hairpin RNA or control small hairpin RNA were employed. As shown in Figure 6a, the knockdown of ezrin in U937 cells led to a nearly twofold increase in UA-mediated apoptosis compared with the vector control cells. Consistent with these findings, UA was considerably more effective in triggering activation of caspase-3, -8 and PARP degradation in ezrin knockdown cells, relative to vector control cells (Figure 6b). Figure 6c illustrates that more than 90% reduction in ezrin levels in U937 cells after infection with ezrin-specific hairpin RNA, UA treatment failed to induce downregulation of ezrin in these cells. Interestingly, treatment with UA resulted in decrease in phosphorylation of ezrin in both vector control and ezrin siRNA cells. As the association of Fas with ezrin is required for proper DISC formation and susceptible to UA-mediated apoptosis, we then measured the extent of DISC formation after exposure of ezrin-knockdown cells to UA. As shown in Figure 6d, both caspase-8 and FADD were recruited to the Fas receptor at a significantly higher level in ezrin siRNA cells than that in control siRNA cells. We also measured polarization and colocalization of Fas-ezrin after exposure of ezrin-knockdown cells to UA. As shown in Figure 6e, Fas-ezrin polarization and colocalization-mediated by UA was significantly increased in ezrin siRNA cells. Therefore, inhibition of ezrin by siRNA led to increased overall formation of DISC and polarization/colocalization, resulting in accelerated activation of caspase-8 and caspase-3 and, ultimately, cell death.
Figure 6.
Effects of genetic interruption of ezrin on UA-induced apoptosis. (a) U937 cells were stably infected with lentivirus-containing small-interfering RNA specific for control or ezrin (siEz). All cells were then treated with 10 μℳ UA for 24 h. After treatment, apoptosis was determined using Annexin V/propidium iodide staining and flow cytometry. ** indicate values for ezrin siRNA cells treated with UA were significantly increased compared with those for control siRNA cells by Student's t-test; P<0.01. (b) Total protein extracts were prepared and subjected to western blot assay using antibodies against PARP, Caspase-8 and C-Caspase-3. (c) Western blot determined cell signaling proteins including p-Ezrin and Ezrin. (d) Cells were treated with 10 μℳ UA for 12 h. Cell lysates were prepared and subjected to immunoprecipitation using antibody against Fas. The associated Caspase-8, FADD and Fas were determined using immunoblotting. (e) Cells were treated with 10 μℳ UA for 12 h, after which Fas (green)/ezrin (red) localization was determined by immunofluorescence analysis, as described in Materials and Methods. Scale bar represents 10 μm. (f) U937 cells were also stably infected with lentivirus containing control or overexpressing ezrin. All cells were then treated with 20 μℳ UA for 24 h. After treatment, apoptosis was determined using Annexin V/propidium iodide staining and flow cytometry. ** indicate values for ezrin-overexpressing cells treated with UA were significantly decreased compared with those for control cells by Student's t-test; P<0.01. (g) Total protein extracts were prepared and subjected to western blot assay using antibodies against PARP, Caspase-8 and C-Caspase-3. (h) Western blot determined cell signaling proteins including p-Ezrin and Ezrin. Each lane was loaded with 15 μg protein. (i) Cells were treated with 20 μℳ UA for 12 h. Cell lysates were prepared and subjected to immunoprecipitation using antibody against Fas. The associated Caspase-8, FADD and Fas were determined using immunoblotting. (j) Cells were treated with 20 μℳ UA for 12 h, after which Fas (green)/ezrin (red) localization was determined by immunofluorescence analysis, as described in Materials and Methods. Scale bar represents 10 μm.
To further evaluate the functional significance of ezrin downregulation in UA-mediated lethality, U937 cells stably overexpressing ezrin were employed. As shown in Figure 6f, a clone displaying overexpression of ezrin was markedly less sensitive to UA-induced apoptosis than control cells (P<0.01). Consistent with these findings, UA was considerably less effective in triggering activation of caspase-3 and caspase-8, and PARP degradation in ezrin overexpressing cells compared with vector control cells (Figure 6g). Western blot analysis displayed marked increase in levels of total and phospho-ezrin in ezrin-overexpressing cells. UA failed to induce dephosphorylation/downregulation of ezrin in these cells (Figure 6h). Finally, we investigated the effects of UA on the formation of DISC and Fas-ezrin polarization and colocalization in ezrin-overexpressing cells. The formation of DISC and Fas-ezrin polarization/colocalization mediated by UA was significantly blocked in ezrin-overexpressing cells compared with vector control cells (Figures 6i–j). All together, these findings suggest that downregulation of ezrin leads to enhanced UA-induced DISC formation and apoptosis in human leukemia U937 cells.
UA inhibits tumor growth of U937 xenograft model accompanied by striking induction of apoptosis and dephosphorylation/downregulation of ezrin
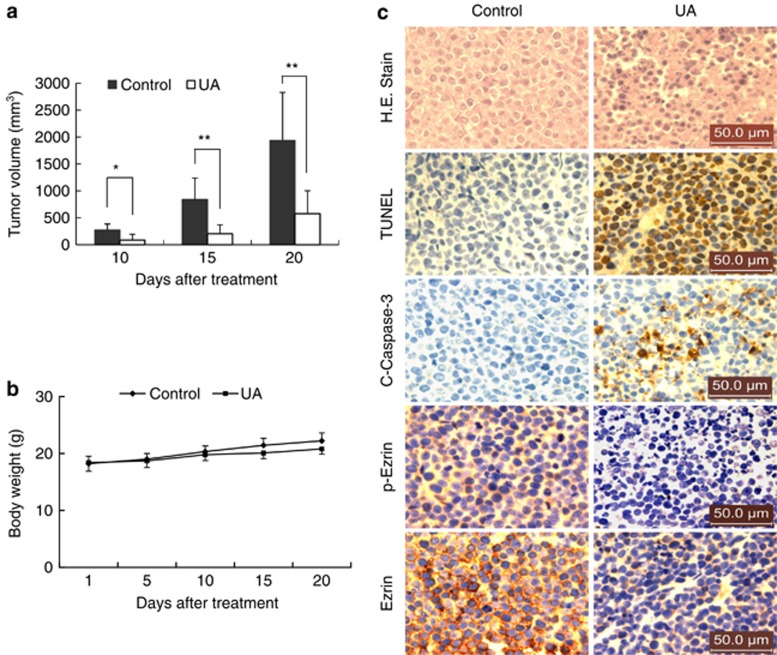
Finally, attempts were made to determine whether the preceding in vitro findings would be operative in vivo. To address this issue, NOD/SCID mice were inoculated subcutaneously with U937 cells, after which mice were received injections with vehicle or UA (50 mg/kg, intraperitoneally) for 20 days. As shown in Figure 7a, treatment with UA resulted in a dramatic suppression of tumor growth, 10 days following drug exposure (P<0.05 versus vehicle control). These events became more apparent 15 and 20 days after drug exposure (P<0.01 between UA treatment and vehicle control). In contrast, no statistically significant change in body weight was noted comparing vehicle control with UA regimen (Figure 7b), indicating that no severe toxicity was observed.
Figure 7.
UA inhibits tumor growth and induces apoptosis in U937 xenograft animal model. (a) Twenty NOD/SCID mice were inoculated with U937 cells (2 × 106 cells per mouse, s.c.) on right flank and randomly divided into two groups (10 per group) for treatment with UA (50 mg/kg, intraperitoneally, daily) or with vehicle control. Data are means±s.d., ** indicate values for tumor volume of U937 xenograft mice treated with UA were significantly decreased compared with vehicle control by Student's t-test, P<0.01. (b) Body weight changes of mice during the 20 days of UA treatment. (c) Tumors were removed from animals 20 days after drug exposure. Tumors were fixed and stained with hematoxylin and eosin stain to examine tumor cell morphology, using TUNEL assay to determine apoptosis, and using immunohistochemistry to determine the levels of C-Caspase-3, p-Ezrin and Ezrin. The sections were lightly counterstained with 4',6-diamidino-2-phenylindole and photographed with a Scan Scope (TCS SP5; Leica Microsystems, Mannhein, Germany). Scale bar represents 50 μm.
We also applied hematoxylin and eosin staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay (TUNEL) to evaluate whether administration of UA results in morphological change and induction of apoptosis in U937 cells in vivo. As shown in Figure 7c, the sections of UA-treated U937 xenograft tumor showed a reduction in densely packed cells and consisted of sparse areas of apoptotic/necrotic cells. Moreover, exposure to UA resulted in a striking induction of apoptosis in tumor cells, with signs of numerous dark brown-colored apoptotic cells. Also, exposure to UA caused a rapid increase in immunoreactivity for cleaved caspase-3, indicative of apoptosis.
We then applied immunohistochemistry analysis to evaluate whether the dephosphorylation/downregulation of ezrin have an important role in UA-mediated lethality in U937 cells in vivo. The result showed that tumors from vehicle-treated control mice stained strongly for phospho-ezrin and ezrin (Figure 7c), which were immunolocalized to the cytomembrane of cancer cells. Treatment with UA resulted in marked decrease in expression of phospho-ezrin and ezrin in tissue sections of tumors. Such findings suggest that UA-mediated antileukemic activity in vivo is associated with dephosphorylation/downregulation of ezrin.
Discussion
In the present study, we demonstrate that UA dramatically induces apoptosis in diverse human leukemia cell lines as well as in primary human AML blast cells in dose- and time-dependent manners. We show for the first time that dephosphorylation/downregulation of ezrin contributes to UA-mediated apoptosis in human leukemia cells through an enhancement of DISC formation. More importantly, the increased association of FADD and caspase-8 with Fas- and UA-mediated cell death caused by ezrin knockdown indicate that ezrin is involved in DISC formation and the subsequent apoptosis. Ezrin is a membrane cytoskeletal cross-linker that participates in several growth factor receptors signaling leading to cell survival, differentiation, motility, invasion and cell adhesion.30, 31 Numerous studies have implicated ezrin in apoptotic events in a variety of cell types. As ezrin participates in cell functions in either activated or an inactivated forms, it is perhaps not surprising that it has been described to both promote and inhibit apoptosis. In T cell, ezrin is essential for Fas-initiated apoptosis.20, 21, 22 However, a more recent study argues that ezrin limits the extent of cell death triggered through Fas activation.23 In T cells stimulated with FasL, ezrin dissociates from Fas, resulting in activation of the DISC apoptotic cascade. Knockdown of ezrin and expression of a dominant-negative ezrin lead to an increase in Fas-mediated cell death, whereas overexpression of ezrin inhibited Fas-initiated apoptosis.23 It has also been shown that the level of ezrin is increased in leukemia cells and that phosphorylation of ezrin promotes proliferation of the leukemic cells in vitro and in vivo. Disruption of ezrin function by the ezrin mutants leads to reduced proliferation and increased death of leukemic cells, suggesting that ezrin may have a critical role in the development of leukemia.17 Consistent with these studies, our results indicate that UA-mediated dephosphorylation/downregulation of ezrin has a critical role in mediating UA lethality. This phenomenon might be explained by the following lines of evidence: first, exposure of leukemia cells to UA resulted in dephosphorylation/downregulation of ezrin, and an enhancement of DISC formation; second, the knockdown of ezrin through ezrin-specific siRNA increased UA-triggered cell death in human leukemia cells; lastly, overexpression of ezrin attenuated UA-induced apoptosis. Our results suggest that ezrin has a critical negative role in UA-mediated cell death.
Although the present study indicates that ezrin has a critical role in UA-mediated cell death, the molecular mechanism how ezrin participates in this event remains unclear. A body of evidence suggests that ezrin can interact with various membrane proteins such as Fas, E-cadherin, CD43 and CD44. These interactions are involved in diverse biological processes including cell death, adherens junctions formation, immunological recognition, as well as membrane domain organization.20, 32, 33, 34 It has been reported that Fas-ezrin association and polarized colocalization are essential for Fas-mediated cell death in T lymphocytes.20 In agreement with these results, our observation indicates that susceptibility to UA-mediated apoptosis in human leukemia cells depends on Fas-ezrin association and polarized colocalization. We also demonstrate here that the association of ezrin with Fas caused by UA is necessary for an optimal DISC formation and subsequent caspase cascade leading to cell death. Interestingly, our results indicate, for the first time, that dephosphorylation/downregulation of ezrin contribute to DISC formation. UA treatment led to a high degree of DISC formation in U937 cells, and this DISC formation was further increased by ezrin knockdown using siRNA approach. In contrast, overexpression of ezrin attenuated UA-mediated apoptosis through inhibition of DISC formation. Our results are consistent with the previous study, indicating that ezrin has a negative role in Fas-mediated cell death through an enhancement of DISC formation.23 The opposite results found that ezrin-mediated cytoskeleton association is necessary for DISC formation and subsequent caspase cascade leading to cell death.20 Therefore, ezrin-mediated Fas association and DISC formation is cell-type dependent.
Induction of caspase activation and apoptosis in human leukemia U937 cells was also associated with the activation of Rho and cleavage/activation of ROCK1. ROCK1 can be activated constitutively by proteolytic cleavage of the inhibitory COOH-terminal domain. The previous studies indicated that ROCK1 is cleaved by caspase-3 at the cleavage site during apoptosis.28, 29 In addition, ROCK1 cleavage by caspase-3 can be inhibited by caspase inhibitors in a variety of apoptotic cells.27, 35 Our present results indicate that UA induces apoptosis by activating caspase-8 and caspase-3, raising the possibility that ROCK1 activation might represent a consequence of engagement of the caspase cascade. In the present study, cotreatment of U937 cells with the pan-caspase inhibitor Z-VAD-FMK, which abrogated UA-induced activation of caspases-3 and caspase-8 and apoptosis, failed to prevent ROCK1 activation, arguing strongly that factors other than caspase-mediated events are involved in this phenomenon. Our findings are in agreement with previous reports, showing that caspase-3-independent cleavage/activation of ROCK1 was involved in cell death mediated by P2X7 ATP receptor, and combination of BGC9331 (a thymidylate synthase inhibitor) and SN-38 (a topoisomerase I inhibitor) in cancer cells.36, 37
It has been shown that ezrin is directly phosphorylated by the serine/threonine kinase ROCK1 in a Rho-dependent manner, which facilitates Fas receptor aggregation as well as activation of caspase-8 and apoptosis induction.21 Similarly, the previous study showed that cisplatin-induced apoptosis involves ezrin phosphorylation through a Rho-ROCK1-dependent pathway.38 The present findings suggest that dephosphorylation/downregulation of ezrin through a Rho-ROCK1-dependent pathway, contributing to UA-mediated lethality, differ from those of previous reports. Most notably, UA exposure resulted in diminished, rather than increased, ezrin phosphorylation. ROCK1 inhibition by Y27632 (ROCK inhibitor) attenuates UA-mediated ezrin dephosphorylation, caspase-8 and caspase-3 activation and apoptosis. It has been reported that phosphatase PP2A, which is regulated through Rho-ROCK1 signaling cascade, is responsible for the dephosphorylation of ezrin.39, 40 Our previous findings indicate that exposure of U937 cells to UA resulted in increase in PP2A levels,4 raising the possibility that UA-mediated ezrin dephosphorylation might be catalyzed by PP2A through Rho-ROCK1 pathway, suggesting that the impact of Rho-ROCK1 signaling on ezrin dephosphorylation may be indirect.
It has been shown that administration of UA exhibits the inhibitory effects on tumor growth in human hepatocellular cancer H22 cell xenograft as well as esophageal carcinoma YES-2 cell xenograft.41, 42 In our in vivo studies using a NOD/SCID mouse xenograft model of leukemia U937 cells, tumor volumes were significantly reduced after exposure to UA, indicating an antileukemic activity of this agent. To understand whether the induction of apoptosis triggered by UA in vitro is identical to those in vivo, we next examined apoptosis in tumor specimens obtained from control and UA-treated animals, using TUNEL staining. The increase of TUNEL-positive cells was detected in the UA-treated group compared with the control group, suggesting the apoptotic evidence in UA-treated U937 xenograft mice. This event was further confirmed by the results indicating that the administration of UA resulted in marked increase in activation/cleavage of caspase-3 in tissue sections of U937 tumor xenograft. To dissect the possible mechanisms of UA-mediated cell death in vivo, we also determined the expression levels of phospho-ezrin and ezrin in tissue sections of U937 xenograft tumor using immunohistochemistry analysis. The observation that disruption of ezrin signaling (for example, dephosphorylation/downregulation of ezrin) dramatically potentiated UA-mediated apoptosis in U937 xenograft model, arguing that such mechanisms do not simply reflect in vitro phenomena, but are also operative in vivo.
In conclusion, our results demonstrate that UA effectively induces apoptosis in human leukemia cells, including primary leukemia blasts, as well as in leukemia xenografts. These effects occur in association with dephosphorylation/downregulation of ezrin, association and polarized-colocalization of Fas and ezrin, as well as formation of DISC. Such findings suggest that treatment with UA may warrant further examination as a novel antileukemic strategy.
Acknowledgments
We thank GeneChem for supplying ezrin lentiviral expression plasmid and lentiviral RNAi. We also thank Fang Wang for FACS assistance, Wei Sun for confocal assistance. This work was supported by Chongqing Natural Science Foundation (cstc2013jjB0181) and National Natural Science Foundation of China (No. 30971288).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Liu J. Pharmacology of oleanolic acid and ursolic acid. J Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Lee HZ, Bau DT, Kuo CL, Tsai RY, Chen YC, Chang YH. Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am J Chin Med. 2011;39:201–213. doi: 10.1142/S0192415X11008750. [DOI] [PubMed] [Google Scholar]
- Lin CC, Kuo CL, Lee MH, Hsu SC, Huang AC, Tang NY, et al. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T- and B-cell proliferation in leukemic mice. In Vivo. 2011;25:633–640. [PubMed] [Google Scholar]
- Gao N, Cheng S, Budhraja A, Gao Z, Chen J, Liu EH, et al. Ursolic acid induces apoptosis in human leukaemia cells and exhibits anti-leukaemic activity in nude mice through the PKB pathway. Br J Pharmacol. 2012;165:1813–1826. doi: 10.1111/j.1476-5381.2011.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuttan G, Pratheeshkumar P, Manu KA, Kuttan R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm Biol. 2011;49:995–1007. doi: 10.3109/13880209.2011.559476. [DOI] [PubMed] [Google Scholar]
- Zhang T, He YM, Wang JS, Shen J, Xing YY, Xi T. Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPbeta expression by ERK pathway activation. Anticancer Drugs. 2011;22:158–165. doi: 10.1097/CAD.0b013e3283409673. [DOI] [PubMed] [Google Scholar]
- Liu XS, Jiang J. Induction of apoptosis and regulation of the MAPK pathway by ursolic acid in human leukemia K562 cells. Planta Med. 2007;73:1192–1194. doi: 10.1055/s-2007-981597. [DOI] [PubMed] [Google Scholar]
- Baek JH, Lee YS, Kang CM, Kim JA, Kwon KS, Son HC, et al. Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int J Cancer. 1997;73:725–728. doi: 10.1002/(sici)1097-0215(19971127)73:5<725::aid-ijc19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Hsu HY, Yang JJ, Lin CC. Effects of oleanolic acid and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997;111:7–13. doi: 10.1016/s0304-3835(96)04481-3. [DOI] [PubMed] [Google Scholar]
- Sultana N. Clinically useful anticancer, antitumor, and antiwrinkle agent, ursolic acid and related derivatives as medicinally important natural product. J Enzyme Inhib Med Chem. 2011;26:616–642. doi: 10.3109/14756366.2010.546793. [DOI] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akisawa N, Nishimori I, Iwamura T, Onishi S, Hollingsworth MA. High levels of ezrin expressed by human pancreatic adenocarcinoma cell lines with high metastatic potential. Biochem Biophys Res Commun. 1999;258:395–400. doi: 10.1006/bbrc.1999.0653. [DOI] [PubMed] [Google Scholar]
- Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 2005;7:R365–R373. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- Monni R, Haddaoui L, Naba A, Gallais I, Arpin M, Mayeux P, et al. Ezrin is a target for oncogenic Kit mutants in murine erythroleukemia. Blood. 2008;111:3163–3172. doi: 10.1182/blood-2007-09-110510. [DOI] [PubMed] [Google Scholar]
- Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, et al. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J. 2000;19:5123–5134. doi: 10.1093/emboj/19.19.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. J Immunol. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, et al. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WC, Yang KT, Hsieh SL, Lai MZ. Ezrin is a negative regulator of death receptor-induced apoptosis. Oncogene. 2010;29:1374–1383. doi: 10.1038/onc.2009.417. [DOI] [PubMed] [Google Scholar]
- Smertenko A, Franklin-Tong VE. Organisation and regulation of the cytoskeleton in plant programmed cell death. Cell Death Differ. 2011;18:1263–1270. doi: 10.1038/cdd.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Matsui T, Tsukita S. Rho-dependent and -independent activation mechanisms of ezrin/radixin/moesin proteins: an essential role for polyphosphoinositides in vivo. J Cell Sci. 2002;115:2569–2580. doi: 10.1242/jcs.115.12.2569. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, et al. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 1983;97:425–432. doi: 10.1083/jcb.97.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–2191. doi: 10.1091/mbc.E02-07-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 2005;24:1287–1300. doi: 10.1038/sj.emboj.7600595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumier A, Olivo-Marin JC, Arpin M, Michel F, Martin M, Mangeat P, et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity. 2001;15:715–728. doi: 10.1016/s1074-7613(01)00225-4. [DOI] [PubMed] [Google Scholar]
- Ueda H, Morishita R, Itoh H, Narumiya S, Mikoshiba K, Kato K, et al. Galpha11 induces caspase-mediated proteolytic activation of Rho-associated kinase, ROCK-I, in HeLa cells. J Biol Chem. 2001;276:42527–42533. doi: 10.1074/jbc.M102529200. [DOI] [PubMed] [Google Scholar]
- Coudray AM, Louvet C, Kornprobst M, Raymond E, Andre T, Tournigand C, et al. Increased anticancer activity of the thymidylate synthase inhibitor BGC9331 combined with the topoisomerase I inhibitor SN-38 in human colorectal and breast cancer cells: induction of apoptosis and ROCK cleavage through caspase-3-dependent and -independent mechanisms. Int J Oncol. 2005;27:553–561. [PubMed] [Google Scholar]
- Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, et al. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebillard A, Jouan-Lanhouet S, Jouan E, Legembre P, Pizon M, Sergent O, et al. Cisplatin-induced apoptosis involves a Fas-ROCK-ezrin-dependent actin remodelling in human colon cancer cells. Eur J Cancer. 2010;46:1445–1455. doi: 10.1016/j.ejca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- Boratko A, Gergely P, Csortos C. Cell cycle dependent association of EBP50 with protein phosphatase 2A in endothelial cells. PLoS One. 2012;7:e35595. doi: 10.1371/journal.pone.0035595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe A, Shiraishi M, Negishi M, Saito N, Tanabe M, Sasaki Y. MARCKS dephosphorylation is involved in bradykinin-induced neurite outgrowth in neuroblastoma SH-SY5Y cells. J Cell Physiol. 2012;227:618–629. doi: 10.1002/jcp.22763. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang F, Yang L, Mei Y, Long H, Zhang X, et al. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J Biomed Biotechnol. 2011;2011:419343. doi: 10.1155/2011/419343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K, et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer. 2009;125:952–960. doi: 10.1002/ijc.24433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.