Abstract
Aggregates of amyloid-beta (Aβ) and tau are hallmarks of Alzheimer's disease (AD) leading to neurodegeneration and synaptic loss. While increasing evidence suggests that inhibition of N-methyl-𝒟-aspartate receptors (NMDARs) may mitigate certain aspects of AD neuropathology, the precise role of different NMDAR subtypes for Aβ- and tau-mediated toxicity remains to be elucidated. Using mouse organotypic hippocampal slice cultures from arcAβ transgenic mice combined with Sindbis virus-mediated expression of human wild-type tau protein (hTau), we show that Aβ caused dendritic spine loss independently of tau. However, the presence of hTau was required for Aβ-induced cell death accompanied by increased hTau phosphorylation. Inhibition of NR2B-containing NMDARs abolished Aβ-induced hTau phosphorylation and toxicity by preventing GSK-3β activation but did not affect dendritic spine loss. Inversely, NR2A-containing NMDAR inhibition as well as NR2A-subunit knockout diminished dendritic spine loss but not the Aβ effect on hTau. Activation of extrasynaptic NMDARs in primary neurons caused degeneration of hTau-expressing neurons, which could be prevented by NR2B–NMDAR inhibition but not by NR2A knockout. Furthermore, caspase-3 activity was increased in arcAβ transgenic cultures. Activity was reduced by NR2A knockout but not by NR2B inhibition. Accordingly, caspase-3 inhibition abolished spine loss but not hTau-dependent toxicity in arcAβ transgenic slice cultures. Our data show that Aβ induces dendritic spine loss via a pathway involving NR2A-containing NMDARs and active caspase-3 whereas activation of eSyn NR2B-containing NMDARs is required for hTau-dependent neurodegeneration, independent of caspase-3.
Keywords: amyloid-beta, Aβ, tau, dendritic spine, neurodegeneration, NMDA receptor
Aggregates of amyloid-beta (Aβ) and tau are hallmarks of Alzheimer's disease (AD). It has been shown that tau may mediate critical pathological effects downstream of Aβ.1, 2, 3, 4, 5 Nevertheless, how extracellular Aβ and intracellular tau pathology is functionally connected remains unclear.
Soluble Aβ can bind to or near NMDARs indicating NMDARs as potential targets of Aβ.6, 7, 8 Preventing synaptic targeting of Aβ9 or blocking NMDAR activation2, 10 can abolish Aβ-induced dendritic spine loss and tau-dependent toxicity. NMDARs can be categorized by subunit composition and by localization. It has been shown that synaptic and extrasynaptic (eSyn) NMDAR signaling is gated by different coagonists,11 has opposite effects on cell survival and that differentially located NMDARs are coupled to different intracellular cascades.12, 13 Some studies showed NR2A-subunit-containing NMDARs incorporated into the synapse whereas NR2B-containing NMDARs were found predominantly at extrasynaptic locations.11, 14, 15, 16 However, others reported NR2A- and NR2B-containing NMDARs at both locations.17, 18 Contrasting data exist on the effect of Aβ on different NMDAR types. Oligomeric Aβ induced neuronal dysfunctions by activation of NR2B-containing NMDARs.19 Further, Aβ caused loss of synaptic proteins PSD-95 and synaptophysin by NR2B-containing NMDAR activation accompanied by the suppression of NR2A-containing NMDAR function.20 In contrast, Aβ particularly activated NR2A-containing NMDARs after heterologous expression in Xenopus oocytes.21
Here, we show that NR2A- and NR2B-subunit containing NMDARs differentially mediate Aβ-induced tau phosphorylation, cell death and dendritic spine loss. The presence of human wild-type tau protein (hTau) was essential for Aβ-induced neurodegeneration. Neuronal death required activation of extrasynaptic NR2B-containing NMDARs followed by increased hTau phosphorylation while dendritic spine loss was mediated by NR2A-containing NMDARs signaling and active caspase-3, independent of tau.
Results
Aβ induces hTau-dependent neurotoxicity and tau-independent dendritic spine loss
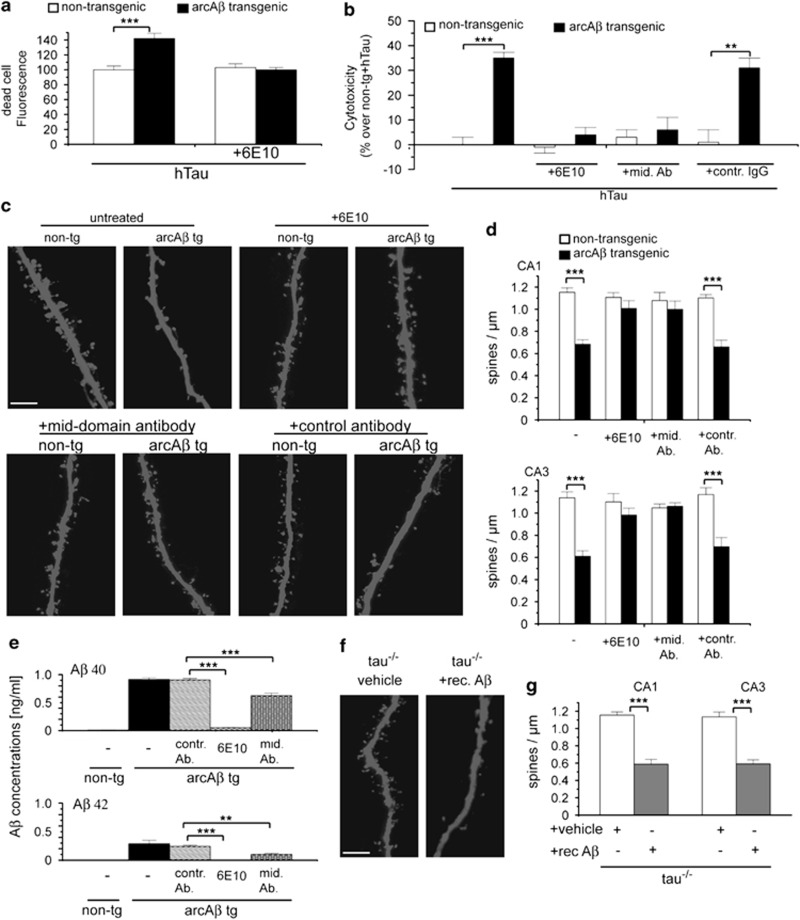
We determined the role of Aβ and tau for neuronal cell death and dendritic spine loss using organotypic hippocampal slice cultures from 7-day-old arcAβ tg mice combined with virus-mediated expression of enhanced green fluorescent protein (EGFP)-coupled 441 residue isoform of hTau or EGFP alone using neurovirulent Sindbis virus. By Live/Dead assays, the proportion of living and dead cells in slice cultures was determined. After 16 days in vitro (DIV), dead cell staining increased significantly after hTau overexpression in arcAβ tg cultures compared with non-tg cultures, which was prevented in the presence of 1 μg/ml of the N-terminal Aβ antibody Aβ antibody 6E10 (Figure 1a). In agreement, hTau overexpression caused cytotoxicity in arcAβ transgenic slice cultures compared with non-tg controls (Figure 1b) as analyzed with Cytotox-Glo assay. Toxicity was completely abolished in the presence of 1 μg/ml of 6E10 or mid-domain Aβ antibody, which does not detect cell-surface amyloid precursor protein (APP) but not with control antibody suggesting that hTau-dependent toxicity in arcAβ cultures was induced by Aβ rather than by APP or any other product of APP processing.
Figure 1.
Aβ induces hTau-dependent neurotoxicity and tau-independent spine loss. (a) Quantification of dead cell fluorescence intensities of hTau-expressing hippocampal slice cultures from arcAβ tg and non-tg mice treated with Aβ antibody 6E10 determined by Live/Dead cell viability/cytotoxicity assay. (b) Cytotoxicity of hTau in arcAβ tg and non-tg control slice cultures treated with 1 μg/ml of Aβ antibody 6E10, a mid-domain Aβ antibody or control antibody measured by Cytotox-Glo assay. (c) Confocal images of apical dendritic segments from CA1 neurons of arcAβ tg and non-tg slices in the presence or absence of Aβ antibody 6E10, mid-domain Aβ antibody or control antibody. (d) Quantification of spine density in cultures from arcAβ tg and non-tg mice. (e) Quantitative bar graphs representing mean values of the amount of Aβ40 and Aβ42 peptides in medium of arcAβ hippocampal slice cultures after treatment with respective Aβ antibodies as determined by ELISA. (f) Representative images of apical dendritic segments from CA1 neurons from tau−/− mice treated with 1 μM recombinant Aβ42. (g) Quantification of spine density in tau−/− cultures treated with recombinant Aβ42. rec. Aβ, recombinant Aβ42; mid. Ab, mid-domain Aβ antibody; contr. Ab, control antibody; mean±S.E.M.; **P<0.01 and ***P<0.001; Mann–Whitney-U-test; n=9–13, n=4 (e) scale bar: 5 μm
To determine the effect of Aβ and tau on dendritic spines, high-resolution imaging of dendritic segments and spines was performed in slice cultures. A strong reduction of dendritic spine numbers by 40–50% was observed in CA1 and CA3 neurons from arcAβ cultures compared with non-tg cultures. Spine loss was completely abolished after treatment with 6E10 or mid-domain Aβ antibody but not by control antibody (Figures 1c and d). Antibody treatment reduced Aβ ELISA signals (Figure 1e) indicating that the removal of Aβ from culture medium is sufficient to prevent hTau-dependent toxicity. The effects of Aβ in cultures from transgenic mice could be confirmed by treatment of wild-type (wt) cultures with recombinant preparations of Aβ4222 (Supplementary Figure S1).
We showed previously that overexpression of wt or mutant hTau in the presence or absence of Aβ did not affect dendritic spine density or morphology.2, 23 Nevertheless, it could not be excluded that endogenous mouse tau mediated Aβ-induced dendritic spine loss. To determine a potential involvement of endogenous tau, organotypic slice cultures were prepared from tau−/− mice and treated with 1 μM recombinant Aβ42, which caused strong reduction in spine density compared with untreated tau−/− cultures (Figures 1f and g). Tau depletion itself did not affect dendritic spine number. The degree of spine loss in tau−/− cultures after Aβ treatment was similar to that observed in cultures expressing endogenous tau, indicating that endogenous tau is not involved in Aβ-induced spine loss in our model.
NR2B-containing NMDARs mediate Aβ-induced hTau-dependent toxicity whereas NR2A-containing NMDARs are involved in dendritic spine loss
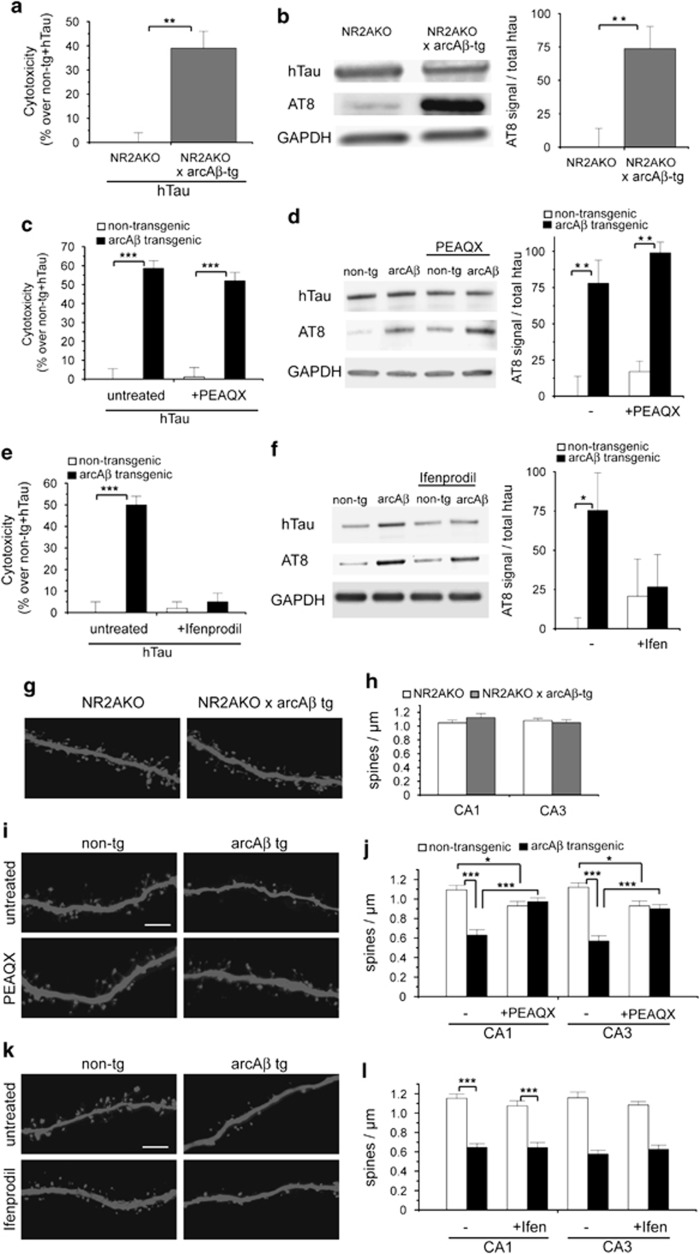
We determined the role of NR2A- and NR2B-containing NMDARs for Aβ-induced neuronal cell death and dendritic spine loss. Although potent NR2B antagonists, for example, Ifenprodil, exist, selectivity of NR2A antagonists is rather low. One of the most selective NR2A antagonists, PEAQX, has been shown to have a 13–130-fold preference for NR1/NR2A over NR1/NR2B receptors.24, 25, 26 However, to avoid any bias due to nonspecific binding of PEAQX, we supported this data by using cultures from NR2A-knockout (NR2AKO) mice in addition. Ifenprodil and PEAQX concentrations were chosen according to previous reports demonstrating highest degree of specificity.19, 26
We found increased toxicity and AT8 phosphorylation of hTau in cultures from NR2AKO × arcAβ tg mice compared with NR2AKO control cultures (Figures 2a and b). In agreement, treatment with NR2A antagonist PEAQX did not prevent toxicity and AT8 phosphorylation of hTau in arcAβ tg cultures (Figures 2c and d). In contrast, treatment with NR2B antagonist Ifenprodil abolished hTau-dependent toxicity (Figure 2e) and reduced AT8 phosphorylation of hTau in arcAβ tg slices (Figure 2f). We further show that activity of GSK-3β, one of the major tau kinases, was increased in arcAβ tg slices. Activity could be reduced by Ifenprodil treatment to control levels (Supplementary Figures S2a and b). In addition, slice cultures were treated with 20 mM lithium (LiCl), known to block GSK-3β activity,27, 28 which prevented hTau toxicity in arcAβ tg slices (Supplementary Figure S2c). This suggests that GSK-3β causes hTau phosphorylation and toxicity downstream of NR2B-containing NMDARs.
Figure 2.
NR2B-containing NMDAR inhibition prevents Aβ-induced hTau-dependent toxicity whereas NR2A knockout or inhibition abolishes dendritic spine loss. (a) Cytotoxicity of hTau in NR2AKO × arcAβ tg and NR2AKO cultures measured by Cytotox-Glo assay. (b) Western blot showing AT8 phosphorylation of hTau in NR2AKO × arcAβ tg and NR2AKO cultures. (c) Cytotoxicity of hTau in arcAβ tg and non-tg control cultures treated with 50 nM PEAQX. (d) Western blot showing expression of hTau and phosphorylation at AT8 epitope after PEAQX treatment. (e) Cytotoxicity of hTau in arcAβ tg and non-tg control cultures treated with 3 μM Ifenprodil. (f) Western blot showing phosphorylation of hTau at AT8 epitope after Ifenprodil treatment. (g) Representative confocal images of apical dendritic segments from NR2AKO and NR2AKO × arcAβ tg cultures. (h) Spine density in NR2AKO and NR2AKO × arcAβ tg cultures. (i) Apical dendritic segments from CA1 neurons of arcAβ tg and non-tg slices in the presence or absence of 50 nM PEAQX. (j) Quantification of spine density after treatment with PEAQX. (k) Apical dendritic segments from CA1 neurons of arcAβ tg and non-tg slices in the presence or absence of 3 μM Ifenprodil. (l) Quantification of spine density after treatment with Ifenprodil. Ifen, Ifenprodil; NR2AKO, NR2A-containing NMDAR knockout; values are shown as mean±S.E.M. with *P<0.05, **P<0.01 and ***P<0.001; Mann–Whitney-U-test; n=6–8 (a, c, e), n=5 (b), n=4 (d), n=6 (f), n=10–13 (h, j, l); scale bar: 5 μm
Opposite effects with respect to NMDA receptor subunit composition were observed on dendritic spine density as both NR2AKO and PEAQX treatment prevented Aβ-induced spine loss while Ifenprodil did not (Figures 2g–l). We confirmed the Ifenprodil data by using a further NR2B inhibitor, Ro 25–6981, which gave identical results (Supplementary Figure S3). Note that NR2A–NMDAR inhibition with PEAQX slightly decreased spine density in control cultures. Our data suggest that Aβ induces tau-dependent cell death and tau-independent loss of dendritic spines by different pathways involving NR2B- or NR2A-containing NMDAR-mediated signaling, respectively.
Activation of extrasynaptic NMDARs induces hTau-dependent toxicity but no spine loss
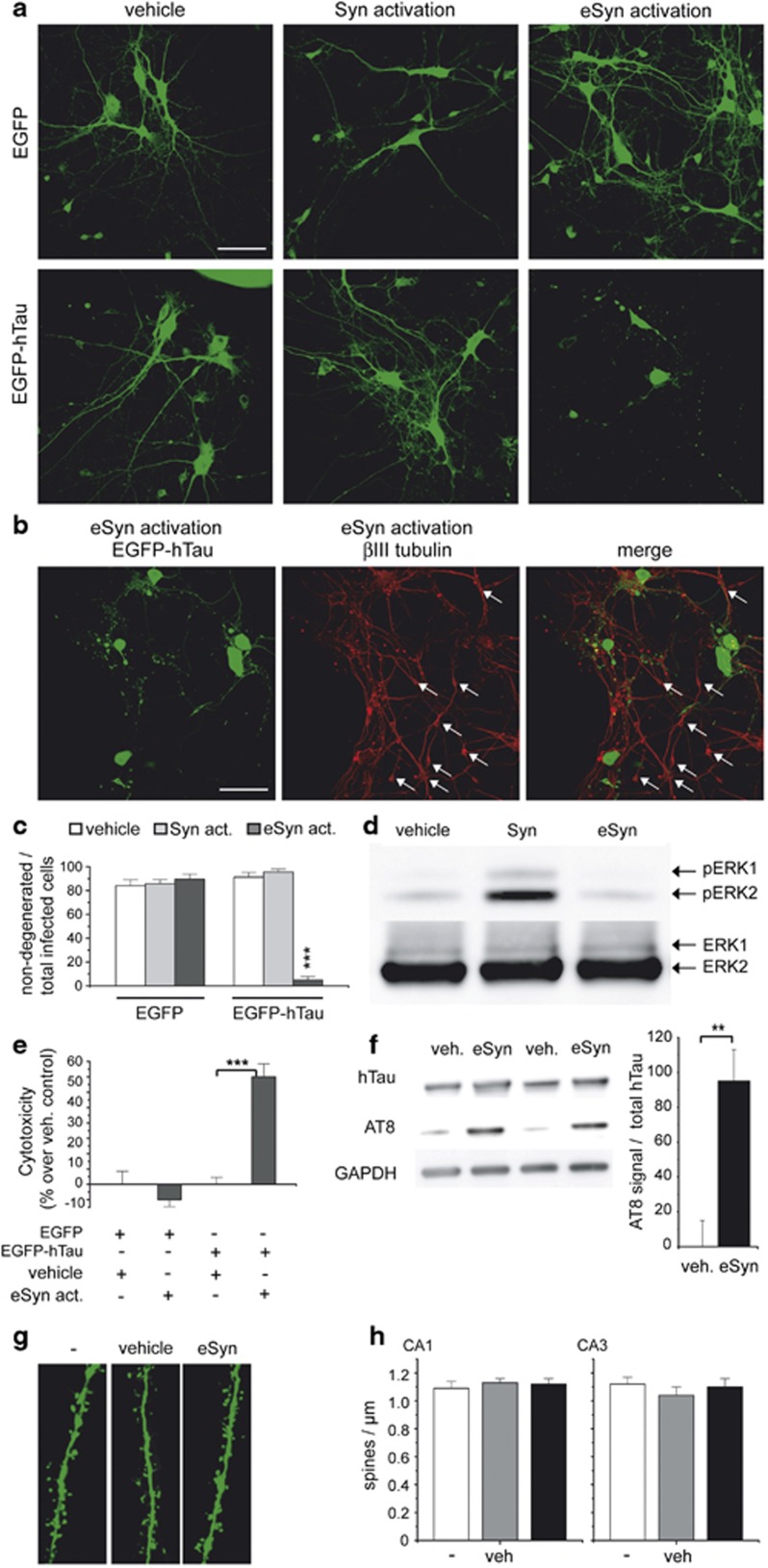
We aimed to determine the role of synaptic and extrasynaptic NMDARs for Aβ-induced hTau toxicity using a protocol that has been shown to selectively activate synaptic versus extrasynaptic NMDARs.12, 29, 30 In primary neuronal cultures from non-tg mice expressing only EGFP, we observed no toxicity after synaptic or extrasynaptic NMDAR activation (Figure 3a upper row, Figure 3c). This is in agreement with a study showing that the use of NMDA for up to 180 min to activate extrasynaptic NMDARs does not induce morphological changes to the neuronal network or increase LDH release from neuronal cultures.30 However, upon hTau expression, the activation of extrasynaptic NMDARs caused a significant increase in toxicity as evidenced by fragmented or beaded neurites and ballooned neurons (Figure 3a lower row, Figure 3c). A ballooned phenotype was already found to be a characteristic for cell death caused by abnormally phosphorylated tau in culture2, 23 and is a histopathological feature of several neurodegenerative diseases, including Pick's disease and AD.31 Immunostaining against βIII tubulin showed no degeneration of non-infected neurons after extrasynaptic activation in primary neuronal cultures (Figure 3b, arrows) confirming that extrasynaptic NMDAR activation causes selective toxicity only in EGFP-hTau-expressing neurons. Increased phosphorylation of ERK kinase after synaptic but not after extrasynaptic activation confirms the selective activation of the respective NMDARs in our protocol.29, 30 Extrasynaptic activation also induced hTau-dependent toxicity and increased AT8 phosphorylation in organotypic hippocampal slices (Figures 3e and f). However, extrasynaptic activation did not affect dendritic spine density in slice cultures (Figures 3g and h). These data suggests a major role of extrasynaptic NMDARs for tau-dependent cell death.
Figure 3.
Extrasynaptic NMDAR activation induces hTau-dependent toxicity in primary neuronal cultures and hippocampal slice cultures. (a) Representative confocal images of primary neurons expressing EGFP or EGFP-hTau after synaptic or extrasynaptic activation. (b) Confocal images of EGFP-hTau-expressing neurons after extrasynaptic activation and immunostaining against βIII tubulin. Arrows mark non-infected neurons. (c) Quantification of hTau-dependent toxicity. Shown is the ratio of non-degenerated infected primary neurons (neurons without fragmented or beaded neurites or ballooned morphology) to total infected neurons. (d) Representative western blot with phospho-ERK 1/2 (pERK) and ERK 1/2 antibodies of lysates from primary neurons after synaptic or extrasynaptic activation. (e) Cytotoxicity of hTau in wt hippocampal slice cultures after extrasynaptic activation measured by Cytotox-Glo assay. (f) Western blot showing AT8 phosphorylation of hTau after extrasynaptic activation in slice cultures. (g) Representative dendritic segments from CA1 neurons of wt slice cultures after extrasynaptic activation. (h) Quantification of spine density in wt slices analyzed 1 day after extrasynaptic activation. eSyn, extrasynaptic; Syn, synaptic activation; veh, vehicle; values are shown as mean±S.E.M. with **P<0.01 and ***P<0.001; Mann–Whitney-U-test; n=10–13 (c), n=3 (d); n=8 (e), n=4 (f); n=10 (h) scale bars: 50 μm (a, b), 5 μm (g)
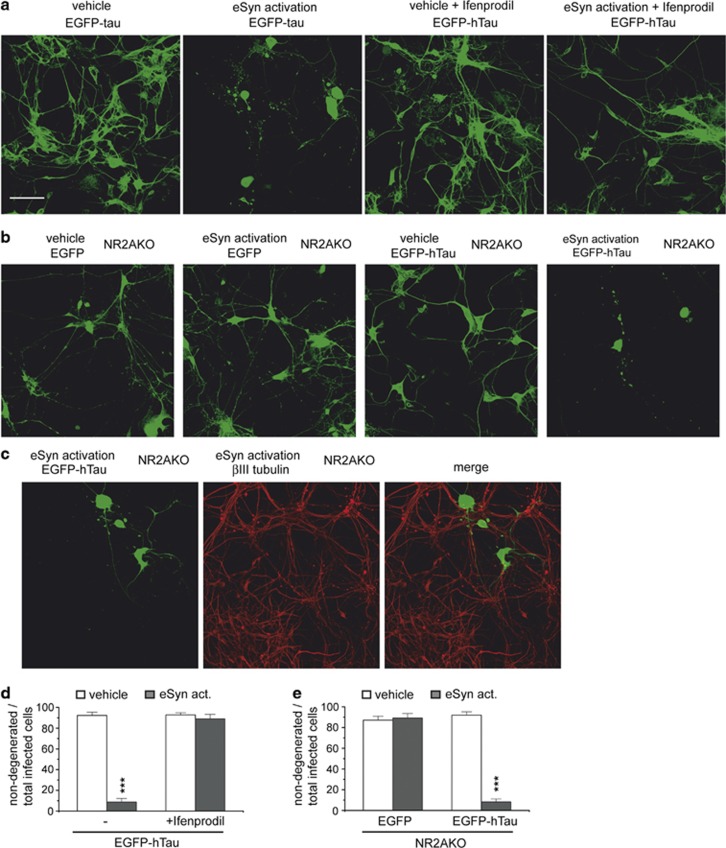
Antagonizing NR2B-containing NMDARs abolishes hTau-dependent toxicity after extrasynaptic NMDAR activation
We showed that hTau-dependent toxicity in arcAβ tg cultures was mediated via NR2B-dependent signaling (Figures 2a and b) and that activation of extrasynaptic NMDARs could mimic the effect of Aβ to induce hTau-dependent toxicity (Figures 3a–c). We next aimed to determine whether extrasynaptically located NR2B-containing NMDARs mediated the induction of hTau-dependent toxicity. Activation of extrasynaptic NMDARs in the presence of Ifenprodil did not induce hTau-dependent toxicity (Figures 4a), while strong degeneration of hTau-expressing neurons was observed after extrasynaptic activation in NR2AKO neurons (Figures 4b and e). This suggests that extrasynaptically localized NR2B- but not NR2A-containing NMDARs mediated hTau-dependent toxicity. The expression of both NR2A- and NR2B-subunits (GRIN2A and GRIN2B) in primary neurons was verified by RT-PCR (Grinschgl et al., unpublished observations).
Figure 4.
NR2B-containing NMDAR inhibition but not NR2A-containing NMDAR knockout abolishes hTau-dependent toxicity after activation of extrasynaptic NMDARs. (a) Representative confocal images of primary neurons expressing EGFP-hTau after extrasynaptic activation in the presence and absence of 3 μM Ifenprodil. (b) Representative confocal images of primary neurons from NR2AKO mice expressing EGFP-hTau after extrasynaptic activation. (c) Confocal images of EGFP-hTau-expressing primary neurons from NR2AKO mice after extrasynaptic activation and immunostaining against βIII tubulin. (d) Quantification of hTau-dependent toxicity in the presence and absence of Ifenprodil. Shown is the ratio of non-degenerated infected neurons (neurons without fragmented or beaded neurites or ballooned morphology) to total infected neurons. (e) Quantification of hTau-dependent toxicity in primary neurons of NR2AKO mice. eSyn act, activated eSyn NMDARs; values are shown as mean±S.E.M. with ***P<0.001; Mann–Whitney-U-test; n=10–14; scale bar: 50 μm
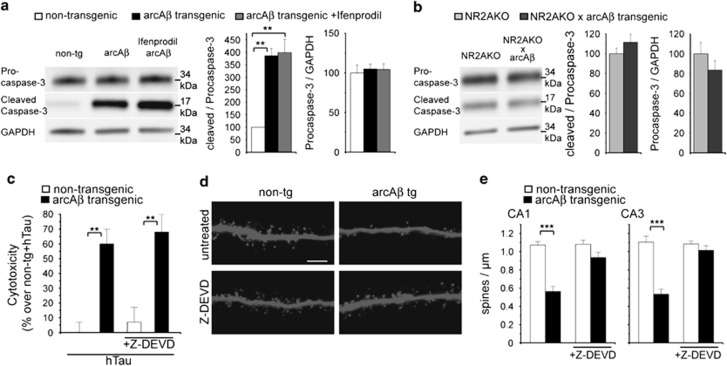
Aβ-induced caspase-3 activity is required for dendritic spine loss
Caspase-3 has been shown to be activated by Aβ20 and to trigger early synaptic dysfunctions in APPSwe tg mice.32 Accordingly, increased proteolytic cleavage of procaspase-3 into a 17-kDa active caspase-3 fragment was observed in arcAβ transgenic slice cultures. Treatment with NR2B antagonist Ifenprodil did not prevent caspase-3 activation whereas increased caspase-3 activation was not observed after NR2A-subunit knockout (Figures 5a and b). To verify the role of active caspase-3 in mediating the effects of Aβ, slices were treated with 10 μM of caspase-3 inhibitor Z-DEVD-FMK. Treatment did not prevent hTau-dependent toxicity in arcAβ tg cultures (Figure 5c) but abolished Aβ-induced spine loss (Figures 5d and e). Thus, active caspase-3 is involved in NR2A-dependent dendritic spine loss, while it is not required for hTau-dependent toxicity induced by Aβ in our model.
Figure 5.
Aβ-induced caspase-3 activation causes dendritic spine loss but not hTau-dependent toxicity. (a) Western blot with antibodies against procaspase-3 and cleaved (activated) caspase-3 in slice cultures from arcAβ tg mice treated with 3 μM Ifenprodil. (b) Western blot with antibodies against procaspase-3 and cleaved caspase-3 in cultured slices from NR2AKO and NR2AKO × arcAβ tg mice. (c) Cytotoxicity of hTau in arcAβ tg and non-tg control slice cultures treated with 10 μM Z-DEVD-FMK measured by Cytotox-Glo assay. (d) Representative confocal images of apical dendritic segments from CA1 neurons of arcAβ tg and non-tg slices treated with 10 μM Z-DEVD-FMK. (e) Quantification of spine density after treatment with Z-DEVD-FMK. Z-DEVD, Z-DEVD-FMK; values are shown as mean±S.E.M. with **P<0.01 and ***P<0.001; Mann–Whitney-U-test; n=3–4 (a, b), n=7–11 (c, e); scale bar: 5 μm
Discussion
The results of this study establish the differential involvement of synaptic and extrasynaptic NR2A- and NR2B-subunit-containing NMDARs in pathways coupling Aβ to loss of dendritic spines and hTau-dependent neurodegeneration. Using selective pharmacological inhibition combined with knockout techniques, we show that Aβ induced hTau-dependent toxicity accompanied by increased hTau phosphorylation via activation of extrasynaptic NR2B-containing NMDARs. In contrast, loss of dendritic spines was mediated by NR2A-containing NMDAR signaling. Importantly, Aβ also induced spine loss in cultures prepared from tau-deficient mice suggesting that loss of postsynaptic spines is not dependent on endogenous mouse tau. In agreement with the missing involvement of endogenous tau for spine loss, overexpression of wt or mutant hTau did also not affect spine number or shape as previously shown.2, 23 However, hTau overexpression in the presence of either recombinant or transgenically expressed Aβ caused cell death with hTau being abnormally phosphorylated at the AT8 epitope. Interestingly, Aβ did not induce detectable toxicity in the presence of endogenous murine tau (mTau). We did not find any effect of Aβ on AT8 phosphorylation of mTau (Supplementary Figure S4), suggesting that hTau may be a better substrate for hyperphosphorylation and induction of cell death than mTau. In agreement, lentiviral expression of hTau accelerated the neurotoxic effect of Aβ dimers compared with endogenous tau in primary neurons.33
Increased activation or expression of several tau kinases, for example GSK-3β,34 as well as reduced activity of tau phosphatases35 has been reported in AD, and toxicity of phosphorylated tau has been shown in various studies.2, 23, 33, 36 Recently, a longitudinal study of 286 participants revealed that Aβ-associated brain volume loss occurs only in the presence of phosphorylated tau in the human brain.37
The mechanisms by which tau causes degeneration of neurons are discussed controversially. Four independently created tau-knockout lines show largely normal behavior,38, 39, 40, 41 indicating that loss of tau function does not cause neurodegeneration. Accumulating evidence points to the possibility that physiological functions of tau may be involved in neuronal excitotoxicity3, 4, 42 or axonal transport dysfunction43 in APP transgenic mice and that tau interactions other than microtubule binding may also be involved.44 Furthermore, several in vitro and in vivo studies support abnormal gain of toxic function for tau caused by hyperphosphorylation.2, 23, 33, 36, 45 Together, both phosphorylation-independent physiological tau functions as well as hyperphosphorylation-induced gain of toxic function may not be mutually exclusive and jointly contribute to neuronal dysfunction in disease.
NMDARs are potential targets of Aβ as soluble Aβ can bind to or near NMDARs.6, 7 Some studies showed NR2A-subunit-containing NMDARs incorporated into the synapse whereas NR2B-containing NMDARs were found predominantly at extrasynaptic locations.14, 15, 16 However, others reported NR2A- and NR2B-containing NMDARs at both locations.17, 18 Signaling via NR2A- or NR2B-containing NMDARs causes different or even opposing effects.46, 47, 48, 49 The same applies for synaptic or extrasynaptic NMDAR activity.12, 29, 30, 50, 51, 52 Thus, specific NMDAR function may depend on both subunit composition and spatial distribution suggesting that NR2A- and NR2B-containing NMDARs at both locations are each coupled to different cascades.
We analyzed the involvement of different NMDAR subtypes and locations for spine loss and tau toxicity in arcAβ tg cultures. Knockout and inhibition of NR2A-containing NMDARs but not of NR2B-containing NMDARs prevented spine loss in arcAβ cultures. In agreement, Aβ has been shown to influence NR2A-containing NMDARs although the results are controversial: in Xenopus oocytes, Aβ directly activated NR2A-containing NMDARs21 whereas Aβ blocked NR2A-containing NMDARs in primary rat neurons leading to loss of PSD-95 and synaptophysin signals.20
We now show for the first time that Aβ-induced hTau phosphorylation and toxicity was mediated by NR2B- but not NR2A-containing NMDAR-dependent signaling and involved NR2B-dependent activation of tau kinase GSK-3β.
An involvement of NR2B-containing NMDARs for tau toxicity was also reported previously53 where Ifenprodil blocked toxicity after tau overexpression in dissociated primary neurons. However, no increased tau phosphorylation was found and tau toxicity was analyzed in the absence of Aβ implying different mechanisms of toxicity in both studies.
In agreement with our data, soluble Aβ oligomers have been shown to excessively activate extrasynaptic NR2B-containing NMDARs in acute slice cultures,19 and activation of NR2B-containing NMDARs by Aβ in primary neurons resulted in a loss of synaptophysin- and PSD-95 signals.20 We show that Aβ increased proteolytic caspase-3 activation via NR2A- but not NR2B-mediated signaling. Further, pharmacological caspase-3 inhibition abolished Aβ-induced dendritic spine loss but not hTau-dependent toxicity indicating that Aβ-induced synaptic loss is mediated by NR2A signaling followed by caspase-3 activation. Caspase-3 activation has been shown to be required for long-term depression (LTD)54 and is also involved in Aβ-induced loss of PSD-95 and synaptophysin signals20 and inhibition of LTP.55 We and others have shown that the calcium-dependent phosphatase calcineurin, a key enzyme in LTD, can trigger Aβ-induced changes and loss of dendritic spines2, 10, 56, 57 via an LTD-like cascade. Calcineurin can be activated by caspase-3 in dendritic spines32 thus linking NR2A-mediated activation of caspase-3 to dendritic spine loss.
In conclusion, our data uncover two independent pathways for neuronal and synaptic loss, both triggered by Aβ, which already differ in their requirement for distinct types of NMDARs. This may explain the difficulty and the big challenge to find appropriate drug targets downstream of Aβ in order to prevent synaptic loss and neuronal cell death in AD.
Materials and Methods
Animals
ArcAβ mice and NR2A–NMDA receptor-knockout mice were obtained as described.58, 59 Tau-deficient B6.129-Mapttm1Hnd/J mice39 were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All animal experiments were performed in accordance with the guidelines of the Swiss veterinary cantonal office.
Organotypic hippocampal slice culture and viral infection
Organotypic hippocampal slice cultures were prepared and cultured as previously described.2 On DIV 12, slice cultures were infected with Sindbis virus using the droplet method. For assessment of dendritic spine density, cultures were infected with Sindbis virus expressing EGFP and were fixed at DIV 15 with 4% paraformaldehyde in PBS containing 4% sucrose for 2 h at 4 °C. After washing with PBS, cultures were mounted with Hydromount (National diagnostics, Atlanta, GA, USA) and coverslipped. For analysis of hTau-dependent toxicity, slices were infected at DIV 12 with Sindbis virus expressing EGFP-coupled human 441 wt tau. At DIV 16, culture medium was harvested for cytotoxicity assays and lysates were prepared for western blot analyses.
Drug treatments
NR2A-subunit-selective NMDAR antagonist PEAQX ([[[(1S)-1-(4-Bromophenyl)ethyl]amino](1,2,3,4-tetrahydro-2,3-dioxo-5-quinoxalinyl)methyl] phosphonic acid tetrasodium hydrate, 50 nM) was purchased from Sigma (Schnelldorf, Germany); NR2B–NMDAR antagonist Ifenprodil ((1S,2S)-threo-2-(4-benzylpiperidino)-1-(4-hydroxyphenyl)-1-propanol hemitartrate, 3 μM), NMDAR antagonist MK801 ((5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate, 5 μM), GABAA antagonist bicuculline ([R-(R*,S*)]-6-(5,6,7,8-tetrahydro-6-methyl-1,3-dioxolo[4,5-g]isoquinolin-5-yl)furo[3,4-e]-1,3-benzodioxol-8(6H)-one), 25 μm), potassium channel blocker 4-aminopyridine (1 mM) and caspase-3 inhibitor Z-DEVD-FMK (benzyloxycarbonyl-Asp(OMe)-Glu(OMe)-Val-Asp(OMe)-fluoromethylketone, 10 μM) were purchased from Tocris (Bristol, UK). We used two antibodies against Aβ, targeting either the N terminus (6E10) or the Aβ mid-domain. The mid-domain antibody binds to both monomeric and aggregated Aβ but not full-length APP. The control antibody was directed against bovine herpes virus. All antibodies were applied at concentrations of 1 μg/ml.
To determine the effect on dendritic spine density, cultures were treated for 7 days with the respective substance. For cell survival analysis, cultures were treated for 4 days.
Preparation of primary neuronal cultures
Neuronal cultures were prepared as described previously.60 On DIV 12, cultures were infected with Sindbis virus expressing EGFP or EGFP-hTau. At 16 h after infection, synaptic/ extrasynaptic activation protocols were performed followed by fixation 24 h after activation.
Immunblot analysis
On DIV 16, hippocampal slices were harvested in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 0.5% deoxycholate and 0.1% SDS, pH 8.0) containing phosphatase inhibitor cocktails 1 and 2 (Sigma) and protease inhibitor cocktail (Roche, Basel, Switzerland) and centrifuged at 5000 × g for 10 min at 4 °C. The supernatant was collected, frozen in liquid nitrogen and stored at −80 °C.
Samples were resolved by 10–20% SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Immunoblotting was performed using primary antibodies anti-GFP (1:1000, Roche) to detect total GFP-coupled hTau, anti phospho-Tau AT8 (1:200, Thermo Fisher, Rockford, IL USA), Tau-5 (1:500, Neo Markers, Fremont, CA, USA), GSK-3β and phospho-GSK-3β (1:1000, Cell Signaling, Danvers, MA, USA), GAPDH (1:5000, Biodesign, Saco, ME, USA) and HRP-conjugated secondary antibody (1:2000, GE Healthcare, Glattbrugg, Switzerland). Immunoreactive bands were detected using the ECL Reagent (Thermo Fisher) or Supersignal Femto Maximum Sensitivity Substrate (Thermo, Rockford, IL, USA) according to the manufacturer's instructions and imaged with Fujifilm Las-3000 (Fujifilm, Dielsdorf, Switzerland). It was verified by software tools that no pixels were saturated. Band intensities were quantified with ImageJ (NIH, Bethesda, MD, USA) corrected by background.
Assessment of cell death
To measure dead cell protease activity in slice cultures, culture medium was harvested at DIV 16, directly frozen in liquid nitrogen and stored at −80 °C for further analysis with CytoTox-Glo assay according to the manufacturer's recommendations. In the case of EGFP-tau overexpression, luminescence signals were normalized to EGFP fluorescence using microplate reader Synergy HT (BioTek, Bad Friedrichshall, Germany).
Cell viability and cytotoxicity were further determined using LIVE/DEAD Viability/Cytotoxicity assay (Molecular Probes, Grand Island, NY, USA) according to the manufacturer's recommendations. EtHD-1-stained slices were imaged using Leica DMIRE2 fluorescence microscope (Leica, Heerbrugg, Switzerland) with excitation filters 470/40 and 545/30, respectively. Images were acquired using identical microscope settings for all conditions devoid of saturation. Integrated fluorescence intensities relative to background fluorescence were determined by ImageJ program.
To analyze the effects of extrasynaptic activation in primary neuronal cultures, EGFP- or EGFP-hTau-expressing neurons were imaged using confocal microscopy. The ratio of non-degenerated infected neurons to total infected neurons was determined by dividing the number of neurons without fragmented or beaded neurites or ballooned morphology by the total number of infected cells.
Dendritic spine analysis
For analysis of dendritic spine density, virus solution was diluted to achieve 1–10 infected neurons per slice. This allowed imaging of single dendritic fragments. Analysis of dendritic spine density was performed using Leica SP2 CLSM equipped with × 63 objective (NA: 1.2) and 488-nm Argon laser. Apical dendritic segments in CA1 and CA3 stratum radiatum were imaged with image size of 30 × 30 μm (512 × 512 pixel, voxel size: 0.05813 × 0.05813 × 0.25 μm). Image stacks were processed to maximum projections, and dendritic spine density was determined as spine counts per μm dendrite using ImageJ.
Activation of synaptic/extrasynaptic NMDARs
For extrasynaptic activation, cultures were exposed to fresh neurobasal medium containing 1 mM 4-AP, 25 μM bicuculline and 5 μM MK801 for 5 min to activate and block synaptic NMDARs. Then, cultures were washed several times with neurobasal medium to remove unbound MK801, followed by incubation in Nb-N1 medium containing 50 μM NMDA for 15 min. Control cultures were treated with Nb-N1 medium containing water and DMSO vehicle.
To stimulate synaptic activation, cultures were exposed to neurobasal medium containing 1 mM 4-AP and 25 μM bicuculline for 15 min.
These protocols have been shown to selectively activate synaptic versus extrasynaptic NMDARs.12, 29, 30
To determine the effect of extrasynaptic NMDAR activation on dendritic spine density in slice cultures, slices were infected with EGFP-expressing virus on DIV 12, activation was carried out on DIV14 as described above and slices were fixed on DIV 15 for analysis of dendritic spine density.
Statistical analysis
Data are presented as mean±S.E.M. Statistical analysis was performed with Statview 5.0 (SAS Institute Inc., Cary, NC, USA) using Mann–Whitney-U-tests. Values of P<0.05 were considered statistically significant.
Acknowledgments
CT was supported by the Deutsche Forschungsgemeinschaft (DFG, Ta762/1-1). SG and AT were supported by the Swiss National Science Foundation (SNF) grant 31003A 130148. ACS was supported by Novartis Stiftung. MCF was supported by Velux Stiftung. RB was supported by the Deutsche Forschungsgemeinschaft (DFG, BR1192/11-2). We thank Lawrence Rajendran for critical reading and valuable comments, and Masayoshi Mishina for kindly providing NR2A–NMDA receptor-knockout mice.
Glossary
- APP
amyloid precursor protein
- Aβ
amyloid β-peptide
- AD
Alzheimer's disease
- EGFP
enhanced green fluorescent protein
- hTau
human wild-type tau protein
- mTau
endogenous murine tau
- NMDAR
N-methyl-𝒟-aspartate receptor
JG is CSO of Neurimmune Holding AG, Wagistrasse 13, 8952 Schlieren, Switzerland.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by A Verkhratsky
Supplementary Material
References
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to beta -amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackenberg C, Brandt R. Divergent pathways mediate spine alterations and cell death induced by amyloid-beta, wild-type tau, and R406W tau. J Neurosci. 2009;29:14439–14450. doi: 10.1523/JNEUROSCI.3590-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;31:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Nussbaum JM, Schilling S, Cynis H, Silva A, Swanson E, Wangsanut T, et al. Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-ß. Nature. 2012;485:651–655. doi: 10.1038/nature11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker H, Jürgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, et al. N-methyl-D-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-ß peptide oligomers. J Neurochem. 2010;115:1520–1529. doi: 10.1111/j.1471-4159.2010.07058.x. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, et al. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, et al. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald F, Schulz TW, Schenker LT, Kennedy MB, Seeburg PH, Köhr G. C-Terminal truncation of NR2A subunits impairs synaptic but not extrasynaptic localization of NMDA receptors. J Neurosci. 2000;20:4573–4581. doi: 10.1523/JNEUROSCI.20-12-04573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MA, Wyllie DJ, Hardingham GE. In developing hippocampal neurons, NR2B-containing N-methyl-D-aspartate receptors (NMDARs) can mediate signaling to neuronal survival and synaptic potentiation, as well as neuronal death. Neuroscience. 2009;158:334–343. doi: 10.1016/j.neuroscience.2008.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Chen HS, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aß oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chang L, Roselli F, Almeida OF, Gao X, Wang X, et al. Amyloid-ß induces caspase-dependent loss of PSD-95 and synaptophysin through NMDA receptors. J Alzheimers Dis. 2010;22:541–556. doi: 10.3233/JAD-2010-100948. [DOI] [PubMed] [Google Scholar]
- Texidó L, Martín-Satué M, Alberdi E, Solsona C, Matute C. Amyloid ß peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49:184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Finder VH, Vodopivec I, Nitsch RM, Glockshuber R. The recombinant amyloid-beta peptide Abeta1-42 aggregates faster and is more neurotoxic than synthetic Abeta1-42. J Mol Biol. 2010;396:9–18. doi: 10.1016/j.jmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Shahani N, Subramaniam S, Wolf T, Tackenberg C, Brandt R. Tau aggregation and progressive neuronal degeneration in the absence of changes in spine density and morphology after targeted expression of Alzheimer's disease-relevant tau constructs in organotypic hippocampal slices. J Neurosci. 2006;31:6103–6114. doi: 10.1523/JNEUROSCI.4245-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE, Monaghan DT. Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol. 2004;141:508–516. doi: 10.1038/sj.bjp.0705644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby Ø, et al. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu F, Tian Q, Yang Y, Wang Q, Wang JZ. Activation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in culture. J Neural Transm. 2006;113:93–102. doi: 10.1007/s00702-005-0303-7. [DOI] [PubMed] [Google Scholar]
- Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci. 2009;29:4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW, Yen SH, Suzuki KI, Davies P, Garcia JH, Hirano A. Ballooned neurons in select neurodegenerative diseases contain phosphorylated neurofilament epitopes. Acta Neuropathol. 1986;71:216–223. doi: 10.1007/BF00688042. [DOI] [PubMed] [Google Scholar]
- D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci. 2011;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ. Soluble amyloid beta-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Laferla FM. Pathways by which Abeta facilitates tau pathology. Curr Alzheimer Res. 2006;3:437–448. doi: 10.2174/156720506779025242. [DOI] [PubMed] [Google Scholar]
- Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Zempel H, Thies E, Mandelkow E, Mandelkow EM. Abeta oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J Neurosci. 2010;30:11938–11950. doi: 10.1523/JNEUROSCI.2357-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, McEvoy LK, Thompson WK, Holland D, Roddey JC, Blennow K, et al. Amyloid-ß associated volume loss occurs only in the presence of phospho-tau. Ann Neurol. 2011;70:657–661. doi: 10.1002/ana.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;269:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Muramatsu K, Hashimoto Y, Uemura T, Kunii M, Harada R, Sato T, et al. Neuron-specific recombination by Cre recombinase inserted into the murine tau locus. Biochem Biophys Res Commun. 2008;370:419–423. doi: 10.1016/j.bbrc.2008.03.103. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Halabisky B, Yoo JW, Yao J, Chin J, Yan F, et al. Amyloid-ß/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Kemper A, Weissmann C, Golovyashkina N, Sebö-Lemke Z, Drewes G, Gerke V, et al. The frontotemporal dementia mutation R406W blocks tau's interaction with the membrane in an annexin A2-dependent manner. J Cell Biol. 2011;192:647–661. doi: 10.1083/jcb.201007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhilb ML, Dias-Santagata D, Fulga T, Felch DL, Feany MB. Tau phosphorylation sites work in concert to promote neurotoxicity in vivo. Mol Biol Cell. 2007;18:5060–5068. doi: 10.1091/mbc.E07-04-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MA, Ryan TJ, Bell KF, Fowler JH, McMahon A, Al-Mubarak B, et al. The subtype of GluN2 C-terminal domain determines the response to excitotoxic insults. Neuron. 2012;74:543–556. doi: 10.1016/j.neuron.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci USA. 2011;108:5855–5860. doi: 10.1073/pnas.1012676108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ß production. J Neurosci. 2010;30:15927–15942. doi: 10.1523/JNEUROSCI.3021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroge CM, Hogins J, Eisenman L, Mennerick S. Synaptic NMDA receptors mediate hypoxic excitotoxic death. J Neurosci. 2012. pp. 6732–6742. [DOI] [PMC free article] [PubMed]
- Li B, Chen N, Luo T, Otsu Y, Murphy TH, Raymond LA. Differential regulation of synaptic and extra-synaptic NMDA receptors. Nat Neurosci. 2002;5:833–834. doi: 10.1038/nn912. [DOI] [PubMed] [Google Scholar]
- Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci USA. 2006;103:2892–2897. doi: 10.1073/pnas.0511065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, et al. Aß(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3ß. Nat Neurosci. 2011;14:545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hudry E, Hashimoto T, Uemura K, Fan ZY, Berezovska O, et al. Distinct dendritic spine and nuclear phases of calcineurin activation after exposure to amyloid-ß revealed by a novel fluorescence resonance energy transfer assay. J Neurosci. 2012;32:5298–5309. doi: 10.1523/JNEUROSCI.0227-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M, Konietzko U, Krebs DC, Nitsch RM. Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging. 2007;28:1297–1307. doi: 10.1016/j.neurobiolaging.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Goodger Z, Rajendran L, Trutzel A, Kohli BM, Nitsch RM, Konietzko U. Nuclear signaling by the APP intracellular domain occurs predominantly through the amyloidogenic processing pathway. J Cell Sci. 2009;122:3703–3714. doi: 10.1242/jcs.048090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.