Abstract
Despite initial and often dramatic responses of epidermal growth factor receptor (EGFR)-addicted lung tumors to the EGFR-specific tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, nearly all develop resistance and relapse. To explore novel mechanisms mediating acquired resistance, we employed non-small-cell lung cancer (NSCLC) cell lines bearing activating mutations in EGFR and rendered them resistant to EGFR-specific TKIs through chronic adaptation in tissue culture. In addition to previously observed resistance mechanisms including EGFR-T790M ‘gate-keeper' mutations and MET amplification, a subset of the seven chronically adapted NSCLC cell lines including HCC4006, HCC2279 and H1650 cells exhibited marked induction of fibroblast growth factor (FGF) 2 and FGF receptor 1 (FGFR1) mRNA and protein. Also, adaptation to EGFR-specific TKIs was accompanied by an epithelial to mesenchymal transition (EMT) as assessed by changes in CDH1, VIM, ZEB1 and ZEB2 expression and altered growth properties in Matrigel. In adapted cell lines exhibiting increased FGF2 and FGFR1 expression, measures of growth and signaling, but not EMT, were blocked by FGFR-specific TKIs, an FGF-ligand trap and FGFR1 silencing with RNAi. In parental HCC4006 cells, cell growth was strongly inhibited by gefitinib, although drug-resistant clones progress within 10 days. Combined treatment with gefitinib and AZD4547, an FGFR-specific TKI, prevented the outgrowth of drug-resistant clones. Thus, induction of FGF2 and FGFR1 following chronic adaptation to EGFR-specific TKIs provides a novel autocrine receptor tyrosine kinase-driven bypass pathway in a subset of lung cancer cell lines that are initially sensitive to EGFR-specific TKIs. The findings support FGFR-specific TKIs as potentially valuable additions to existing targeted therapeutic strategies with EGFR-specific TKIs to prevent or delay acquired resistance in EGFR-driven NSCLC.
Keywords: gefitinib, EGFR, FGFR1, FGF2, acquired resistance, NSCLC
Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer related deaths in the United States and despite recent advances in treatment and diagnosis, the 5-year survival remains at ∼16%.1 This poor outcome is largely due to the advanced disease stage and degree of metastasis at diagnosis. Although treatment of lung cancers with standard cytotoxic chemotherapies has been optimized for efficacy,2 recent approaches to NSCLC therapeutics are based on classification of NSCLC into molecular subsets based on their distinct oncogene driver. These molecular drivers of NSCLC can be attacked therapeutically with targeted agents directed against the specific oncogenes.
The epidermal growth factor receptor (EGFR) is highly expressed or amplified in many NSCLC patients,3, 4 although clinical investigation with EGFR-specific tyrosine kinase inhibitors (TKIs) identified patients whose tumors bear gain-of-function EGFR mutations as the subset with the best response.5, 6, 7 Although, these patients initially respond to EGFR-targeted therapies, all will eventually relapse, a common theme observed in all targeted therapies (as reviewed by refs 4, 5, 8, 9, 10). Overall, the median time to progression on EGFR-targeted therapies is 8–10 months.11, 12 Multiple mechanisms of acquired resistance to EGFR-targeted inhibitors have been discovered and validated in patients. Secondary gate-keeper mutations, which increase EGFR-ATP binding affinity, occur in 50% of patients whose tumors progress on EGFR-specific TKIs.13 In addition, MET amplification following treatment with EGFR inhibitors has been reported in ∼5–15% of NSCLC patients.12, 14, 15 EGFR-T790M and MET-amplified tumor cells can be detected in tumors before EGFR-targeted therapies, suggesting these cells are selectively enriched upon treatment. Furthermore, detection of either T790M or amplified MET with HGF expression before EGFR TKI treatment is associated with decreased duration of response to EGFR-targeted treatments.16, 17 Interestingly, both of these resistance mechanisms retain downstream signaling through the AKT pathway. Also, Sequist et al.12 found a small percentage of patients who acquire PI3KCA mutations post EGFR TKI therapy, further highlighting the PI3K-AKT pathway as a survival pathway in NSCLC with gain-of-function EGFR. Importantly, this same study failed to detect acquired mutations in ∼30% of TKI-resistant lung tumors. We hypothesize that alternative receptor tyrosine kinases that are neither mutated nor amplified may contribute to acquired resistance to EGFR-targeted therapies. As precedent, induction of AXL has been recently demonstrated during in vitro and in vivo acquired resistance to EGFR-specific TKIs in lung cancer.18, 19
Alternative receptor tyrosine kinases, also referred to as ‘bypass pathways', have been identified as mechanisms of both intrinsic and acquired resistance to targeted therapeutics including EGFR TKIs.20, 21, 22, 23, 24 Compared with resistance via acquisition of gate-keeper mutations, acquired resistance mechanisms involving induction of distinct signaling pathways lacking genetic alterations are less documented in the literature. To date, the insulin-like growth factor 1 receptor and AXL has been demonstrated to have a role in acquired resistance to gefitinib.18, 19, 25, 26 Recently, we reported the protective role of rapidly upregulated fibroblast growth factor receptor 2 (FGFR2) and FGFR3 in response to gefitinib treatment in NSCLC with either wild-type or mutant EGFR.27 In the present study, we deployed standard chronic adaptation techniques previously described in the literature12, 14, 15 to develop EGFR-mutant NSCLC cell lines with acquired resistance to gefitinib. Herein, we demonstrate that FGFR1 and FGF2 are induced during chronic acquisition of resistance to gefitinib, highlighting FGFR1 as an additional candidate for a bypass mechanism contributing to EGFR inhibitor resistance.
Results
Establishment and characterization of gefitinib-resistant NSCLC cell lines
In vitro modeling of acquired resistance to EGFR-specific TKIs has identified resistance mechanisms also observed in patients upon tumor progression on erlotinib and gefitinib. For example, HCC827 cells undergo MET amplification and AXL induction upon adaption to gefitinib14, 15, 17, 19 and PC9 cells acquire the T790M mutation in EGFR that confers resistance to erlotinib and gefitinib.13 In addition, studies with TKI-resistant tumor specimens suggest alternative mechanisms that remain to be defined.12 To further explore mechanisms which mediate gefitinib resistance, a panel of eight NSCLC cell lines (Supplementary Table S1) with EGFR-activating mutations rendering them sensitive to EGFR-targeted therapies were adapted to increasing concentrations of gefitinib until they could be cultured in 3 μℳ gefitinib (see Materials and methods). In addition, H1975 cells, which express EGFR bearing the activating L858R mutation and the T790M gate-keeper mutation, were selected for resistance to the irreversible EGFR inhibitor, BIBW2992.28 All TKI-resistant and passage control cell lines were submitted to DNA fingerprint analysis to verify authenticity, both before and after adaptation. As shown in Supplementary Figure S1 and Supplementary Table S2, the gefitinib-adapted cell lines exhibited IC50s to EGFR TKIs that were several orders of magnitude larger than that exhibited by the DMSO-cultured control cell lines. Of note, gefitinib-resistant cultures of HCC2935 cells were not obtained after two-independent attempts and this cell line was not studied further. In general, gefitinib-resistant cell lines demonstrated decreased phospho-EGFR expression as compared with passage controls cells, although total EGFR did not change significantly (Figure 1c). In addition, EGFR mRNA sequences were amplified by PCR to verify retention of EGFR gain-of-function mutations (E746-A750 del, L747-A750 del, L858R) and to detect any acquired T790M mutations. Consistent with published studies, our findings reveal that gefitinib-resistant PC9 cells acquired a T790M gate-keeper mutation and gefitinib-resistant HCC827 cells exhibited markedly increased MET expression levels13, 14, 15, 17 (Supplementary Figures S2 and S3). Moreover, gefitinib-resistant PC9 cells retained sensitivity to BIBW2992 as predicted (Supplementary Figure S2) and gefitinib-resistant HCC827 cells exhibited acquired sensitivity to the MET inhibitor, crizotinib, as assessed by inhibition of phospho-MET and reduction of anchorage-independent growth (Supplementary Figure S3). Interestingly, H1650, H1975, HCC2279, HCC4006 and HCC4011 cells neither acquired T790M mutations nor exhibited MET amplification (data not shown).
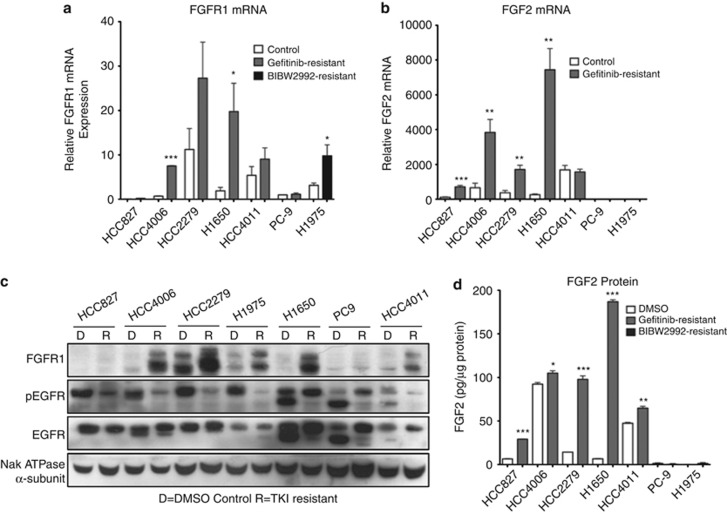
Figure 1.
FGFR1 and FGF2 are induced following adaption of EGFR-dependent NSCLC cell lines to EGFR-specific TKIs. (a, b) Total RNA was purified from the indicated cell lines following chronic adaption to a reversible EGFR inhibitor (gefitinib 3 μℳ) or irreversible EGFR inhibitor (BIBW2992 2 μℳ) that inhibits EGFR-T790M and submitted to quantitative RT–PCR analysis of (a) FGFR1 or (b) FGF2. The data are presented as relative expression following normalization for GAPDH expression. Statistical analysis by t-test revealed significant increases in mRNA expression where *indicates P<0.05, **indicates P<0.01 and ***indicates P<0.001. (c) Cell lysates from the indicated NSCLC cell lines resistant to 3 μℳ gefitinib or 2 μℳ BIBW2292 (R) or DMSO-treated passage control (d) were immunoblotted for FGFR1, phospho-EGFR-Y1046, EGFR and the α subunit of the NaK-ATPase as a loading control. (d) The cell extracts from panel C were submitted to measurement of FGF2 protein by ELISA using a commercially available kit (R&D Systems, Minneapolis, MN, USA). The levels of FGF2 are presented as picograms per μg of cellular protein.
RNA-seq reveals FGF2 and FGFR1 as a novel pathway of acquired resistance to EGFR-specific TKIs
To explore novel mechanisms involved in EGFR-specific TKI resistance, mRNA from control and adapted HCC4006 and HCC827 cells was submitted to RNA-seq analysis by genome analyzer-based deep sequencing. The resulting analysis confirmed the marked increase in MET mRNA expression in gefitinib-resistant cultures of HCC827,13, 14, 15, 17 but not HCC4006 cells (Supplementary Table S3). However, RNA-seq analysis of the HCC4006 cells revealed 2.5- and 9-fold increases in the expression of FGF2 and FGFR1 mRNAs in gefitinib-resistant cells, respectively. Neither MET nor ErbB family members, EGFR and ErbB2, exhibited any changes in expression (Supplementary Table S2). A recent report identifies amplification of MAPK1 as a putative mechanism of resistance to EGFR TKIs.29 Our data indicate a modest increase (∼2-fold) in MAPK1 (ERK2) mRNA in gefitinib-resistant HCC4006 cells, although this was accompanied by a reduction in MAPK3 (ERK1) mRNA expression (Supplementary Table S3). Moreover, no significant increases in ERK2 protein or activity are apparent in our TKI-adapted cell lines (see Figure 2, Supplementary Figures S2 and S6). To test if induction of FGFR1 and FGF2 occurred in additional cell lines, the panel of control and gefitinib-resistant NSCLC cell lines was submitted to quantitative RT–PCR and immunoblot analyses for FGFR1 and FGF2 mRNA (Figures 1a and b) and protein levels (Figures 1c and d). TKI-resistant cultures of HCC4006, HCC2279, H1650 and H1975 cells, and to a lesser extent, HCC4011 cells, exhibited significantly increased levels of FGFR1 mRNA (Figure 1a) and protein (Figure 1c). Of these, adapted cultures of HCC4006, HCC2279, H1650 and HCC4011 cells also exhibited increased expression of FGF2 mRNA (Figure 1b) and protein (Figure 1d). Combined, the findings suggest that FGF2 and FGFR1 may comprise an acquired autocrine growth loop in gefitinib-resistant cultures of HCC4006, HCC2279, HCC4011 and H1650 cells. Although gefitinib-resistant H1975 cells exhibit increased FGFR1 levels, FGF2 mRNA was not detected.
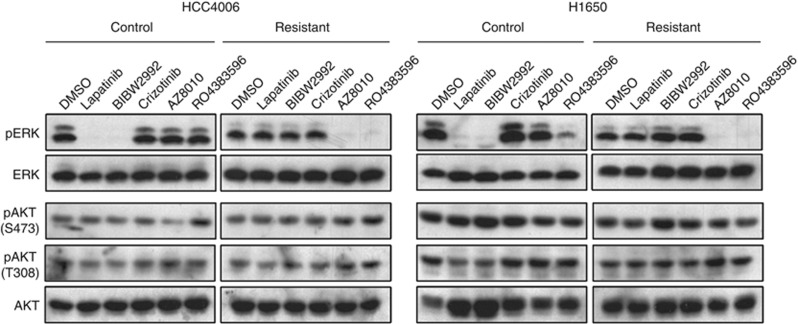
Figure 2.
Effect of TKIs on ERK and AKT phosphorylation in control and gefitinib-resistant HCC4006 and H1650 cells. (a, b) HCC4006 and H1650 control (DMSO) or gefitinib-resistant cells were treated for 2 h with inhibitors: Reversible EGFR inhibitor (lapatinib/gefitinib, 1 μℳ), irreversible EGFR inhibitor (BIBW2992, 0.1 μℳ), FGFR inhibitors (AZD8010, 0.3 μℳ, RO4383596, 1 μℳ) or Met inhibitor (crizotinib, 0.2 μℳ). Cell lysates were immunoblotted for phospho-ERK and phospho-AKT-T308 and phospho-AKT-S473 as indicated. The filters were stripped and reprobed for total ERK1/2 and AKT as loading controls. The phospho-AKT-T308 blot was submitted to densitometry and a graphical presentation is shown in Supplementary Figure S4.
Gefitinib-resistant HCC4006, HCC2279 and H1650 cells are FGFR1-dependent for growth and proliferation
To analyze the dominant growth pathways in gefitinib-resistant NSCLC cell cultures, control and gefitinib-resistant cultures of HCC4006, HCC2279 and H1650 were treated for 2 h with inhibitors of EGFR (lapatinib, gefitinib), EGFR-T790M (BIBW2992), FGFRs (AZ8010 and R0438359630, 31) or MET (crizotinib) and phospho-ERK and phospho-AKT were measured as downstream signaling targets. In the DMSO-adapted cell cultures, phospho-ERK and phospho-AKT-T308 were decreased or abolished following treatment with the EGFR-targeted TKIs, lapatinib, gefitinib and BIBW2992 (Figure 2, Supplementary Figures S4 and S6). In addition, phosphorylation of EGFR on Y1068 was also inhibited (Supplementary Figures S5 and S6). By contrast, these TKIs had no effect on phospho-ERK or phospho-AKT-T308 levels in the gefitinib-resistant cell lines (Figure 2, Supplementary Figure S6). Instead, phospho-ERK in the gefitinib-resistant cultures of HCC4006, HCC2279 and H1650 cells was reduced or abolished by the FGFR1-specific TKI, AZ8010, and the FGFR-active TKI, RO4383596 (Figure 2 and Supplementary Figure S4). Importantly, FGFR inhibitors failed to effect phosphorylation of either T308 or S473 on AKT, indicating that the induced FGFR1 pathway does not significantly engage the PI3K/AKT signal pathway. In addition, the FGFR inhibitor, AZ8010, did not inhibit ERK phosphorylation in gefitinib-resistant PC9 (Supplementary Figure S2) or HCC827 cells (Supplementary Figure S3), consistent with EGFR-T790M or MET functioning as the major drivers in these two cell lines.
Next, control and gefitinib-resistant HCC4006 cells were submitted to anchorage-independent growth assays and proliferation assays in the presence of lapatinib, BIBW2992 and AZ8010. Anchorage-independent growth of control HCC4006 cells was potently inhibited by lapatinib and BIBW2992 (Figure 3a, Supplementary Figure S1), but not by AZ8010. By contrast, gefitinib-resistant HCC4006 cells were highly sensitive to AZ8010, but the two EGFR inhibitors were without effect (Figure 3a, Supplementary Figure S1). The FGF trap, FP-1039,32 completely inhibited growth of gefitinib-resistant HCC4006 cells, but not control cells (Figure 3b), indicating a requirement for induced FGF2 for growth of the gefitinib-adapted cell cultures. As a molecular test of the requirement for FGFR1 in gefitinib-resistant HCC4006 cells, both control and gefitinib-resistant HCC4006 cells were transiently transfected with an FGFR1-targeting small interfering RNA (siRNA) or a non-silencing control siRNA. The data in Supplementary Figure S7 demonstrate effective silencing of FGFR1 mRNA, but not FGFR2 mRNA. Moreover, significant inhibition of cell proliferation was observed in response to transfection of the FGFR1 siRNA into gefitinib-resistant, but not control HCC4006 cells. Combined, the data indicate that HCC4006 cells switch from EGFR dependency to dependency on FGFR1 and FGF2 following acquired resistance to gefitinib.
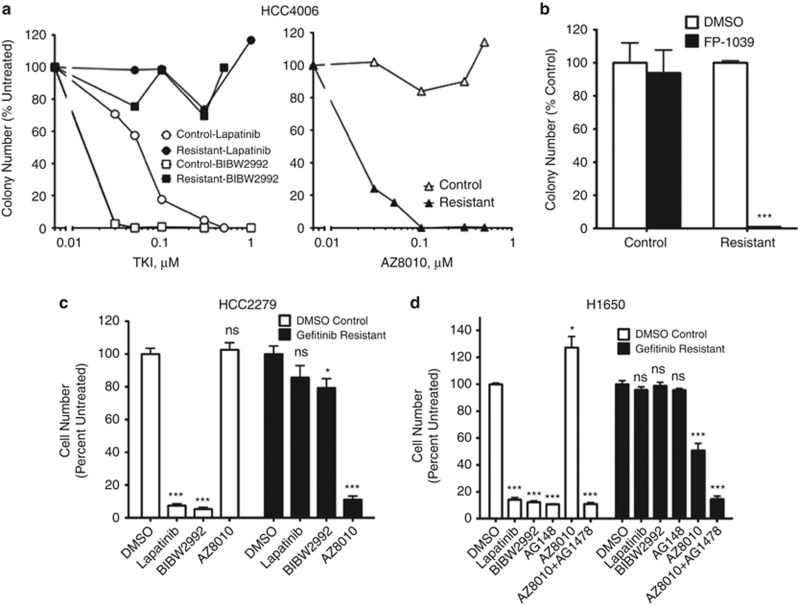
Figure 3.
Gefitinib-resistant HCC4006, H1650 and HCC2279 cell cultures are dependent on FGFR signaling for cell growth. (a) HCC4006 control and gefitinib-resistant cells were analyzed for anchorage-independent growth. Cells were treated with increasing concentrations of the EGFR reversible inhibitor, lapatinib (0, 0.03, 0.05, 0.1, 0.3, 1 μℳ), the irreversible EGFR inhibitor, BIBW2992 (0, 0.03, 0.05, 0.1, 0.3, 0.5 μℳ), and the FGFR inhibitor AZ8010 (0, 0.03, 0.05, 0.1, 0.3, 0.5 μℳ). (b) HCC4006 control and gefitinib-resistant cells were analyzed for anchorage-independent growth with 0.1 μg/ml FGF-ligand trap (FP-1039). (c) HCC2279 control (DMSO) and gefitinib-resistant cells were analyzed for cell proliferation by cell counts after 7 days of treatment with DMSO or inhibitors: (1 μℳ, lapatinib, 0.1 μℳ BIBW2992 or 0.3 μℳ AZ8010. D) H1650 control (DMSO) and gefitinib-resistant cells were analyzed for proliferation as above except with the addition of EGFR inhibitor AG1478 (0.1 μℳ). Statistical analysis by two-way ANOVA revealed significant decreases in cell growth where *indicates P<0.05, **indicates P<0.005 and ***indicates P<0.001.
We similarly tested the sensitivity of the panel of control and gefitinib-resistant cell lines to gefitinib/BIBW2992 and AZ8010. The findings in Figures 3c and d, Supplementary Figure S1 and Supplementary Table S2 demonstrate that HCC2279 and H1650 also undergo a switch in sensitivity from EGFR-specific TKIs to the FGFR-specific TKIs. Control HCC4011 cells were already somewhat sensitive to AZ8010 (IC50=84 nℳ) and gefitinib-resistant HCC4011 cells were only slightly more sensitive (IC50=33 nℳ), a finding that is consistent with detectable levels of FGFR1 and FGF2 in control HCC4011 cells (Figure 1). To test whether treatment with FGFR inhibitors induced measures of apoptosis, control and adapted HCC827, HCC4006 and H1650 cells were cultured with gefitinib or AZ8010 for 3 days and cells were collected for measurement of caspase 3 activity as a biochemical measure of apoptosis. The data in Supplementary Figure S8 demonstrate a marked increase (∼12-fold) in caspase 3 activity in DMSO control cultures of HCC827 cells treated with gefitinib, but only a 1.5-fold increase in caspase 3 activity in gefitinib-resistant HCC827 cells treated with gefitinib. Neither DMSO control cultures of H1650 nor HCC4006 cells exhibited evidence for induction of apoptosis following treatment with gefitinib. Gefitinib-resistant cultures of H1650 cells exhibited a modest (∼1.3-fold) increase in caspase 3 activity in response to AZ8010 treatment, but gefitinib-resistant cultures of HCC4006 cells showed no increase in caspase 3 activity following FGFR inhibitor treatment (Supplementary Figure S8). Thus, the findings are consistent with a cytostatic, not a proapoptotic, activity of FGFR inhibitors in gefitinib-adapted cell lines.
Evidence for cellular reprogramming in gefitinib-resistant NSCLC cells with induced FGFR1
In addition to upregulation of FGFR1 and FGF2, RNA-seq analysis of HCC4006 cells revealed decreased mRNA expression of the epithelial genes E-cadherin (CDH1) and junctional plakoglobin (JUP), as well as increased expression of the mesenchymal markers, vimentin, ZEB1, ZEB2 and N-cadherin (CDH2), in gefitinib-resistant cultures relative to control cells (Supplementary Table S3). This presumptive epithelial to mesenchymal transition (EMT) is not universal to the acquired resistance response as these changes were not observed in TKI-adapted HCC827 cells (Supplementary Table S3). However, reduced E-cadherin protein expression was observed in gefitinib/BIBW2992-resistant cultures of HCC4006, H1650 and H1975 cells (Figure 4a). HCC2279 cells lacked E-cadherin protein before adaptation. In addition, vimentin protein levels were increased in gefitinib-resistant cultures of HCC4006, HCC2279, H1650 and HCC4011 cells. These changes in EMT markers correlate with phenotypic differences in HCC4006 control and gefitinib-resistant cells grown in Matrigel containing medium, where gefitinib-resistant HCC4006 cells form unorganized structures in Matrigel relative to organized spheres produced by control HCC4006 cells (Figure 4b). Thus, the findings in Supplementary Table S3 and Figure 4 indicate that acquisition of gefitinib resistance in a subset of the EGFR-specific TKI-resistant cell lines is accompanied by an EMT program that includes induction of FGFR1 and FGF2 expression.
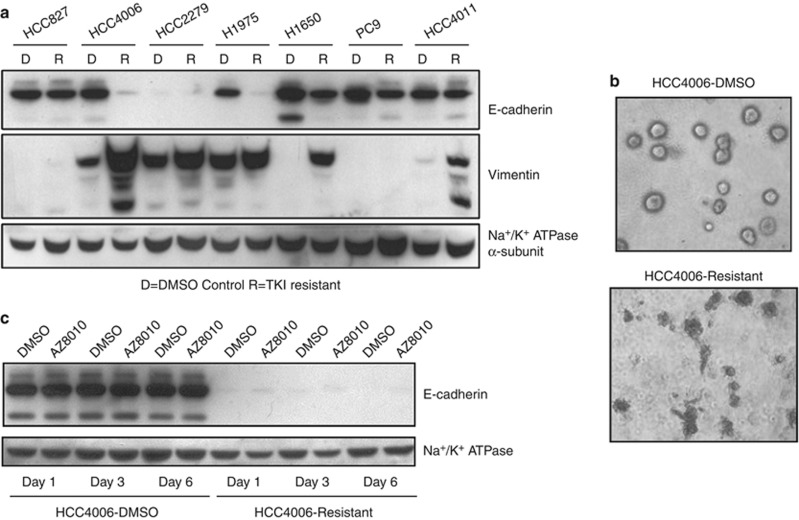
Figure 4.
Induction of EMT in EGFR TKI-resistant NSCLC cell lines is associated with induction of FGFR1. (a) Cell lysates from the indicated NSCLC cell lines resistant to 3 μℳ gefitinib or 2 μℳ BIBW2292 (R) or DMSO-treated passage control (d) were immunoblotted for E-cadherin, vimentin and the α subunit of the NaK-ATPase as a loading control. (b) HCC4006 DMS0 and gefitinib-resistant cells were plated in media containing 2% Matrigel and allowed to form colonies over 7 days. Colonies were imaged at × 100 magnification using a Nikon Eclipse TS100 camera. (c) HCC4006 control and gefitinib-resistant cells were treated 1, 3 or 6 days with DMSO or 0.3 μℳ AZ8010. Cell lysates were immunoblotted for E-cadherin and the α-subunit of NaK-ATPase as a loading control.
To test the role of FGFR1 signaling as the potential driver of the EMT program in these cells, HCC4006 control and gefitinib-resistant cell cultures were treated with AZ8010 or DMSO for 1, 3 or 6 days and E-cadherin protein expression was measured. As shown in Figure 4c, AZ8010 treatment did not restore E-cadherin expression in the gefitinib-adapted HCC4006 cultures, indicating that FGFR1 signaling is not directly regulating E-cadherin expression. In addition, H1975 cells rendered resistant to BIBW2992 also undergo an EMT response (Figure 4a and Sequist et al.12), but do not establish a functional FGFR1-driven autocrine loop. Therefore, FGFR1, FGF2, E-cadherin and vimentin are likely to be co-regulated in a transcriptional program mediating resistance and suggest potential value for identifying patients who might respond to FGFR-targeted therapies following progression on EGFR inhibitors. Further supporting our findings of co-regulated FGFR1 and mesenchymal genes, a study examining metastasis in NSCLC found FGFR1 expression to be a poor prognostic marker for metastasis (hazard ratio of >3).33 Another group found both FGFR1 and vimentin to positively correlate with ZEB1 expression in NSCLC,34 consistent with the gene changes reported in our study (Supplementary Table S3).
Finally, a prediction of the results presented thus far is that combined treatment of EGFR-driven NSCLC cell lines with gefitinib and an FGFR-specific TKI would yield superior growth inhibition. As shown in Supplementary Figure S9, a clonogenic growth assay shows greater reduction in the emergence of TKI-resistant HCC4006 cells following treatment with gefitinib plus AZD4547, an FGFR-specific TKI,35 relative to gefitinib alone. Likewise, inhibition of anchorage-independent growth of H1650 cells by gefitinib was markedly enhanced by inclusion of AZ8010. The data support the increased efficacy of combinations of EGFR and FGFR-specific TKIs for inhibiting growth of EGFR-mutant cell lines that employ FGFR1 as an acquired resistance mechanism.
Discussion
Acquired resistance has emerged as a major limitation of monotherapy with TKIs.22, 36 EGFR-targeted therapies can significantly improve disease control in EGFR-mutant NSCLC patients, but the response is short-lived. To this end, many studies defining mechanisms of resistance to EGFR-targeted therapies have been reported, and technological advances in genome-based medicine have led to the identification of distinct resistance mechanisms including acquisition and amplification of EGFR-T790M mutations, selection for PIK3CA mutations, and MET and MAPK1 amplification.12, 29, 37 However, ∼30% of acquired resistance cases lack identifiable mutations12 and thus, may rely on mutation-independent mechanisms of resistance. In support of this hypothesis, we used a previously validated approach involving chronic adaption of NSCLC cell lines to increasing concentrations of gefitinib12, 14, 15 and identified a subgroup of EGFR-mutant NSCLC cell lines that undergo cellular reprogramming accompanied by induction of an FGFR1-FGF2 autocrine signaling pathway. FGFR signaling is sufficient to drive transformed growth as evidenced by the findings that gefitinib-resistant cells are growth arrested by FGFR-specific TKIs, an FGF-ligand trap and RNAi-mediated FGFR1 silencing. Notably, the failure of FGFR-specific TKIs to markedly increase apoptosis in lung cancer cell lines bearing an induced FGF2-FGFR1 pathway suggests that FGFR inhibitors may yield stable disease, not tumor regressions, if these drugs are deployed to target this mechanism of resistance to EGFR-specific TKIs.
Using a chronic resistance model, we demonstrate that co-induction of FGFR1 and FGF2 occur in concert with dramatic cellular reprogramming leading to multiple EMT-associated gene changes (Figure 4 and Supplementary Table S3). This finding is in support of previous observations that an EMT phenotype is associated with both intrinsic and acquired resistance to EGFR-specific TKIs in NSCLC cell lines (including H1975, HCC4006 and H1650).12, 21, 38, 39, 40 Moreover, we have significantly expanded these observations by demonstrating that FGFR1 and FGF2 function as the ‘driver' of growth, but not EMT, in these adapted cell lines. Thus, these findings provide an example distinct from MET of how an alternative receptor tyrosine kinase can serve as a bypass growth pathway in NSCLC cell lines rendered resistant to EGFR-specific TKIs. Similar to our findings demonstrating induction of FGFR1 in gefitinib-resistant HCC4006, HCC2279 and H1650, AXL has also been shown to be induced in gefitinib-resistant HCC827 cells and in human tumors that have progressed on erlotinib.19 Although we observe induction of MET, but not AXL, in our cultures of gefitinib-resistant HCC827 cells (Supplementary Figure S10), we detect induction of AXL mRNA in HCC4006, HCC2279, H1650, PC9 and H1975 cells. Except for PC9, induction of AXL mRNA following acquired resistance to EGFR-specific TKIs is associated with an EMT program. Importantly, Byers et al.18 have demonstrated that AXL induction is also associated with an acquired resistance-induced EMT program. Despite the induction of AXL simultaneous with FGFR1, our data support FGFR1 as the dominant bypass growth driver in HCC4006, HCC2279 and H1650 cells based on sensitivity to FGFR inhibitors and the lack of growth and signaling inhibition by crizotinib (Figure 2, Supplementary Figures S2 and S6), a TKI which exhibits activity against AXL.41, 42 Clearly, elucidation of the underlying molecular pathways and their prevalence in human lung tumor biopsies post progression with EGFR-specific TKIs will be critical for a full understanding of acquired resistance. Evidence of cellular reprogramming as a mechanism to relieve EGFR dependency during EGFR-targeted therapy has been observed in clinical samples.12 In fact, it may be a common means to escape multiple types of treatment. For example, analysis of multiple serial biopsies of one patient demonstrated transdifferentiation from EGFR-dependent NSCLC to small-cell lung cancer following erlotinib therapy and back to EGFR-dependent NSCLC following treatment with a cytotoxic therapy appropriate for small-cell lung cancer.12 The tumor re-sensitized to EGFR-targeted therapy only after treatment with chemotherapy and radiation for small-cell lung cancer revealing the ability of cancer cells to reprogram as a mechanism for cell survival. The EGFR mutation was identified in all biopsies indicating the tumors arose from a common clone.12
In support of epigenetic alterations as common mechanisms of resistance, Sharma et al.26 demonstrated that drug resistant persister cells readily arise in vitro following treatment, regardless of the oncogene being targeted. Importantly, an HDAC inhibitor reduced the outgrowth of drug resistant persister cells, presumably through disrupting the necessary chromatin remodeling-dependent gene expression changes. The EMT phenotype observed upon acquired resistance to EGFR-targeted therapy appears to be exclusively observed in the subset lacking identified mutations.12, 39 Therefore, HCC4006, H1650 and HCC2279 cells could be potentially used to develop an expression signature predictive of tumors that undergo epigenetic reprogramming as a mechanism of resistance and as a biomarker for patients that would benefit from EGFR–FGFR combination therapies.
Although there is increasing support that epigenetic mechanisms of resistance are prevalent in response to targeted therapies, it is well known that other cancer cells are predisposed to pathways involving genetically acquired resistance mechanisms (mutation and amplifications). In fact, germline mutations not specific to tumor cells that occur in the host can be important in predicting response. A germline deletion of the proapoptotic protein BIM poises tumor cells for survival when confronted by the relevant TKI in CML and NSCLC, thus serving a crucial role in how a tumor responds to treatment.43 Therefore, a more thorough understanding of a patient tumor's mutation status (germline and somatic) and epigenetic volatility will be relevant in designing treatment regimens to prevent resistance to targeted therapies. Although FGFR1 and FGF2 induction as a mechanism of resistance has not yet been verified in clinical samples from matched pre- and post-treatment biopsies, in vitro studies with NSCLC cell lines thus far have been consistent with established in vivo mechanisms of resistance.15, 44, 45 A phase I clinical trial (NCT01515969) combining erlotinib and the FGFR TKI, dovitinib, in metastatic NSCLC will give retrospective information on patients who respond to EGFR–FGFR combination treatment. The molecular heterogeneity of oncogene drivers in NSCLC, which demands flexibility in treatment regimens, will likely be recapitulated in resistance mechanisms to targeted therapies. Therefore, rational treatment regimens entailing combinations of TKIs must be founded on extensive molecular understanding of the tumors. Finally, the history of therapeutics for infectious diseases such as HIV and TB demonstrates that long-term disease management occurs by preventing resistance rather than treating resistance.22 Consistent with the advantages of treating with combinations of inhibitors at onset, clinical benefit from treating lung cancer patients that progress on erlotinib with irreversible EGFR inhibitors or MET inhibitors has been rather modest.46, 47 Based on the findings in this study and the increased efficacy of combined EGFR and FGFR inhibitors on specific NSCLC cell lines (Supplementary Figure S8), if supported by future demonstration of increased FGF2 and FGFR1 in lung tumor biopsies following progression on EGFR inhibitors, we suggest that treatment with combinations of EGFR and FGFR inhibitors may be superior to sequential treatment with an FGFR inhibitor.
Materials and methods
Cell culture
H1650, H1975, HCC827, HCC2279, HCC4006 and PC9 cells were obtained from the University of Colorado Cancer Center Tissue Culture Core and HCC4011 and HCC2935 cells were purchased from ATCC (Manassas, VA, USA). The passage controls and TKI-adapted cell lines used in this study were submitted to fingerprint analysis to verify their authenticity relative to established sources and to demonstrate that the control and TKI-resistant cultures were genetically equivalent. All cell lines were routinely cultured in RPMI-1640 growth medium supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, USA) at 37 °C in a humidified 5% CO2 incubator. Resistant cell population were cultured with the addition of 3 μℳ gefitinib or 2 μℳ BIBW2992.
In vitro adaptation of gefitinib-resistant cell lines
Cells were serially passaged initially with low concentrations of gefitinib or BIBW2992 (5 nℳ) and sequentially cultured in increasing concentrations of these TKIs. Cells were considered resistant when they could be routinely cultured in growth medium containing 3 μℳ gefitinib or 2 μℳ BIBW2992.
Three-dimensional matrigel growth
For three-dimensional growth experiments, 5000 control and gefitinib-resistant HCC4006 cells were suspended in RPMI-1640 media containing 2% Matrigel and overlaid on a base layer of 50% Matrigel containing RPMI-1640 media in 8-well Tissue Tek II chamber slides. Wells were covered with 100 μl growth media and allowed to form colonies over a week. Colonies were imaged at × 100 magnification using a Nikon Eclipse TS100 camera.
RNA-seq analysis
Total RNA purified from two-independent cultures of DMSO passaged control and gefitinib-resistant cultures of HCC4006 cells and HCC827 cells was used to prepare cDNA for sequencing with the Illumina Genome Analyzer IIx using the Illumina mRNA sequencing preparation kit (San Diego, CA, USA). Sequenced reads were mapped against hg19 using Tophat and transcripts were assembled against ensemble reference using Cufflinks. Expression is reported as fragments per kilobase transcript per million reads (FPKM). Data were submitted to applied bias correction and quartile normalization.
Quantitative Real-Time PCR (RT–PCR)
Total RNA (5 μg) was reverse transcribed in a volume of 20 μl using Maxima First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD, USA). Aliquots (1 μl) of 5-fold diluted reverse transcription reactions were subjected to PCR in 25 μl reactions with SYBR green Jumpstart Taq Readymix (Sigma) and primers previously described for FGFR1 and FGF224 using a My iQ real-time PCR detection system (BioRad, Hercules, CA, USA). AXL mRNA was measured by quantitative RT–PCR using a TaqMan Gene Expression Assay primer/probe set (no.HS01064444) from Applied Biosystems (Carlsbad, CA, USA). GAPDH mRNA levels were measured by quantitative RT–PCR in replicate samples as a housekeeper gene for normalization and the data are presented as ‘Relative Expression'.
Anchorage-independent growth and cell proliferation assays
For measurement of anchorage-independent cell growth, 50 000 cells (HCC4006, control or gefitinib resistant) were suspended in 1.5 ml RPMI 1640 containing 10% fetal bovine serum and 0.35% Difco agar noble (Becton Dickinson and Co., Sparks, MD, USA) and overlaid on base layers containing 1.5 ml RPMI 1640 containing 10% fetal bovine serum and 0.5% agar noble in six-well plates. The wells were covered with 2 ml growth medium containing drugs. This media was replaced with fresh media containing drugs every 7 days. The plates were incubated in a 37 °C CO2 incubator for 21 days after which viable colonies were stained for 24 h with 200 μl of 1 mg/ml nitroblue tetrazolium. Following digital photography, the colony number was quantified using the MetaMorph imaging software program.
For measurement of proliferation, 10 000 control or gefitinib-resistant cells were plated in 500 μl RPMI 1640 containing 10% fetal bovine serum in 24-well plates or 20 000 HCC2279 control or gefitinib-resistant cells were plated in 1 ml RPMI 1640 containing 10% fetal bovine serum in 12-well plates. Cells were allowed to attach overnight, then treated with the indicated drug treatments and cultured over 7 days. Following trypsinization, viable cells were counted with a Cellometer (Nexcelom, Lawrence, MA, USA) and compared with untreated cell number for each cell line and presented as percent control. In some experiments (Supplementary Figure S1), cell proliferation was determined using the CyQUANT assay. DMSO and resistant cells were seeded at 100 cells per well in 96-well tissue culture plates and treated in triplicate with inhibitors at various doses for 7 days. Relative cell numbers were measured using a CyQUANT Direct Cell Proliferation Assay (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions.
RNAi-mediated silencing of FGFR1
Control and gefitinib-resistant HCC4006 cells were plated at 100 000 cells per 35 mm dish. The next day, the cells were transfected (16 h) with 12 nℳ of an FGFR1-targeting siRNA (5′-phos TGGTATTAACTCCAGCAGTCTTCAAGA-3′) or a non-silencing control siRNA using HiPerfect reagent from Qiagen (Valencia, CA, USA). The cells were fed with fresh growth medium and total RNA was prepared following 3 days of culture and FGFR1 mRNA was measured by quantitative RT–PCR. Replicate dishes were further cultured for a total of 7 days and cell number was determined by direct cell counting.
Immunoblot analyses
For analysis of specific phosphorylation status of signaling proteins in response to drug treatment, cells were seeded in six-well dishes to allow cell attachment. After 24 h, cells were treated with DMSO, 1 μℳ lapatinib or gefitinib, 0.1 μℳ BIBW2992, 0.3 μℳ AZ8010, 1 μℳ RO4383596 or 0.2 μℳ crizotinib for 2 h. Cells were collected in 1 ml phosphate-buffered saline, centrifuged at 1000 g for 5 min, lysed in MAP kinase lysis buffer (MKLB; 0.5% Triton X-100, 50 mℳ β-glycerophosphate (pH 7.2), 0.1 mℳ Na3VO4, 2 mℳ MgCl2, 1 mℳ EGTA, 1 mℳ DTT, 0.3 ℳ NaCl, 2 μg/ml leupeptin and 4 μg/ml aprotinin) and centrifuged (5 min at 13 000 r.p.m.). The particulate fractions were discarded, aliquots of the extracts were mixed with SDS sample buffer and submitted to SDS–PAGE. Following electrophoretic transfer onto nitrocellulose, the filters were blocked in 3% bovine serum albumin (Cohn Fraction V, ICN Biomedicals, Inc., Aurora, OH, USA) in Tris-buffered saline with 0.1% Tween 20 (TTBS) and then incubated with antibodies to phospho-ERK, phospho-AKT-T308, phospho-AKT-S473, phospho-EGFR-Y1068 or phospho-MET-Y1234/1235 (Cell Signaling Technology, Inc.; Danvers, MA, USA) for 16 h at 4 °C. The filters were washed thoroughly in TTBS, then incubated with alkaline phosphatase coupled goat anti-rabbit or mouse antibodies and developed with LumiPhos reagent (Pierce, Rockford, IL, USA) according to the manufacturer's instructions. The filters were subsequently stripped and reprobed for total ERK1 and ERK2, AKT, EGFR or MET. For immunoblot analysis of FGFR1, the α subunit of the NaK-ATPase, vimentin and E-cadherin, NSCLC cells were collected in phosphate-buffered saline, centrifuged (5 min, 1000 g) and suspended in MKLB. Aliquots of the cell lysate preparations containing 150 μg of protein were submitted to SDS–PAGE and immunoblotted for FGFR1 (Origene, Rockville, MD, USA no.TA301021), α-subunit NaK-ATPase (Santa Cruz Biotechnology, Dallas, TX, USA), vimentin (Sigma, no.V6630), or E-cadherin (BD Biosciences, San Jose, CA, USA, no.610182).
Acknowledgments
This research was supported by NIH grants P50 CA58187 and R01 CA127105. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. DNA sequencing and cell line fingerprinting were performed through the University of Colorado Cancer Center Molecular Pathology shared resource facility. The completion of the deep sequencing for the RNA-seq analysis was performed by the University of Colorado Next-Generation Sequencing Development Core. Investigational drugs were provided through materials transfer agreements with AstraZeneca and Five Prime Therapeutics.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- American Cancer Society . Cancer Facts & Figures. American Cancer Society, Atlanta, GA, USA; 2012. [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA, Di Maria MV, Veve R, Bremmes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Ann Rev Pathol. 2011;6:49–69. doi: 10.1146/annurev-pathol-011110-130206. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Janne PA. Mechanisms of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2008;14:2895–2899. doi: 10.1158/1078-0432.CCR-07-2248. [DOI] [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- Camp ER, Summy J, Bauer TW, Liu W, Gallick GE, Ellis LM. Molecular mechanisms of resistance to therapies targeting the epidermal growth factor receptor. Clin Cancer Res. 2005;11:397–405. [PubMed] [Google Scholar]
- Riely GJ, Politi KA, Miller VA, Pao W. Update on epidermal growth factor receptor mutations in non-small cell lung cancer. Clin Cancer Res. 2006;12:7232–7241. doi: 10.1158/1078-0432.CCR-06-0658. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Lynch TJ. EGFR tyrosine kinase inhibitors in lung cancer: an evolving story. Annu Rev Med. 2008;59:429–442. doi: 10.1146/annurev.med.59.090506.202405. [DOI] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino A, Kitao H, Hirano S, Uchida A, Ishiai M, Kozuki T, et al. Emergence of epidermal growth factor receptor T790M mutation during chronic exposure to gefitinib in a non small cell lung cancer cell line. Cancer Res. 2007;67:7807–7814. doi: 10.1158/0008-5472.CAN-07-0681. [DOI] [PubMed] [Google Scholar]
- Bean J, Brennan C, Shih JY, Riely G, Viale A, Wang L, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- Su KY, Chen HY, Li KC, Kuo ML, Yang JC, Chan WK, et al. Pretreatment epidermal growth factor receptor (EGFR) T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-small-cell lung cancer. J Clin Oncol. 2012;30:433–440. doi: 10.1200/JCO.2011.38.3224. [DOI] [PubMed] [Google Scholar]
- Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition (EMT) gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2012;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgillo F, Kim WY, Kim ES, Ciardiello F, Hong WK, Lee HY. Implication of the insulin-like growth factor-IR pathway in the resistance of non-small cell lung cancer cells to treatment with gefitinib. Clin Cancer Res. 2007;13:2795–2803. doi: 10.1158/1078-0432.CCR-06-2077. [DOI] [PubMed] [Google Scholar]
- Thomson S, Petti F, Sujka-Kwok I, Epstein D, Haley JD. Kinase switching in mesenchymal-like non-small cell lung cancer lines contributes to EGFR inhibitor resistance through pathway redundancy. Clin Exp Metastasis. 2008;25:843–854. doi: 10.1007/s10585-008-9200-4. [DOI] [PubMed] [Google Scholar]
- Glickman MS, Sawyers CL. Converting cancer therapies into cures: lessons from infectious diseases. Cell. 2012;148:1089–1098. doi: 10.1016/j.cell.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono SA, Marshall ME, Ware KE, Heasley LE. The fibroblast growth factor receptor signaling pathway as a mediator of intrinsic resistance to EGFR-specific tyrosine kinase inhibitors in non-small cell lung cancer. Drug Resist Updat. 2009;12:95–102. doi: 10.1016/j.drup.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE, et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol. 2009;75:196–207. doi: 10.1124/mol.108.049544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118:2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, et al. Rapidly acquired resistance to egfr tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One. 2010;5:e14117. doi: 10.1371/journal.pone.0014117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, et al. Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Shao L, Yu W, Gavine P, Ittmann M. Targeting fibroblast growth factor receptor signaling inhibits prostate cancer progression. Clin Cancer Res. 2012;18:3880–3888. doi: 10.1158/1078-0432.CCR-11-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott LA, Simcox M, Higgins B, Nevins T, Kolinsky K, Smith M, et al. RO4383596, an orally active KDR, FGFR, and PDGFR inhibitor: synthesis and biological evaluation. Bioorg Med Chem. 2005;13:4835–4841. doi: 10.1016/j.bmc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Marshall ME, Hinz TK, Kono SA, Singleton KR, Bichon B, Ware KE, et al. Fibroblast growth factor receptors are components of autocrine signaling networks in head and neck squamous cell carcinoma cells. Clin Cancer Res. 2011;17:5016–5025. doi: 10.1158/1078-0432.CCR-11-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Tidow C, Diederichs S, Bulk E, Pohle T, Steffen B, Schwable J, et al. Identification of metastasis-associated receptor tyrosine kinases in non-small cell lung cancer. Cancer Res. 2005;65:1778–1782. doi: 10.1158/0008-5472.CAN-04-3388. [DOI] [PubMed] [Google Scholar]
- Gemmill RM, Roche J, Potiron VA, Nasarre P, Mitas M, Coldren CD, et al. ZEB1-responsive genes in non-small cell lung cancer. Cancer lett. 2011;300:66–78. doi: 10.1016/j.canlet.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavine PR, Mooney L, Kilgour E, Thomas AP, Al-Kadhimi K, Beck S, et al. AZD4547: an orally bioavailable, potent and selective inhibitor of the fibroblast growth factor receptor tyrosine kinase family. Cancer Res. 2012;72:2045–2056. doi: 10.1158/0008-5472.CAN-11-3034. [DOI] [PubMed] [Google Scholar]
- Dannenberg JH, Berns A. Drugging drug resistance. Cell. 2010;141:18–20. doi: 10.1016/j.cell.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Janne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- Frederick BA, Helfrich BA, Coldren CD, Zheng D, Chan D, Bunn PA, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non-small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683–1691. doi: 10.1158/1535-7163.MCT-07-0138. [DOI] [PubMed] [Google Scholar]
- Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, et al. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fenoglio S, Gao DC, Camiolo M, Stiles B, Lindsted T, et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc Natl Acad Sci USA. 2010;107:15535–15540. doi: 10.1073/pnas.1009472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui JJ, Tran-Dube M, Shen H, Nambu M, Kung PP, Pairish M, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK) J Med Chem. 2011;54:6342–6363. doi: 10.1021/jm2007613. [DOI] [PubMed] [Google Scholar]
- Mollard A, Warner SL, Call LT, Wade ML, Bearss JJ, Verma A, et al. Design, Synthesis and Biological Evaluation of a Series of Novel Axl Kinase Inhibitors. ACS Med Chem Lett. 2011;2:907–912. doi: 10.1021/ml200198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sawyers CL. In cancer drug resistance, germline matters too. Nat Med. 2012;18:494–496. doi: 10.1038/nm.2725. [DOI] [PubMed] [Google Scholar]
- Ercan D, Zejnullahu K, Yonesaka K, Xiao Y, Capelletti M, Rogers A, et al. Amplification of EGFR T790M causes resistance to an irreversible EGFR inhibitor. Oncogene. 2010;29:2346–2356. doi: 10.1038/onc.2009.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25:587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- Ayoola A, Barochia A, Belani K, Belani CP. Primary and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small cell lung cancer: an update. Cancer Invest. 2012;30:433–446. doi: 10.3109/07357907.2012.666691. [DOI] [PubMed] [Google Scholar]
- Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–1622. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.