Abstract
Neurite outgrowth, a cell differentiation process involving membrane morphological changes, is critical for neuronal network and development. The membrane lipid, phosphatidylinositol (PI) 4,5-bisphosphate (PIP2), is a key regulator of many important cell surface events of membrane signaling, trafficking and dynamics. This lipid is produced mainly by the type I PI 4-phosphate 5-kinase (PIP5K) family members. In this study, we addressed whether PIP5Kα, an isoform of PIP5K, could have a role in neurite outgrowth induced by nerve growth factor (NGF). For this purpose, we knocked down PIP5Kα in PC12 rat pheochromocytoma cells by stable expression of PIP5Kα microRNA that significantly reduced PIP5Kα expression and PIP2 level. Interestingly, NGF-induced neurite outgrowth was more prominent in PIP5Kα-knockdown (KD) cells than in control cells. Conversely, add-back of PIP5Kα into PIP5Kα KD cells abrogated the effect of NGF on neurite outgrowth. NGF treatment activated PI 3-kinase (PI3K)/Akt pathway, which seemed to be associated with reactive oxygen species generation. Similar to the changes in neurite outgrowth, the PI3K/Akt activation by NGF was potentiated by PIP5Kα KD, but was attenuated by the reintroduction of PIP5Kα. Moreover, exogenously applied PIP2 to PIP5Kα KD cells also suppressed Akt activation by NGF. Together, our results suggest that PIP5Kα acts as a negative regulator of NGF-induced neurite outgrowth by inhibiting PI3K/Akt signaling pathway in PC12 cells.
Keywords: Akt, nerve growth factor, neurite outgrowth, PI3K, phosphatidylinositol 4, 1-phosphatidylinositol-4-phosphate 5-kinase, 5-bisphosphate
Introduction
Neurite outgrowth is a cellular process involved in neuronal migration, differentiation and plasticity.1 Neurite outgrowth is propagated through multiple steps of membrane remodeling such as formations of membranes protrusion and lammelipodia.2 These membrane structures are supported by actin cytoskeletal rearrangements. The Rho family of small guanosine triphsopahatases, RhoA, Rac1 and Cdc42 that have critical roles in actin polymerization, function as primary regulators of neurite outgrowth.3, 4 In addition, a number of studies have demonstrated that multiple signaling events, including phosphatidylinositol (PI) 3-kinase (PI3K) and its downstream effector Akt, MAPK and reactive oxygen species (ROS) generation, participate in the mediation of neurite outgrowth.5, 6, 7, 8, 9
Nerve growth factor (NGF) is a neurotrophin crucial for neuronal growth and survival. NGF is also a potent inducer of neurite outgrowth.1, 10 NGF binds to the tyrosine kinase receptor TrkA, triggering activation of various signaling pathways including PI3K/Akt, phospholipase C and Ras/Raf/MAPK cascades.8, 10, 11, 12, 13 PC12 cells derived from pheochromocytoma of the rat adrenal medulla have been widely used as a model system for studies of NGF-induced neurite outgrowth. Following NGF treatment, these cells stop dividing and show terminally differentiated neuronal phenotype.
PI 4,5-bisphosphate (PIP2), a membrane lipid enriched in the plasma membrane, is generated mainly by the type I PI 4-phosphate 5-kinase (PIP5K) family members comprising three isoforms, PIP5Kα, PIP5Kβ and PIP5Kγ.14, 15 PIP2 is a key regulator of membrane signaling and trafficking, and actin cytoskeletal reorganization.14, 16, 17 It was previously shown that overexpression of PIP5Kβ (in this study, the previous mouse and rat PIP5Kβ is referred to as PIP5Kα, and vice versa, according to the revised nomenclature in the current GenBank database17) in mouse N1E-115 neuroblastoma cells induced neurite retraction and cell rounding, while overexpression of its catalytically inactive mutant promoted neurite extension.18, 19 The signaling pathway of RhoA and its downstream effector p160 Rho-associated coiled-coil-forming protein kinase (ROCK) is known to mediate neurite retraction. RhoA/ROCK functioned upstream of PIP5Kβ in the PIP5Kβ-induced neurite retraction.18, 19
However, a functional role of PIP5K and PIP2 in NGF-dependent neurite growth remains unaddressed. In this study, we aimed to determine whether PIP5Kα, another isoform of PIP5K, has a regulatory role in neurite outgrowth elicited by NGF. Here, we present evidence that PIP5Kα acts to inhibit NGF-induced neurite outgrowth by negatively regulating PI3K/Akt signaling pathway in a PIP2-dependent manner.
Materials and methods
Materials
Most research chemicals, including Dulbecco's modified Eagle's medium, blasticidin, N-acetyl-ℒ-cysteine and paraformaldehyde, were purchased from Sigma-Aldrich (St Louis, MO, USA). Fetal bovine serum and penicillin/streptomycin were obtained from Hyclone (Logan, UT, USA). NGF (murine 2.5S) was purchased from Promega (Wisconsin, MI, UAS). LY294002 was from Biomol (Plymouth Meeting, PA, USA). Goat polyclonal antibodies to PIP5Kα and β-actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibodies to Akt and phospho-Akt (Ser473), and mouse mAb to Myc were obtained from Cell Signaling Technology (Beverly, MA, USA). Lipofectamine 2000, Opti-MEM I, dihydroethidium (DHE) and horse serum were from Invitrogen (Carlsbad, CA, USA). PC12 rat pheochromocytoma cell line was a gift from Haeyoung Suh-Kim (Ajou University). Expression plasmids of Myc–PIP5Kα and monomeric red fluorescence protein (mRFP)–PIP5Kα were described previously.20
Cell culture and treatment
PC12 rat pheochromocytoma cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 5% horse serum and penicillin/streptomycin at 37 °C in a humidified atmosphere of 5% CO2 in air. Cells were serum starved overnight and then treated with 100 ng ml−1 NGF. In case of experiments with LY294002 or N-acetyl-ℒ-cysteine, cells were pretreated with the chemicals before NGF treatment.
Stable knockdown (KD) of PIP5Kα
A PC12 cell line stably expressing PIP5Kα microRNA (miR) was generated using BLOCK-iT Pol II miR RNAi Expression Vector Kits (Invitrogen) according to the manufacturer's instructions. Synthesized oligonucleotides (Genotech, Daejeon, Korea) harboring the rat PIP5Kα sequence (NM_001042621, 546 amino acids), 5′-TGCTGAACAGGTGAACCCTCACTTATGTTTTGGCCACTGACTGACATAAGTGAGTTCACCTGTT-3′ (top strand) and 5′-CCTGAACAGGTGAACTCACTTATGTCAGTCAGTGGCCAAAACATAAGTGAGGGTTCACCTGTTC-3′ (bottom strand), were cloned into the pcDNA 6.2-GW/Emerald green fluorescent protein (EmGFP)-miR expression vector. PC12 cells were transfected with the PIP5Kα miR expression plasmid by Amaxa Nucleofection using a Cell Line Nucleofector Kit V (Amaxa Biosystems, Cologne, Germany) following the manufacturer's protocol and cultured in growth medium supplemented with 5 μg ml−1 blasticidin. Blasticidin-resistant cells expressing EmGFP, visualized by Axiovert 200M inverted microscope (Carl Zeiss Microimaging, Göttingen, Germany), were selected for 3 weeks. As a negative control, a pcDNA 6.2-GW/EmGFP-miR-neg control plasmid, supplied by the manufacturer, was stably expressed in the same manner.
Western blot analysis
Cell lysates (30–40 μg) prepared in a lysis buffer (50 mℳ Tris pH 7.4, 150 mℳ NaCl, 1 mℳ EDTA, 1 mℳ EGTA, 1 mℳ dithiothreitol, 1 mℳ Na3VO4, 5 mℳ NaF and 1% Triton X-100) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Following blocking with 5% nonfat milk solution, membrane blots were probed with primary antibodies against PIP5Kα, Akt, phospho-Akt (Ser473), Myc tag or β-actin and then with horseradish peroxidase-conjugated secondary antibodies (Zymed Laboratories, San Francisco, CA, USA). The immune complexes were visualized using enhanced chemiluminescence detection system (Santa Cruz Biotechnology).
Neurite outgrowth
PC12 cells were seeded into 6-well plates at a low density and then serum starved overnight. After NGF treatment under the indicated time periods, bright field images were acquired using an Axiovert 200M inverted microscope for the detection of neurites. Neurite length was determined by image analysis using AxioVision LE software (Carl Zeiss Microimaging).
PIP5Kα transfection
Myc–PIP5Kα, mRFP–PIP5Kα or corresponding empty vectors were mixed with Lipofectamine 2000 in Opti-MEM I according to the supplier's protocol, and added to the PIP5Kα KD cells. Twenty-four hours post transfection, Myc–PIP5Kα and mRFP–PIP5Kα transfection samples were processed for western blot analysis and cell imaging, respectively. For imaging of mRFP–PIP5Kα, cells were washed twice with filtered phosphate-buffered saline and fixed with 4% paraformaldehyde for 15 min at ambient temperature. mRFP fluorescence was visualized using an Axiovert 200M inverted microscope.
PIP2 immunostaining
PIP2 immunocytochemistry was performed as described previously.20 Briefly, cells were fixed with 4% paraformaldehyde and permeabilized with 0.5% saponin for 15 min each at room temperature. After blocking with 10% goat serum in theta burst stimulation for 30 min at 37 °C, the cells were sequentially incubated with anti-PIP2 mouse IgM mAb (Echelon Biosciences, Salt Lake City, UT, USA), biotinylated goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Alexa Fluor 594-conjugated streptavidin (Invitrogen). Each immunostaining step was performed for 1 h at 37 °C and followed by washing with theta burst stimulation containing 1% goat serum. Cells were visualized using an Axiovert 200M inverted microscope
Detection of intracellular ROS
Cells were preincubated with DHE, a ROS-specific fluorescent dye at 37 °C. After washing three times with phosphate-buffered saline, fresh growth media containing NGF were added to the cells. Then, the DHE fluorescent images were immediately captured using Axiovert 200M inverted microscope.
Intracellular delivery of PIP2
Intracellular delivery of PIP2 was performed as described previously.20 In brief, dioctanoyl–PIP2, a water-soluble analog of PIP2, was mixed with histone as a shuttle PIP carrier (Echelon Biosciences) at a 1:1 molar ratio for 20 min at room temperature. The mixture was diluted with serum-free medium and added to the serum-starved PIP5Kα KD cells for 1 h before NGF treatment.
Reverse transcription–PCR
Complementary DNA was synthesized from isolated total RNA according to the previously described procedures.20 The sense and antisense primers of rat PIP5Kα and glyceraldehyde-3-phosphate dehydrogenase21 were used for PCR amplification. The following specific oligonucleotide primers (Bioneer, Daejeon, Korea) were used: 5′-GTGAAGGGAGCAATCTGACCC-3′ (sense) and 5′-CGGCAGCAC GTTGTTCATCAC-3′ (antisense) for rat PIP5Kβ 5′-GTACTCACTCTGCAACGAGCC-3′ (sense) and 5′-GTCAGAGTCCAGTAGCAGCC-3′ (antisense) for rat PIP5Kγ. Amplified PCR products were separated by electrophoresis on 1.5% agarose gels and detected under ultraviolet light. Gel images were obtained using the Gel Doc molecular imaging system (Bio-Rad Laboratories, Hercules, CA, USA).
Density quantification and statistical analysis
The band intensities of western blots and PCR products were measured using NIH ImageJ software (NIH, Bethesda, MD, USA). Statistical significance of data shown in the graphs was determined using an unpaired two-tailed t-test (Graphpad Software, San Diego, CA, USA) and data are presented as mean±s.e.m.
Results
PIP5Kα KD promotes NGF-induced neurite outgrowth
To examine a role for PIP5Kα in NGF-induced neurite outgrowth, we first developed PIP5Kα-KD PC12 cell lines using miR expression system. Complementary oligonucleotides corresponding to the PIP5Kα target sequence (1844–1864 bp) were cloned into a pcDNA 6.2-GW/EmGFP-miR expression vector that contains EmGFP, a variant of enhanced GFP, as a reporter. As a negative control, a pcDNA 6.2-GW/EmGFP-miR-neg control plasmid harboring complementary oligonucleotides that do not target mammalian genes was also used. PC12 cells transfected with the PIP5Kα miR- or negative control miR-expressing plasmids were selected by treatment with blasticidin. Stable expression of both plasmids was confirmed by the presence of EmGFP fluorescence (Figure 1a). PIP5Kα miR significantly reduced of protein PIP5Kα mRNA (Figures 1b and d) and protein (Figures 1c and d) expression levels compared with the control miR, as demonstrated by reverse transcription–PCR and western blot analysis, respectively. PIP5Kβ and PIP5Kγ mRNA levels were not affected by PIP5Kα miR (Figure 1b), indicating its specific action on PIP5Kα.
Figure 1.
Stable PIP5Kα KD in PC12 cells. (a) Stable PC12 cells expressing control miR or PIP5Kα miR were generated using a vector-based miR expression system harboring an EmGFP reporter. Stable cells and EmGFP were visualized in the bright field and fluorescein isothiocyanate channels, respectively, using fluorescence microscopy. Scale bar, 20 μm. (b) mRNA expression levels of PIP5Ks in control and PIP5Kα KD cells were examined by reverse transcription–PCR with their specific PCR primers. (c) PIP5Kα protein expression in control and PIP5Kα KD cells was analyzed by western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (b) or β-actin (c) was included as a loading control. (d) Changes in mRNA and protein expression levels of PIP5Kα in PIP5Kα KD cells were normalized to those in control cells. Values are presented as mean±s.e.m. *P<0.01.
We then measured neurite outgrowth in the absence or presence of NGF in the control and PIP5Kα KD cells. As expected, neurite outgrowth was clearly observed 24 and 48 h after NGF treatment in a time-dependent manner (Figure 2a). Notably, we found that NGF-induced neurites were more branched and longer in PIP5Kα KD cells, compared with those in control cells (Figures 2a and b). These results suggest that PIP5Kα acts to downregulate neurite outgrowth by NGF.
Figure 2.
Effect of PIP5Kα KD on nerve growth factor (NGF)-induced neurite outgrowth. (a) Control and PIP5Kα KD cells were treated with or without NGF (100 ng ml−1) for 24 or 48 h. Bright field images of neurite outgrowth were obtained using an inverted microscope. Scale bar, 20 μm. (b) Lengths of the longest neurites were measured from more than 40 different cells in each group. Values are mean±s.e.m. *P<0.01. miRNA, microRNA.
PIP5Kα KD facilitates NGF-induced activation of PI3K/Akt pathway and ROS generation
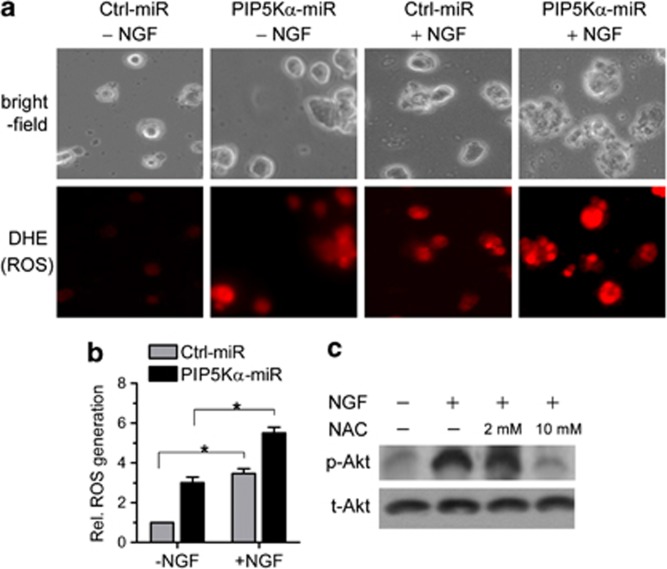
We tested whether Akt activation was responsible for the enhanced neurite outgrowth observed in PIP5Kα KD cells. Phosphorylation of Akt (Ser473) by NGF in PIP5Kα KD cells was much higher than that in control cells (Figures 3a and b). PIP5Kα KD also led to significant increase in Akt phosphorylation even in the NGF-untreated condition (Figures 3a and b). As Akt activation is dependent on PI3K activity, we tested the effect of a PI3K inhibitor LY294002 on the NGF-induced Akt phosphorylation. Pretreatment of PIP5Kα KD cells with LY294002 strongly inhibited Akt phosphorylation by NGF (Figure 3c). These results suggest that PI3K activation potentiated by PIP5Kα KD is involved in the Akt activation by NGF.
Figure 3.
Changes in nerve growth factor (NGF)-induced phosphatidylinositol (PI)3K/Akt activation by PIP5Kα KD. Control (a) and PIP5Kα KD (a, c) cells were treated with or without NGF (100 ng ml−1) for 15 min. (c) LY294002 (20 μℳ) was added for 1 h before NGF treatment as indicated. (a, c) Phosphorylated and total levels of Akt were measured by western blotting. (b) The phosphorylation levels of Akt by NGF in (a) were quantified as fold-change over those in NGF-stimulated control cells. Values are mean±s.e.m. *P<0.01. miRNA, microRNA.
Previously, it was reported that NGF treatment increased ROS generation that was a cause of induction of neurite outgrowth.22, 23 We measured changes in ROS generation with DHE, a ROS-specific probe that emits red fluorescence upon oxidation by intracellular ROS. NGF treatment induced a rapid ROS generation in both control and PIP5Kα KD cells (Figures 4a and b). Measurements of florescent intensities of DHE showed that ROS generation by NGF in PIP5Kα KD cells was higher than that in control cells (Figures 4a and b). In addition, ROS generation was also increased by the PIP5Kα KD in NGF-untreated condition (Figures 4a and b). These observations were somewhat similar to the changes in phosphorylated Akt levels in the NGF-treated and -untreated conditions (Figure 3b). We then examined the possibility that NGF-induced Akt phosphorylation and ROS generation were related to each other. Pretreatment of PIP5Kα KD cells with the antioxidant N-acetyl-ℒ-cysteine significantly suppressed NGF-induced Akt phosphorylation in the concentration-dependent manner (Figure 4c), supporting that ROS generation acts upstream of PI3K/Akt signaling pathway upon NGF stimulation.
Figure 4.
Nerve growth factor (NGF)-induced reactive oxygen species (ROS) generation and its effect on Akt phosphorylation. (a) Control and PIP5Kα KD cells were incubated in the presence of 5 μℳ dihydroethidium (DHE), a fluorescent probe for ROS, for 20 min and then washed out with phosphate-buffered saline. Cells were further treated with or without NGF (100 ng ml−1) for additional 10 min. The red fluorescence of the probe owing to its oxidation was monitored using fluorescent microscopy. Cells were visualized on bright field channel. LED channel. (b) The fluorescent intensities of DHE were determined by image analysis and quantified as fold-change over those in unstimulated control cells. Values are mean±s.e.m. *P<0.01 (c) PIP5Kα KD cells were pretreated with N-acetyl-ℒ-cysteine (NAC) for 30 min and then treated with or without NGF (100 ng ml−1) for 15 min as indicated. Changes in phosphorylated and total levels of Akt were measured by western blotting.
Reconstituted expression of PIP5Kα attenuates NGF-induced neurite outgrowth and Akt activation
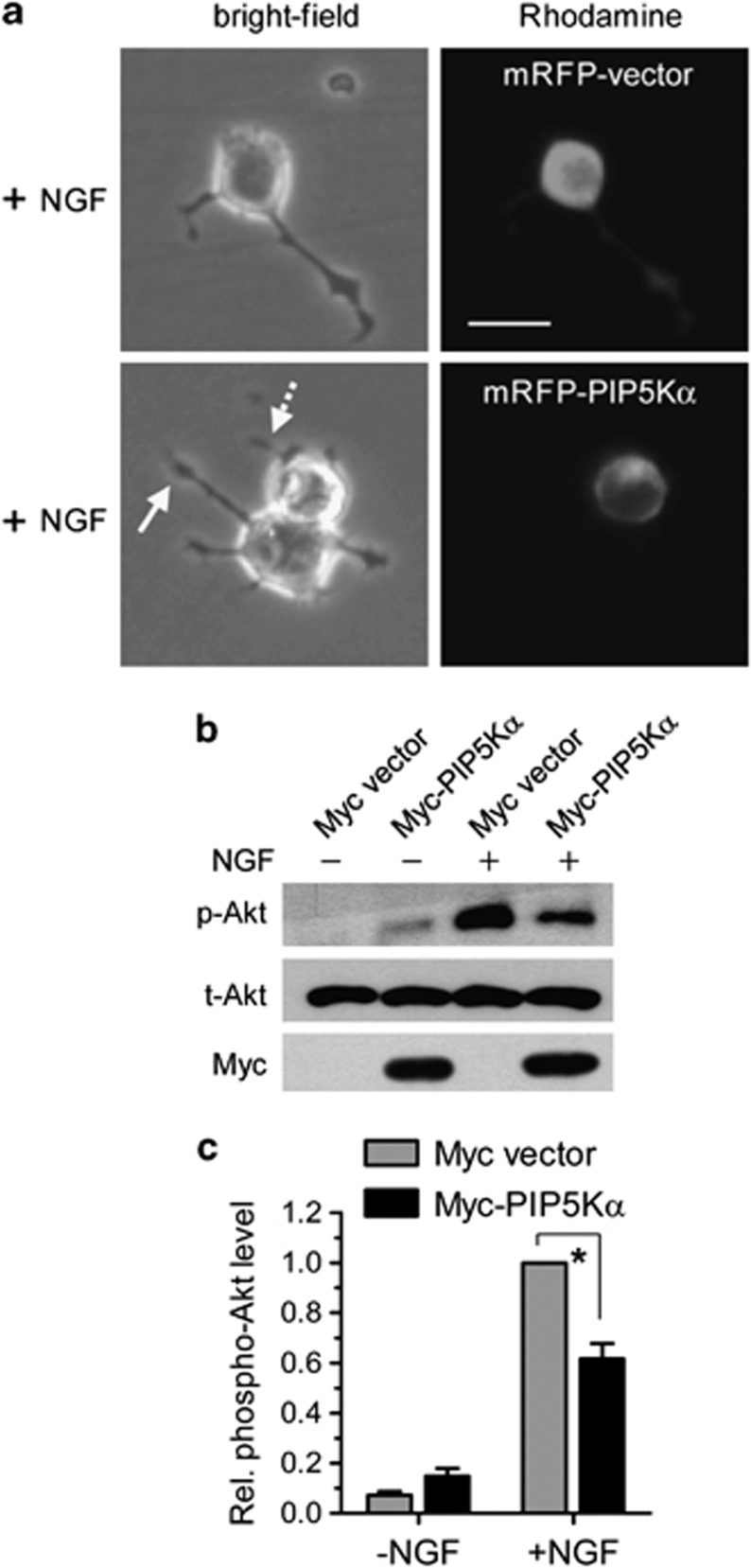
Next, we examined whether complementation of PIP5Kα KD cells with PIP5Kα could inhibit NGF-induced neurite outgrowth. To test this, we transfected PIP5Kα KD cells with mRFP empty vector or mRFP–PIP5Kα and measured changes in neurite outgrowth in the absence or presence of NGF. As expected, NGF-induced neurite outgrowth was observed in the mRFP-transfected and mRFP–PIP5Kα-untransfected cells (Figure 5a). In contrast, however, expressed mRFP–PIP5Kα that partially localized to the cell surface15 significantly blocked neurite outgrowth by NGF (Figure 5a). We then measured the effect of reconstituted expression of PIP5Kα on Akt activation by NGF. PIP5Kα KD cells were transfected with Myc–PIP5Kα or Myc control vector. Overexpression of Myc–PIP5Kα was confirmed by Myc tag immunoreactivity (Figure 5b). The magnitude of NGF-induced increase in Akt phosphorylation was much lower in the Myc–PIP5Kα-transfected cells than in the vector-transfected cells (Figures 5b and c). These results indicate an inhibitory role of PIP5Kα in NGF-induced neurite outgrowth and Akt activation.
Figure 5.
Effects of reconstituted PIP5Kα expression on nerve growth factor (NGF)-induced neurite outgrowth and Akt phosphorylation. PIP5Kα KD cells were transfected with monomeric red fluorescence protein (mRFP)–PIP5Kα (a) or Myc–PIP5Kα (b) for 24 h. mRFP or Myc empty vector was transfected as a corresponding control. (a) Following NGF treatment for 24 h, neurite outgrowth (bright field channel) and mRFP expression (Rhodamine channel) were detected by fluorescence microscopy. Note the difference in neurite length between the mRFP–PIP5Kα non-transfected cell (straight line arrow) and mRFP–PIP5Kα-transfected cell (dotted line arrow). Scale bar, 20 μm. (b) After treatment with or without NGF for 15 min, Akt phosphorylation was analyzed by western blotting. Myc–PIP5Kα expression was ascertained by anti-Myc western blot. (c) The phosphorylation levels of Akt in (b) were calculated as fold-change over that in NGF-stimulated vector-transfected condition. Values are mean±s.e.m. *P<0.01.
Delivery of PIP2 interferes with NGF-induced Akt phosphorylation
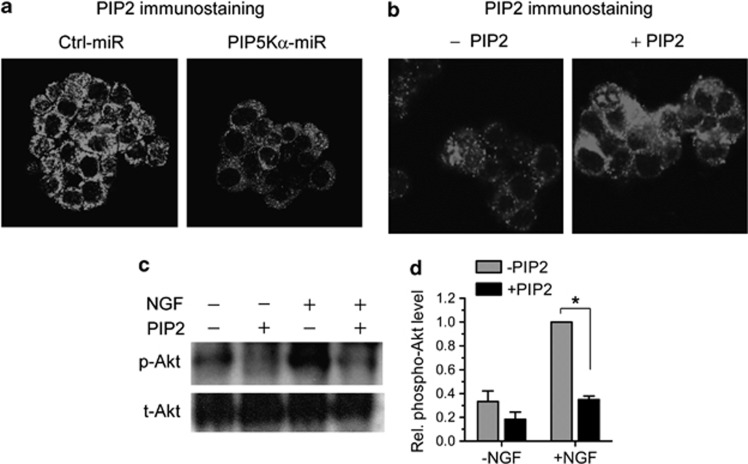
We then examined whether the inhibitory effects of PIP5Kα on NGF-induced Akt phosphorylation could be attributable to its lipid product, PIP2. We first measured difference in PIP2 levels in control and PIP5Kα KD cells using immunocytochemistry with an antibody against PIP2. As expected, the plasma membrane PIP2 levels were significantly reduced in PIP5Kα KD cells compared with those in control cells (Figure 6a). Then, we supplemented the PIP2 levels by employing a method of intracellular deliver of PIP2. Exogenous sources of PIP2 mixed with shuttle carriers such as polyamine and histone were successfully internalized into mammalian cells across the plasma membrane.24 We added a similar complex of dioctanoyl–PIP2 and histone as a carrier to PIP5Kα KD cells before NGF treatment. As demonstrated by the PIP2 immunostaining result, the PIP2 delivery resulted in increased PIP2 levels (Figure 6b). The exogenously applied PIP2 also resulted in a blunted Akt phosphorylation by NGF (Figures 6c and d).
Figure 6.
Effect of phosphatidylinositol (PI) 4,5-bisphosphate (PIP2) delivery on PIP2 levels and nerve growth factor (NGF)-induced Akt phosphorylation. (a) Control and PIP5Kα KD cells were examined for PIP2 levels by PIP2 immunostaining with a PIP2-specific antibody. Cells were further stained with biotin-labeled secondary antibody and then with Alexa Fluor 594-conjugated streptavidin. The resulting immune complexes were visualized by fluorescence microscopy. (b and c) PIP5Kα KD cells were preincubated with an equimolar complex of PIP2 and histone (final 10 μℳ each, +PIP2) or with histone only (−PIP2) for 1 h. (b) Changes in PIP2 levels were assayed by the PIP2 imaging in the same manner as described in (a). (c) Cells were further treated with or without NGF (100 ng ml−1) for 15 min under the indicated conditions. Akt phosphorylation was measured by western blot analysis. (d) The Akt phosphorylation levels in (c) were calculated as fold change over that in NGF-stimulated condition without PIP2. Values are mean±s.e.m. *P<0.01. miRNA, microRNA.
Discussion
In this study, we pursued functional roles for PIP5Kα and PIP2 in NGF-dependent neurite growth using a PC12 cell model. Gene KD or overexpression of PIP5Ks that causes changes in plasma membrane PIP2 levels has been widely used for demonstrating their physiological roles in a variety of biological events. Using such manipulation of PIP5Kα, we found that PIP5Kα KD increased and reconstituted PIP5Kα expression decreased the neuritogenic activity of NGF in PC12 cells. We further showed that PIP5Kα deficiency potentiated NGF-induced Akt phosphorylation, which was reversed by the reconstituted PIP5Kα expression. Thus, our results support that PIP5Kα has a potential to limit neurite outgrowth by NGF. The antineuritogenic effect of PIP5Kα is likely dependent on PIP2, as demonstrated by the results that directly added PIP2 also decreased Akt activation by NGF.
PI3K/Akt pathway functions as a signal transducer in growth factor signaling. We confirmed that NGF acted specifically through PI3K to promote Akt phosphorylation, which was in agreement with the previous findings. Our results support an idea that newly synthesized PIP2 by PIP5Kα in the plasma membrane may desensitize PI3K/Akt signaling pathway, which in turn negatively regulates NGF-induced neurite outgrowth. Phosphorylation and activation of Akt that is mediated via phosphoinositide-dependent kinase 1 contributes to NGF-induced neurite outgrowth.8 PI3K-catalyzed generation of PI 3,4,5-trisphosphate (PIP3) from PIP2 is required for the phosphoinositide-dependent kinase 1-mediated Akt phosphorylation. PI3K/Akt signaling has an important role in neurite branching and elongation.4, 8 An increase in local PIP3 production in the plasma membrane through a positive-feedback loop between PI3K and Rac1/Cdc42 was important for neurite outgrowth induced by NGF.4, 25
Generally, PIP3 remains very low in the resting condition and undergoes elevation upon stimulation. The increased PIP3 level is rapidly hydrolyzed back to PIP2 by the PTEN (phosphate and tensin homolog) that acts as a PIP3 3-phosphatase. In addition, SHIP2 (Src homology 2 domain-containing inositol phosphatase 2) and PIPP (proline-rich inositol polyphosphate 5-phosphatase) that mediate PIP3 hydrolysis to PI 3,4-bisphosphate also reduce PIP3 level. PTEN as well as SHIP2 and PIPP were shown to prevent the NGF-induced neurite outgrowth in PC12 cells,4, 26, 27 indicating a positive correlation between PIP3 level and neurite outgrowth. Interestingly, accumulating evidence has demonstrated that PTEN harbors a PIP2-binding motif in the N-terminal region, and the interaction with PIP2 induces its conformational change, promoting membrane targeting and the lipid phosphatase activity of PTEN.28, 29, 30, 31 It is well established that PTEN negatively regulates PI3K/Akt signaling pathway and neurite outgrowth by reducing PIP3 level. Moreover, the increased Akt activity by heat shock treatment was reduced by the presence of PIP2.32 Overexpression of PIP5K-like1 (the fourth member of the type I PIP5Ks) showed an inhibitory effect on Akt phosphorylation.33 Given these reports, one may consider the possibility that PIP5Kα-dependent PIP2 formation allows PTEN to become more active, resulting in decrease in PIP3 level, which attenuates NGF-induced PI3K/Akt signaling and neurite outgrowth. The detailed molecular mechanisms by which PIP5Kα-derived PIP2 mediates inhibitory effects on PI3K/Akt signaling pathway require further investigation.
Phosphoinositides and their metabolizing enzymes are key factors for controlling cell polarity involving membrane remodeling processes, such as neurite outgrowth and chemotaxis. In chemotaxis of neutrophils and Dictyostelium, PTEN and PIP2 localize to the rear of cells, whereas PIP3 and PI3K are spatially restricted to the front of the leading edges.34, 35 Activation of phospholipase C-mediated PIP2 degradation resulted in downregulation of PIP2-dependent PTEN activity, resulting in increased PIP3 level at the leading edge in the chemotaxing cells.34, 36, 37, 38 In this context, it is plausible that PIP5Kα KD, which can also deplete PIP2 level like the action of phospholipase C, can enhance neurite outgrowth. Rho family small guanosine triphsopahatases that coordinate cytoskeletal membrane remodeling also have an important role in the neurite outgrowth. Rac1 and Cdc42, the critical regulators of actin and microtubule dynamics, localize to the tips of axons together with PI3K and induce axonal elongation.2, 39 On the other hand, activation of RhoA and ROCK strongly inhibits neurite outgrowth.40 Accordingly, inhibition of the RhoA/ROCK pathway has been recognized as a promising therapeutic target for inducing axonal growth.41 Moreover, PTEN is activated by RhoA/ROCK pathway during membrane polarization.42, 43 PIP5Kγ-mediated PIP2 production associated with RhoA/ROCK signaling pathway participated in the rear retraction during neutrophil chemotaxis.44 All these observations support distinct roles for PIP2 and PIP3 in the neurite regulation, where PIP3 accelerates neurite outgrowth but PIP2 contributes to neurite retraction.
We found a casual correlation between changes in ROS levels and activation of Akt, and an inhibitory effect of N-acetyl-ℒ-cysteine on Akt phosphorylation in NGF-treated control and PIP5Kα KD cells. These suggest that Akt activation was at least in part owing to the ROS generation. In addition, such a casual correlation was also observed in the resting (NGF-untreated) control and PIP5Kα KD cells. The physiologically relevant range of intracellular ROS levels was reported to be important for actin polymerization that was associated with neurite outgrowth.22 ROS scavenging or inhibiting NADPH oxidase-specific ROS that reduced ROS levels suppressed neurite extension. Epidermal growth factor-induced activation of Akt was dependent on increased intracellular ROS.45 NGF stimulation also evoked ROS, and the NGF-induced neurite outgrowth was prevented by N-acetyl-ℒ-cysteine in PC12 cells.23, 46 PIP2 formation by PIP5K activity was decreased in response to hydrogen peroxide treatment in the sarcolemma plasma membranes.47 Conversely, PIP5Kα KD decreased gene expression of antioxidant enzymes such as heme oxygenase-1, suggesting that PIP2 may retain an ability to suppress intracellular ROS.48 Overall, our results suggest that increase in NGF-induced ROS underlies activation of Akt, which can be facilitated by deficiency of PIP5Kα.
Previously, PIP5Kβ was demonstrated to act as a downstream effector of RhoA/ROCK signaling pathway that makes a primary contribution to the neurite retraction.18, 19 The antineuritogenic effect of PIP5Kβ depended on its lipid kinase activity. Considering previous observations that the type I PIP5K family members are activated by RhoA,15, 49 we cannot exclude the possibility that PIP5Kα-specific PIP2 pool also participates in the RhoA/ROCK-mediated neurite retraction. In this regard, it is intriguing to consider that a deficiency of PIP5Kα-lowering PIP2 level renders RhoA/ROCK signaling pathway ineffective, thereby promoting neurite outgrowth. On the other hand, whether PIP5Kβ has a similar role to PIP5Kα in the regulation of NGF-induced neurite outgrowth needs to be studied.
In the central nervous system, embryonic neurons are morphologically changed into a specialized form having multiple dendrites and an axon. The neuronal differentiation involving neurite outgrowth is important for synapse formation during development and also for axon regeneration following neuronal damages.2, 50 In response to diverse extracellular cues such as chemoattractants and chemorepellents, axons undergo directional elongations or retractions that are related to their maturation and polarization.2, 40 Although further studies will be necessary to fully elucidate underlying mechanisms of the antineuritogenic actions of PIP5Kα-driven PIP2, this study provides novel insight into the regulatory roles of PIP5Kα in NGF-induced neurite outgrowth.
Acknowledgments
We thank Dr Haeyoung Suh-Kim (Ajou University, Korea) for providing PC12 cells. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0013962).
References
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Aoki K, Nakamura T, Inoue T, Meyer T, Matsuda M. An essential role for the SHIP2-dependent negative feedback loop in neuritogenesis of nerve growth factor-stimulated PC12 cells. J Cell Biol. 2007;177:817–827. doi: 10.1083/jcb.200609017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DM, Tucker BA, Rahimtula M, Mearow KM. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem. 2003;86:1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288 (Pt 2:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read DE, Gorman AM. Involvement of Akt in neurite outgrowth. Cell Mol Life Sci. 2009;66:2975–2984. doi: 10.1007/s00018-009-0057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi Z, Igarashi T, Shibayama N, Inanobe S, Sakurai K, Yamaguchi H, et al. Phospholipase Cdelta3 regulates RhoA/Rho kinase signaling and neurite outgrowth. J Biol Chem. 2011;286:8459–8471. doi: 10.1074/jbc.M110.171223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Willard MD, Willard FS, Li X, Cappell SD, Snider WD, Siderovski DP. Selective role for RGS12 as a Ras/Raf/MEK scaffold in nerve growth factor-mediated differentiation. EMBO J. 2007;26:2029–2040. doi: 10.1038/sj.emboj.7601659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Mao YS, Yin HL. Regulation of the actin cytoskeleton by phosphatidylinositol 4-phosphate 5 kinases. Pflugers Arch. 2007;455:5–18. doi: 10.1007/s00424-007-0286-3. [DOI] [PubMed] [Google Scholar]
- Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- van den Bout I, Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- van Horck FP, Lavazais E, Eickholt BJ, Moolenaar WH, Divecha N. Essential role of type I(alpha) phosphatidylinositol 4-phosphate 5-kinase in neurite remodeling. Curr Biol. 2002;12:241–245. doi: 10.1016/s0960-9822(01)00660-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Miyazaki H, Watanabe H, Sasaki T, Maehama T, Frohman MA, et al. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J Biol Chem. 2002;277:17226–17230. doi: 10.1074/jbc.M109795200. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim B, Jeong HK, Min KJ, Liu T, Park JY, et al. Enhanced phosphatidylinositol 4-phosphate 5-kinase alpha expression and PI(4,5)P2 production in LPS-stimulated microglia. Neurochem Int. 2010;57:600–607. doi: 10.1016/j.neuint.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kim B, Yoon S, Kim YJ, Liu T, Woo JH, et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is induced in ganglioside-stimulated brain astrocytes and contributes to inflammatory responses. Exp Mol Med. 2010;42:662–673. doi: 10.3858/emm.2010.42.9.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnamalai V, Suter DM. Reactive oxygen species regulate F-actin dynamics in neuronal growth cones and neurite outgrowth. J Neurochem. 2009;108:644–661. doi: 10.1111/j.1471-4159.2008.05787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, et al. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- Ozaki S, DeWald DB, Shope JC, Chen J, Prestwich GD. Intracellular delivery of phosphoinositides and inositol phosphates using polyamine carriers. Proc Natl Acad Sci USA. 2000;97:11286–11291. doi: 10.1073/pnas.210197897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosker KE, Eickholt BJ. Phosphoinositide 3-kinase signalling events controlling axonal morphogenesis. Biochem Soc Trans. 2007;35:207–210. doi: 10.1042/BST0350207. [DOI] [PubMed] [Google Scholar]
- Musatov S, Roberts J, Brooks AI, Pena J, Betchen S, Pfaff DW, et al. Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc Natl Acad Sci USA. 2004;101:3627–3631. doi: 10.1073/pnas.0308289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooms LM, Fedele CG, Astle MV, Ivetac I, Cheung V, Pearson RB, et al. The inositol polyphosphate 5-phosphatase, PIPP, Is a novel regulator of phosphoinositide 3-kinase-dependent neurite elongation. Mol Biol Cell. 2006;17:607–622. doi: 10.1091/mbc.E05-05-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RB, Liu F, Ross AH. Allosteric activation of PTEN phosphatase by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2003;278:33617–33620. doi: 10.1074/jbc.C300296200. [DOI] [PubMed] [Google Scholar]
- Redfern RE, Redfern D, Furgason ML, Munson M, Ross AH, Gericke A. PTEN phosphatase selectively binds phosphoinositides and undergoes structural changes. Biochemistry. 2008;47:2162–2171. doi: 10.1021/bi702114w. [DOI] [PubMed] [Google Scholar]
- Wang X, Jiang X. Post-translational regulation of PTEN. Oncogene. 2008;27:5454–5463. doi: 10.1038/onc.2008.242. [DOI] [PubMed] [Google Scholar]
- Rahdar M, Inoue T, Meyer T, Zhang J, Vazquez F, Devreotes PN. A phosphorylation-dependent intramolecular interaction regulates the membrane association and activity of the tumor suppressor PTEN. Proc Natl Acad Sci USA. 2009;106:480–485. doi: 10.1073/pnas.0811212106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Konishi H, Tanaka M, Ono Y, Takenawa T, Watanabe Y, et al. Isolation of the active form of RAC-protein kinase (PKB/Akt) from transfected COS-7 cells treated with heat shock stress and effects of phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 4,5-bisphosphate on its enzyme activity. FEBS Lett. 1996;396:305–308. doi: 10.1016/0014-5793(96)01120-9. [DOI] [PubMed] [Google Scholar]
- Shi L, Zhao M, Luo Q, Ma YM, Zhong JL, Yuan XH, et al. Overexpression of PIP5KL1 suppresses cell proliferation and migration in human gastric cancer cells. Mol Biol Rep. 2010;37:2189–2198. doi: 10.1007/s11033-009-9701-5. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- Janetopoulos C, Firtel RA. Directional sensing during chemotaxis. FEBS Lett. 2008;582:2075–2085. doi: 10.1016/j.febslet.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]
- Kortholt A, King JS, Keizer-Gunnink I, Harwood AJ, Van Haastert PJ. Phospholipase C regulation of phosphatidylinositol 3,4,5-trisphosphate-mediated chemotaxis. Mol Biol Cell. 2007;18:4772–4779. doi: 10.1091/mbc.E07-05-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keizer-Gunnink I, Kortholt A, Van Haastert PJ. Chemoattractants and chemorepellents act by inducing opposite polarity in phospholipase C and PI3-kinase signaling. J Cell Biol. 2007;177:579–585. doi: 10.1083/jcb.200611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Key regulators in neuronal polarity. Neuron. 2005;48:881–884. doi: 10.1016/j.neuron.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Hata K, Kubo T, Yamaguchi A, Yamashita T. Signaling mechanisms of axon growth inhibition. Drug News Perspect. 2006;19:541–547. doi: 10.1358/dnp.2006.19.9.1050423. [DOI] [PubMed] [Google Scholar]
- Kubo T, Hata K, Yamaguchi A, Yamashita T. Rho-ROCK inhibitors as emerging strategies to promote nerve regeneration. Curr Pharm Des. 2007;13:2493–2499. doi: 10.2174/138161207781368657. [DOI] [PubMed] [Google Scholar]
- Meili R, Sasaki AT, Firtel RA. Rho Rocks PTEN. Nat Cell Biol. 2005;7:334–335. doi: 10.1038/ncb0405-334. [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- Lokuta MA, Senetar MA, Bennin DA, Nuzzi PA, Chan KT, Ott VL, et al. Type Igamma PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol Biol Cell. 2007;18:5069–5080. doi: 10.1091/mbc.E07-05-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, et al. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic Biol Med. 2006;41:1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kamata H, Tanaka C, Yagisawa H, Matsuda S, Gotoh Y, Nishida E, et al. Suppression of nerve growth factor-induced neuronal differentiation of PC12 cells. N-acetylcysteine uncouples the signal transduction from ras to the mitogen-activated protein kinase cascade. J Biol Chem. 1996;271:33018–33025. doi: 10.1074/jbc.271.51.33018. [DOI] [PubMed] [Google Scholar]
- Mesaeli N, Tappia PS, Suzuki S, Dhalla NS, Panagia V. Oxidants depress the synthesis of phosphatidylinositol 4,5-bisphosphate in heart sarcolemma. Arch Biochem Biophys. 2000;382:48–56. doi: 10.1006/abbi.2000.2012. [DOI] [PubMed] [Google Scholar]
- Mellman DL, Gonzales ML, Song C, Barlow CA, Wang P, Kendziorski C, et al. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature. 2008;451:1013–1017. doi: 10.1038/nature06666. [DOI] [PubMed] [Google Scholar]
- Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, et al. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J Biol Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]