Abstract
Background
Exercise is known to reduce disability and improve quality of life in people with Parkinson disease (PD). Although barriers to exercise have been studied in older adults, barriers in people with chronic progressive neurological diseases, such as PD, are not well defined.
Objective
The purpose of this study was to identify perceived barriers to exercise in people with PD.
Design
The study had a cross-sectional design.
Methods
People who had PD, dwelled in the community, and were at stage 2.4 on the Hoehn and Yahr scale participated in this cross-sectional study (N=260; mean age=67.7 years). Participants were divided into an exercise group (n=164) and a nonexercise group (n=96). Participants self-administered the barriers subscale of the Physical Fitness and Exercise Activity Levels of Older Adults Scale, endorsing or denying specific barriers to exercise participation. Multivariate logistic regression analysis was used to examine the contribution of each barrier to exercise behavior, and odds ratios were reported.
Results
Three barriers were retained in the multivariate regression model. The nonexercise group had significantly greater odds of endorsing low outcome expectation (ie, the participants did not expect to derive benefit from exercise) (odds ratio [OR]=3.93, 95% confidence interval [CI]=2.08–7.42), lack of time (OR=3.36, 95% CI=1.55–7.29), and fear of falling (OR=2.35, 95% CI=1.17–4.71) than the exercise group.
Limitations
The cross-sectional nature of this study limited the ability to make causal inferences.
Conclusions
Low outcome expectation from exercise, lack of time to exercise, and fear of falling appear to be important perceived barriers to engaging in exercise in people who have PD, are ambulatory, and dwell in the community. These may be important issues for physical therapists to target in people who have PD and do not exercise regularly. The efficacy of intervention strategies to facilitate exercise adherence in people with PD requires further investigation.
Exercise decreases disability and improves quality of life in people with Parkinson disease (PD).1,2 Studies have revealed that gait, balance, strength, flexibility, and cardiovascular fitness improve in people who have PD and participate in exercise.3–8 Advances in medical management have resulted in increased survival of people with PD; therefore, management of this disease and related sequelae must occur over the course of many years.9–11 Although the short-term benefits of exercise are well documented, the long-term benefits of exercise for people with PD are not well established.12 Sedentary lifestyles and limited adherence to continuous exercise contribute to the challenges of evaluating the long-term benefits of exercise in people with PD.13,14 Understanding the factors that limit sustained participation in exercise over the long term is essential to understanding the potential of continuous exercise to mitigate disability in people with PD.
Adhering to exercise on a continuous basis is a widespread challenge for older adults who are healthy as well as for older adults who have chronic disabilities and live in the community. Researchers have examined many factors that may influence exercise adherence in older adults and have identified the term “motivators” to describe factors that promote adherence and the term “barriers” to describe factors that limit adherence. For older adults, perceived barriers to exercise are more predictive of exercise behavior than perceived motivators.15,16 In a study examining exercise adherence during outpatient physical therapy, 60% of people who did not show exercise adherence but only 13% of people who did show exercise adherence reported at least 1 barrier to exercise.16 Well-documented perceived barriers to exercise in older adults include lack of interest, poor health, weakness, fear of falling, pain, bad weather, lack of time, and limited access to exercise resources.15,17,18 In addition, an association between low outcome expectation from exercise (ie, people did not expect to derive benefit from exercise) and poor exercise adherence was demonstrated in older adults who had impaired balance and were discharged from physical therapy.15 Furthermore, reports have shown that participation in regular physical activity declines with increasing age and that women who are more than 65 years old participate less than men in that age group.17,19
Perceived barriers to exercise also vary by age group. Younger adults most commonly report lack of time as the main perceived barrier to regular physical activity and exercise, whereas older adults frequently indicate poor health.15,17,20 Schmidt et al21 described a community exercise intervention for people 70 years of age or older in which participants who dropped out of the program early were distinguishable from participants who dropped out later or completed the program. Participants who dropped out early were characterized by poorer perceived health status, poorer physical performance, and greater disease burden (particularly musculoskeletal problems) than participants who dropped out later.21 It appears that people who may benefit most from exercise have more difficulty adhering to regular exercise over time.22
Few studies have examined barriers to exercise in people with neurological disorders. Whereas O'Neill and Reid23 reported that 87% of 199 older adults who were healthy had at least 1 perceived barrier to exercise, Rimmer et al24 found that nearly 50% of 83 adults with unilateral stroke reported at least 5 perceived barriers to exercise. The 5 most common perceived barriers for these adults with stroke were the cost of a health promotion program, lack of awareness of a nearby fitness center, lack of transportation, lack of knowledge of how to exercise, and lack of knowledge of where to exercise.24 In a study of 93 people with multiple sclerosis, items related to physical exertion (eg, “exercise tires me” and “exercise is hard work for me”) were the most highly ranked barriers to participation in exercise.25 Ablah et al26 examined barriers to exercise in adults with epilepsy and found that the most common perceived barriers were lack of motivation, personal safety concerns, insufficient time, lack of an exercise partner, excessive pain, lack of transportation, side effects of medication, fear of seizures, and limited access to exercise facilities. These adults experienced many of the same barriers as adults who were healthy in addition to barriers that were disease specific.26
Little is known about barriers to exercise in people with PD. Although people with PD may have barriers to exercise in common with older adults who are healthy, some barriers may be more relevant than others. For example, fear of falling may be an important barrier to exercise because previous studies revealed an association between restricted activity and fear of falling in people with PD.14,27,28 In addition, given the progressive nature of the disease and the associated decline in physical function, people with PD may not expect to derive benefit from exercise (low outcome expectation).29 Furthermore, exercise is not routinely recommended by neurologists for people with PD early in the course of the disease; this factor further limits perceptions regarding the value of exercise in improving outcomes.30 A lack of a patient-centered approach to prescribing exercise may further limit motivation and outcome expectations.31
The purpose of the present study was to identify barriers to exercise in people who have PD and dwell in the community. On the basis of our review of the literature, in addition to studies indicating that perceived barriers are more predictive of exercise behavior than perceived motivators in older adults, we hypothesized that low outcome expectation from exercise and fear of falling would distinguish people who have PD and do not engage in long-term, continuous exercise from those who have PD and exercise regularly. Given that previous research identified age and sex as factors influencing participation in exercise among older adults,17,19 we conducted secondary analyses to determine whether these factors were important to consider in people with PD. In addition, given that people with PD tend to become less active with increasing disease severity,14 we examined whether disease severity influenced the determination of barriers to exercise. Knowledge of modifiable, perceived barriers to exercise could help physical therapists develop strategies to promote long-term adherence to exercise in people with PD.
Method
Study Design and Population
In a parent longitudinal study, the 2-year trajectory of disablement in a cohort of people with PD was investigated. Participants were examined every 6 months over a 2-year period. The present study consisted of a cross-sectional analysis of the baseline data from 260 participants in the parent longitudinal investigation.32 Baseline data were collected at 4 outpatient settings between July 2009 and July 2010. Participants were recruited from movement disorders clinics and local support groups at Boston University, University of Utah, Washington University in St Louis, and the University of Alabama at Birmingham. All participants met the following inclusion criteria: diagnosis of idiopathic PD, as defined by the UK Brain Bank Criteria33; stages 1 to 4 on the modified Hoehn and Yahr scale (H&Y)34; age of 40 years or older; Mini-Mental State Examination score of 24 or greater out of 3035,36; living in the community (not in an institution); and able to attend assessment sessions and provide consent. Potential participants were excluded if they had a diagnosis of atypical parkinsonism, were at H&Y stage 5, or had previous surgical management of their PD. All participants provided informed consent.
All evaluators were provided with a standard operating procedures manual and an instructional video that described the protocol for administering and scoring each instrument for 2 participants with PD. Evaluators rated both participants on 2 occasions separated by 1 week. Within-site and between-site coefficients of variation were calculated for all instruments and ranged from 0.08% to 5%. Participants were examined over a 2.5-hour period and were tested while in the “on” medication state.
Outcome Variables
For the purpose of comparison, participants were divided into an exercise group and a nonexercise group, as determined with the Stages of Readiness to Exercise Questionnaire.37 Internal consistency (0.76) has been established, and this tool was shown to reliably differentiate people at different stages (F=36.57; df=4,369; P=.001) of changes in exercise behavior in a sample of adults who were healthy.38,39 This instrument includes 5 statements describing exercise frequency and ranging from “I currently do not exercise, and I do not intend to start exercising in the next 6 months” to “I currently exercise regularly (3 or more times per week for 20 minutes or more each time) and have done so for longer than 6 months.” Participants selected the statement that best described their exercise behavior. Participants who indicated that they had exercised at least 3 or more times per week for 20 minutes or more each time throughout the preceding 6 months or longer were assigned to the exercise group, and participants who reported that they had not exercised regularly over the preceding 6 months or had not exercised at all were assigned to the nonexercise group.
The exercise behavior designations were validated on the basis of survey data from the Physical Activity Scale for the Elderly (PASE) and performance data from the StepWatch 3 Activity Monitor (SAM) (Orthocare Innovations, Mountlake Terrace, Washington). The PASE is a self-administered survey used to measure physical activity levels (eg, sports, jogging, swimming, strengthening and endurance exercises) and durations of leisure activity, household activity, and work-related activity during the preceding 7-day period. The PASE has been validated in people 65 years of age or older.40,41 The PASE scores range from 0 to 360, with higher scores indicating higher physical activity levels.41 The SAM device is attached to the ankle and captures free-living ambulatory activity. It is able to detect strides taken by the leg to which it is attached on the basis of a combination of acceleration, position, and timing. The SAM has good test-retest reliability (intraclass correlation coefficient=.84) and 96% accuracy in older adults42,43 and people with PD.44 A subset of 100 participants wore the SAM during customary activity, including exercise, 24 hours per day for 7 consecutive days, except during showering or swimming. Validation procedures are further described elsewhere.32
Measures
Baseline characteristics, including age, sex, ethnicity, duration of PD, comorbid health conditions, working status, occurrence of falls (self-report over preceding 6 months), and H&Y stage, were collected through self-report questionnaires, participant interviews, and physical examinations. The Geriatric Depression Scale (GDS) was used to identify depression-related symptoms.45 The GDS is a 30-item self-report questionnaire developed to assess depression in older adults. Scores range from 0 to 30, with higher scores indicating more depression-related symptoms.45
The barriers subscale of the Physical Fitness and Exercise Activity Levels of Older Adults Scale was used to assess the participants' perceived barriers to regular exercise behavior.46 The test-retest reliability (.751, P<.0001) and internal consistency (alpha coefficient=.727) have been established for this subscale in older adults who are healthy.46 The barriers subscale includes 13 statements (Tab. 1), and each statement is rated on a 4-point Likert scale reflecting how strongly a person agrees with the statement, ranging from “strongly agree” to “strongly disagree.” Responses were dichotomized as endorsed (“strongly agree” or “agree”) or denied (“disagree” or “strongly disagree”) and entered into a logistic regression model.
Table 1.
Regression Analysisa
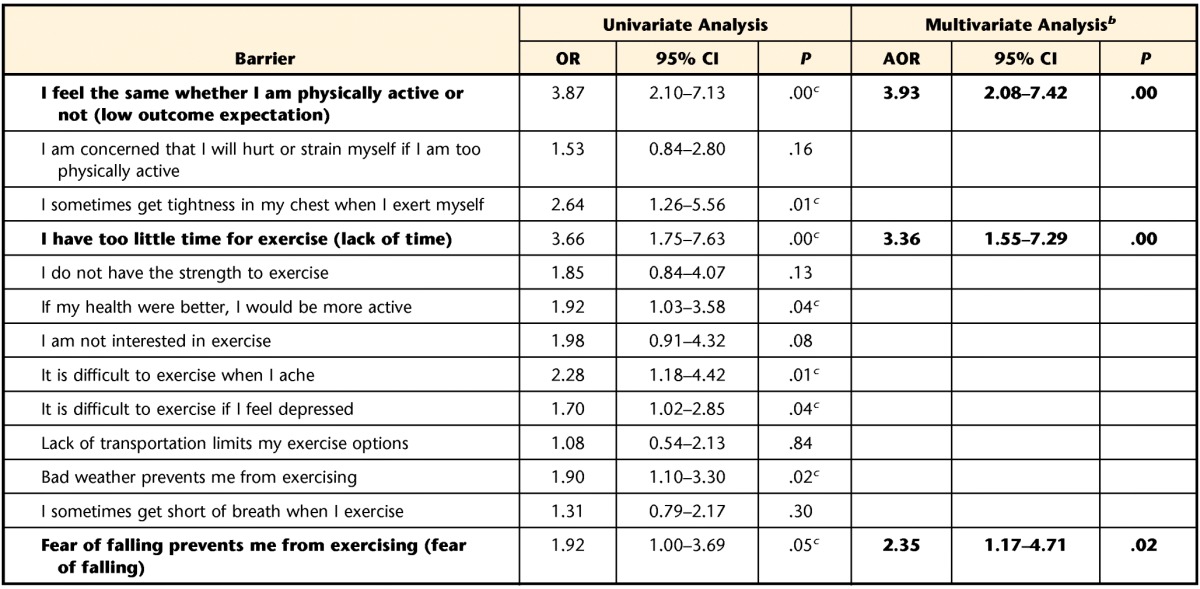
Odds ratio (OR) and adjusted odds ratio (AOR) describe the odds that a particular barrier would be endorsed by the nonexercise group compared with the exercise group. Barriers (and data) in bold type distinguished the nonexercise group from the exercise group in the multivariate logistic regression analysis with backward elimination. CI=confidence interval.
b Data for items remaining in the final multivariate regression model are shown.
c Barrier associated with the nonexercise group in the univariate logistic regression analysis.
Data Analysis
Descriptive statistics.
The means and standard deviations for age, H&Y stage, duration of PD, number of comorbidities, and GDS scores were calculated, and the frequencies (ie, percent occurrence) for sex, ethnicity, working status, and occurrence of falls were determined to describe the characteristics of the total sample as well as the exercise and nonexercise groups (ie, separate groups). Two-tailed independent t tests or chi-square tests were used, as appropriate, to assess differences between the 2 groups.
Determination of barriers to exercise behavior.
Univariate logistic regression was used to examine the contribution of each potential barrier to exercise behavior for the entire sample. A multivariate logistic regression analysis was then applied to examine the association between barriers and exercise behavior while controlling for confounding variables. A backward elimination procedure was used to remove the least significant barriers 1 at a time until only barriers with P values of .05 or less remained. Odds ratios were used to represent the odds that a barrier would be endorsed by the nonexercise group compared with the exercise group. Given that sex, age, and disease severity have been shown to influence exercise behavior, we conducted secondary analyses by using an identical multivariate regression analysis with a backward elimination procedure for subsets of men, women, participants less than 65 years of age, participants 65 years of age or older, participants in H&Y stages 0 to 2, and participants in H&Y stages 2.5 to 4.14,15,17,19–21 Study data were managed with the Research Electronic Data Capture (REDCap) tools hosted at the University of Utah.47 All data were analyzed with the statistical software program SPSS 16.0 (SPSS Inc, Chicago, Illinois).
Role of the Funding Source
Funding for this project was provided by the Davis Phinney Foundation, the Parkinson's Disease Foundation, and NIH K12 Building Interdisciplinary Research in Women's Health (HD43444).
Results
Exercise Behavior Designation and Validation
Of the 260 participants, 164 (63%) were assigned to the exercise group and 96 (37%) were assigned to the nonexercise group. These designations were validated by the PASE scores, which were significantly higher (P=.02) in the exercise group, indicating a higher level of physical activity during the week of assessment (Tab. 2). Furthermore, the SAM data showed that the exercise group had a significantly higher (P=.02) number of average steps per day than the nonexercise group (Tab. 2).
Table 2.
Sample Characteristicsa
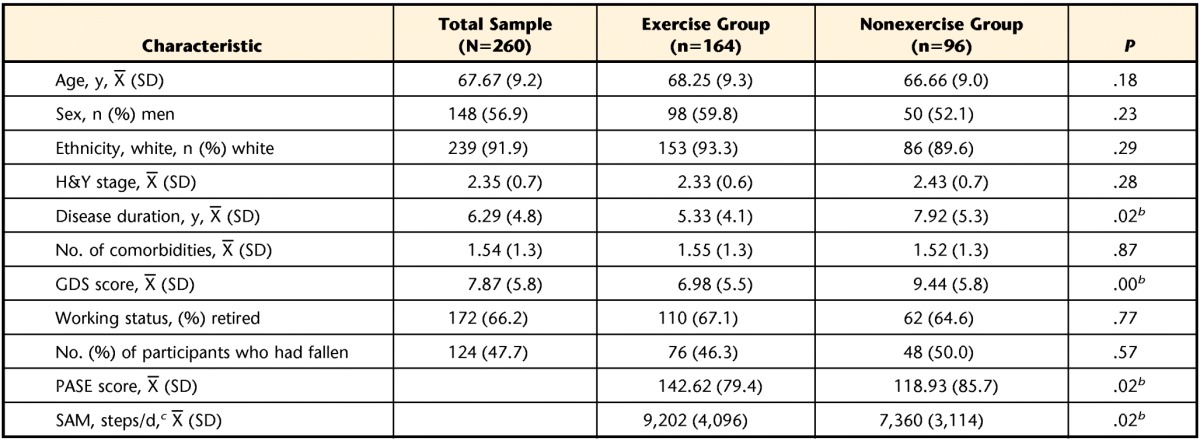
H&Y=Hoehn and Yahr Scale, GDS=Geriatric Depression Scale, PASE=Physical Activity Scale for the Elderly, SAM=StepWatch 3 Activity Monitor.
b Significantly different between the exercise group and the nonexercise group.
c The SAM data were collected from a subset of 100 participants (73 in the exercise group and 27 in the nonexercise group).
Sample Characteristics
The sample characteristics are shown in Table 2. The sample included 260 participants with predominantly mild to moderate disease severity: 7.7% were considered to be in H&Y stages 1 and 1.5, 66.4% were in stages 2 and 2.5, 20.1% were in stage 3, and 5.8% were in stage 4. The exercise group had a significantly shorter average disease duration (P=.02) and significantly lower average GDS scores (P=.00) than the nonexercise group. The exercise and nonexercise groups did not differ in age, sex, H&Y stage, number of comorbidities, working status, or number of participants who had fallen.
Barriers to Exercise
In the univariate logistic regression analysis (Tab. 1), 8 barriers—low outcome expectation, tightness in chest, lack of time, perceived health, discomfort with exercise, depression, bad weather, and fear of falling—were significantly associated with the nonexercise group. After the multivariate regression analysis with backward elimination, low outcome expectation, lack of time, and fear of falling remained in the final regression model and were quantified as odds ratios (Tab. 1). The nonexercise group had 3.93 times the odds of endorsing low outcome expectation as the exercise group. In addition, the nonexercise group had 3.36 times the odds of endorsing lack of time and 2.35 times the odds of endorsing fear of falling as the exercise group.
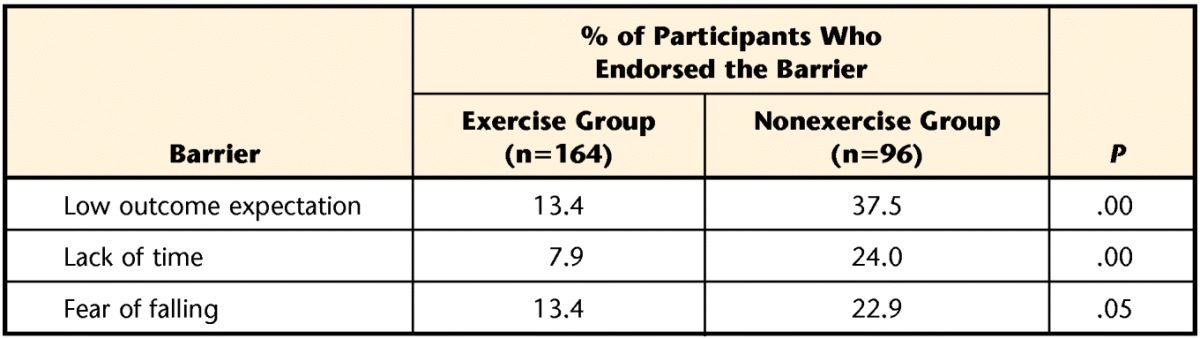
The percentages of the exercise and nonexercise groups endorsing each barrier are shown in Table 3. In the secondary analyses, there were no significant differences in barriers to exercise across sex, age groups dichotomized at 65 years, or disease severity classified by H&Y stage.
Table 3.
Percentages of Participants in the Exercise and Nonexercise Groups Who Endorsed Each Barrier
Discussion
In the present study, we examined perceived barriers to exercise in people who had PD and dwelled in the community by identifying the barriers that distinguished participants who engaged in regular exercise (exercise group) from participants who did not exercise regularly (nonexercise group). Perceived barriers that limited exercise participation included low outcome expectation from exercise, lack of time to exercise, and fear of falling. These results are in agreement with our hypothesis identifying low outcome expectation and fear of falling as potential barriers to exercise in people with PD. Lack of time was also identified as an additional important barrier in the nonexercise group. These perceived barriers to exercise are potentially modifiable and important for physical therapists to consider in their treatment plans.
The most salient barrier identified in the multivariate regression analysis was low outcome expectation from exercise. This concept was represented by the statement, “I feel the same whether I am physically active or not.” Participants who endorsed this statement may not have perceived a benefit of exercise, manifested as a barrier to engaging in regular physical activity. Burton et al48 examined factors associated with performing “brisk physical activity,” such as “walking briskly, gardening, or heavy housework,” at least 3 times per week in older adults who had PD and dwelled in the community. Older adults who believed that physical activity promoted better health were more than twice as likely to initiate physical activity.48 Therefore, educating people about the health-related benefits of exercise is important and may alter exercise outcome expectations.20 Indeed, many older adults who have PD may lack knowledge of how exercise could affect their symptoms—particularly in the context of a chronic progressive neurological disease.
Other methods, in addition to education, may further enhance outcome expectations. For example, cognitive-behavioral strategies targeting exercise outcome expectations, such as goal setting, feedback, and relapse prevention training, have been shown to increase regular exercise participation in older adults more than health education or exercise instruction alone.49 The extent to which such strategies may be effective for people with PD warrants further investigation.
Perceived lack of time also was associated with the nonexercise group. People may be engaged in vocational or social activities that take priority over exercise. This barrier also may represent a perceived lack of time because of the cognitive changes associated with PD. The lack of time to exercise, identified in the present study, may reflect difficulty prioritizing and planning daily activities. Koerts et al50 reported that people with PD planned and executed portions of a multitask test sequentially rather than simultaneously, highlighting the limitations associated with executive function. In a community sample of adults with long-standing PD, more than 50% of the adults had cognitive impairment, and 30% of those adults had a dominant executive function impairment.51 Like low outcome expectation, lack of time may be addressed with cognitive-behavioral strategies, including prioritizing activities and scheduling exercise as part of one's daily routine, with the assistance of caregivers for reinforcing these strategies.52
Fear of falling, the third perceived barrier associated with the nonexercise group, was previously associated with activity restriction in older adults who were healthy as well as those with PD.27,53 Furthermore, people with PD were shown to have more fear of falling than people who were healthy.54,55 Fear of falling has been described as an independent risk factor for falls in people with PD.55,56 Therefore, increasing postural stability may not decrease a person's fear of falling, so that fear of falling must be specifically addressed. Interventions targeting fear of falling in older adults have included education about a realistic self-assessment of the risk of falling and environmental safety factors, risk-taking training to learn to seek assistance in situations in which they were fearful, and promotion of physical fitness.53 One randomized controlled trial demonstrated improvements in fear of falling in people who had PD and completed an 8-week exercise program including incremental speed-dependent treadmill training.57 Experiencing successful mobility during daily activities may build confidence and reduce fear of falling.53
In addition to minimizing barriers to exercise, interventions may also focus on increasing people's ability to successfully exercise even in the presence of barriers. Our results revealed that although a greater percentage of participants in the nonexercise group endorsed low outcome expectation, lack of time, and fear of falling than participants in the exercise group, some participants in the exercise group (albeit fewer) also endorsed these barriers but still managed to successfully engage in regular exercise. Previous research identified an association between self-efficacy and participation in exercise in the general population, in people with chronic disabilities, and in people with PD.18,58,59 Self-efficacy related to exercise refers to people's judgment of their capability to exercise successfully.60 People with high self-efficacy are more likely to engage in exercise even in the presence of perceived barriers.25 Behavioral change interventions targeting self-efficacy (eg, goal setting, overcoming barriers to exercise, social support, and monitoring progress) have been shown to increase physical activity in people with chronic disabilities, such as multiple sclerosis,61 and show promise as potential effective interventions in people with PD.62 Other studies of people with PD have suggested that a patient-centered approach, in which therapists work in partnership with people to facilitate a better understanding of the goals and outcomes expected from exercise, also may help to increase self-efficacy.31,63
The results of the present study may have been influenced by differences in the characteristics of the exercise and nonexercise groups. For example, the nonexercise group had a longer duration of PD; however, disease severity, as measured by H&Y stage, did not differ between the exercise group and the nonexercise group. In addition, the nonexercise group had higher GDS scores, suggesting a greater prevalence of depression in the nonexercise group than in the exercise group. However, the GDS scores of both groups were below the point range associated with mild to moderate depressive symptoms in people with PD.64 Disease severity and depression were assessed indirectly in the logistic regression analysis by the respective barriers “If my health were better, I would be more active” and “It is difficult to exercise if I feel depressed.” Neither of these barriers remained significant in the multivariate analysis. Furthermore, our secondary analyses did not reveal any differences in barriers to exercise according to sex, disease severity, or age. These data suggested that low outcome expectation, lack of time, and fear of falling were important barriers to exercise irrespective of sex, age, or disease severity.
There are several limitations of the present study. First, we are presenting the results of a cross-sectional analysis; therefore, a causal relationship between barriers to exercise and exercise participation cannot be assumed. Second, our participants were predominantly white, had a high socioeconomic status, were healthy, and exercised; these factors may limit the generalization of our results. Although the nonexercise group comprised 96 people, this number represented only 37% of our participants—a percentage considerably lower than the 64% of participants who had PD and were sedentary in the ParkFit study.65 Participants in the present study tended to be active in the PD community, having participated in local support groups, wellness programs, or other research activities; these factors may have led to potential selection bias. The proportion of people with PD in the general population who do not exercise likely is greater. Third, assignment to the exercise group or the nonexercise group was based on a retrospective self-report scale; however, data from the PASE and SAM were used to validate this distinction. Fourth, we did not categorize whether participants engaged in prescribed exercise, self-directed exercise, or group exercise. This distinction may have influenced exercise behavior.
Most studies, including the present study, have identified barriers to exercise in a cross-sectional manner. A distinction between barriers that interfere with initiating exercise and those that interfere with maintaining exercise is often not made when a behavior is observed at only 1 point in time and previous exercise participation is not clearly defined. It is plausible that barriers that limit sustained exercise in people who already exercise may differ from barriers that interfere with initiating exercise. A recent study of people with chronic health conditions began to make this distinction and explicitly set out to examine barriers to maintaining exercise in people who already engaged in regular exercise.66 Additional studies are needed to examine this distinction further to determine whether the targets of interventions differ. Moreover, controlled trials are needed to identify the most effective intervention strategies for reducing barriers to exercise and for facilitating changes in exercise behavior in people with PD and in people with other chronic disabling conditions.
Conclusions
Exercise is known to reduce disability and improve quality of life in people with PD. However, limited participation in exercise reduces the potential of continuous exercise to mitigate disability in people with PD. The purpose of the present study was to identify barriers to exercise in people who have PD and dwell in the community. Our results revealed that low outcome expectation from exercise, lack of time to exercise, and fear of falling were important perceived barriers to engaging in exercise in people who have PD, are ambulatory, and dwell in the community. These may be important issues for rehabilitation providers to target in people who have PD and are not exercising regularly.
The Bottom Line
What do we already know about this topic?
Exercise is known to reduce disability and improve quality of life in people with Parkinson disease. Many individuals with Parkinson disease, however, do not adhere to exercise over the long-term, reducing the potential benefit. Although barriers to exercise have been investigated in adults who are healthy, little is known about the barriers to exercise in people with Parkinson disease.
What new information does this study offer?
This study revealed that low outcome expectation of exercise, lack of time to exercise, and fear of falling appear to be important barriers to engaging in exercise among ambulatory, community-dwelling people with Parkinson disease.
If you're a patient, what might these findings mean for you?
When developing your exercise program, your physical therapist can target these barriers in order to help you engage in regular exercise.
Footnotes
Dr Ellis, Dr Cavanaugh, Dr Ford, Dr Foreman, and Dr Dibble provided concept/idea/research design. Dr Ellis, Dr Boudreau, Dr Cavanaugh, Dr Ford, Dr Foreman, and Dr Dibble provided writing. All authors provided data collection. Dr Ellis, Dr Boudreau, Dr Ford, and Dr Foreman provided data analysis. Dr Ellis, Dr DeAngelis, Dr Foreman, and Dr Dibble provided project management. Dr Ellis, Dr Ford, Dr Foreman, and Dr Dibble provided fund procurement. Dr Ellis, Dr DeAngelis, Dr Earhart, Dr Foreman, and Dr Dibble provided study participants. Dr Ellis, Dr Earhart, Dr Ford, Dr Foreman, and Dr Dibble provided facilities/equipment. Dr Ford provided institutional liaisons. Dr DeAngelis provided clerical support. Dr DeAngelis, Dr Earhart, Dr Foreman, and Dr Dibble provided consultation (including review of manuscript before submission).
This study was approved by the institutional review boards of all of the institutions.
This work was previously presented in a Poster Presentation titled “Barriers to Exercise in Persons with Parkinson Disease” at the American Physical Therapy Association Combined Sections Meeting; February 11, 2012; Chicago, Illinois.
Funding for this project was provided by the Davis Phinney Foundation, the Parkinson's Disease Foundation, and NIH K12 Building Interdisciplinary Research in Women's Health (HD43444).
References
- 1. Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640 [DOI] [PubMed] [Google Scholar]
- 2. Keus SH, Bloem BR, Hendriks EJ, et al. Evidence-based analysis of physical therapy in Parkinson's disease with recommendations for practice and research. Mov Disord. 2007;22:451–460 [DOI] [PubMed] [Google Scholar]
- 3. Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson's disease: a meta-analysis of the effect of exercise and motor training. Mov Disord. 2011;26:1605–1615 [DOI] [PubMed] [Google Scholar]
- 5. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schenkman M, Cutson TM, Kuchibhatla M, et al. Exercise to improve spinal flexibility and function for people with Parkinson's disease: a randomized, controlled trial. J Am Geriatr Soc. 1998;46:1207–1216 [DOI] [PubMed] [Google Scholar]
- 7. Schenkman M, Hall DA, Barón AE, et al. Exercise for people in early- or mid-stage Parkinson disease: a 16-month randomized controlled trial. Phys Ther. 2012;92:1395–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dibble LE, Hale TF, Marcus RL, et al. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. 2006;21:1444–1452 [DOI] [PubMed] [Google Scholar]
- 9. Marras C, McDermott MP, Rochon PA, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93 [DOI] [PubMed] [Google Scholar]
- 10. Morgante L, Salemi G, Meneghini F, et al. Parkinson disease survival: a population-based study. Arch Neurol. 2000;57:507–512 [DOI] [PubMed] [Google Scholar]
- 11. Willis AW, Schootman M, Evanoff BA, et al. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011;77:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tomlinson CL, Patel S, Meek C, et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database Syst Rev. 2012;(7):CD002817. [DOI] [PubMed] [Google Scholar]
- 13. Ashburn A, Fazakarley L, Ballinger C, et al. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Nimwegen M, Speelman AD, Hofman-van Rossum EJ, et al. Physical inactivity in Parkinson's disease. J Neurol. 2011;258:2214–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Forkan R, Pumper B, Smyth N, et al. Exercise adherence following physical therapy intervention in older adults with impaired balance. Phys Ther. 2006;86:401–410 [PubMed] [Google Scholar]
- 16. Sluijs EM, Kok GJ, van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73:771–782 [DOI] [PubMed] [Google Scholar]
- 17. Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39:1056–1061 [DOI] [PubMed] [Google Scholar]
- 18. Trost SG, Owen N, Bauman AE, et al. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001 [DOI] [PubMed] [Google Scholar]
- 19. Schoenborn CA, Vickerie JL, Powell-Griner E. Health characteristics of adults 55 years of age and over: United States, 2000–2003. Adv Data. 2006;370:1–31 [PubMed] [Google Scholar]
- 20. Resnick B, Palmer MH, Jenkins LS, Spellbring AM. Path analysis of efficacy expectations and exercise behaviour in older adults. J Adv Nurs. 2000;31:1309–1315 [DOI] [PubMed] [Google Scholar]
- 21. Schmidt JA, Gruman C, King MB, Wolfson LI. Attrition in an exercise intervention: a comparison of early and later dropouts. J Am Geriatr Soc. 2000;48:952–960 [DOI] [PubMed] [Google Scholar]
- 22. Dishman RK, Sallis JF, Orenstein DR. The determinants of physical activity and exercise. Public Health Rep. 1985;100:158–171 [PMC free article] [PubMed] [Google Scholar]
- 23. O'Neill K, Reid G. Perceived barriers to physical activity by older adults. Can J Public Health. 1991;82:392–396 [PubMed] [Google Scholar]
- 24. Rimmer JH, Wang E, Smith D. Barriers associated with exercise and community access for individuals with stroke. J Rehabil Res Dev. 2008;45:315–322 [DOI] [PubMed] [Google Scholar]
- 25. Stroud N, Minahan C, Sabapathy S. The perceived benefits and barriers to exercise participation in persons with multiple sclerosis. Disabil Rehabil. 2009;31:2216–2222 [DOI] [PubMed] [Google Scholar]
- 26. Ablah E, Haug A, Konda K, et al. Exercise and epilepsy: a survey of Midwest epilepsy patients. Epilepsy Behav. 2009;14:162–166 [DOI] [PubMed] [Google Scholar]
- 27. Nilsson MH, Drake AM, Hagell P. Assessment of fall-related self-efficacy and activity avoidance in people with Parkinson's disease. BMC Geriatr. 2010;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bloem BR, Grimbergen YA, Cramer M, et al. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001;248:950–958 [DOI] [PubMed] [Google Scholar]
- 29. Shulman LM, Gruber-Baldini AL, Anderson KE, et al. The evolution of disability in Parkinson disease. Mov Disord. 2008;23:790–796 [DOI] [PubMed] [Google Scholar]
- 30. Keus SH, Bloem BR, Verbaan D, et al. Physiotherapy in Parkinson's disease: utilisation and patient satisfaction. J Neurol. 2004;251:680–687 [DOI] [PubMed] [Google Scholar]
- 31. Quinn L, Busse M, Khalil H, et al. Client and therapist views on exercise programmes for early-mid stage Parkinson's disease and Huntington's disease. Disabil Rehabil. 2010;32:917–928 [DOI] [PubMed] [Google Scholar]
- 32. Dibble LE, Cavanaugh JT, Earhart GM, et al. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486 [DOI] [PubMed] [Google Scholar]
- 34. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442 [DOI] [PubMed] [Google Scholar]
- 35. Dick JP, Guiloff RJ, Stewart A, et al. Mini-Mental State Examination in neurological patients. J Neurol Neurosurg Psychiatry. 1984;47:496–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamei S, Hara M, Serizawa K, et al. Executive dysfunction using behavioral assessment of the dysexecutive syndrome in Parkinson's disease. Mov Disord. 2008;23:566–573 [DOI] [PubMed] [Google Scholar]
- 37. Courneya KS. Understanding readiness for regular physical activity in older individuals: an application of the theory of planned behavior. Health Psychol. 1995;14:80–87 [DOI] [PubMed] [Google Scholar]
- 38. Marcus BH, Simkin LR. The stages of exercise behavior. J Sports Med Phys Fitness. 1993;33:83–88 [PubMed] [Google Scholar]
- 39. Guirao-Goris JA, Cabrero-García J, Moreno Pina JP, Muñoz-Mendoza CL. Structured review of physical activity measurement with questionnaires and scales in older adults and the elderly [in Spanish]. Gac Sanit. 2009;23:334.e1–334.e17 [DOI] [PubMed] [Google Scholar]
- 40. Washburn RA, McAuley E, Katula J, et al. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651 [DOI] [PubMed] [Google Scholar]
- 41. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162 [DOI] [PubMed] [Google Scholar]
- 42. Resnick B, Nahm ES, Orwig D, et al. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9:275–290 [PubMed] [Google Scholar]
- 43. Bergman RJ, Bassett DR, Jr, Muthukrishnan S, Klein DA. Validity of 2 devices for measuring steps taken by older adults in assisted-living facilities. J Phys Act Health. 2008;5(suppl)1:S166–S175 [DOI] [PubMed] [Google Scholar]
- 44. Schmidt AL, Pennypacker ML, Thrush AH, et al. Validity of the StepWatch Step Activity Monitor: preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J Geriatr Phys Ther. 2011;34:41–45 [DOI] [PubMed] [Google Scholar]
- 45. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49 [DOI] [PubMed] [Google Scholar]
- 46. Devereaux Melillo K, Williamson E, Futrell M, Chamberlain C. A self-assessment tool to measure older adults' perceptions regarding physical fitness and exercise activity. J Adv Nurs. 1997;25:1220–1226 [DOI] [PubMed] [Google Scholar]
- 47. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Burton LC, Shapiro S, German PS. Determinants of physical activity initiation and maintenance among community-dwelling older persons. Prev Med. 1999;29:422–430 [DOI] [PubMed] [Google Scholar]
- 49. King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults: a critical review and recommendations. Am J Prev Med. 1998;15:316–333 [DOI] [PubMed] [Google Scholar]
- 50. Koerts J, Van Beilen M, Tucha O, et al. Executive functioning in daily life in Parkinson's disease: initiative, planning and multi-task performance. PLoS One. 2011;6:e29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson's disease: progression to dementia. Mov Disord. 2006;21:1343–1349 [DOI] [PubMed] [Google Scholar]
- 52. Bassett SS. Cognitive impairment in Parkinson's disease. Prim Psychiatry. 2005;12:50–55 [Google Scholar]
- 53. Legters K. Fear of falling. Phys Ther. 2002;82:264–272 [PubMed] [Google Scholar]
- 54. Franchignoni F, Martignoni E, Ferriero G, Pasetti C. Balance and fear of falling in Parkinson's disease. Parkinsonism Relat Disord. 2005;11:427–433 [DOI] [PubMed] [Google Scholar]
- 55. Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson's disease. Mov Disord. 2003;18:496–502 [DOI] [PubMed] [Google Scholar]
- 56. Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson's disease: a 1-year prospective study. J Neurol. 2009;256:1689–1695 [DOI] [PubMed] [Google Scholar]
- 57. Cakit BD, Saracoglu M, Genc H, et al. The effects of incremental speed-dependent treadmill training on postural instability and fear of falling in Parkinson's disease. Clin Rehabil. 2007;21:698–705 [DOI] [PubMed] [Google Scholar]
- 58. Motl RW, Snook EM, McAuley E, et al. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006;32:154–161 [DOI] [PubMed] [Google Scholar]
- 59. Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91:1838–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Worth Publishers; 1997 [Google Scholar]
- 61. Motl RW, Dlugonski D. Increasing physical activity in multiple sclerosis using a behavioral intervention. Behav Med. 2011;37:125–131 [DOI] [PubMed] [Google Scholar]
- 62. Ellis T, Latham NK, DeAngelis TR, et al. Feasibility of a virtual exercise coach to promote walking in community-dwelling persons with Parkinson disease. Am J Phys Med Rehabil. Epub ahead of print April 2, 2013. doi: 10.1097/PHM.0b013e31828cd466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ene H, McRae C, Schenkman M. Attitudes toward exercise following participation in an exercise intervention study. J Neurol Phys Ther. 2011;35:34–40 [DOI] [PubMed] [Google Scholar]
- 64. Rojo A, Aguilar M, Garolera MT, et al. Depression in Parkinson's disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10:23–28 [DOI] [PubMed] [Google Scholar]
- 65. van Nimwegen M, Speelman AD, Smulders K, et al. Design and baseline characteristics of the ParkFit study, a randomized controlled trial evaluating the effectiveness of a multifaceted behavioral program to increase physical activity in Parkinson patients. BMC Neurol. 2010;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Malone LA, Barfield JP, Brasher JD. Perceived benefits and barriers to exercise among persons with physical disabilities or chronic health conditions within action or maintenance stages of exercise. Disabil Health J. 2012;5:254–260 [DOI] [PubMed] [Google Scholar]


