Abstract
Objective
To identify subsets of chronic obstructive pulmonary disease (COPD) patients who are more protected from exacerbations with the use of an inhaled corticosteroid/long-acting β2 agonist (ICS/LABA) combination, compared with the use of LABA monotherapy.
Design
Post hoc cluster analysis of patients from two randomised clinical trials of salmeterol/fluticasone propionate (SFC) and salmeterol (SAL) that had primary endpoints of moderate/severe exacerbation rates.
Setting
Centres in North America.
Participants
1543 COPD patients were studied.
Interventions
SFC 50/250 µg or SAL 50 µg, twice daily.
Primary and secondary outcome measures
The analysis identified clusters of COPD patients more responsive to SFC versus SAL with respect to the annual rate of moderate/severe exacerbations and compared their baseline clinical characteristics.
Results
Overall, SFC significantly reduced the annual rate of moderate/severe exacerbations as compared with SAL alone (rate ratio (RR)=0.701, p<0.001). Three-patient clusters were identified: COPD patients receiving diuretics (RR=0.56, p<0.001); patients not receiving diuretics but with forced expiratory volume in 1 s (FEV1) reversibility ≥12% (RR=0.67, p<0.001) exhibited a substantial reduction in the annual rate of moderate/severe exacerbations relative to SAL. A third cluster, consisting of patients not receiving diuretics and without FEV1 reversibility, demonstrated no difference for SFC versus SAL. Patients receiving diuretics had a significantly higher prevalence of comorbid cardiovascular disease.
Conclusions
COPD patients receiving diuretics and those not receiving diuretics but with FEV1 reversibility >12% at baseline were significantly more likely to experience a reduction in COPD-associated exacerbations with SFC versus SAL alone.
Trial registration
Keywords: Exacerbation, Long acting beta2 agonist, inhaled corticosteroid
Article summary.
Article focus
This paper describes a cluster analysis of a pooled cohort of chronic obstructive pulmonary disease (COPD) patients receiving salmeterol (SAL) alone or in combination with fluticasone propionate (SFC) for 1 year. The analysis sought to identify clusters of patients who could benefit most from the addition of fluticasone propionate to their long-acting bronchodilator therapy based on the annual rates of moderate/severe exacerbations.
Key message
Three clusters were identified. Two clusters, including patients receiving diuretics and those not receiving diuretics but with baseline bronchodilator reversibility of ≥12%, exhibited a significantly greater reduction in exacerbations when treated with SFC versus SAL. No difference was seen between treatments in the third patient cluster—persons without bronchodilator reversibility and not receiving diuretics. These analyses highlight two strata of COPD patients who may be more likely to benefit from inhaled corticosteroid therapy combined with a long-acting β2 agonist bronchodilator.
Strengths and limitations of this study
Pooled and systematically collected data from >1500 well-characterised patients from two randomised controlled trials were used in the analysis, which was validated using half of the study population. The conclusions are limited by the uncertainty of extrapolating results derived from participants enrolled in a randomised clinical trial in which exacerbation in the previous year was an entry requirement for COPD patients in the general population.
Introduction
The Global Strategy for the Diagnosis, Management and Prevention of COPD (GOLD) was revised in 2011 to reflect that Forced Expiratory Volume in 1 s (FEV1) alone is an insufficient measure of disease severity.1 Importantly, the revised GOLD strategy document also recommends therapy with an inhaled corticosteroid/long-acting β2 agonist (ICS/LABA) combination, or a long-acting muscarinic antagonist, for patients at risk of two or more exacerbations per year, even in the presence of mild airflow limitation. This recommendation reflects the established associations between frequent exacerbations, more rapid decline in lung function2 and greater impairment of health status.3 4
Chronic obstructive pulmonary disease (COPD) is a complex and heterogeneous disease with pulmonary and extra-pulmonary manifestations.5 Significant inroads have recently been made in understanding clinical subtypes and their pathophysiology,6 and how they may contribute to the development of a customised approach to therapeutic intervention based on the patient's individual COPD phenotype.7 Han et al7 have advocated the following process for selection of a COPD phenotype: identify a candidate phenotype, determine its relevance to clinical outcomes and then validate it with longitudinal data collection in carefully characterised patient groups. An example of such a phenotype established through this process is that of the ‘frequent exacerbator’ identified in the ECLIPSE cohort. In that analysis, the presence of two or more exacerbations in the previous year was shown to strongly predict the occurrence of an exacerbation in the coming year.8
Statistical techniques may assist in the identification of COPD phenotypes, with cluster analysis being the most commonly used approach.9–12 Cluster analysis uses algorithms to group a patient population, without an a priori hypothesis, into cohorts where those in the same group are more similar to each other than they are to those in other groups. This is in contrast to subgroup analysis, where populations are predefined and statistical testing is applied to identify differences.13
In the present study, cluster analysis was conducted using data pooled from two clinical trials that studied differences in exacerbation rates in COPD patients randomly assigned either to LABA (salmeterol (SAL)) or to ICS/LABA (salmeterol/fluticasone propionate (SFC)).14 15 The objective of this cluster analysis was to identify patients who benefit most from the addition of ICS to bronchodilator therapy in terms of the reduction of the mean annual rate of moderate/severe exacerbations for SFC compared with SAL.
Methods
Clinical study design and subjects
The methodology for the two clinical trials has been previously published.14 15 These were randomised, double-blind, parallel group studies comparing twice-daily SFC 50/250 µg (Seretide, Advair, GlaxoSmithKline, Research Triangle Park, North Carolina, USA) with SAL 50 µg via DISKUS (Serevent, GlaxoSmithKline, Research Triangle Park, North Carolina, USA) on the annual rate of moderate/severe exacerbations in patients with COPD.
Subjects in the USA and Canada were aged 40 years or more, with a clinical history of COPD, a pre-bronchodilator FEV1 ≤50% of predicted, a pre-bronchodilator FEV1/forced vital capacity ratio of ≤70%, a cigarette smoking history of ≥10 pack-years, and a documented history of at least one moderate or severe COPD exacerbation in the year prior to screening. A moderate exacerbation was defined as one requiring outpatient antibiotic and/or oral corticosteroid use, and a severe exacerbation was defined as one requiring hospitalisation. Current and former smokers were included. The key exclusion criteria were a current diagnosis of asthma based on the American Thoracic Society standards for diagnosis,16 other active chronic respiratory disorders apart from COPD, a moderate/severe exacerbation that had not resolved prior to visit 1, or concurrent use of anticholinergics, theophyllines and leukotriene modifiers, or a history or current significant health conditions that could affect subject safety or effectiveness evaluation if the condition exacerbates during the study. Participants with a history of or current clinically significant cardiac arrhythmias, uncontrolled/unstable congestive heart failure, uncontrolled hypertension or unstable angina were excluded from the study. Participants (n=36) with protocol violations or missing data required for the primary model were excluded (n=1543 analysed vs n=1579).
Cluster analysis methodology
The annual moderate/severe exacerbation rate was entered into a cluster analysis using an interaction tree algorithm to maximise the identification of subgroups showing differences in their response to SFC and SAL treatment.13
The cluster analysis aimed to find subgroups in the study subjects that had similar baseline characteristics and with maximum treatment differences for mean yearly moderate/severe exacerbation rate ratio (RR).
Subjects included in the cluster analysis were required to have the following baseline variables: FEV1% predicted, FEV1 reversibility stratum (yes/no for ≥12% improvement and ≥200 ml), time on treatment and geographical region. Reversibility following the administration of four puffs of albuterol was determined prior to randomisation to treatment, following completion of the 4-week FSC 250/50 run-in period. Missing values for the remaining baseline variables were imputed during cluster analysis as the median for continuous/ordinal variables or as the most frequent value for categorical variables. The baseline characteristics are listed in table 1. Baseline medications were classified by the Anatomical Therapeutic Chemical (ATC) Classification System, controlled by the World Health Organization. Medications are classified based on the organ or system they affect and/or their therapeutic and chemical characteristics.17 When medications could have more than one ATC code, the second-level ATC code corresponding to the patient-supplied indication was evaluated to classify the medication (eg, aspirin as a platelet inhibitor vs analgesic would be assigned to B01 Antithrombotic vs N02 Analgesic).
Table 1.
Baseline characteristics and other variables employed in the cluster analysis
| Variable | |
|---|---|
| Demographics | Age (years) |
| Gender | |
| Smoking status (current/former) | |
| Pack-years | |
| Body mass index (m/kg2) | |
| Lung function/QOL | FEV1% predicted |
| FEV1% reversibility | |
| FEV1/FVC ratio postalbuterol | |
| FVC % predicted | |
| SGRQ Activity score | |
| SGRQ Impact score | |
| SGRQ Symptom score | |
| SGRQ Total score | |
| COPD history | Duration of COPD (years) |
| Chronic bronchitis (self-reported, yes/no) | |
| Emphysema (self-reported, yes/no) | |
| Exacerbations requiring hospitalisation (past 12 months) | |
| Exacerbations requiring OCS/antibiotics (past 12 months) | |
| Gold Stage indicator variables based on lung function (II, III/IV) | |
| Medications (ATC classification) | Agents acting on the renin-angiotensin system (angiotensin converting enzyme inhibitors) |
| Anti-anaemic preparations | |
| Anti-haemorrhagics | |
| Anti-histamines | |
| Anti-hypertensives | |
| Anti-thrombotics | |
| Anti-inflammatory and antirheumatic products | |
| β-blockers | |
| Bone disease (including muscle pain) medications | |
| Calcium channel blockers | |
| Cardiac therapies | |
| Diabetes medications | |
| Diuretics | |
| Lipid-modifying agents | |
| Psychoanaleptics | |
| Psycholeptics | |
| Vasodilators | |
ATC, Anatomical Therapeutic Chemical; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; OCS, oral corticosteroid; QOL, quality of life; SGRQ, St George's Respiratory Questionnaire.
Baseline characteristics were examined before inclusion in the model to ensure that there was no significant co-linearity that may influence the cluster analysis. Co-linearity was assessed by creating a correlation tree, and any two variables with an R2 ≥0.7 were examined. The variables considered most clinically relevant were retained. St George's Respiratory Questionnaire Impact and Activity scores were removed from the cluster analysis since they correlated highly with the total score.
Modelling to define the tree: supervised analysis
Modified recursive partitioning techniques were used to perform the supervised subgroup analysis. The frequency of each variable was examined to identify sparse values prior to inclusion in the tree. As the minimal subgroup size (terminal node) was set at 100, all categorical variables were required to have at least 100 participants in a response category in order to be considered for the recursive partitioning algorithm. Variables with several responses were collapsed into fewer categories as appropriate, such that all categories had at least 100 participants (eg, exacerbations requiring hospitalisation in the previous year) or were eliminated from consideration during the cluster analysis (race, anti-haemorrhagics, anti-hypertensives (eg, anti-adrenergics and smooth muscle agents), vasodilators and vasoprotectives (eg, topical haemorrhoid treatments and anti-varicose therapy)).
The best split of the tree was determined by maximising the subgroups according to the treatment interaction effect, and the subgroup membership was then assigned to each patient based on the selected tree. Internal validation was performed by using a split sample, so that a random sample of 50% of the patients was selected to create the tree and the remaining half was used for the computation of rate ratios and CIs to test statistical significance.
Generalised linear models using a negative-binomial function were used to compare the likelihood of having an exacerbation by examining the treatment by subgroup interaction. The model was adjusted for study baseline FEV1% predicted, FEV1 reversibility stratum (yes/no for ≥12% and 200 ml post-bronchodilator change), time on treatment and geographical region (eight regions), which was considered a random effect. The algorithm used in the study maximises treatment differences (mean moderate/severe exacerbation rates for SFC vs SAL) among subgroups. The RR for each cluster was estimated using linear contrast, and rate ratios plus 95% CIs were used to estimate the differences in annual mean moderate/severe exacerbation rates for each cluster. The programme was completed in the R statistical package.18
Clusters were clinically characterised based on the tree. Descriptive statistics were used to present the baseline differences in clinical features among clusters; proportions were used for categorical variables, and medians with interquartile ranges (IQRs) were used for continuous variables. The χ2 test was used to examine the statistical differences among the subgroups for categorical variables and the non-parametric Wilcoxon rank sum test was performed to test the statistical differences among the subgroups for continuous variables.
Results
Pooled demographics and efficacy
The baseline characteristics of the pooled population were well matched between those receiving SFC and those receiving SAL (table 2). The majority of patients reported a moderate and not a severe exacerbation in the 12 months prior to study. Thirty-seven per cent of patients had two or more moderate exacerbations and 2% had two or more severe exacerbations.
Table 2.
Demographic and baseline clinical characteristics of participants participating in the primary clinical studies of SFC versus SAL (cluster analysis population)
| Demographic characteristics | SFC 50/250 µg | SAL | Total |
|---|---|---|---|
| N=771 | N=772 | N=1543 | |
| Age, median years (IQR) | 65 (59–72) | 65 (59–71.5) | 65 (59–72) |
| Gender, male/female ratio | 54/46 | 54/46 | 54/46 |
| Race, n (%) | |||
| Caucasian | 94 | 94 | 94 |
| Non-Caucasian | 6 | 6 | 6 |
| Body mass index, mean m/kg2 (IQR) | 27 (23–31) | 27 (23–30) | 27 (23–31) |
| Smoking history (%) | |||
| Former | 59 | 59 | 59 |
| Current | 41 | 41 | 41 |
| Exacerbations requiring hospitalisation (past year) (%) | |||
| 0 | 78 | 76 | 77 |
| 1 | 20 | 22 | 21 |
| ≥2 | 3 | 2 | 2 |
| Exacerbations requiring oral steroids/antibiotics (past year) (%) | |||
| 0 | <1 | 1 | 1 |
| 1 | 65 | 60 | 63 |
| 2 | 20 | 24 | 22 |
| ≥3 | 14 | 14 | 15 |
| FEV1% predicted (IQR) | 33.1 (25.1–41.8) | 33.8 (24.9–41.9) | 33.6 (25.0–41.9) |
| FEV1% reversibility (IQR) | 20.1 (9.1–33.4) | 18.6 (8.5–30.5) | 18.9 (8.9–31.7) |
| Reversibility stratum* [no/yes] (%) | 58/42 | 61/39 | 60/40 |
| SGRQ Total, mean (IQR) | 46.60 (35.88–59.41) | 48.67 (36.60–60.34) | 47.5 (36.1–59.9) |
*Reversibility based on change in FEV1 from baseline following four puffs (360 µg) albuterol, defined as a ≥12% and ≥200 ml increase.
FEV1, forced expiratory volume in 1 s; IQR, interquartile range; SAL, salmeterol; SFC, salmeterol/fluticasone propionate; SGRQ, St Georges Respiratory Questionnaire.
In the primary studies,14 15 the annual combined moderate or severe exacerbation rates were significantly lower with SFC (1.10 and 1.06) than with SAL (1.59 and 1.53). The risk of a moderate or severe exacerbation among SFC users in the pooled study population was decreased by 30% as compared with those using SAL alone (RR=0.701, p<0.001).
Cluster analysis results
Supervised cluster analysis identified four distinct clusters based on the use of diuretics and the extent of FEV1 reversibility, expressed solely as a percentage of the pre-bronchodilator value. Reversibility was categorised initially into three levels: <11.5%, 11.5–28% and >28%. When maximising differences in response to therapy with SFC versus SAL (data not shown), we pruned the tree at the ≥12% reversibility threshold as 11.5% was close to the ≥12% component of the ERS/ATS threshold for reversibility, although it should be noted that this definition also requires a volume response of ≥200 ml.19 The initial reversibility clusters were otherwise similar with respect to baseline characteristics (data not shown). The final model used to generate the clusters had adjusted for the baseline FEV1% predicted, FEV1 reversibility stratum (yes/no for ≥12% and 200 ml post-bronchodilator change) and region. Only the baseline FEV1% predicted was statistically significant (p<0.01) in the final model.
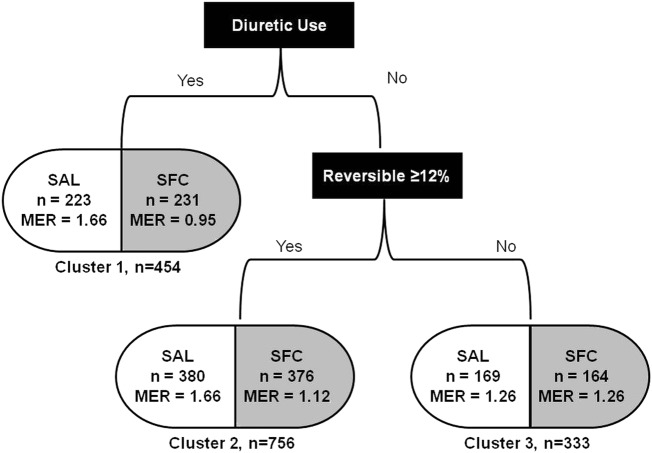
Three final COPD clusters were defined (figure 1) based on the use of diuretics and the presence or absence of ≥12% FEV1 reversibility based on the model. The first cluster (cluster 1) identified participants treated with diuretics (predominantly furosemide). Approximately half of the diuretic use reported (n=282) was for hypertension, the remaining use was for unspecified oedema, coronary artery disease and/or congestive heart failure. No other subpopulations were identified in this cluster. In patients not using diuretics at baseline, two further clusters were defined based on the presence or absence of FEV1 reversibility. Cluster 2 patients exhibited reversibility, defined as a post-bronchodilator change of ≥12%. Cluster 3 patients did not exhibit reversibility, that is, a post-bronchodilator change in FEV1 of <12%. Compared with SAL, significant reductions in the rate of moderate/severe exacerbations were observed with SFC therapy in cluster 1 (44% reduction) and cluster 2 (33% reduction). Similar reductions were not observed in cluster 3 (figure 2).
Figure 1.
Interaction tree generated by supervised cluster analysis. MER, mean annual rate of moderate/severe exacerbations; SAL, salmeterol; SFC, salmeterol/fluticasone propionate combination.
Figure 2.
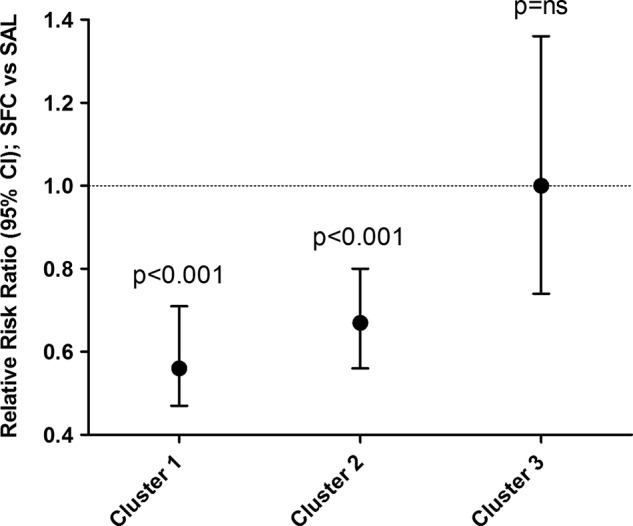
Pooled analysis of SFC effect on the mean annual moderate/severe exacerbation rate by cluster. Ns, not significant (p>0.05); SAL, salmeterol; SFC, salmeterol/fluticasone propionate combination.
Baseline demographics that were significantly different across clusters are presented in table 3. Participants in cluster 1 tended to be older, had a higher body mass index (BMI), were more likely to be former smokers than current smokers and had the greatest smoking pack-year history. Participants in cluster 1 also had a higher prevalence of treatment for comorbidities (eg, cardiovascular disease (CVD), hypertension and diabetes) than those in clusters 2 and 3. Subjects in cluster 3 had a higher % predicted FEV1 compared with those in clusters 1 and 2, whereas those in cluster 2 had the lowest % predicted FEV1. No difference was observed in the baseline incidence of moderate/severe exacerbations between the clusters.
Table 3.
Baseline characteristics of interest according to the cluster group
| Covariate | Cluster 1: diuretic (N=454) | Cluster 2: reversible, no diuretic (N=756) | Cluster 3: not reversible, no diuretic (N=333) | p Value |
|---|---|---|---|---|
| Age, median years (IQR) | 67 (62–74) | 64 (58–70) | 65 (59–71) | <0.0001 |
| Body mass index, median m/kg2 (IQR) | 28 (25–34) | 26 (23–30) | 25 (22–29) | <0.0001 |
| Smoking status (%) | ||||
| Former | 65 | 58 | 53 | 0.0024 |
| Current | 35 | 42 | 47 | |
| Smoking, mean pack-years (IQR) | 52 (40–77) | 50 (37–70) | 48.5 (36–70) | 0.0401 |
| FEV1% predicted (SD) | 33.9 (25.1–42.6) | 31.3 (23.9–39.4) | 37.7 (29.0–44.6) | <0.0001 |
| FEV1% reversibility (SD) | 18.55 (7.40–31.70) | 26.25 (18.60–38.20) | 4.50 (-1.00–8.70) | <0.0001 |
| Reversibility stratum* [no/yes] (%) | 60/40 | 41/59 | 100/0 | <0.0001 |
| Exacerbations requiring hospitalisation (past year) (%) | ||||
| 0 | 73.8 | 78.9 | 76.3 | ns |
| 1 | 23.6 | 19.5 | 19.8 | |
| 2 | 2.6 | 1.6 | 3.9 | |
| Exacerbations requiring oral steroids/antibiotics (past year) (%) | ||||
| 1 | 62.6 | 61.1 | 67.0 | ns |
| 2 | 20.0 | 25.0 | 19.2 | |
| 3 | 8.1 | 7.7 | 7.2 | |
| Baseline medications (%) | ||||
| Diuretics | 100 | 0.0 | 0.0 | <0.0001 |
| Anti-thrombotics | 50.7 | 32.0 | 40.2 | <0.0001 |
| ACE inhibitors | 50.0 | 26.7 | 30.6 | <0.0001 |
| Lipid modifiers | 49.3 | 28.7 | 33.6 | <0.0001 |
| Calcium channel blockers | 33.5 | 16.3 | 14.1 | <0.0001 |
| Psycholeptics | 32.6 | 21.4 | 24.0 | <0.0001 |
| Anti-histamines | 30.4 | 22.4 | 23.7 | 0.0062 |
| β-blockers | 24.0 | 10.8 | 12.9 | <0.0001 |
| Cardiac therapy | 23.1 | 8.6 | 7.2 | <0.0001 |
| Diabetes | 17.4 | 7.8 | 8.4 | <0.0001 |
| Anti-anaemics | 13.2 | 5.8 | 3.6 | <0.0001 |
| Anti-hypertensives | 7.3 | 3.2 | 1.5 | <0.0001 |
*Reversibility based on change in FEV1 from baseline following four puffs (360 µg) albuterol, defined as a ≥12% and ≥200 ml increase.
ACE, angiotensin converting enzyme; FEV1, forced expiratory volume in 1 s; IQR, interquartile range; ns, not significant; SD, standard deviation.
Discussion
This study identified three clusters: cluster 1: diuretic users with treatment for cardiovascular comorbidity; cluster 2: reversible, not taking diuretics; cluster 3: not reversible, not taking diuretics. Participants in clusters 1 and 2 benefited from receiving combination therapy with SFC, compared with SAL alone, with a greater reduction in exacerbations. This exercise identified two groups that are more likely to respond to SFC. The largest benefit with SFC was observed in cluster 1, but the difference in RR between clusters 1 and 2 did not quite reach statistical significance.
A number of hypotheses can be put forward to explain the lower exacerbation rates with SFC relative to SAL among diuretic users. The use of diuretics may identify a group of patients with, or at risk of, CVD, such as those with hypertension or heart failure, though with the limited data available from the source studies, this cannot be confirmed. There was a significantly higher use of CVD medications in cluster 1, which suggests a preponderance of CVD diagnosis in this group; it may also suggest the presence of metabolic syndrome, as more patients in cluster 1 were in receipt of statins and ACE inhibitors than those in other clusters; furthermore, the proportion of patients with diabetes was greater in cluster 1 than in other clusters, as was the baseline BMI. Metabolic syndrome is more frequent among COPD than non-COPD patients, reflecting CVD and diabetes to be concurrent with airway obstruction.20
ICS (FP) could exert a benefit on exacerbations in COPD patients with CVD if (1) CVD comorbidity reflects an increased inflammatory state related to COPD21 and (2) if CVD is a driver for COPD exacerbation occurrence22 and severity,23 as has been reported. It is therefore plausible to conjecture that subjects with CVD would exhibit higher levels of inflammation than those without CVD. Inflammation, as demonstrated by the elevated C reactive protein or fibrinogen, increases the risk of a COPD exacerbation,8 24 and this logic supports the value of the addition of an ICS (FP) in cluster 1.
The heterogeneity of COPD is well established6 and it has recently been suggested, through use of a rigorous assessment of comorbidity, that patients with CVD and metabolic syndrome form discrete clusters of COPD patients12 which are represented in our analysis in cluster 1. Another assessment of comorbidity in COPD has found that certain CVDs increase the risk of all-cause mortality in COPD.25 Although the TORCH study failed to show a significant effect (p=0.052) of FP/SAL versus placebo for all-cause mortality,26 a subsequent analysis of CV-related mortality and AEs found a positive effect of FP/SAL versus SAL in terms of CV-related outcomes,27 which further implies a potential benefit of ICS in COPD patients with comorbid CVD.
It is also possible that participants in cluster 1 (diuretic users) were pre-disposed to an increased exacerbation risk as a consequence of heart failure, especially as compared with clusters 2 and 3, more patients in cluster 1 were in receipt of antithrombotics, β-blockers and cardiac therapy, all of which suggest a greater degree of heart failure in cluster 1 compared with the other clusters. Heart failure can be aggravated by increased aortic stiffness, a marker of cardiovascular risk found in greater prevalence among COPD patients than in the general population.28 Dransfield et al29 recently found that SFC lowered aortic pulse wave velocity (aPWV), a marker of aortic stiffness, in COPD patients with elevated aPWV.
Another possible explanation of the lower rate in moderate/severe exacerbations with SFC over SAL in cluster 1 reflects the direct activity of the concomitant diuretic therapy. Recent studies have examined the effectiveness of diuretics in the treatment of chronic respiratory diseases, in particular furosemide (which was the predominantly used diuretic in cluster 1). Mechanistically, furosemide inhibits inflammatory cytokines30 and enhances the anti-inflammatory impact of ICS.31 Clinically, it has been shown to alleviate exertional dyspnoea in COPD32 and to protect against bronchoconstriction in asthma.33–35
The findings in cluster 1 relating to comorbidity in COPD are of particular interest, given the recent publications on the prevalence and impact of comorbidity in COPD12 25 36 and the concept of multiple morbidities and their impact on clinical practice.37 Certainly, our findings and those of others11 12 suggest that patients with multiple diseases may benefit from a different approach to management than those with a single disease, a concept which has recently been raised as a major issue in primary care.38
In cluster 2 (reversibility ≥12%), a significant effect of SFC was also observed over SAL in terms of a lower rate of moderate/severe exacerbations. There was also reversibility in cluster 1 (median, 18.6%). Participants exhibiting reversibility have been shown to have greater improvement in lung function compared with those without reversibility, which could explain the significant effect of SFC relative to SAL in terms of a lower rate of moderate/severe exacerbations.39 Recent data suggest that an improvement in the lung function of 100 ml relates to a reduction in the exacerbation rate of 12%,40 while a 12% increase in the exacerbation rate has been reported for each 100 ml loss of lung function.8 The effect of SFC in COPD41 has been shown to provide a significantly greater effect on lung function in reversible versus irreversible participants. This suggests a potential mechanism for the lower rate of moderate/severe exacerbations in clusters 1 and 2 for patients receiving SFC. The rationale for this is that a greater improvement in lung function is typically associated with a greater effect on exacerbations (eg, Jones et al40).
The role of reversibility as a distinguishing feature in COPD has recently been questioned. While it is apparent that COPD subjects can be more or less reversible, there is considerable within-patient variability both in single testing39 and in testing on multiple occasions.42 Subject reversibility will vary over time, such that over 1 year only 4% of patients were reversible on every occasion tested.42 As such, it has been shown that while the percentage of reversible patients is between 20% and 30% in any given population at any time (as was the case in these studies), the actual patients who are reversible may change. Despite the limitations with reversibility, there is evidence that participants who are more reversible are likely to have a more robust bronchodilator response to treatment than those who are less reversible.39 41 None of the participants in cluster 3 exhibited reversibility by definition, and this, together with the highest prevalence of current smokers (which is known to attenuate ICS effects in COPD),43 may explain why no difference was observed between SFC and SAL in this cluster.
Although the studies excluded participants with current asthma based on the investigator's judgement, participants could have had a history of asthma but not a diagnosis of active asthma. It has been suggested that asthma and COPD form part of the same disease continuum,44 and though this is a controversial concept, the idea of an asthma–COPD overlap syndrome may give insight into the response to combination ICS/LABA.45 46 Forty per cent and 59% of patients in clusters 1 and 2, respectively, were ≥12% reversible and had an increase of ≥200 ml in FEV1. However, the reversibility stratum (≥12% and ≥200 ml) was adjusted for in the overall negative binomial model examining the mean annual exacerbation rates and was found to be not statistically significant, suggesting that any impact of reversibility as determined by both ≥12% and ≥200 ml was minimal.
A number of recent studies have investigated COPD heterogeneity, and have identified independent factors such as dyspnoea, airway inflammation and asthma-like features,10 47 or subgroups associated with differential outcome.48 49 However, only one11 has validated the COPD subtypes identified against clinically meaningful outcomes. Garcia-Aymerich et al11 identified three clusters of subjects, comprising those with severely impaired lung function, those with more mildly impaired lung function and, importantly, those with more mildly impaired lung function and evidence of cardiovascular disorders, obesity, diabetes and systemic inflammation. The clusters identified in the present study align to some extent with those already identified, such as increased reversibility and the presence of CVD. This suggests a convergence of COPD subtypes that warrants further examination.
Cluster analysis is limited due to its retrospective nature and the fact that it is limited to assessing only the categorical variables collected at baseline. In addition, the splitting into groups is automated by the computer-driven algorithm to maximise treatment differences, and is not necessarily robust; thus, external validation is warranted. As this analysis includes only those patients with a history of exacerbation, it is also difficult to generalise to participants with COPD who do not have a history of exacerbation.
In conclusion, a cluster analysis of participants taking part in two exacerbation studies of SFC versus SAL identified three distinct groups of COPD subjects based on diuretic use and reversibility. These participants varied in their response with participants in two of the three groups experiencing a greater reduction in the annual rate of moderate/severe exacerbations with SFC versus SAL. Those in the remaining group received no additional benefit in terms of reduction in the annual moderate/severe exacerbation rate over that provided by SAL alone. This study highlights the future potential for a personalised medicine approach to the treatment of patients with COPD. It additionally suggests how this methodology can be used to generate potential hypotheses for future studies.
Supplementary Material
Acknowledgments
Editorial support in the form of development of the manuscript's first draft under the guidance of the corresponding author and coauthors, editorial suggestions to draft versions of this paper in collaboration with the corresponding author and coauthors, assembling tables and figures, collating author's comments, copyediting, fact checking, referencing and graphic services was provided by Geoff Weller, PhD, at Gardiner-Caldwell Communications. Amanda Emmett, an employee of GlaxoSmithKline, provided consultation regarding the clinical trials data and statistical review of the results and subsequent manuscript. Ms Emmett was the primary statistician on the two COPD exacerbation randomised control trials.
Footnotes
Contributors: All the listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. All authors helped to develop the design and concept of this analysis, had full access to and interpreted the data, and critically reviewed the manuscript and revised it for important intellectual content. All authors vouch for the accuracy and completeness of the data and the data analysis. RLD co-wrote the protocol, led the outline and editing of the manuscript. HL co-wrote the protocol, conducted the analyses and provided the methodological expertise. DBR was involved in planning the analyses and interpreting the results. DAS was also involved in planning the analyses and interpreting the results. All authors have read and approved the final manuscript.
Funding: Funded by GlaxoSmithKline.
Competing interests: All the co-authors are employees and shareholders of GlaxoSmithKline.
Ethics approval: Institutional Review Board approval was granted for the original studies analysed here.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management and prevention of COPD. 2011. http://www.goldcopd.org/ (accessed 1 Jun 2012).
- 2.Celli BR, Thomas NE, Anderson JA, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med 2008;178:332–8 [DOI] [PubMed] [Google Scholar]
- 3.Spencer S, Calverley PM, Burge PS, et al. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J 2004;23:698–702 [DOI] [PubMed] [Google Scholar]
- 4.Jones PW, Tabberer M, Chen WH. Creating scenarios of the impact of COPD and their relationship to COPD Assessment Test (CAT™) scores. BMC Pulm Med 2011;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med 2005;99:670–82 [DOI] [PubMed] [Google Scholar]
- 6.Agusti A, Calverley P, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010;182:598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst JR, Vestbo J, Anzueto A. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New Engl J Med 2010;363:1128–38 [DOI] [PubMed] [Google Scholar]
- 9.Cho MH, Washko GR, Hoffmann TJ, et al. Cluster analysis in severe emphysema subjects using phenotype and genotype data: an exploratory investigation. Respir Res 2010;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy K, Smith J, Kolsum U, et al. COPD phenotype description using principal components analysis. Respir Res 2009;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Aymerich J, Gomez F, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD subtypes). Thorax 2011;66:430–7 [DOI] [PubMed] [Google Scholar]
- 12.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:728–35 [DOI] [PubMed] [Google Scholar]
- 13.Su X, Tsai C-L, Wang H, et al. Tree-based subgroup analysis via recursive partitioning. J Mach Learn Res 2009;10:141–58 [Google Scholar]
- 14.Anzueto A, Feldman G, Chinsky K, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. J COPD 2009;6:320–9 [DOI] [PubMed] [Google Scholar]
- 15.Ferguson GT, Anzueto A, Fei R, et al. Effect of fluticasone propionate/salmeterol (250/50 mcg) or salmeterol (50 mcg) on COPD exacerbations. Respir Med 2008;102:1099–108 [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society Standards for diagnosis and care of patients with chronic obstructive pulmonary disease and asthma. Am Rev Respir Dis 1987;136:225–44 [DOI] [PubMed] [Google Scholar]
- 17.WHO Collaborating Centre for Drug Statistics Methodology Structure and principles of the Anatomical Therapeutic Chemical (ATC) classification system. http://www.whocc.no/atc/structure_and_principles/ (accessed 20 Feb 2013).
- 18.Everitt B. An R and S-PLUS companion to multivariate analysis. London: Springer-Verlag, 2005 [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005;26:948–68 [DOI] [PubMed] [Google Scholar]
- 20.Poulain M, Doucet M, Drapeau V, et al. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis 2008;5:35–41 [DOI] [PubMed] [Google Scholar]
- 21.Man SF, Leipsic JA, Man JP, et al. Is atherosclerotic heart disease in COPD a distinct phenotype? Chest 2011;140:569–71 [DOI] [PubMed] [Google Scholar]
- 22.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest 2007;131:20–8 [DOI] [PubMed] [Google Scholar]
- 23.Chang CL, Robinson SC, Mills GD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011;66:764–8 [DOI] [PubMed] [Google Scholar]
- 24.Groenewegen KH, Postma DS, Hop WC, et al. Increased systemic inflammation is a risk factor for COPD exacerbations. Chest 2008;133:350–7 [DOI] [PubMed] [Google Scholar]
- 25.Divo M, Cote C, de Torres JP, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:155–61 [DOI] [PubMed] [Google Scholar]
- 26.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–89 [DOI] [PubMed] [Google Scholar]
- 27.TORCH CV, Calverley PM, Anderson JA, et al. Cardiovascular events in patients with COPD: TORCH study results. Thorax 2010;65:719–25 [DOI] [PubMed] [Google Scholar]
- 28.Sabit R, Bolton CE, Edwards PH, et al. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:1259–65 [DOI] [PubMed] [Google Scholar]
- 29.Dransfield MT, Cockcroft JR, Townsend RR, et al. Effect of fluticasone propionate/salmeterol on arterial stiffness in patients with COPD. Respir Med 2011;105:1322–30 [DOI] [PubMed] [Google Scholar]
- 30.Yuengsrigul A, Chin TW, Nussbaum E. Immunosuppressive and cytotoxic effects of furosemide on human peripheral blood mononuclear cells. Ann Allergy Asthma Immunol 1999;83:559–66 [DOI] [PubMed] [Google Scholar]
- 31.Prandota J. Furosemide: progress in understanding its diuretic, anti-inflammatory, and bronchodilating mechanism of action, and use in the treatment of respiratory tract diseases. Am J Ther 2002;9:317–28 [DOI] [PubMed] [Google Scholar]
- 32.Jensen D, Amjadi K, Harris-McAllister V, et al. Mechanism of dyspnoea relief and improved exercise after furosemide inhalation in COPD. Thorax 2008;63:606–13 [DOI] [PubMed] [Google Scholar]
- 33.Roger A, Botey J, Esevrri JL, et al. Prevention of exercise-induced asthma in children using low doses of inhaled furosemide. J Invest Allergol Clin Immunol 1993;3:300–3 [PubMed] [Google Scholar]
- 34.Novembre E, Frongia G, Lombardi E, et al. The preventative effect and duration of action of two doses of inhaled furosemide on exercise-induced asthma in children. J Allergy Clin Immunol 1995;96:906–9 [DOI] [PubMed] [Google Scholar]
- 35.Melo RE, Sole D, Naspitz CK. Comparative efficacy of inhaled furosemide and disodium cromoglycate in the treatment of exercise-induced asthma in children. J Allergy Clin Immunol 1997;99:204–9 [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J 2009;33:1165–85 [DOI] [PubMed] [Google Scholar]
- 37.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012; 380:37–43 [DOI] [PubMed] [Google Scholar]
- 38.Guthrie B, Payne K, Alderson P, et al. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012;345:e6341. [DOI] [PubMed] [Google Scholar]
- 39.Hanania N, Celli BR, Donohue JF, et al. Bronchodilator reversibility in COPD. Chest 2011;140:1055–63 [DOI] [PubMed] [Google Scholar]
- 40.Jones PW, Donohue JF, Nedelman J, et al. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res 2011;12:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleecker ER, Emmett A, Crater G, et al. Lung function and symptom improvement with fluticasone propionate/salmeterol and ipratropium bromide/albuterol in COPD: response by beta-agonist reversibility. Pulm Pharmacol Ther 2008;21:682–8 [DOI] [PubMed] [Google Scholar]
- 42.Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax 2012;68:701–8 [DOI] [PubMed] [Google Scholar]
- 43.Soriano JB, Sin DD, Zhang X, et al. A pooled analysis of FEV1 decline in COPD patients randomized to inhaled corticosteroids or placebo. Chest 2007;131:682–9 [DOI] [PubMed] [Google Scholar]
- 44.Postma DS, Boezen HM. Rationale for the Dutch hypothesis. Allergy and airway hyperresponsiveness as genetic factors and their interaction with environment in the development of asthma and COPD. Chest 2004;126(Suppl 2):96S–104S [DOI] [PubMed] [Google Scholar]
- 45.de Oca MM, Halbert RJ, Lopez MV, et al. The chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 2012;40:28–36 [DOI] [PubMed] [Google Scholar]
- 46.Soler-Cataluña JJ, Cosío B, Izquierdo JL, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012;48:331–7 [DOI] [PubMed] [Google Scholar]
- 47.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011;184:662–71 [DOI] [PubMed] [Google Scholar]
- 48.Burgel P-R, Paillasseur J-L, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J 2010;36:531–9 [DOI] [PubMed] [Google Scholar]
- 49.Weatherall M, Travers J, Shirtcliffe PM, et al. Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Repir J 2009;34:812–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.