Abstract
Objective
To assess whether colon cancer follow-up can be organised by general practitioners (GPs) without a decline in the patient's quality of life (QoL) and increase in cost or time to cancer diagnoses, compared to hospital follow-up.
Design
Randomised controlled trial.
Setting
Northern Norway Health Authority Trust, 4 trusts, 11 hospitals and 88 local communities.
Participants
Patients surgically treated for colon cancer, hospital surgeons and community GPs.
Intervention
24-month follow-up according to national guidelines at the community GP office. To ensure a high follow-up guideline adherence, a decision support tool for patients and GPs were used.
Main outcome measures
Primary outcomes were QoL, measured by the global health scales of the European Organisation for Research and Treatment of Cancer QoL Questionnaire (EORTC QLQ C-30) and EuroQol-5D (EQ-5D). Secondary outcomes were cost-effectiveness and time to cancer diagnoses.
Results
110 patients were randomised to intervention (n=55) or control (n=55), and followed by 78 GPs (942 follow-up months) and 70 surgeons (942 follow-up months), respectively. Compared to baseline, there was a significant improvement in postoperative QoL (p=0.003), but no differences between groups were revealed (mean difference at 1, 3, 6, 9, 12, 15, 18, 21 and 24-month follow-up appointments): Global Health; Δ−2.23, p=0.20; EQ-5D index; Δ−0.10, p=0.48, EQ-5D VAS; Δ−1.1, p=0.44. There were no differences in time to recurrent cancer diagnosis (GP 35 days vs surgeon 45 days, p=0.46); 14 recurrences were detected (GP 6 vs surgeon 8) and 7 metastases surgeries performed (GP 3 vs surgeon 4). The follow-up programme initiated 1186 healthcare contacts (GP 678 vs surgeon 508), 1105 diagnostic tests (GP 592 vs surgeon 513) and 778 hospital travels (GP 250 vs surgeon 528). GP organised follow-up was associated with societal cost savings (£8233 vs £9889, p<0.001).
Conclusions
GP-organised follow-up was associated with no decline in QoL, no increase in time to recurrent cancer diagnosis and cost savings.
Trial registration
ClinicalTrials.gov identifier NCT00572143.
Keywords: colorectal cancer, follow-up, health service research, Surgery
Article summary.
Article focus
Intensive follow-up after curative colon cancer resection is associated with improved overall survival of 5–10%.
No international consensus exists regarding the detailed content of a follow-up programme for colorectal cancer.
Quality of life (QoL), cost-effectiveness and patient safety in a general practitioner (GP)-organised follow-up programme are unknown.
Key messages
GP-organised colon cancer follow-up is associated with no decline in QoL, no increase in time to recurrent cancer diagnosis and cost savings.
Strengths and limitations of this study
·Intention to treat analyses with high adherence to the national follow-up programme.
·First trial assessing cost-effectiveness of a GP-organised colon cancer follow-up programme.
·The trial was stopped after 1884 patient follow-up months owing to no impact of the intervention on QoL global health status.
Fifty two per cent of the included patients were followed for 2 years. This limits the interpretation of recurrence, as 80% of the colon cancer recurrences occur within 3 years.
Background
Colon cancer is the third most common cancer in the western world, and surgery is the only curative treatment. Around one-third of the patients resected of colon cancer will experience recurrence of the disease with less than 2 years expected survival.1 2 Despite the generally poor outcomes among patients with recurrent disease, most patients treated with curative intent are included in some form of surveillance programme involving periodic evaluation. Reviews comparing various follow-up programmes have suggested that more intensive strategies tend to increase 5-year survival by detecting relapse about 6 months earlier than less intensive strategies—at a point where the patient will be more likely to be considered a candidate for potentially curative metastases surgery.2–4 However, wide consensus has not been reached regarding what an intensive follow-up strategy should entail.5–8 New surveillance trials in progress are not likely to fully settle the issue.9–12 What none of the available clinical recommendations for follow-up have addressed adequately is the setting where this follow-up should occur: conducted by specialists who originally treated the cancer at hospitals, or in the offices of local GPs.2 Increasingly, the benefits of greater involvement of primary care providers in the ongoing management of chronic illnesses are recognised.13 The level of follow-up care may greatly influence the quality of life (QoL) and costs, especially in rural areas with long distances to travel for hospital services. However, such considerations must be balanced against the imperative that colon cancer survivors receive the best care available. Recently, the UK’s National Cancer Survivorship Initiative recognised the need to develop new models of cancer care that support patient self care, care planning and making the best out of resources.14 In Norway, similar national initiatives have been launched. In this trial, we tested the main hypothesis that patients with colon cancer followed up by their GP would experience similar or higher scores on QoL measures at a lower cost than alternative hospital controls. The other aims were to test for differences of harms and benefits in a follow-up programme, that is, the rate of serious clinical events (SCE), time to diagnosis of SCE and cancer recurrence and frequency of metastases surgery.
Methods
This was a randomised controlled multicentre trial carried out in a North Norway Health Authority trust using a previously published protocol.15 The first patient was included on 1 June 2007, while the last patient was included on 15 December 2011. Interim analyses were performed in June 2012.
Ethics and trial registration
The Regional Committee for Medical Research Ethics, North Norway approved this protocol in 2006 (P REK NORD 79/2006). Patients provided written consent before entering the trial. The trial was registered at ClinicalTrials.gov with identifier NCT00572143. Owing to organisational delay, the trial was registered on 11 December 2007; the specified study start in ClinicalTrials.gov was June 2007.
Inclusion and exclusion criteria
Inclusion criteria were age less than 75 years with recent surgery for colon cancer at Dukes’ stage A, B or C. Patients receiving postsurgical adjuvant chemotherapy (some Dukes’ B and all Dukes’ C) were also eligible. Exclusion criteria were patients older than 75 years, patients belonging to healthcare trust not participating in the trial or those not able to provide informed consent and cancer stage Dukes’ D.
Hospitals, primary and secondary care professionals
Three local hospitals and one university hospital participated. Approximately 100 patients with colon cancer are surgically treated annually at these four hospitals. All 550 GPs in the region received written information, while 448 GPs consented to participate in the trial.
Objective and hypotheses
The primary objective was to compare patients’ QoL and costs of follow-up by their local GP or at the surgical outpatient clinic. The primary hypothesis was that the patients followed up by their GPs would experience similar or better QoL scores (on the global health scale) at a lower cost. The secondary objective was to test whether the incidence of SCE would be similar for patients followed up by their GPs or hospital surgeons the secondary hypothesis being that patients followed up by their GPs would have no delay in detection of relapse and the same frequency of SCEs as controls.
Description of intervention
We defined this as a complex intervention, consisting of several interconnecting parts.16 To ensure high follow-up guideline adherence by patients allocated to GPs’ follow-ups, we used a decision support tool as part of the intervention.17 Thus, the intervention consisted of the following parts:
GP organised colon cancer follow-up: The patients were referred to their GP for postoperative follow-up according to the national guidelines (table 1). Information was given to the GP about surgery, any complications, Dukes’ staging, time and location of chemotherapy (for Dukes’ C patients), and risk of recurrence.
Patient decision-support pamphlet: Received at the baseline consultation, containing information about: (1) their own disease, tumour stage and risk of recurrence; (2) the aim and objective of the trial; (3) the current national follow-up guidelines, that is, schedule and location of carcinoembryonic antigen (CEA) measurements, chest x-ray, contrast-enhanced liver ultrasound, colonoscopy and clinical examination; (4) a detailed description of signs and symptoms of potential recurrence of colon cancer and (5) in case of a SCE between appointments, relevant phone numbers and contact information were given.
GP decision-support pamphlet: Sent at the time of baseline appointment to all GPs who had a patient allocated to their practice. This pamphlet contained information similar to what the patient received, that is, information about follow-up guidelines, signs and symptoms of recurrence and behavioural strategy in the case of suspicion of a recurrence. In case of questions regarding the follow-up, relevant contact information was given.
Table 1.
Norwegian Gastrointestinal Cancer Group (NGICG) 2007 surveillance programme
| Examination | Follow-up cycle (month postoperative) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | |
| Chest x-ray | X | X | X | X | X | X | X | ||||||||
| Liver ultrasonography | X | X | X | X | X | X | X | ||||||||
| Colonoscopy | X | X | |||||||||||||
| CEA measurement | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Clinical examination | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
CEA, carcinoembryonic antigen; red, length of trial participation (24 months, 9 follow-up cycles).
Patients allocated to GP follow-up could be referred back to any surgical clinic at any time during the study period. Similarly, patients in the hospital follow-up group were free to consult their GP at any time. National follow-up guidelines were applied in both study arms and patients were followed for up to 2 years (table 1).
Randomisation
At study entry, patients were seen for a baseline visit by a local trial investigator at the hospital where they received the surgical treatment, approximately 3–4 weeks postoperatively. At this visit, a clinical examination was performed and information about the histology and results of the surgery was shared with each patient. If the patients provided informed consent, they were randomised to follow-up either by their GP (intervention) or at the surgical outpatient clinic (controls) using a web-based randomisation service managed by the Norwegian University of Science and Technology (http://www.ntnu.no). The randomisation ratio was 1:1; patients were stratified according to the Dukes’ staging (A, B and C) and on whether they had a stoma. The local trial investigator was not involved in the subsequent follow-up appointments in any way. Recruited patients were not informed about the other patients recruited in the same trial. Similarly, no information regarding trial progress and allocation was revealed to the participating GPs or surgeons. However, as GP organised follow-up represented a new practice, blinding was not possible in the intervention arm.
Primary outcome measures
Quality of life
The primary outcome measure in this trial was the global health status on the European Organisation for Research and Treatment of Cancer QoL Questionnaire (EORTC QLQ C-30). QoL measurements were collected at baseline and at 3, 6, 9, 12, 15, 18, 21 and 24 months, that is,
European Organisation for Research and Treatment of Cancer QoL Questionnaire: EORTC QLQ C-30 incorporates nine multi-item scales: five functional scales (physical, role, cognitive, emotional and social); three symptom scales (fatigue, pain and nausea/vomiting) and a global health status/QOL scale. Six single-item scales are also included (dyspnoea, insomnia, appetite loss, constipation, diarrhoea and financial difficulties).18
EuroQol-5D (EQ-5D; EuroQol Group, Rotterdam, The Netherlands): EuroQol-5D is a standardised generic QoL instrument. EQ-5D measures five dimensions of health-related QoL (HRQoL): mobility, self care, usual activities, pain/discomfort and anxiety/depression. Each dimension is rated at three levels: no problems (1), some problems (2) and major problems (3).19 Based on preferences elicited from a general population, EQ-5D health states (eg, 1–1–2–1–3) may be converted into utility scores (=index scores, IS). In this trial, we used preferences elicited from a UK population, as no similar Norwegian preferences exist.20
EQ visual analogue scale (EQ VAS) records the respondent’s self-rated health status on a vertically graduated (0–100) visual analogue scale.
Secondary outcome measures
Cost-effectiveness
Resources used (baseline to 24 months) were registered prospectively based on reports by the patients and on hospital electronic medical record (EMR) review. The cost elements included costs related to hospital visits, GP visits, laboratory tests, radiology examinations, colonoscopy, examinations owing to suspected relapse (radiology, colonoscopy, CT of thorax and/or abdomen and positron emission tomography scan), treatment of recurrence, travelling/transportation, production losses, copayments and other patient/family expenses.
Time to cancer diagnosis
Time to cancer diagnosis was defined as the time from occurrence of an SCE (dated in the GP referral or hospital EMR record) to the date of diagnosis of an SCE. An SCE was defined as an episode in which cancer recurrence was suspected. An SCE can be triggered either by symptoms reported (at follow-up or in between follow-ups), clinical findings at follow-up or findings by a screening test. Symptoms and clinical findings initiating a diagnostic check-up were defined as follows: cancer suspect lesion revealed at colonoscopy, increase in CEA measurements shown by repeated measurements, blood in stool detected by the Hemofec (FOB) test, unexplained abdominal pain, unexplained weight loss of 5 kg during the last 3 months, cancer-suspect lesions detected by rectal examination, palpable lymphadenopathy, metastatic suspect lesions shown by chest x-ray, ultrasound of liver or CT scan, cancer-suspect findings at clinical examination and occurrence of cancer-related symptoms.
Data collection
At the baseline appointment, patients recruited received nine questionnaires (as part of the patient decision-support pamphlet) corresponding with the nine follow-up cycles (table 1). The questionnaires contained questions about QoL, patient satisfaction and cost and resource utilisation. Questionnaires were returned by mail every 3 months by the patients to the trial centre until 24 months postoperatively. These questionnaires were optically readable, being consecutively registered in the trial database. A research assistant was responsible for data collection, database input and patient reminders when questionnaires were missing. The reminders were sent to the participating patients when the questionnaires were 3 months overdue (beyond the estimated follow-up schedule). All questionnaires were dated and we could thus monitor trial progression. In case of missing information about cost elements, we either reviewed the hospital EMR or performed telephone interviews with participating surgeons, GPs or patients.
Sample size calculation
In June 2007, sample size calculations were based on a significance level of 5% and power set at 80%, which indicated that we needed 136 patients to detect a 10 units QoL difference (ie, a small to moderate improvement) on the EORTC QLQ C-30 Global Health score with an SD of 20. Definitions of a small-to-moderate improvement on QoL (ie, 10 units on the global health score), and SD estimates of QoL (patients with localised colon cancer) were retrieved from previous published publications.21 22
Economic analysis
BMJ guidelines for economic analyses alongside randomised controlled trials were employed.23 As the trial revealed no difference in QoL, a cost-minimisation analysis was carried out. The economic evaluation had a societal perspective. A 3% discount rate was used to discount future costs and benefits. For this publication, cost elements have been converted from Norwegian kroner (NOK) into British Pounds at the rate of GBP £1=NOK 9.39 NOK as of the Norwegian National Bank on 27 June 2012. Details of the unit costs assigned to healthcare resource use are shown in table 2.
Table 2.
Details of the unit costs assigned to healthcare resource use data
| Variable | Unit cost (£)* | Sensitivity analyses (%) |
|---|---|---|
| Cost of travel | ±25 | |
| Mean costs of hospital travel | 88† | |
| Hotel overnight | 74‡ | |
| Private car rates | 0.2/km§ | |
| Parking | 10.6‡ | |
| Taxi | 1.3/km§ | |
| Bus | 2.6§ | |
| Cost of GP consultation | ±25–40 | |
| GP consultation 20 min | 18.5¶ | |
| Phone consultation GP 10 min | 5.3¶ | |
| Emergency consultation GP 30 min | 26¶ | |
| Cost of surgeon outpatient consultation | ±25–40 | |
| Surgeon outpatient consultation 30 min | 69** | |
| Phone consultation surgeon 15 min | 10.6†† | |
| Emergency outpatient consultation 30 min | 69** | |
| Cost of follow-up tests | ±25–40 | |
| Blood samples | 5¶ | |
| Chest x-ray | 25‡‡,§§ | |
| Contrast-enhanced ultrasound of liver | 153‡‡,§§ | |
| CT abdomen | 105‡‡,§§ | |
| CT thorax | 105‡‡,§§ | |
| Colonoscopy | 293**,§§ | |
| PET scan | 2662‡‡ | |
| Cost related to sick leave | ±25 | |
| Governmental reimbursement 1 day work absence | 102¶¶ | |
| Costs related to metastases surgery | ±25 | |
| Cost of abdominal surgery | 14176** | |
| Cost of liver surgery | 11596** | |
| Cost of lung surgery | 13061** | |
*Exchange rate on 29 June 2012: £1=9.36 Norwegian kroner: http://www.dnb.no/en/currencylist?la=EN&site=DNB_NO
†Personal communication North Norwegian Health Administration (JN): 5 400 000 NOK budgeted annual travel expenses/950 000 annual patient travels=£88 per travel.
‡Local data.
§Norwegian National Bureau of Patient Travels: http://www.pasientreiser.no/andre-spraak/english
¶The Norwegian Medical Association: Norwegian Policy Document for Governmental Reimbursements in Primary Care (Fastlegetariffen) 2011: http://www.legeforeningen.no/normaltariff/Fastlegetariff_2010.pdf
Cost of GP consultation: 136 NOK (20 min consultation)+386 NOK per patient annually. Assuming 10 consultations per patient annually=38 NOK/consultation. In total, 174 NOK per consultation=£18.5.
**Norwegian Health Authorities. Reimbursement and DRG weighting in Norwegian Hospitals 2012: http://www.helsedirektoratet.no/publikasjoner/regelverk-innsatsstyrt-finansiering-2012/Sider/default.aspx
1 DRG weight: 38 209NOK. Surgeon outpatient consultation (day and night-time): DRG 923 O, weight 0.017. Colonoscopy: DRG 710 O, weight 0.072. Abdominal surgery: DRG 170, weight 3.484. Liver surgery: DRG 201, weight 2.850. Lung surgery: DRG 76, weight 3.21.
††Statistics in Norway 2011: Average annual salary 750 000 NOK (£80 000) hospital consultant.
‡‡Cost rates Department of Radiology and Nuclear Medicine University Hospital North Norway.
§§Korner et al.33
¶¶Estimated from a median income of 350 000 NOK/year/patient as reported by patient subsample in regular work at the time of surgery.
PET, positron emission tomography.
Economic evaluation data are invariably positively skewed, and they require an alternative analysis. We used a bootstrapping technique, which makes no assumptions regarding the equality, variance or shape of the distribution, and takes skewness into account.24 25 To adjust for the skewness, costs were bootstrapped with 1000 replications to estimate bias corrected CI. The bootstrapping technique was undertaken using IBM SPSS Statistics V.19.0.
A one-way sensitivity analysis was used to assess the robustness of the results and the impact of variance. The societal cost of 24-month follow-up was assessed for low, base and high-input values, and the result was expressed as a many inputs, one output tornado chart. To increase the generalisability of cost between countries, unit costs from the UK were included in the sensitivity analyses. The costs for GP consultation and diagnostic testing have been reported to be 30–40% higher than the unit cost applied in this trial and the relevant cost elements were increased accordingly in sensitivity analyses.26
Statistics
Descriptive statistics were performed by percentages, 2×2 contingency tables, χ2, Fisher's exact test and t test. The base case analyses (n=110, 600 complete follow-up questionnaires/cycles) were performed on the intention to treat principle. Treatment arms were compared with respect to potential covariates using continuous and categorical univariable analyses. The main analyses examined whether significant differences existed in QoL outcome measures between baseline and 3, 6, 9, 12, 15, 18, 21 and 24 months (EORTC QLQ C-30 and EQ-5D). A general linear model was employed, where time (1–24 months) and intervention group (GPs vs surgeons) were predictors in analyses of variance (between groups ANOVA). Missing data were imputed by the last observation carried forward (LOCF) when there were missing QoL items in a form, and when the QoL form was missing. Conditional power (CP) was defined as the chance of getting statistically significant results at the end of the trial given the data so far.27 28 We defined a CP < 15% as a sufficient threshold to stop early.29 Results were expressed as the mean differences for continuous outcomes with corresponding SD, 95% CIs, and associated p values. p Values were reported with two decimal places with p-values less than 0.001 being reported as p<0.001. For all tests, we used the p=0.05 level of significance. All analyses were performed with the IBM SPSS Statistics V.19.0 (IBM Company SPSS 2010) and Microsoft Excel for Mac 2011.
Results
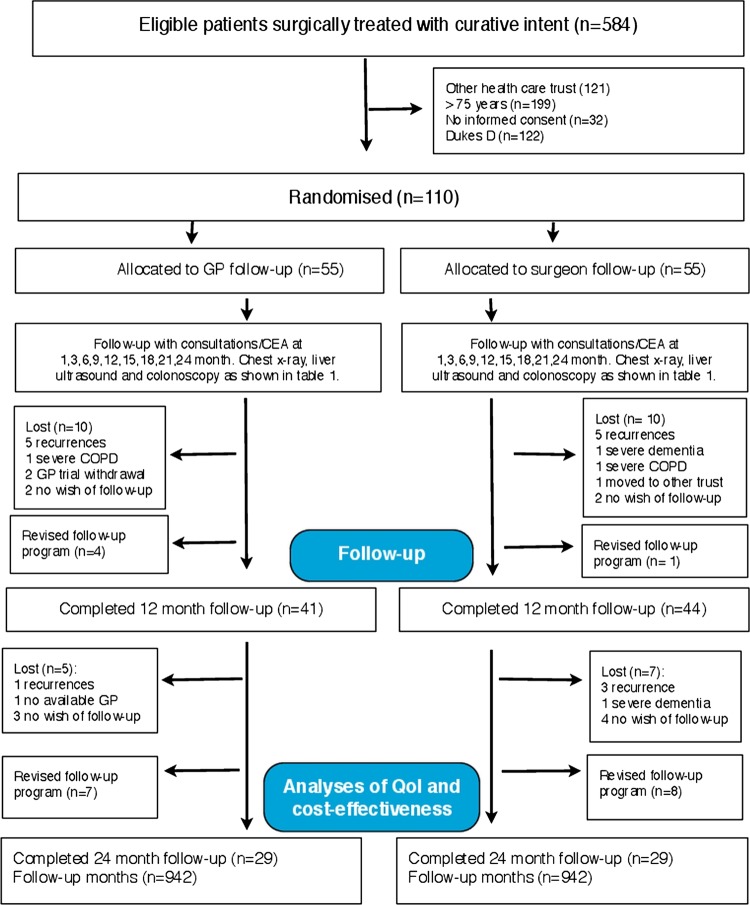
A total of 110 patients surgically treated for colon cancer met the inclusion criteria and agreed to participate in the survey (figure 1). The control and intervention groups were matched at baseline for demographic and medical characteristics and there were no significant differences between groups (table 3).
Figure 1.
Flow of participants. Patients were enrolled in the 2007 NGICG (Norwegian Gastrointestinal Cancer Group, table 1) follow-up programmes in both trial arms. The programmes are divided in 3-month cycles, that is, clinical examination at 1 (baseline), 3, 6, 9, 12, 15, 18, 21 and 24 months, carcinoembryonic antigen (CEA) measurement at 3-month intervals, chest x-ray and contrast-enhanced liver ultrasound every 6 months and colonoscopy once during 24 months (table 1).
Table 3.
Baseline demographics and clinical characteristics
| Variable | Surgeon (%) n=55 | GP (%) n=55 | Total (%) n=110 | p Value |
|---|---|---|---|---|
| Age group | ||||
| <50 | 2 (3.6) | 6 (10.9) | 7 (6.3) | 0.10 |
| 50–59 | 8 (14.5) | 6 (10.9) | 14 (12.7) | 0.56 |
| 60–69 | 23 (41.8) | 24 (43.6) | 47 (42.7) | 0.84 |
| 70–75* | 22 (40.0) | 19 (34.5) | 41 (38.0) | 0.55 |
| Mean age (SD) | 66.7 (7.3) | 64.0 (8.7) | 65.4 (8.1) | 0.09 |
| Gender | ||||
| Male | 32 (58.2) | 33 (60.0) | 65 (59.1) | 0.84 |
| Female | 23 (41.8) | 22 (40.0) | 45 (40.9) | 0.84 |
| Education | ||||
| Primary | 20 (36.3) | 18 (32.7) | 38 (34.5) | 0.68 |
| Secondary | 21 (38.1) | 25 (45.4) | 46 (41.8) | 0.49 |
| University <4 years | 8 (14.5) | 5 (9.0) | 13 (11.8) | 0.37 |
| University >4 years | 6 (10.9) | 7 (12.7) | 13 (11.8) | 0.76 |
| Income level | ||||
| Median (£) | 32–42 000 | 32–42000 | 32–42000 | |
| Main activity | ||||
| Employment | 12 (21.8) | 17 (30.9) | 29 (26.3) | 0.27 |
| Home | 3 (5.4) | 9 (16.3) | 11 (10.0) | 0.06 |
| Out of work | 0 (0) | 1 (1.8) | 1 (0.9) | |
| Pensioner | 40 (72.7) | 28 (50.9) | 68 (61.8) | 0.01 |
| Location of surgery | ||||
| University hospital (n=1) | 34 (61.8) | 37 (67.3) | 71 (64.5) | 0.55 |
| Local hospital (n=3) | 21 (38.1) | 18 (32.7) | 39 (35.4) | 0.55 |
| Clinical characteristics | ||||
| Tumour location | ||||
| Cøkum | 13 (23.6) | 13 (23.6) | 26 (23.6) | 1.0 |
| Ascendens | 9 (16.3) | 5 (9.1) | 14 (12.7) | 0.25 |
| Transversum | 4 (7.2) | 5 (9.1) | 9 (8.1) | 0.72 |
| Decendens | 1 (1.8) | 4 (1.8) | 5 (4.5) | 0.15 |
| Sigmoid | 28 (50.9) | 28 (50.9) | 56 (50.9) | 1.0 |
| Elevated preoperative CEA | 19 (34.5) | 23 (41.8) | 42 (38.1) | 0.55 |
| Type of surgery | ||||
| Laparoscopic surgery | 14 (25.5) | 11 (20.0) | 25 (22.7) | 0.49 |
| Open surgery | 41 (74.5) | 44 (80.0) | 85 (77.3) | 0.49 |
| Tumour stage | ||||
| Dukes A | 12 (21.8) | 11 (20.0) | 24 (21.8) | 0.81 |
| Dukes B | 25 (45.5) | 30 (54.5) | 55 (50.0) | 0.34 |
| Dukes C | 18 (32.7) | 14 (25.5) | 32 (29.0) | 0.40 |
| New surgery owing to complications | 6 (10.9) | 9 (16.4) | 15 (13.6) | 0.40 |
| Permanent stoma | 8 (14.5) | 7 (12.7) | 15 (13.6) | 0.78 |
| 6 months chemotherapy regime | 18 (32.7) | 14 (25.5) | 32 (29.1) | 0.40 |
*Patients <75 years were included in the survey. p Values calculated with χ square, t test and Fisher's exact test when appropriate.
GP, general practitioner.
Trial flow and dropouts
Eighty five patients (75%) (GP 41 vs surgeon 44) were followed for 12 months, and 58 patients (52%) (GP 29 vs surgeon 29) for 24 months. Twenty patients (surgeon 9 vs GP 11) were transferred to the new national colon cancer surveillance programme (figure 1).
Response rate
We received 636 of the expected 657 questionnaires (response rate 96%), of which 600 (91%) (GP 299 vs surgeon 301) were included in the final cost and QoL analyses. A total of 21 (4%) questionnaires (surgeon 11 vs GP 10) were not returned and 36 questionnaires (surgeon 18 vs GP 18) were excluded from the analyses owing to insufficient identification.
Interim analyses
New national colon cancer surveillance guidelines were gradually implemented from 2010, with a different frequency of consultations (3 vs 6 months interval) and radiological modalities (chest x-ray vs chest CT).7 This could bias the cost-effectiveness and QoL analyses, and an interim analysis was performed in June 2012 (80% of preplanned recruitment, 1884 follow-up months). There was at this point a 4% probability (ie, conditional power) of showing a significant impact of the intervention on QoL global health score, which meant that further trial continuation were not justified.
Quality of life
There was no significant effect on the QoL main outcome measures. However, on the EORTC QLQ C-30 subscales, there were significant effects in favour of GP follow-up, that is, role functioning (p=0.02), emotional functioning (p=0.01) and pain (p=0.01; table 4 and figure 2A–C).
Table 4.
Health-related quality of life (ERTOC QLQ-C30, European Organisation for Research and Treatment of Cancer QoL Questionnaire; and EQ-5D, EuroQol-5D) outcome variables and estimated differences
| Outcome variable | Mean (SD) |
Estimated mean difference (95% CI) | p Value* | ||
|---|---|---|---|---|---|
| Baseline | 12 months | 24 months | |||
| Global health status | |||||
| Surgeon | 70.7 (22.5) | 75.9 (19.2) | 85.0 (16.8) | ||
| GP | 70.4 (20.8) | 81.3 (17.0) | 86.5 (16.2) | −2.23 (−5.7 to 1.2) | 0.20 |
| Physical functioning | |||||
| Surgeon | 80.5 (23.6) | 88.8 (15.0) | 88.0 (17.0) | ||
| GP | 74.5 (24.9) | 90.6 (16.6) | 93.3 (16.0) | −2.4 (−5.7 to 0.8) | 0.14 |
| Role functioning | |||||
| Surgeon | 62.5 (37.3) | 83.8 (26.5) | 90.3 (18.6) | ||
| GP | 62.7 (37.5) | 91.6 (22.1) | 93.7 (20.7) | −5.1 (−9.7 to (−0.5)) | 0.02 |
| Emotional functioning | |||||
| Surgeon | 87.4 (18.1) | 87.7 (16.1) | 87.7 (16.9) | ||
| GP | 85.8 (23.2) | 91.9 (15.8) | 94.4 (17.3) | −3.7 (−6.8 to (−0.6)) | 0.01 |
| Cognitive functioning | |||||
| Surgeon | 87.0 (20.6) | 86.5 (22.8) | 90.3 (15.0) | ||
| GP | 72.4 (31.8) | 91.1 (17.0) | 93.0 (21.3) | −1.7 (−5.0 to 1.4) | 0.27 |
| Social functioning | |||||
| Surgeon | 70.7 (30.5) | 87.0 (23.8) | 90.4 (15.6) | ||
| GP | 72.4 (31.8) | 91.6 (17.3) | 93.0 (21.3) | −4.2 (−8.4 to (−0.009)) | 0.04 |
| Fatigue | |||||
| Surgeon | 32.3 (26.1) | 19.2 (17.1) | 14.6 (23.4) | ||
| GP | 36.9 (28.0) | 22.2 (19.9) | 18.3 (20.8) | 0.24 (−3.7 to 4.2) | 0.9 |
| Nausea and vomiting | |||||
| Surgeon | 6.0 (12.4) | 2.8 (8.5) | 0.9 (3.9) | ||
| GP | 6.5 (14.1) | 3.5 (9.9) | 4.3 (10.3) | −0.8 (−2.8 to 1.2) | 0.4 |
| Pain | |||||
| Surgeon | 22.3 (26.6) | 11.1 (21.9) | 9.6 (16.9) | ||
| GP | 19.1 (28.2) | 9.3 (14.0) | 2.8 (14.7) | 4.5 (0.8 to 8.2) | 0.01 |
| Dyspnoea | |||||
| Surgeon | 18.1 (26.3) | 14.2 (20.2) | 10.5 (19.4) | ||
| GP | 24.0 (32.7) | 12.1 (23.3) | 7.2 (21.2) | 3.0 (−1.2 to 7.2) | 0.1 |
| Insomnia | |||||
| Surgeon | 22.9 (25.4) | 18.5 (25.7) | 17.5 (25.7) | ||
| GP | 28.6 (34.5) | 14.7 (23.4) | 23.6 (25.0) | 2.9 (−1.7 to 7.5) | 0.2 |
| Appetite loss | |||||
| Surgeon | 15.5 (23.1) | 3.7 (10.6) | 1.7 (7.6) | ||
| GP | 20.9 (31.7) | 1.9 (7.9) | 4.1 (11.2) | 0.8 (−2.9 to 3.9) | 0.6 |
| Constipation | |||||
| Surgeon | 27.4 (32.0) | 21.2 (29.9) | 10.5 (19.4) | ||
| GP | 18.6 (33.5) | 7.8 (16.5) | 15.2 (19.6) | 5.1 (0.8 to 9.4) | 0.01 |
| Diarrhoea | |||||
| Surgeon | 24.4 (29.6) | 21.2 (25.3) | 24.5 (24.4) | ||
| GP | 31.0 (33.6) | 22.5 (26.8) | 23.6 (28.6) | −1.0 (−5.7 to 3.5) | 0.6 |
| Financial difficulties | |||||
| Surgeon | 9.8 (26.2) | 9.2 (20.4) | 7.0 (21.0) | ||
| GP | 6.9 (21.2) | 1.9 (7.9) | 4.1 (11.2) | 2.7 (−0.4 to 5.8) | 0.08 |
| EQ-5D Index score | |||||
| Surgeon | 0.83 (0.16) | 0.85 (0.20) | 0.90 (0.14) | ||
| GP | 0.79 (0.22) | 0.87 (0.18) | 0.89 (0.13) | −0.10 (−0.039 to 0.018) | 0.48 |
| EQ-5D VAS score | |||||
| Surgeon | 72.2 (18.9) | 78.2 (16.2) | 82.4 (16.6) | ||
| GP | 67.4 (17.4) | 79.0 (14.6) | 83.5 (14.8) | −1.10 (−3.9 to 1.7) | 0.44 |
*Adjusted general linear model from 1800 follow-up months, ie, 600 quality of life questionnaires (GP 299 vs surgeon 301).
EQ-5D, EuroQol-5D; GP, general practitioner; VAS, visual analogue scale.
Figure 2.
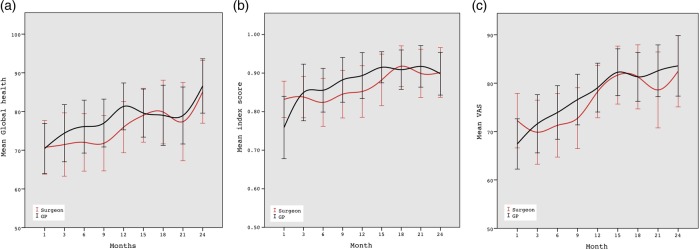
A–C. Health-related quality of life 1–24 postoperative months. European Organisation for Research and Treatment of Cancer QoL Questionnaire (EORTC QLQ C-30) Global Health, EuroQol-5D (EQ-5D) index score and EQ-5D visual analogue scale.
Cost-effectiveness
There were no significant differences in primary QoL measures (global health status), and cost minimisation analyses were performed. A total of 778 travels (consultations, radiological investigations and colonoscopy) to the hospital were registered, 528 in the surgeon group and 250 in the GP group, respectively. A total of 1186 healthcare contacts (regular appointments, emergency appointments and phone consultations) were registered, 678 in the GP group versus 508 in the surgeon group (table 5). The mean cost of follow-up per patient per follow-up cycle was £292 in the GP group and £351 in the surgeon group (p=0.02) (figure 3). Overall, the mean societal cost per patient for 24 months follow-up was £9889 in the surgeon group and £8233 in the GP group (p<0.001, table 6).
Table 5.
Resource use in a colon cancer follow-up programme
| Cost variable | Surgeon n=55 |
GP n=55 |
Total n=110 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | n/cycle | cost/cycle | n | n/cycle | cost/cycle | n | n/cycle | cost/cycle | |
| Follow-up months | 903 | 897 | 1800 | ||||||
| Hospital travels | |||||||||
| Car | 189 | 0.62 | * | 113 | 0.37 | * | 302 | 0.50 | * |
| Taxi | 37 | 0.12 | 22 | 0.07 | 59 | 0.09 | |||
| Bus | 96 | 0.31 | 33 | 0.11 | 129 | 0.21 | |||
| Airplane | 0 | 0 | 8 | 0.02 | 8 | 0.01 | |||
| Express boat | 43 | 0.14 | 12 | 0.04 | 55 | 0.09 | |||
| Extra travel owing to poor logistics | 104 | 0.34 | 52 | 0.17 | 156 | 0.26 | |||
| Travel assistant | 59 | 0.19 | 10 | 0.03 | 69 | 0.11 | |||
| Hotel | 7 | 0.02 | 1.7 (11) | 8 | 0.02 | 2.0 (12) | 15 | 0.02 | 1.8 (11.6) |
| Total | 528* | 1.75 | 250* | 0.83 | 778* | 1.29 | |||
| Mean cost £ (SD) | 156.9 (145.0) | 76.7 (160.1, p<0.001) | 117.1 (157.7) | ||||||
| GP office travels | |||||||||
| Car | 155 | 0.51 | † | 317 | 1.06 | † | 472 | 0.78 | † |
| Taxi | 7 | 0.02 | 14 | 0.05 | 21 | 0.03 | |||
| Bus | 17 | 0.06 | 35 | 0.12 | 52 | 0.08 | |||
| Travel assistant | 0 | 0 | 15 | 0.05 | 15 | 0.02 | |||
| Total | 179 | 0.59 | 381 | 1.27 | 560 | 0.93 | |||
| Mean cost £ (SD) | 4.1 (7.9) | 9.0 (9.1, p<0.001) | 6.6 (8.9) | ||||||
| Out-of-pocket expenses | |||||||||
| Mean cost £ (SD) | 2.7 (7.7) | 4.3 (15.0, p=0.10) | 3.5 (11.9) | ||||||
| Healthcare contacts | |||||||||
| GP consultations | 156 | 0.52 | 9.6 (17.8) | 329 | 1.10 | 20.6 (19.9) | 485 | 0.80 | 15.1 (19.6) |
| GP phone consultation | 61 | 0.20 | 1.0 (3.9) | 94 | 0.31 | 1.7 (4.3) | 155 | 0.25 | 1.4 (4.1) |
| GP emergency consultations | 23 | 0.08 | 1.9 (12.2) | 37 | 0.12 | 3.2 (14.4) | 60 | 0.1 | 2.6 (13.3) |
| Surgeon outpatient consultations | 227 | 0.75 | 52.3 (93.8) | 185 | 0.61 | 43.3 (104.1) | 412 | 0.68 | 47.8 (99.0) |
| Surgeon phone consultations | 41 | 0.14 | 1.45 (5.7) | 33 | 0.11 | 1.2 (4.4) | 74 | 0.12 | 1.32 (5.1) |
| Total | 508 | 1.68 | 678 | 2.26 | 1186 | 1.97 | |||
| Mean cost £ (SD) | 66.4 (100.1) | 70.1 (112.2, p=0.67) | 68.2 (106.1} | ||||||
| NGICG follow-up tests | |||||||||
| Blood samples | 203 | 0.67 | 3.3 (5.1) | 300 | 1.0 | 5.1 (6.8) | 503 | 0.83 | 4.2 (6.0) |
| Chest x-ray | 150 | 0.50 | 12.2 (12.2) | 128 | 0.43 | 10.6 (12.1) | 278 | 0.46 | 11.4 (12.2) |
| CEUS | 110 | 0.37 | 56.2 (74.0) | 99 | 0.33 | 51 (72.5) | 209 | 0.34 | 53.8 (73.2) |
| Colonoscopy | 50 | 0.17 | 49.2 (110.3) | 65 | 0.22 | 65.1 (122) | 115 | 0.19 | 57.1 (116.7) |
| Total | 513 | 1.70 | 592 | 1.97 | 1105 | 1.84 | |||
| Mean cost £ (SD) | 121.1 (152.8) | 132.2 (166.7, p=0.39) | 126.6 (159.8) | ||||||
| Work loss | |||||||||
| Patients in paid work (n) | 17 | 12 | 29 | ||||||
| Days off work mean (SD) | 215 (168) | 198 (190, p=0.79) | 208 (219) | ||||||
| Mean cost £ (SD)‡ | 2440 (1906) | 1884 (2092, p=0.45) | 2086 (2014) | ||||||
| Serious clinical events | |||||||||
| Number of events | 22 | 26 | 48 | ||||||
| Mean cost £ (SD)§ | 261.6 (157.7) | 573.1 (838.9, p=0.14} | 444.0 (662.4) | ||||||
| Metastases surgeries | |||||||||
| Cancer recurrences | 8 | 6 | 14 | ||||||
| Metastases surgeries | 4 | 3 | 7 | ||||||
| Mean cost £ (SD)¶ | 9037.2 (5117.5) | 13316.0 (1489.0, p=0.22) | 10871.0 (4366.3) | ||||||
*Mean travel cost for hospital travels (see table 2).
†Values calculated with a median distance general practitioner office 30 km.
‡Value represents the mean cost (SD) relating to the subsample (surgeon) who were in paid work at the time of surgical treatment.
§Value represent the mean cost (SD) of work-up tests (CEA, chest x-ray, colonoscopy) relating to the subsample who experienced a serious clinical event.
¶Value represent the mean cost (SD) relating to the subsample (surgeon) who performed metastases surgery.
CEUS, contrast-enhanced liver ultrasound; GP, general practitioner; NGICG, Norwegian Gastrointestinal Cancer Group.
Figure 3.

Health care cost of follow-up per 3 month follow-up cycle.
Table 6.
Cost of colon cancer follow-up
| Cost variable (mean, £) | Surgeon n=55 | GP n=55 | Total n=110 | p Value |
|---|---|---|---|---|
| Healthcare cost/follow-up cycle | 351 | 292 | 324 | 0.02 |
| Bootstrapped 95% CI | 315 to 386 | 255 to 327 | 296 to 348 | |
| Mean difference £ | 58 | |||
| Healthcare cost/24-month follow-up | 3178 | 2651 | 2917 | 0.03 |
| Bootstrapped 95% CI | 2833 to 3485 | 2228 to 3006 | 2660 to 3147 | |
| Mean difference (£) | 529 | |||
| Societal cost/follow-up cycle | 1098 | 914 | 1007 | <0.001 |
| Bootstrapped 95% CI | 1062 to 1139 | 877 to 954 | 981 to 1034 | |
| Mean difference (£) | 184 | |||
| Societal cost/24-month follow-up | 9889 | 8233 | 9068 | <0.001 |
| Bootstrapped 95% CI | 9569 to 10194 | 7904 to 8619 | 8823 to 9320 | |
| Mean difference (£) | 1656 | |||
In estimation of healthcare and societal cost, cycles with complete cost data (n=600, ie, 1800 follow-up months) were included in analyses (as defined in table 1). Cost data from 57 follow-up cycles were excluded from analyses (incomplete ID or not returned forms). Cost of sick leave was adjusted for baseline characteristic. CI based on 1000 stratified bootstrap samples.
Sensitivity analyses
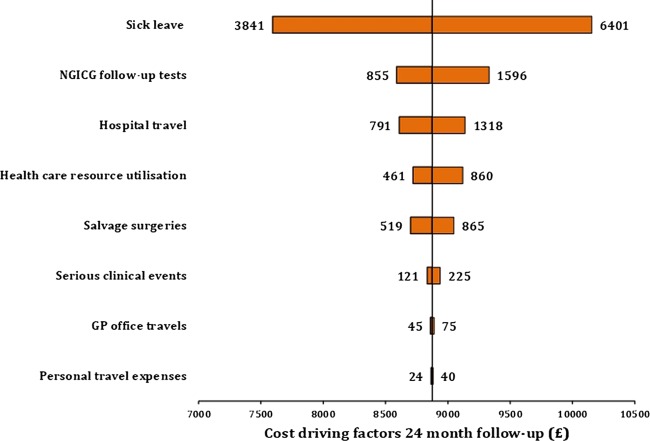
The single factor with the greatest impact on overall societal costs was length of sick-leave, followed by cost of follow-up tests and cost of hospital travels. Variances in costs related to GP office travels and follow-up appointments had a minor impact on overall cost in a follow-up programme (figure 4).
Figure 4.
Sensitivity analyses of cost-driving elements in surveillance. Societal cost per patient (£) for a 24-month colon cancer follow-up. Most critical variable in terms of impact is listed at the top of the graph, and the rest ranked according to their impact thereafter. As unit cost from the UK, like cost for GP consultation and diagnostic testing, has been reported to be 30–40% higher than units cost applied in this trial, relevant cost elements were increased accordingly. Cost values for serious clinical events, metastases surgeries and sick leave were adjusted for baseline characteristics.
Time to cancer diagnoses
A total of 48 SCEs occurred; the mean time until diagnosis of an SCE was 45 days in the surgeon group and 35 days in the GP group (p=0.46). Of the patients with SCE, 14 had cancer recurrence and 7 (50%) were offered metastases surgery. Five patients died (all deaths caused by disseminated colon cancer) during the follow-up period (GP 1 vs surgeon 4).
Discussion
Summary of findings
A representative population of patients surgically treated for colon cancer participated in this trial, with an expected normal variance of demographic factors and colon cancer severity. We have shown that a decentralised colon cancer follow-up programme will not impair QoL; on the contrary, we observed a significant improvement in the following QoL subscales: role functioning, emotional functioning and pain. This is the first trial evaluating the economical implications of a GP organised follow-up programme after curative resection for colon cancer. Despite a higher frequency of healthcare contacts in primary care, a decentralised GP organised follow-up programme was associated with total cost savings owing to the decreased costs of primary care consultations and less hospital travels. Importantly, our result shows that GP follow-up was not associated with increased time to diagnosis of SCE, and thus cancer recurrence (35 vs 45 days, p=0.46), and the frequency of an SCE was similar in both groups.
Comparison with existing literature and ongoing trials
Although intensive follow-up is associated with improved survival, there are still international controversies on how to best organise follow-up of colon cancer patients. These controversies are mirrored in the wide variation of national follow-up guidelines.4–7 Two systematic reviews, comparing follow-up trials, have been published.2 3 Owing to the variation in the follow-up programmes included in these reviews, it is not possible to infer the best combination of consultations, blood tests, colonoscopy, radiological investigations and level of care to maximise the outcomes.2 Large randomised trials are under way (COLOFOL, GILDA and FACS), but the results are most likely years away.9–11 Few published surveys have evaluated the effect of a GP organised follow-up programme. Two surveys have reported on QoL in a primary care-based follow-up programme, and a single cost-effectiveness analysis of intensified hospital-based follow-up was published in 2004.30–32 Surveys have assessed the costs of follow-up in a Norwegian setting. In a retrospective survey, 314 patients were assessed with regard to the cost, compliance and success rate of curative surgery. It was concluded that the cost of one successful curative surgery was US$25 289, and that further implementation of such a programme should be debated.33 The harms and unintended effects of a follow-up programme are poorly explored. The rate of false positive tests in a follow-up programme is especially unknown. Current surveillance is often based on serial CEA measurements; this biomarker has several pitfalls and shortcomings. In a recent survey, it was shown that the diagnostic accuracy of the serial measurement of CEA is low, and is impacted by the cut-off value.34 These aspects are of high importance when designing a follow-up programme, as a false-positive test probably has a negative impact on the patient’s QoL. Finally, there exists a considerable variance in follow-up strategies, internationally and at a national level.35 This makes the comparison of outcomes between different follow-up strategies challenging.
For other cancer conditions, more cost-effective ways of organising follow-up are extensively described and evaluated. For breast cancer patients, nurse lead telephone and GP organised follow-up are cost-effective36––38 with no increase in the frequency of SCE.39 Nevertheless, the quality of primary care cancer management is still debated.40–42
Strengths and limitations
Our trial has several strengths. First, this is the first randomised trial addressing the economical implications and time to recurrent cancer diagnoses in a GP-organised colon cancer follow-up programme. We have shown that GP-organised follow-up, even with the increased frequency of healthcare contacts, was associated with cost savings and no decline in QoL. Second, poor guideline compliance has been shown to represent a problem in cancer follow-up programmes.43 However, tools to support decision-making in cancer are in progress. In this study, a decision support pamphlet was part of the intervention, and the patient and the GP organising the follow-up received a decision support tool. We believe that this decision support tool contributed to a high follow-up guideline adherence (table 6, GP 592 tests vs surgeon 513 tests). Third, we have shown that the rate of SCE and time to diagnosis of cancer recurrence are comparable between groups. In our opinion, this is an indicator of adequate quality in a GP organised follow-up programme.
There exist limitations. First, it might be argued that we were missing important information by choosing another endpoint than survival. However, this trial was designed primarily to evaluate whether general practice follow-up results in effects on patient-specific QoL and cost-effectiveness. We acknowledge that this choice of endpoint might impact the observed frequency of SCEs and time to cancer diagnoses, as a higher number of SCE and cancer recurrences would have occurred with a longer follow-up time. Similarly, societal costs will be impacted by a longer follow-up time. However, when healthcare cost of follow-up is analysed separately (table 5, figure 3), cost spendings are significantly lower in the GP group compared to the surgeon group. Second, generalisability and cost transferability across jurisdictions might be challenging, as elements of cost data may vary from place to place.44 It might be argued that this is a single country trial with limited generalisability. However, we do not think this is the case. Comparable follow-up trials have been performed in countries like the USA, Canada, the UK, Australia and the Netherlands.30 38 39 45 These surveys are commonly cited and thus accepted as generalisable. In Norway, the GP has a traditional gatekeeper function and plays a central role managing resource usage in secondary care. Similarly, many European countries have a healthcare organisation where the GP plays a central role as gatekeeper to the access of secondary healthcare service. In our trial, guidelines for dealing with aspects of generalisability and transferability were applied, and variations in unit costs were included in the sensitivity analyses (see figure 3).44
Finally, the trial was stopped after 1884 follow-up months owing to no significant effect of the intervention on global health score and implementation of a new national follow-up programme. This is a limitation, as it will impact the interpretation of cancer recurrence. However, it would have been unethical to spend large resources over years to complete an intervention with a 4% probability of showing a significant impact on global health score.
Implication for patients, decision makers and clinicians
Colon cancer in numbers is the third largest cancer type worldwide and a considerable number of patients are enrolled in a postsurgical surveillance programme, resulting in significant societal cost. However, as there is no evidence-based consensus on how to design cost-effective follow-up programmes, the differences in tests, test frequency and level of care will have a high impact on societal cost spending. Therefore, the cost-driving elements in a colon cancer follow-up programme have to be critically evaluated.
From a societal perspective, this survey has important implications. It may be argued that there are limited benefits from having GPs organising the follow-up programmes, as the radiological examinations and the colonoscopy have to be performed inhospital anyway. However, we believe that the most important factors causing a less costly GP follow-up are better coordination of care: as shown in table 5, GP organised follow-up leads to fewer hospital travels. We believe this is mainly caused by improved coordination of care, for instance by performing multiple radiological tests at the same hospital visit. Interestingly, the GP group had fewer extra travels (GP 52 travels vs surgeon 102 travels) owing to poor logistics (table 5). Cost of GP consultation versus hospital consultation: the societal cost of GP consultations is lower compared to cost of hospital consultations, owing to a more costly hospital infrastructure. Complex and chronic conditions: patients surgically treated often have other chronic illnesses, and there is a trend towards higher involvement of primary care in treating these conditions as described in the chronic care model.13 Sick leave: although not statistically significant, patients in the GP group return to work 17 days (mean) earlier compared to patients in the surgeon group.
From a hospital perspective, a transfer of follow-up programmes to primary care has economical and organisational implications. GP organised follow-up may be an effective way of reducing the burden on busy hospital clinics.
Conclusion
The present study suggests that a colon cancer follow-up can safely be performed by GPs, with no negative impact on QoL and at a lower cost. However, there exist limitations. Thirteen per cent (n=14) of patients had colon cancer recurrence; this low recurrence rate is most likely caused by limited long-term follow-up as most recurrences occur within 3 years. Furthermore, the best combination of consultations, radiological test, blood samples and colonoscopy that optimises cancer survival is unknown. We therefore argue that the cost-driving elements of colon cancer surveillance should be critically evaluated, when implementing follow-up programmes, as cancer surveillance represents a huge financial burden for the society. Finally, little is known about the potential harms of follow-up, especially when it comes to the impact of false positive tests. Further research is needed to settle these controversies, and new methods of decision-analytic modelling in combination with emerging data from ongoing randomised trials must be applied.46
Supplementary Material
Acknowledgments
We thank Trine Hansen at the Norwegian Centre for Integrated Care and Telemedicine for administrative support and for maintaining the research database. We thank Professor Roar Johnsen, Department of Public Health and General Practice, Norwegian University of Science and Technology, for assistance in protocol writing and design of the trial. We thank Professor Lars Vatten, Department of Epidemiology, Norwegian University of Science and Technology, for assistance in prestudy sample size calculations. We thank Johnie Rose, MD, PhD, Department of Family Medicine and Community Health, Case Western Reserve University, for valuable comments on our research and manuscript. We thank Dr Caroline Sagatun (Surgical Department, Bodø Regional Hospital) and Dr Henriette Fagertun (Surgical Department, Harstad Hospital) for comments on the study protocol and identification of the potential trial participants. We thank Frank Hauboff (Surgical Outpatient Clinic, University Hospital of North Norway) for assistance in randomisation and identification of potential trial participants. We thank Berit Marianne Bjelkåsen, Norwegian University of Science and Technology, for assistance with the web-based randomisation service.
Footnotes
Contributors: KMA and ROL conceived and designed the research idea, and were responsible for the overall administration and direction of the study, as well as the analysis and interpretation of the data. KMA and SOS designed the statistical analyses, while KMA did the statistical analyses. KMA did the economic analysis with assistance from JN, who contributed to the design, data analysis and interpretation of the findings. TN, RA and SD helped with patient recruitment and randomisation, and with the trial and interpretation of the findings. UR advised on the trial protocol, unit cost and reimbursement practice in primary care. BV advised on protocol writing and on the pretrial sample size calculations and manuscript revision. KMA wrote the first draft. All authors read and approved the final manuscript. KMA had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: Northern Norway Health Authorities Research Fund.
Competing interests: http://www.icmje.org/coi_disclosure.pdf None.
Patient consent: Obtained.
Ethics approval: IRB board University Hospital North Norway.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Larsen IK. Cancer in Norway 2009. Oslo, Norway: Cancer Registry of Norway, 2011:1–169 [Google Scholar]
- 2.Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev 2007; doi:10.1002/14651858.CD002200.pub2 [DOI] [PubMed] [Google Scholar]
- 3.Tjandra JJ, Chan MKY. Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007;50:1783–99 [DOI] [PubMed] [Google Scholar]
- 4.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer 2003;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666e690. doi:10.1136/gut.2009. 179804 [DOI] [PubMed] [Google Scholar]
- 6.Bülow S. Retningslinier for diagnostik og behandling af kolorektal cancer (Danish Guidelines). Danish Colorectal Cancer Group, 2009;4:1–176 [Google Scholar]
- 7.Vonen B. Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft i tynntarm og endetarm (Norwegian Guidelines). Norwegian Gastrointestinal Cancer Group NGICG, 2010:1–162 [Google Scholar]
- 8.Engstrom PF. NCCN clinical practice guidelines in oncology colon cancer V.2.2009. National Comprehensive Cancer network, 2009;2:1–71 www.nccn.org [DOI] [PubMed] [Google Scholar]
- 9.Follow-up study of patients who have undergone surgery for stage I, stage II, or stage III colorectal cancer. clinicaltrials.gov 2009:1–4
- 10.Grossmann EM, Johnson FE, Virgo KS, et al. Follow-up of colorectal cancer patients after resection with curative intent—the GILDA trial. Surg Oncol 2004;13:119–24 [DOI] [PubMed] [Google Scholar]
- 11.Wille-Jørgensen P, Laurberg S, Carriquiry L, et al. An interim analysis of recruitment to the COLOFOL trial. Colorect Dis 2009;11:756–8 [DOI] [PubMed] [Google Scholar]
- 12.Wille-Jørgensen P, Balleby L. Follow-up in colorectal cancer: questions to be answered. Colorectal Dis 2011;13:959–60 [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA 2002;288:1775–9 [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Survivor Initiative Department of Health, Macmillan Cancer Support and NHS Improvement, London, UK, 2010;1–82 [Google Scholar]
- 15.Augestad KM, Vonen B, Aspevik R, et al. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost-effectiveness and serious clinical events. BMC Health Serv Res 2008;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell NC. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto K. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprangers MA, Cull A, Bjordal K, et al. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC study group on quality of life. Qual Life Res 1993;2:287–95 [DOI] [PubMed] [Google Scholar]
- 19.Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095–108 [DOI] [PubMed] [Google Scholar]
- 20.Dolan P. The time trade-off method: results from a general population study. Health Econ 2006;5:1–14 [DOI] [PubMed] [Google Scholar]
- 21.Cambell M. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ 1995;311:1145–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King M. The interpretation of scores from the EORTC quality of life questionnaire QLQ-C30. Qual Life Res 2004;5:555–67 [DOI] [PubMed] [Google Scholar]
- 23.Drummond M. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond M, Sculpher MJ, Torrance GW. Methods for the economic evaluation of health care programmes. New York: Oxford University Press Inc, 2005
- 25.Desgagné A, Castilloux A, Angers J, et al. The use of the bootstrap statistical method for the pharmacoeconomic cost analysis of skewed data. Pharmacoeconomics 1998;13:487–97 [DOI] [PubMed] [Google Scholar]
- 26.Hill JC, Whitehurst D, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet 2011;378:1560–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lachin JM. Futility interim monitoring with control of type I and II error probabilities using the interim Z-value or confidence limit. Clin Trials 2009;6:565–73 [DOI] [PubMed] [Google Scholar]
- 28.Lachin JM. A review of methods for futility stopping based on conditional power. Stat Med 2005;24:2747–64 [DOI] [PubMed] [Google Scholar]
- 29.Jitlal M, Khan I, Lee SM, et al. Stopping clinical trials early for futility: retrospective analysis of several randomised clinical studies. Br J Cancer 2012;107:910–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer 2006;94:1116–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renehan AG, O'Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 2004;328:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gall CA, Weller D, Esterman A, et al. Patient satisfaction and health-related quality of life after treatment for colon cancer. Dis Colon Rectum 2007;50:801–9 [DOI] [PubMed] [Google Scholar]
- 33.Körner H, Søreide K, Stokkeland PJ, et al. Systematic follow-up after curative surgery for colorectal cancer in Norway: a population-based audit of effectiveness, costs, and compliance. J Gastroint Surg 2005;9:320–8 [DOI] [PubMed] [Google Scholar]
- 34.Körner H, Søreide K, Stokkeland PJ, et al. Diagnostic accuracy of serum-carcinoembryonic antigen in recurrent colorectal cancer: a receiver operating characteristic curve analysis. Ann Surg Oncol 2006;14:417–23 [DOI] [PubMed] [Google Scholar]
- 35.Søreide K, Traeland JH, Stokkeland PJ, et al. Adherence to national guidelines for surveillance after curative resection of nonmetastatic colon and rectum cancer: a survey among Norwegian gastrointestinal surgeons. Colorectal Dis 2012;14: 320–4 [DOI] [PubMed] [Google Scholar]
- 36.Beaver K, Hollingworth W, McDonald R, et al. Economic evaluation of a randomized clinical trial of hospital versustelephone follow-up after treatment for breast cancer. Br J Surg 2009;96:1406–15 [DOI] [PubMed] [Google Scholar]
- 37.Grunfeld E. Follow-up of breast cancer in primary care vs specialist care: results of an economic evaluation. Br J Cancer 1999;79:1227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimman ML, Dirksen CD, Voogd AC, et al. Economic evaluation of four follow-up strategies after curative treatment for breast cancer: results of an RCT. Eur J Cancer 2011;47:1175–85 [DOI] [PubMed] [Google Scholar]
- 39.Grunfeld E. Randomized trial of long-term follow-up for early-stage breast cancer: a comparison of family physician versus specialist care. J Clin Oncol 2006;24:848–55 [DOI] [PubMed] [Google Scholar]
- 40.Editorial Cancer detection and primary care revisited. Lancet Oncol 2012;13:559. [DOI] [PubMed] [Google Scholar]
- 41.Rubin G, Lyratzopoulos G, Abel G, et al. Cancer detection in primary care. Lancet Oncol 2012;13:e325–6 [DOI] [PubMed] [Google Scholar]
- 42.Lyratzopoulos G, Neal R, Barbiere J, et al. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 national cancer patient experience survey in England. Lancet Oncol 2012;13:353–65 [DOI] [PubMed] [Google Scholar]
- 43.Cooper G, Kou T, Reynolds H. Receipt of guideline-recommended follow-up in older colorectal cancer survivors. Cancer 2008;113:2029–37 [DOI] [PubMed] [Google Scholar]
- 44.Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR good research practices task force report. Value Health 2009;12:409–18 [DOI] [PubMed] [Google Scholar]
- 45. doi: 10.1136/bmj.313.7058.665. Grunfeld E. Routine follow up of breast cancer in primary care: randomised trial. BMJ 1996;313:665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RA. Mathematical models and cost-effective screening strategies for colorectal cancer. CMAJ 2010;182:1283–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.