Abstract
Objectives
Little is known regarding the ‘false-negative’ or ‘false-positive’ striatal dopamine transporter binding on SPECT for the diagnosis of dementia with Lewy bodies (DLB). We explored the clinical course in patients fulfilling the criteria for clinical DLB with a normal (123I)FP-CIT SPECT (ie, SPECT scan negative, clinical features positive (S−CF+)) and patients not fulfilling DLB criteria with an abnormal scan (S+CF−).
Design
Longitudinal case study over 2–5 years.
Setting
Consecutive referrals of patients with mild dementia to dementia clinics in western Norway.
Participants
50 patients (27 men and 23 women; mean age at baseline of 74 (range 52–88)) with (123I)FP-CIT SPECT images underwent cluster analysis: 20/50 patients allocated to a ‘DLB’ and 8 to a ‘non-DLB’ cluster were included.
Outcome measures
Scores on standardised clinical rating scales for hallucinations, parkinsonism, fluctuations, rapid eye movement (REM) sleep behaviour disorder and visually rated (123I)FP-CIT SPECT.
Results
During the follow-up period, in the S+CF− group (n=7), frequency and severity of DLB symptoms tended to increase, particularly parkinsonism (7/7) and cognitive fluctuations (7/7), while severity of visual hallucinations and REM sleep behaviour disorder remained stable. The S−CF+ (n=3) fulfilled the operationalised criteria for probable DLB both at baseline and at the end of the follow-up.
Conclusions
The findings suggest that systematic visual analyses of (123I)FP-CIT SPECT can detect people with DLB prior to the development of the full clinical syndrome. In addition, the study indicates that some patients fulfilling clinical criteria for probable DLB have a normal scan, and further studies are required to characterise these patients better.
Article summary.
Article focus
(123I)FP-CIT SPECT is an established biomarker for dementia with Lewy bodies (DLB) versus Alzheimer's disease.
Little is known about the clinical course of patients with symptoms of DLB and a normal (123I)FP-CIT SPECT and patients not fulfilling the DLB criteria with an abnormal scan.
We performed a detailed clinical follow-up of such patients.
Key messages
Patients not fulfilling the DLB criteria with an abnormal scan developed over time typical DLB clinical features.
Patients with symptoms of DLB and a normal (123I)FP-CIT SPECT continued to fulfil clinical criteria for DLB, that is, were false negative according to clinical criteria.
(123I)FP-CIT SPECT is a helpful investigation in an early stage of DLB.
Strengths and limitations of this study
Limitations include the small number of patients included and the lack of autopsy.
Strengths include the objective clinical classification based on cluster analysis and the long duration of follow-up and the use of standardised procedures during the study period.
Introduction
Dementia with Lewy bodies (DLB) is the second most frequent neurodegenerative dementia after Alzheimer's disease (AD). In addition to cognitive decline, frequent clinical symptoms of DLB are parkinsonism, hallucinations and other psychiatric symptoms, fluctuating attention and autonomous dysfunction including orthostatic hypotension and falls.1 DLB patients have more reduced quality of life, higher costs and higher mortality than patients with AD.
DLB is often underdiagnosed especially in the early stages when the frequency of presenting core symptoms is low.2 Early and accurate diagnosis is, however, important for informing patient and relatives about key treatment decisions as the disease course and prognosis differ between the dementia types. In addition, diagnosing DLB is meaningful for avoiding antipsychotic drugs due to the sensitivity for side effects in this patient group. Diagnosis is particularly problematic in people who have some symptoms of DLB, but do not fulfil the criteria for probable DLB. The clinical diagnosis of DLB has a high specificity (approximately 95%), but low sensitivity (30%) mainly based on the consensus criteria presented in 1996,3 indicating that the diagnosis is often missed.
Dopaminergic nigrostriatal degeneration is common in DLB and (123I)FP-CIT SPECT imaging is able to detect this dopaminergic deficit. This imaging technique is an established biomarker for the in vivo detection of nigrostriatal degeneration, which is a typical feature of Parkinson's disease (PD) also. (123I)FP-CIT SPECT uses a 123I-labeled tracer that binds with high affinity to the dopamine transporter (DAT). A high correlation between abnormal DAT binding and a clinical diagnosis of probable DLB has been shown; in a pivotal multicentre study, abnormal scans had a mean sensitivity of 78% for distinguishing clinically probable DLB from AD, with a specificity of 90% for excluding non-DLB.4 Decreased striatal DAT binding is listed as one of the suggestive features in the consensus criteria for the clinical diagnosis of DLB.1
Very little is known regarding patients fulfilling clinical DLB criteria with a negative (123I)FP-CIT SPECT scan (S−CF+) or patients with an abnormal scan not fulfilling clinical DLB criteria (S+CF−). In contrast, considerable research has been conducted on PD patients with negative DAT scans, that is, ‘scans without evidence of dopaminergic deficit’. Repeated DAT imaging showed a normal scan up to 4 year follow-up and this group did not benefit from antiparkinson medication.5 This demonstrates that the causes other than nigrostriatal degeneration can cause parkinsonism, such as, for example, cerebrovascular disease (vascular parkinsonism) and drugs with antidopaminergic activity.6
It has been shown in a trial cohort that (123I)FP-CIT SPECT can distinguish DLB from AD even before the full syndrome has emerged. Indeed, this imaging marker is particularly clinically useful in diagnostically uncertain cases, and patients with ‘S+CF−’ may actually represent early cases who will later develop a full DLB syndrome.7 8 However, the nosological status and course of the S−CF+ and S+CF− group in the context of DLB have not been reported in a clinical cohort.
This study aims to explore the clinical characteristics and course of dementia patients who underwent (123I)FP-CIT SPECT imaging. A group of such patients from the Norwegian DemWest cohort was followed according to a standardised and prospective research protocol. We hypothesised that ‘S+CF−’ cases would develop a clinical profile more consistent with the diagnostic criteria for DLB, and that ‘S−CF+’ cases may develop a clinical phenotype that differed from the typical pattern of DLB patients.
Materials and methods
Participants were selected from the DemWest cohort, which includes patients from dementia clinics with a first time diagnosis of mild dementia who are followed annually.9 Patients were assessed with medical exam and routine blood tests, and standardised clinical assessments and neuropsychological tests were administered, such as the motor subscale of the Unified Parkinson’s Disease Rating Scale (UPDRS) for parkinsonism, Neuropsychiatric Inventory (NPI) to assess psychiatric symptoms including visual hallucinations (VHs) and the Clinician Assessment of Cognitive Fluctuations (COGA)10 or Mayo fluctuation Questionnaire11 for cognitive fluctuations. Sleep disturbances including rapid eye movement (REM) sleep behaviour disorder (RBD) were monitored with the Mayo sleep Questionnaire.12 Neuroleptic sensitivity was classified as previously reported.13 Exclusion criteria were acute delirium or confusion, terminal illness, recently diagnosed major somatic illness, previous bipolar disorder or psychotic disorder. More details regarding the selection and diagnostic procedures are provided elsewhere.9
Continuous scores for the core and suggestive DLB features were calculated: for VHs (frequency × intensity) using the NPI scale item 2 with a range of 0–12; parkinsonism on the UPDRS motor subscale (0–108); fluctuating cognition by the COGA (0–16) (a subgroup on the Mayo fluctuation questionnaire (0–4)) and combined as previously described.14 RBD was determined with the Mayo sleep questionnaire (0–4). To select DLB patients, we used UPDRS-motor subscale cut-off score of >9 and at least 1 within other scales.14
Imaging
From the DemWest database 50 patients underwent (123I)FP-CIT SPECT imaging on the discretion of the clinician considered DLB to be a differential diagnosis between March 2005 and May 2010 (27 men and 23 women, mean age at baseline of 74 (range 52–88). Initial diagnosis of possible or probable DLB was made using clinical judgement.1 Subsequent classification of patients was undertaken based upon a cluster analysis of symptoms (see analysis section below for details).The average time between initial clinical diagnosis and date of (123I)FP-CIT SPECT imaging was 7 months.
SPECT imaging with the well-validated radiotracer (123I)FP-CIT (N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane; DaTscan, GE Healthcare) was performed according to the clinical routine at each of the three centres. An intravenous injection of about 185 MBq was administered and the images were acquired 3–4 h after injection on a multidetector or multiheaded γ camera with LEHR collimators, a time point at which the specific binding ratio of this tracer to the DAT is stable.15 Subsequently, images were reconstructed using filtered back projection with a Butterworth filter with a 0.55 cut-off and an order of 10. Chang’s attenuation correction was applied with an attenuation coefficient of 0.11/cm.16
Representative transversal images through the basal ganglia were visually analysed by an external nuclear medicine specialist, experienced in DAT imaging (JB), who did not have access to clinical information. The visual analysis consisted of separate evaluations of the left and right caudate nucleus and putamen divided in normal, abnormal and strongly abnormal.
In addition, MRI was acquired at baseline at the three centres using 1.5 Tesla MRI: a Philips Intera scanner with fast field echo protocol (repetition time (TR)/echo time (TE)/flip angle (FA)=10 ms/4.6 ms/30°,ST=2.0 mm, number of excitations (NEX)=2.0, matrix=256×256 or TR/TE/FA=20 ms/4.6 ms/30°,ST=1.0 mm, NEX=2.0, matrix=256×256) and GE Signa Excite scanner with fast spoiled gradient recalled (FSPGR) protocol (TR/TE/FA=20 ms/3.1 ms/7°,ST=1.0 mm, NEX=1.0, matrix=256×256). Details of the harmonisation have been reported previously.17 Basal ganglia hyperintensities were scored using the basal ganglia part of the Scheltens scale, a semiquantitative rating scale (0–30) including separate assessment (0–6) of the caudate nucleus, putamen, globus pallidus, thalamus and the internal/external capsule.18 One patient (case 9) did not receive an MRI due to metal prostheses in both ears.
Analysis
For an objective, quantitative classification of cases, cluster analysis was applied based on the scores on the four DLB symptom scales as previously reported.14 The patients were classified in four clusters with the help of SPSS V.18. In brief, the two-step cluster analysis was performed with four continuous variables (ie, parkinsonism, hallucinations, cognitive fluctuations and RBD) and log-likelihood.14 Missing values analysis with expectation-maximisation algorithm was performed when scores at one of the four symptom scales was missing. The four clusters included a ‘DLB’ cluster, with high scores for hallucinations and parkinsonism and cognitive fluctuations, a ‘non-DLB’ cluster with low values on all DLB symptom scales, one cluster included patients with high scores for RBD, and one with high values of cognitive fluctuations. In this study, we only considered patients classified in the ‘DLB’ and the ‘non-DLB’ clusters.14 Based on the (123I)FP-CIT SPECT scan results, patients were classified as S−CF+ (ie, DLB-cluster and normal scan) or S+CF− (ie, non-DLB cluster and abnormal scan).
Design
Patients were followed with annual assessments using the same assessment battery at baseline performed by trained research physicians and research nurses. Subsequently, core and suggestive DLB features (parkinsonism, VH, cognitive fluctuations, neuroleptic hypersensitivity and RBD) and their progress were rated by an experienced research clinician (AR), taking into account both research data and transcripts from the medical records, but blinded to all information of (123I)FP-CIT SPECT scan information and clinical diagnosis. The blinding was achieved by actively removing all information about the scans from the transcripts. The rater noted whether symptoms were present and their severity (no, mild-to-moderate and moderate-to-severe) at baseline, and whether they increased or decreased (mild or marked) or remained stable during the follow-up period.
Ethics approval
The regional ethics committee and the Norwegian authorities approved the DemWest study for the collection of medical data. The patients provided written consent to participate in the study after a thorough explanation of the procedure to the patient and the caregiver.
Results
There were 20 patients in the DLB cluster and 8 patients in the non-DLB cluster (the remaining 22 patients were included in the other two clusters, with subsequently n=5 with S− and n=7 with S+ in the RBD cluster and n=6 with S− and n=4 with S+ in the cognitive fluctuation cluster). Nine of the 50 patients with (123I)FP-CIT SPECT had a missing value for one of the four symptom scores, and therefore the previously mentioned missing value analysis was performed.
Scans of three patients in the DLB cluster were classified as normal, and these three were consequently classified as S−CF+. Seven patients in the non-DLB cluster had an abnormal scan, and were classified as S+CF−. An example of the (123I)FP-CIT SPECT scan for the S−CF+ group (left) and S+CF− group (right) is shown in figure 1. OTable 1 shows the characteristics of the two groups.
Figure 1.
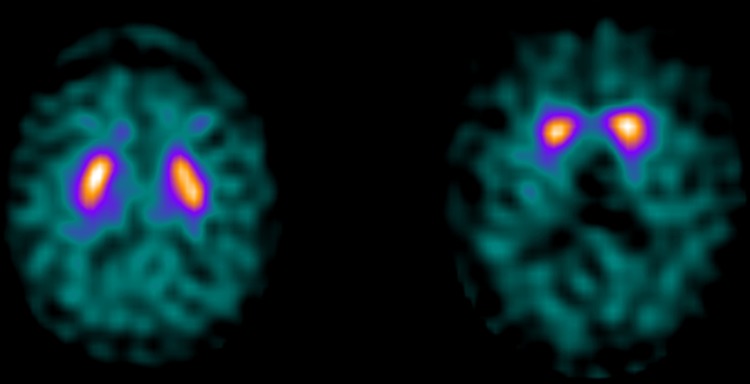
Transversal (123I)FP-CIT SPECT image from a patient in the dementia with Lewy bodies (DLB) cluster with a normal scan (left: case 2) and a patient with an abnormal scan in the non-DLB cluster (right: case 4).
Table 1.
Clinical characteristics
| Characteristics | S−CF+ (n=3) | S+CF− (n=7) | |
|---|---|---|---|
| Gender (M:F) | 3:0 | 4:3 | |
| MMSE | 22 (20–26) | 25 (16–27) | |
| Age at baseline (years) | 79 (72–88) | 71 (52–80) | |
| Observation time (years) | 3.0 (2–4) | 3.4 (2–5) | |
| Medication | |||
| Antiparkinson (yes/no) | 0:3 | 1:6 | |
| Antipsychotics (yes/no) | 2:1 | 1:6 | |
| Antidepressants (yes/no)\ | 1:2 | 3:4 | |
| Antidementia (yes/no) | 1:2 | 6:1 |
Numbers represent median (range) or number of patients.
CF, clinical features; MMSE, mini mental state examination; S, (123I)FP-CIT SPECT.
Detailed information about the visual assessment of the (123I)FP-CIT SPECT scans regarding DAT binding in the caudate nucleus bilaterally, and the putamen bilaterally, as well as hyperintensities based on MRI, are shown in Otable 2.
Table 2.
Visual assessment of (123I)FP-CIT SPECT and MRI
| (123I)FP-CIT SPECT |
MRI |
||||||
|---|---|---|---|---|---|---|---|
| Caudate right | Putamen left | Putamen right | Final evaluation | Scheltens score (0–30) | details | ||
| S−CF+ | |||||||
| 1 | 0 | 0 | 0 | 0 | Normal | 3 | Right capsula interna/externa |
| 2 | 0 | 0 | 0 | 0 | Normal | 0 | |
| 3 | 0 | 0 | 0 | 0 | Normal | 8 | Left thalamus, right capsula interna/externa |
| S+CF− | |||||||
| 4 | 0 | 0 | 2 | 2 | Abnormal | 0 | |
| 5 | 0 | 2 | 0 | 2 | Abnormal | 0 | |
| 6 | 2 | 2 | 2 | 2 | Abnormal | 0 | |
| 7 | 0 | 1 | 2 | 2 | Abnormal | 0 | |
| 8 | 0 | 0 | 2 | 1 | Abnormal | 4 | Left caudate, right capsula externa |
| 9 | 2 | 2 | 2 | 2 | Abnormal | − | Not performed |
| 10 | 0 | 1 | 0 | 1 | Abnormal | 6 | Putamen bilateral, left capsula externa |
0=normal, 1=abnormal, 2=strongly abnormal on (123I)FP-CIT SPECT.
CF, clinical features; S, (123I)FP-CIT SPECT.
It can be seen that in the S−CF+ group, the (123I)FP-CIT SPECT scans were normal, by definition, in all studied striatal subareas. In the S+CF− group, putamen DAT binding was abnormal bilaterally in most cases (5/7). None of the cases shows infarcts in the basal ganglia on MRI. Some patients had white matter lesions, but these were usually in the low-to-moderate range.
During follow-up, no other diseases were detected which could explain the symptoms. OTable 3 shows the core and suggestive symptoms at baseline and their development during follow-up.
Table 3.
Status of main dementia with Lewy bodies symptoms at baseline and during follow-up
| Visual hallucinations |
REM sleep behaviour disorder |
Cognitive fluctuations |
Parkinsonism |
|||||
|---|---|---|---|---|---|---|---|---|
| Bsl | Fu | Bsl | Fu | Bsl | Fu | Bsl | Fu | |
| S−CF+ | ||||||||
| 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 |
| 2 | 1 | −1 | 1 | 0 | 0 | 1 | 1 | 2 |
| 3 | 1 | 0 | 1 | −1 | 0 | 2 | 0 | 1 |
| S+CF− | ||||||||
| 4 | 1 | 0 | 2 | −1 | 1 | 2 | 0 | 2 |
| 5 | 2 | 1 | 0 | 1 | 0 | 2 | 1 | 2 |
| 6 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| 7 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 2 |
| 8 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 |
| 9 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 |
| 10 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 |
Baseline: 0=not present, 1=mild/moderate, 2=moderate/marked; follow-up: –2=significant decrease, –1=decrease, 0=no change, 1=increase, 2=significant increase.
Bsl, baseline; CF, clinical features; Fu, follow-up; REM, rapid eye movement; S, (123I)FP-CIT SPECT.
In the S+CF− group, it can be seen that some DLB core symptoms were present at baseline in some patients. These were usually of mild-to-moderate severity and did not reach the cut-off values that emerged from the cluster analysis.14 All seven had a moderate-to-severe increased parkinsonism during follow-up, although 3/7 had no parkinsonism at baseline. In addition, all seven S+CF− developed marked worsening in cognitive fluctuations, whereas six were without these symptoms at baseline. Less marked changes were noted for VH.
In the S−CF+ group, all three cases had VH at baseline that remained stable (n=2) or decreased (n=1) in severity during the follow-up period. In contrast, two cases showed a moderate-to-severe worsening in parkinsonism and two cases had worsening of cognitive fluctuations.
There were no remarkable changes in the progression of the severity of RBD that was also rare at baseline. Three patients (2 S−CF+, 1 S+CF−) received antipsychotics, but hypersensitivity reactions to these drugs were not observed.
All cases fulfilled the criteria for probable DLB at the end of the follow-up period without taking the (123I)FP-CIT SPECT scan results into account, that is, they had at least two core features or one core and one clinical suggestive feature.
Discussion
In this study, we report longitudinal findings in patients who, based on cluster analysis of symptom scores were classified as ‘DLB’ or ‘non-DLB’, and who had (123I)FP-CIT SPECT results that were inconsistent with these findings, that is, ‘S−CF+’ or ‘S+CF−’. Our main findings are that S+CF− patients tend to develop core or suggestive features of DLB, in particular parkinsonism and cognitive fluctuations, that is, they represent DLB in the early stage. In the S−CF+ group, more various DLB symptoms were shown at baseline with mainly a stable or increased severity at follow-up, and all cases fulfilled the criteria for probable DLB at the end of the follow-up period.
Very few longitudinal studies of DLB patients in light of (123I)FP-CIT SPECT have been reported. In contrast, the results of (123I)FP-CIT SPECT imaging and the discrepancy with clinical diagnosis have been extensively studied in PD. The Clinically Uncertain Parkinsonism Syndrome study considered patients with uncertain but clinically suspect PD. (123I)FP-CIT SPECT imaging was inconsistent with initial diagnosis in 36% of the patients with a clinical diagnosis of presynaptic parkinsonism and 54% with non-presynaptic parkinsonism. After 2 years, however, the clinical diagnosis was established and the rate of agreement between the diagnosis at follow-up and the initial imaging results was 90%, indicating that the initial DAT SPECT scan is of value in the diagnostic follow-up of the patients with clinically uncertain PD.19 This is in line with the current study showing that all seven FP cases developed increased parkinsonism and cognitive fluctuations consistent with a diagnosis of DLB. Our findings are also consistent with a previous study demonstrating that in cases with ‘possible DLB’, that is, not fulfilling criteria for probable DLB, the scan differentiated between those who developed probable DLB and those who did not after 1 year.7
An explanation for this could be that the (123I)FP-CIT SPECT scan detects nigrostriatal degeneration before the full clinical syndrome has been developed. This is supported by the knowledge of PD that 80% of the striatal dopamine neurons need to be lost before PD symptoms are present.20 In addition, it is shown that by using a radioligand for DAT nigrostriatal damage can be detected years before the onset of motor signs of PD.21
Three of our cases (3 of 50; 6% of all scans) were in the S−CF+ group, and searching for possible explanations is important. It is well known that 10–15% of patients with clinical PD have a normal DAT scan, and it is suggested that this subgroup have no involvement of the dopaminergic nigrostriatal pathway.6 In the S−CF+ group, the cases may have a true negative (123I)FP-CIT SPECT, that is, they have DLB, but without the involvement of dopaminergic neurons in the substantia nigra. Some DLB patients indeed do not develop parkinsonism.22 Pathological classification of DLB identifies three types: brainstem predominant, limbic and neocortical, assuming that the substantia nigra is first affected, following by the amygdalae and limbic cortex and subsequently the neocortex.23 However, it has been reported that in some cases, Lewy-body pathology can be found in the cortex and higher brain stem, but not in the lower brain stem,24 suggesting that in some patients the pathological process starts in the neocortex, and then progresses towards the brain stem. Our findings in the S−CF+ group are consistent with this hypothesis, in that parkinsonism mainly developed later on. Unfortunately, a follow-up scan or neuropathology was not available to address this hypothesis. Interestingly, a recent clinicopathological study, which included seven autopsy proven DLB cases, showed that the antemortem (123I)FP-CIT SPECT scan was normal in two of these cases. Importantly, in these two cases, the number of nigrostriatal dopaminergic neurons was also within the normal range.25 This observation may support the hypothesis that in some patients the pathological process may start in the neocortex.
Another possible explanation for the S−CF+ cases is the pathological heterogeneity in DLB. Whereas some patients have a ‘pure’ DLB, the majority in addition may have AD-type changes such as amyloid plaques and even tangle pathology.1 In total, 5–10% of patients with clinical dementia have intermediate (123I)FP-CIT SPECT scans, that is, abnormal DAT binding, but not as low as typical of DLB. These intermediate scans could represent cases with mixed DLB/AD pathology.26 DLB cases with AD pathology have lower prevalence of core DLB symptoms than ‘pure’ DLB.6 27 It is possible that mixed cases in an early stage may have subtle or no nigrostriatal dopaminergic pathology leading to a normal DAT scan. In these cases parkinsonism may be caused by Alzheimer-type or even LB-type degeneration in the striatum itself rather than by dopaminergic nigrostriatal neurodegeneration.28
Another possible explanation is that S−CF+ cases have another type of dementia than DLB. For example, parkinsonism is not uncommon in AD, particularly in the later stages,29 and it can be seen in other conditions such as vascular parkinsonism and frontotemporal dementia. However, both vascular parkinsonism and frontotemporal dementia may have abnormal scans, although less common than in DLB.30 31 The score for hyperintensities on MRI is in the range from 0 to 8 (see table 2). These relatively low scores show that cerebrovascular is an unlikely cause of parkinsonism in our cases, also since none of the subjects had lacunar infarcts in the basal ganglia.
Drug-induced parkinsonism is also common,6 and may take several weeks to resolve after drug discontinuation, and complete resolution may take over a year.32 These patients usually have a normal DAT SPECT scan,33 and thus may be misinterpreted as ‘S−CF+’. In our S−CF+ group, case 1 received antipsychotics during 5 months and discontinued 1 month before scanning; in case three, it was discontinued directly at baseline. However, in both cases, parkinsonism increased during follow-up. Drug-induced parkinsonism is therefore not a likely cause of the ‘S−CF+’ patients in our study.
Finally, several patients were treated with drugs such as antidepressants, antipsychotics, L-DOPA and antidementia drugs, and scanned while on medication, which may influenced the interpretation of the (123I)FP-CIT SPECT scans.34
The antidopaminergic effect of antipsychotic drugs may increase the synthesis and release of dopamine in the striatum, leading to a potential competition between the DAT ligand (123I)FP-CIT and synaptic dopamine. Indeed, high doses of the neuropsychotic haloperidol resulted in a reduction in striatal (123I)FP-CIT binding by 25% in rats.35 However, in another rat study, this could not be replicated.36 Also, it has recently been discussed that even if neuroleptics will induce changes in DAT imaging, such changes will presumably not be large enough to influence the visual assessments of (123I)FP-CIT SPECT studies.34 Nevertheless, in the present study, one patient in the S+CF− group had used an antipsychotic, and we cannot totally exclude that this may have led to a false-positive scan.
Although antiparkinsonian medications influence the dopaminergic transmission, they do not seem to affect the visual interpretation and quantification of DAT imaging.6 The use of Levodopa, for example, did not change the striatal (123I)FP-CIT binding significantly.37
The acetylcholinesterase inhibitors, such as donepezil and rivastigmine, may reduce striatal dopaminergic transmission, but did not show significant effects on striatal (123I)FP-CIT binding.38
Antidepressants such as the selective serotonin reuptake inhibitor (SSRI) paroxetine have been shown to induce a small, but significant increase in striatal (123I)FP-CIT binding ratios.39 On the other hand, the SSRI citalopram showed a reduction in striatal (123I)FP-CIT binding ratios, and blocking of the serotonin transporter (SERT) may lead to high plasma (123I)FP-CIT concentrations.40 DAT and SERT are important for the termination of dopaminergic and serotonergic transmission, respectively, by the reuptake of dopamine and serotonin from the synaptic cleft. FP-CIT binds to DAT and SERT with affinities in the nanomolar range, although the affinity to SERT is lower than that to DAT.34 Therefore, the quantification of (123I)FP-CIT SPECT is susceptible to SERT-blocking pharmaceuticals.40 However, as recently discussed, a small change in the quantification in striatal FP-CIT binding, and with the same change of binding in both the caudate nucleus and putamen, is unlikely to result in false-negative and false-positive results when a visual assessment is used.34 One of our S−CF+ and two of the S+CF− patients had used an SSRI, but it is unlikely that this has influenced the interpretation of our scan results. Two of the antidepressant users showed a decrease in VHs during follow-up, cases 2 and 5 (table 3). Nevertheless, more studies are needed to explore how central nervous system-active drugs frequently used by DLB-patients may influence DAT imaging interpretation.
Some limitations of this study need to be acknowledged. First, the limited number of patients in the S−CF+ and S+CF− group and the difference in follow-up time ranging from 2 to 5 years, dependent on the moment of entrance in the study should be mentioned. Also, no repeat imaging was performed. A prolonged follow-up time in a larger study group with follow-up DAT scan may have provided additional information. Second, the NPI item 2 includes hallucinations from different modalities and not only visual. However, we have previously shown that of those with hallucinations in the overall cohort, nearly 80% had VHs.41 In addition, we did verify through the medical record transcripts that the patients with hallucinations in fact had VHs. RBD was diagnosed based only on clinical assessment. Videopolysomnograpphy is the preferred diagnostic method, but not widely available; acceptable sensitivity and specificity have been reported for the Mayo sleep scale.42 Finally, scans were only analysed visually. However, in previous studies, this approach has shown to be as accurate as semiquantitative analyses to differentiate normal from abnormal (123I)FP-CIT SPECT scans.4
The golden standard for diagnosing DLB is the brain pathology analysis postmortem. Unfortunately, autopsy of the FP and FN cases in this study was not available to confirm diagnosis. However, all seven available autopsy diagnoses from the DemWest study were consistent with the clinical diagnosis including two AD and five DLB patients. In addition, a relative objective method of cluster analysis was performed based on symptom scores for the main DLB symptoms in this study, and the symptoms of interest were rated by trained research clinicians using standardised and validated instruments.
Conclusion
These data support the notion that (123I)FP-CIT SPECT imaging can identify DLB patients before the full syndrome has developed, supporting the usefulness of (123I)FP-CIT SPECT as part of the routine clinical work up in cases with suspect DLB. A minority of patients fulfilling clinical DLB criteria have a normal (123I)FP-CIT SPECT scan, and further studies are needed to characterise such cases.
Supplementary Material
Acknowledgments
The authors acknowledge the coinvestigators involved in the large dementia study in western Norway and especially K Brønnick for the advice and contribution. In addition, they thank the National Institute for Health Research (UK) for supporting the work of Clive Ballard through the Biomedical Research Unit for Dementia and the Biomedical Research Centre for Mental Health at King's College London.
Footnotes
Contributors: FJS and DA were responsible for the study concept, design, data analysis, writing of the manuscript and provided input during the whole process of the study. AR was involved in the development of the study concept, clinical patient evaluation and data analysis. TCB was involved in the image selection and reconstruction and provided main input during data acquisition. MKB and JB were responsible for image analysis and interpretation of results. CGB was involved in the concept and evaluation of results. All authors were involved in critical review of the draft and approval of the version to be published.
Funding: This study was supported by GE Healthcare. Françoise Siepel is supported by a grant from Western Norway regional health authority (grant number Helse Vest 911714).
Competing interests: JB is consultant of GE Healthcare.
Ethics approval: Regional committee for medical researchetics west Norway (REK 167.04).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–72 [DOI] [PubMed] [Google Scholar]
- 2.Tiraboschi P, Salmon DP, Hansen LA, et al. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain 2006;129(Pt 3):729–35 [DOI] [PubMed] [Google Scholar]
- 3.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–24 [DOI] [PubMed] [Google Scholar]
- 4.McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol 2007;6:305–13 [DOI] [PubMed] [Google Scholar]
- 5.Marek K, Jennings D, Seibyl J. Long-term follow-up of patients with scans without evidence of dopaminergic deficit (SWEDD) in the ELLDOPA study (abstract). Neurology 2005;64(Suppl 1):A274 [Google Scholar]
- 6.Cummings JL, Henchcliffe C, Schaier S, et al. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain 2011;134(Pt 11):3146–66 [DOI] [PubMed] [Google Scholar]
- 7.O'Brien JT, McKeith IG, Walker Z, et al. Diagnostic accuracy of 123I-FP-CIT SPECT in possible dementia with Lewy bodies. Br J Psychiatry 2009;194:34–9 [DOI] [PubMed] [Google Scholar]
- 8.Papathanasiou ND, Boutsiadis A, Dickson J, et al. Diagnostic accuracy of 123I-FP-CIT (DaTSCAN) in dementia with Lewy bodies: a meta-analysis of published studies. Parkinsonism Relat Disord 2012;18:225–9 [DOI] [PubMed] [Google Scholar]
- 9.Aarsland D, Rongve A, Nore SP, et al. Frequency and case identification of dementia with Lewy bodies using the revised consensus criteria. Dement Geriatr Cogn Disord 2008;26:445–52 [DOI] [PubMed] [Google Scholar]
- 10.Walker MP, Ayre GA, Cummings JL, et al. The Clinician Assessment of Fluctuation and the One Day Fluctuation Assessment Scale. Two methods to assess fluctuating confusion in dementia. Br J Psychiatry 2000;177:252–6 [DOI] [PubMed] [Google Scholar]
- 11.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004;62:181–7 [DOI] [PubMed] [Google Scholar]
- 12.Boeve BF, Ferman TJ, Silber MH, et al. Validation of a questionnaire for the diagnosis of REM sleep behavior disorder. Neurology 2002;58(Suppl 3):A509 [Google Scholar]
- 13.Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson's disease and parkinsonian dementias. J Clin Psychiatry 2005;66:633–7 [PubMed] [Google Scholar]
- 14.Rongve A, Bronnick K, Ballard C, et al. Core and suggestive symptoms of dementia with Lewy bodies cluster in persons with mild dementia. Dement Geriatr Cogn Disord 2010;29:317–24 [DOI] [PubMed] [Google Scholar]
- 15.Booij J, Hemelaar TG, Speelman JD, et al. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson's disease by (123I]FPCIT SPECT. J Nucl Med 1999;40:753–61 [PubMed] [Google Scholar]
- 16.Darcourt J, Booij J, Tatsch K, et al. EANM procedure guidelines for brain neurotransmission SPECT using (123)I-labelled dopamine transporter ligands, version 2. Eur J Nucl Med Mol Imaging 2009;37:443–50 [DOI] [PubMed] [Google Scholar]
- 17.Lebedev AV, Beyer MK, Fritze F, et al. Cortical changes associated with depression in Alzheimer's disease: an MRI surface-based morphometric study (abstract). Eur Psychiatry 2012;27:792. [DOI] [PubMed] [Google Scholar]
- 18.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci 1993;114:7–12 [DOI] [PubMed] [Google Scholar]
- 19.Tolosa E, Borght TV, Moreno E, et al. Accuracy of DaTSCAN (123I-Ioflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord 2007;22:2346–51 [DOI] [PubMed] [Google Scholar]
- 20.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 1991;114(Pt 5):2283–301 [DOI] [PubMed] [Google Scholar]
- 21.Ponsen MM, Stoffers D, Booij J, et al. Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann Neurol 2004;56:173–81 [DOI] [PubMed] [Google Scholar]
- 22.Ballard CG, Aarsland D, McKeith I, et al. Fluctuations in attention: PD dementia vs DLB with parkinsonism. Neurology 2002;59:1714–20 [DOI] [PubMed] [Google Scholar]
- 23.Braak H, Bohl JR, Muller CM, et al. Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 2006;21:2042–51 [DOI] [PubMed] [Google Scholar]
- 24.Halliday GM, McCann H. The progression of pathology in Parkinson's disease. Ann N Y Acad Sci 2010;1184:188–95 [DOI] [PubMed] [Google Scholar]
- 25.Colloby SJ, McParland S, O'Brien JT, et al. Neuropathological correlates of dopaminergic imaging in Alzheimer's disease and Lewy body dementias. Brain 2012;135(9):2798–808 [DOI] [PubMed] [Google Scholar]
- 26.Kemp PM, Holmes C. Imaging in dementia with Lewy bodies: a review. Nucl Med Commun 2007;28:511–19 [DOI] [PubMed] [Google Scholar]
- 27.Ballard CG, Jacoby R, Del Ser T, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with Lewy bodies. Am J Psychiatry 2004;161:843–9 [DOI] [PubMed] [Google Scholar]
- 28.Duda JE, Giasson BI, Mabon ME, et al. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol 2002;52:205–10 [DOI] [PubMed] [Google Scholar]
- 29.Starkstein SE, Merello M, Brockman S, et al. Apathy predicts more severe parkinsonism in Alzheimer's disease. Am J Geriatr Psychiatry 2009;17:291–8 [DOI] [PubMed] [Google Scholar]
- 30.Benitez-Rivero S, Marin-Oyaga VA, Garcia-Solis D, et al. Clinical features and 123I-FP-CIT SPECT imaging in vascular parkinsonism and Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;84:122–9 [DOI] [PubMed] [Google Scholar]
- 31.Morgan S, Kemp P, Booij J, et al. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. J Neurol Neurosurg Psychiatry 2012;83:1063–70 [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Sendon JL, Mena MA, De Yebenes JG. Drug-induced parkinsonism in the elderly: incidence, management and prevention. Drugs Aging 2012;29:105–18 [DOI] [PubMed] [Google Scholar]
- 33.Diaz-Corrales FJ, Sanz-Viedma S, Garcia-Solis D, et al. Clinical features and 123I-FP-CIT SPECT imaging in drug-induced parkinsonism and Parkinson's disease. Eur J Nucl Med Mol Imaging 2010;37:556–64 [DOI] [PubMed] [Google Scholar]
- 34.Booij J, Kemp P. Dopamine transporter imaging with ((123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging 2008;35:424–38 [DOI] [PubMed] [Google Scholar]
- 35.Nikolaus S, Antke C, Kley K, et al. Pretreatment with haloperidol reduces (123)I-FP-CIT binding to the dopamine transporter in the rat striatum: an in vivo imaging study with a dedicated small-animal SPECT camera. J Nucl Med 2009;50:1147–52 [DOI] [PubMed] [Google Scholar]
- 36.Lavalaye J, Knol RJ, De Bruin K, et al. (123I)FP-CIT binding in rat brain after acute and sub-chronic administration of dopaminergic medication. Eur J Nucl Med 2000;27:346–9 [DOI] [PubMed] [Google Scholar]
- 37.Schillaci O, Pierantozzi M, Filippi L, et al. The effect of levodopa therapy on dopamine transporter SPECT imaging with(123)I-FP-CIT in patients with Parkinson's disease. Eur J Nucl Med Mol Imaging 2005;32:1452–6 [DOI] [PubMed] [Google Scholar]
- 38.Knol RJ, De Bruin K, Van Eck-Smit BL, et al. No significant effects of single intravenous, single oral and subchronic oral administration of acetylcholinesterase inhibitors on striatal (123I)FP-CIT binding in rats. Eur J Nucl Med Mol Imaging 2008;35:598–604 [DOI] [PubMed] [Google Scholar]
- 39.Booij J, De Jong J, De Bruin K, et al. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med 2007;48:359–66 [PubMed] [Google Scholar]
- 40.Ziebell M, Holm-Hansen S, Thomsen G, et al. Serotonin transporters in dopamine transporter imaging: a head-to-head comparison of dopamine transporter SPECT radioligands 123I-FP-CIT and 123I-PE2I. J Nucl Med 2010;51:1885–91 [DOI] [PubMed] [Google Scholar]
- 41.Bjoerke-Bertheussen J, Ehrt U, Rongve A, et al. Neuropsychiatric symptoms in mild dementia with Lewy bodies and Alzheimer's disease. Dement Geriatr Cogn Disord 2012;34:1–6 [DOI] [PubMed] [Google Scholar]
- 42.Boeve BF, Molano JR, Ferman TJ, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in an aging and dementia cohort. Sleep Med 2011;12:445–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


