SUMMARY
We have previously demonstrated associations between fetal growth in late pregnancy and postnatal bone mass. However, the relationships between the intrauterine and early postnatal skeletal growth trajectory remains unknown. We addressed this in a large population-based mother-offspring cohort study. 628 mother-offspring pairs were recruited from the Southampton Women’s Survey. Fetal abdominal circumference (AC) was measured at 11, 19 and 34 weeks gestation using high resolution ultrasound with femur length (FL) assessed at 19 and 34 weeks. Bone mineral content (BMC) was measured postnatally in the offspring using DXA at birth and 4 years; postnatal linear growth was assessed at birth, 6, 12, 24, 36 and 48 months. Late pregnancy AC growth (19-34 weeks) was strongly (p<0.01) related to bone mass at birth, but less robustly associated with bone mass at 4 years. Early pregnancy growth (11-19 weeks) was more strongly related to bone mass at 4 years than at birth. Postnatal relationships between growth and skeletal indices at 4 years were stronger for the first and second postnatal years, than the period aged 2-4 years. The proportion of children changing their place in the distribution of growth velocities progressively reduced with each year of postnatal life. The late intrauterine growth trajectory is a better predictor of skeletal growth and mineralisation at birth, while the early intrauterine growth trajectory is a more powerful determinant of skeletal status at age 4 years. The perturbations in this trajectory which influence childhood bone mass warrant further research.
Keywords: Epidemiology, osteoporosis, programming, developmental origins, fetal growth
INTRODUCTION
We have previously demonstrated that fetal growth in late pregnancy is a determinant of skeletal size and density, assessed by DXA, in childhood1. Other work suggests important relationships between growth trajectories in utero and those experienced in the first few years of postnatal life2, 3. There are, however, relatively few longitudinal data relating patterns of intrauterine growth using objective measures such as gestational ultrasound to bone mineral accrual in childhood. The seminal studies by Tanner and colleagues, using linear external measurements during pregnancy, have suggested that fetal growth can be divided into early and late phases2, 3. The later phase, when growth velocity is at its peak, accounts for the accrual of 80% of neonatal bone mineral4. Growth during the second half of pregnancy is thus particularly susceptible to environmental influences, for example nutrition (placental transfer) and maternal constraint, such that heredity only accounts for a small proportion of the variance in birthweight5-7. These findings are broadly supported by more recent studies using gestational ultrasound scanning8, 9. Existing models of pre and postnatal growth suggest that maternal influences can lead to a temporary reduction of growth velocity in late pregnancy, and that postnatally the infant tends to revert to the original early fetal trajectory if allowed sufficient nutrition2, 3 in the first two years of life. Historically there has been very little objective longitudinal evidence to confirm these clinical models, particularly in relation to skeletal outcomes. At conception the genotype is set, and epigenetic influences occur all through gestation10. We have previously shown that factors such as maternal diet, lifestyle, body build and physical activity11-14 during pregnancy are all associated with offspring bone mineral content, and appear to exert most effect in late pregnancy. Additionally, in an earlier study1, late pregnancy growth in fetal femur length and abdominal circumference positively predicted measures of bone size and density at 4 years old. We therefore aimed to investigate how offspring growth velocity at periods from early fetal life to 4 years related to their skeletal development utilising an ongoing prospective population-based mother-offspring cohort.
METHODS
Participants
The Southampton Women’s Survey is a unique, prospective cohort study of 12,583 women aged 20-34 years recruited from the general population15. Assessments of lifestyle and anthropometry were performed at study entry and then in early (11 weeks) and late (34 weeks) gestation in those women who became pregnant. Maternal height was measured with a stadiometer, weight with calibrated digital scales, and skin folds (biceps, triceps, subscapular and suprailiac regions) with Harpenden callipers. The research nurses carrying out the measurements underwent regular assessment and re-training during the study to optimise consistency. The women were asked to characterise their current walking speed into one of five groups (very slow, stroll at an easy pace, normal speed, fairly brisk or fast). The women’s own birth-weight was recorded (by recall, checked by asking her own parents).
Prenatal ultrasound scanning
3,156 singleton pregnancies were followed. As this was a population survey, no inclusion criteria were set for the pregnancy study other than singleton pregnancy and ability to provide informed consent. At 11, 19 and 34 weeks gestation, the women underwent high-resolution ultrasound scanning using a Kretz Voluson® 730 (GE Kretz Ultrasound, Austria) or Acuson Sequoia® 512 (Siemens, USA) system, which were cross-calibrated. After establishing correct positioning according to standard anatomical landmarks, measurements of abdominal circumference (a composite of adiposity and liver size, obtained at all three timepoints) and femur length (obtained at 19 and 34 weeks) were made on the frozen images using electronic callipers by the two operators (PM and CN), according to internationally accepted and validated methodology 16,17. Each measurement was performed in triplicate and the mean value used for analysis.
Neonatal DXA assessment
Mothers registered with specific GP practices were invited to participate in the bone component of the SWS. These practices were selected to avoid the mothers participating in more than one substudy, and were representative of the population of Southampton as a whole. At birth, the babies were weighed on calibrated digital scales (Seca, UK), and crown-heel length measured using a neonatometer (CMS Ltd, UK). The mother was asked to agree to her baby undergoing assessment of bone mass and body composition within 2 weeks of birth, using a Lunar DPX-L instrument with specific paediatric software (paediatric small scan mode, v 4.7c, GE Corporation, Madison, Wisconsin, USA). The instrument underwent daily quality assessment, and was calibrated against a water phantom weekly. 1003 neonates underwent DXA assessment. The mothers could attend either as inpatients, or return from home within the two-week time period. At the visit to the scan room, the baby was pacified and fed if necessary, undressed completely, and then swaddled in a standard towel. It was placed on a waterproof sheet in a standard position on the scanner for measurement of whole body bone area, bone mineral content and body composition, using specific software protocols. The baby was kept in position using rice bags placed over the bottom end of the towel. The short-term and long-term coefficients of variation (CV) for adult whole body BMD for the DXA instrument were 0.8% and 1.4% respectively. It was not possible to scan neonates repeatedly to establish precision values in the study group, however, the ability of DXA to measure bone mass in small subjects has been demonstrated by Abrams et al18 using miniature piglets, where the correlation between DXA-derived BMC and ashed calcium content was 0.90 (p<0.001). The radiation exposure to the baby was estimated as a maximum of 8.9 microsieverts for whole body measurement, which is equivalent to around 3 day’s exposure to normal background radiation. All scan results were checked independently by two trained operators and agreement reached as to their acceptability. 31 scans showing unacceptable movement artefact were excluded.
Postnatal growth
Children were assessed at 6 months, 1, 2 and 3 years with a home visit from a research nurse. Information on diet, lifestyle, illness and medication was collected and anthropometric measurements were performed. Height was measured using a Leicester height measurer (Seca Ltd, UK) and weight, in underpants only, using calibrated digital scales (Seca Ltd, UK).
4 year DXA assessment
A subset of 900 participants was recruited sequentially from the SWS cohort. The mother (or father/ guardian) and child were invited to visit the Osteoporosis Centre at Southampton General Hospital for assessment. At this visit written informed consent for the DXA scan was obtained from the mother or father/ guardian. The child’s height (using a Leicester height measurer [Seca Ltd, UK]) and weight (in underpants only, using calibrated digital scales [Seca Ltd, UK]) were measured. Abdominal circumference was not measured at this age as it was not a requirement for the DXA assessment. A whole body DXA scan was obtained, using a Hologic Discovery instrument (Hologic Inc., Bedford, MA, USA) in paediatric scan mode. To encourage compliance, a sheet with appropriate coloured cartoons was laid on the couch first; to help reduce movement artefact, the children were shown a suitable DVD cartoon. The total radiation dose for the scans was 4.7 microsieverts for whole body measurement (paediatric scan mode). 32 scans were found to have unacceptable movement artefact so were excluded. The manufacturer’s CV for the instrument was 0.75 % for whole body bone mineral density, and the experimental CV when a spine phantom was repeatedly scanned in the same position 16 times was 0.68%.
Statistical analysis
All women had a reliable date of last menstrual period. All variables were checked for normality. Non-normally distributed variables were transformed logarithmically. The ultrasound derived measures of fetal size were adjusted for the gestational age at which the measurement was taken and within group z-scores were subsequently created. Conditional models of change were built using linear regression analysis: abdominal circumference (AC) is used as an example. AC z-score at 11 weeks is the starting point. Conditional change in AC z-score from 11 to 19 weeks is equivalent to the standardised residuals resulting from the linear regression model of AC z-score at 19 weeks on AC z-score at 11 weeks. Accordingly, the conditional change in AC z-score from 19 to 34 weeks is given as the standardised residuals obtained from regressing AC z-score at 34 weeks on both AC z-score at 19 weeks and AC z-score at 11 weeks simultaneously. This process is continued for each subsequent timepoint, resulting in measures of conditional growth which are mutually uncorrelated. Abdominal circumference measurements were available at all timepoints up to and including 3 years old; length assessment comprised femur length at 19 and 34 weeks gestation, crown-heel length at birth, 6 and 12 months, and height at 2-4 years.
Correlation and linear regression methods were used to explore the relationship between the growth measurements and bone size and density at birth and 4 years, using Stata V11.0 (Statacorp, Texas, USA). At birth, whole body bone area (BA), mineral content (BMC) and areal mineral density (aBMD) were used as the primary skeletal outcomes. At 4 years the same DXA indices were used but for the whole body minus head site.
The study had full approval from the Southampton and Southwest Hampshire Local Research Ethics Committee and all participants gave written informed consent.
RESULTS
There were 628 children (330 boys) with complete pregnancy ultrasound, post-natal growth and 4 year DXA data. The characteristics of the mothers and children are shown in table 1
Table 1.
Characteristics of mothers and children.
| A) Mothers | ||
|---|---|---|
| Maternal Characteristics | ||
| n=628 | Median | IQR |
| Age at child’s birth (years) | 30.8 | 27.9-33.6 |
| Triceps skinfold 34 weeks (mm) | 20.8 | 16.9-25.9 |
| BMI pre-pegnancy (kg/m2) | 24.2 | 22.2-27.2 |
| Mean | SD | |
|
| ||
| Height (cm) | 163.9 | 6.6 |
| n | % | |
|
| ||
| Walking speed at 34 weeks | ||
| Very slow | 95 | 15.3 |
| Stroll at an easy pace | 337 | 54.2 |
| Normal speed | 157 | 25.2 |
| Fairly brisk | 32 | 5.1 |
| Fast | 1 | 0.2 |
| Parity | ||
| 0 | 298 | 47.5 |
| 1+ | 330 | 52.5 |
| Smoking before pregnancy | ||
| No | 496 | 79 |
| Yes | 132 | 21 |
| Smoke in late pregnancy | ||
| No | 557 | 89.5 |
| Yes | 65 | 10.5 |
| B) Children | |||||
|---|---|---|---|---|---|
| Boys, n=330 | Girls, n=298 | ||||
| Mean | SD | Mean | SD | P diff | |
| 11 week: Mean AC (cm) | 5.6 | 0.7 | 5.6 | 0.7 | 0.97 |
| 19 week: Mean AC (cm) | 14.6 | 0.8 | 14.4 | 0.9 | 0.11 |
| 34 week: Mean AC (cm) | 30.8 | 1.4 | 30.7 | 1.4 | 0.53 |
| Birth: Mean AC (cm) | 31.8 | 1.8 | 31.9 | 1.9 | 0.86 |
| 1 year: Mean AC (cm) | 50 | 3 | 49.4 | 3.4 | 0.17 |
| 2 years: Mean AC (cm) | 49.3 | 2.8 | 49.1 | 3.1 | 0.52 |
| 3 years: Mean AC (cm) | 51.7 | 3 | 51.2 | 2.9 | 0.23 |
| 19 week scan: Femur length (cm) | 3.1 | 0.2 | 3.1 | 0.2 | 0.69 |
| 34 week scan: Femur length (cm) | 6.5 | 0.3 | 6.6 | 0.3 | 0.02 |
| Birth: Crown-heel length (cm) | 50.5 | 2 | 50.1 | 1.8 | 0.04 |
| 1 year: Crown-heel length (cm) | 76.3 | 2.3 | 75.2 | 2.6 | <0.001 |
| 2 years: Height (cm) | 87.2 | 2.8 | 86.5 | 3.1 | 0.02 |
| 3 years: Height (cm) | 96.4 | 3.2 | 96.1 | 3.7 | 0.39 |
| 4 years: Height (cm) | 104.3 | 3.6 | 104.3 | 4.3 | 0.95 |
| Birthweight (g) | 3576 | 475 | 3507 | 463 | 0.067 |
| Gestational age (weeks) | 40.0 | 1.2 | 40.3 | 1.2 | 0.009 |
| 4 year DXA | |||||
| WB BA (cm2) | 748.4 | 44 | 768.9 | 51.1 | <0.001 |
| WB BMC (g) | 371.1 | 42.2 | 378.8 | 48.3 | 0.037 |
| WB aBMD (g/cm2) | 0.50 | 0.03 | 0.49 | 0.04 | 0.23 |
AC= Abdominal circumference, BA = Bone area, BMC= Bone mineral content; aBMD = Areal bone mineral density
Compared with mothers of children born to the SWS during the same time frame, but who did not have DXA scans at 4 years, the mothers of children who did have DXA assessments were, on average, slightly older at the birth of their child (mean age 31.2 years vs 30.6 years respectively, p=0.007), better educated (24.8% with higher degree vs 20.6% respectively, p=0.002) and smoked less (8.5% smoked before pregnancy vs 17.4% respectively, p<0.001).
Relationships between intrauterine growth and postnatal skeletal development
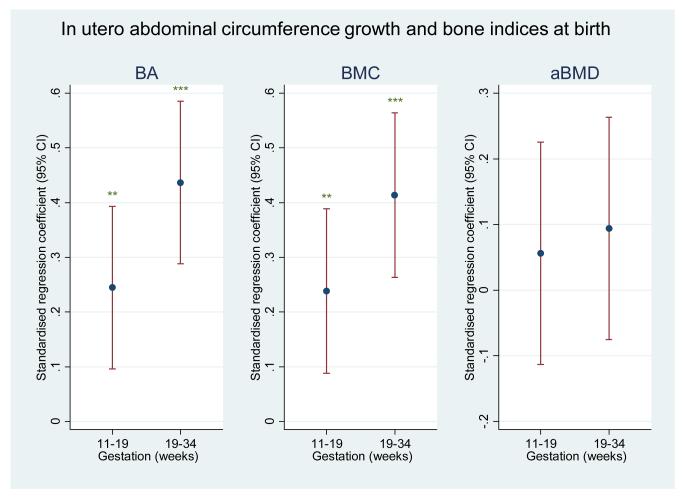
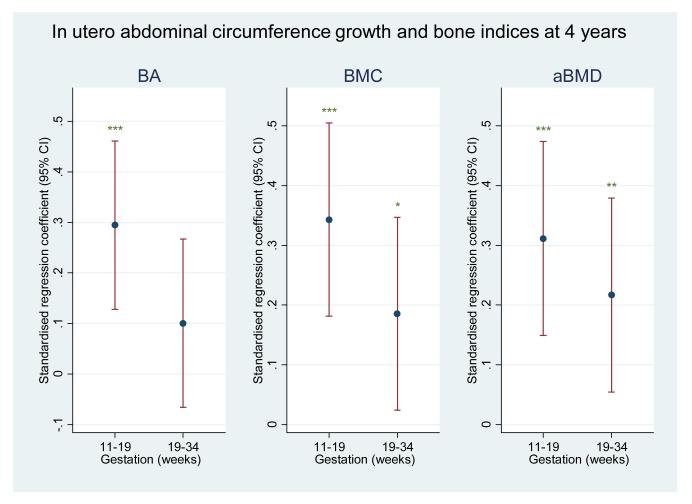
In the subset of children who had undergone DXA assessment at birth, we observed that conditional AC growth in early pregnancy strongly and statistically significantly predicted bone mineral measures at four years; these associations appeared less marked for bone mineral measures at birth. In contrast, conditional AC growth in late gestation appeared to be more strongly related to bone mineral measures at birth than at four years of age (Figure 1). However, on formal statistical testing, the differences in regression coefficients for early and late growth with bone outcomes at birth or 4 years did not attain statistical significance.
Figure 1a.
Plot of standardised regression coefficients for whole body minus head BA, BMC and aBMD at birth on conditional growth in abdominal circumference from 11-19 weeks (early) and 19-34 weeks (late). Growth variables are all independent. *=p<0.05; **=p<0.01; ***=p<0.001
Patterns of fetal and infant growth as determinants of bone indices at 4 years
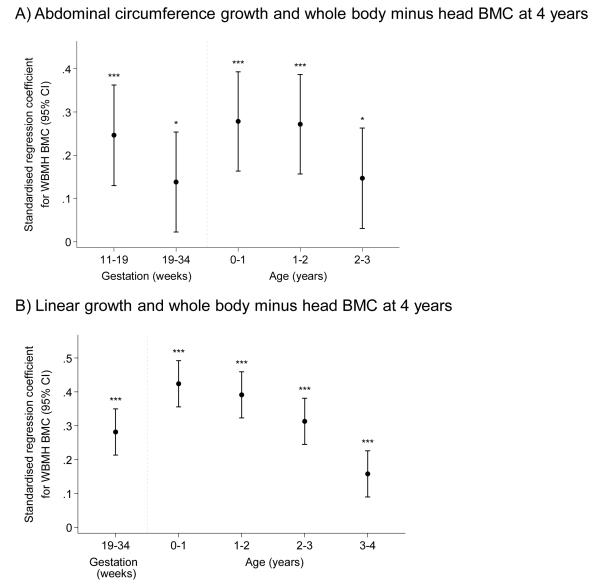
Table 2 summarises the conditional analyses of growth from early gestation to the end of the fourth year of postnatal life as determinants of bone indices at 4 years, for both AC and length. It clearly demonstrates significant influences of these growth measures at each time interval over this period, but considerable heterogeneity in effect size for each stage. Figure 2 demonstrates these associations for BMC. Thus growth at all time intervals (except 19-34 week AC growth for BA) predicted positively and statistically significantly whole body minus head BA, BMC and aBMD, but not estimated vBMD at 4 years. As in the subset with both neonatal and 4 year DXA, in this larger group AC growth in early pregnancy was more strongly associated with bone outcomes at 4 years than was that in late pregnancy. The pattern of postnatal growth was similar for both abdominal circumference and length, and for BA, BMC and aBMD, with weaker relationships for estimated vBMD. Thus the associations between growth and bone indices at 4 years were stronger for growth at 0-1 year and 1-2 years than at 2-3 years and 3-4 years.
Table 2.
(a) Conditional growth in abdominal circumference (AC) from 11 weeks to 3 years and bone indices at 4 years old.
| BA (SD) | BMC (SD) | aBMD (SD) | ||||
|---|---|---|---|---|---|---|
| Δ AC z-score | β | p | β | p | β | p |
| 11-19 weeks | 0.17 | 0.008 | 0.23 | <0.001 | 0.23 | <0.001 |
| 19-34 weeks | 0.05 | 0.418 | 0.15 | 0.017 | 0.21 | 0.001 |
| 0-1 year | 0.20 | 0.003 | 0.26 | <0.001 | 0.26 | <0.001 |
| 1-2 years | 0.21 | 0.001 | 0.26 | <0.001 | 0.25 | <0.001 |
| 2-3years | 0.11 | 0.09 | 0.13 | 0.029 | 0.12 | 0.043 |
| R2 | 0.19 | 0.27 | 0.26 | |||
(b) Conditional growth in length from 19 weeks to 4 years and bone indices at 4 years.
| BA (SD) | BMC (SD) | aBMD (SD) | ||||
|---|---|---|---|---|---|---|
| Δ Length z-score | β | p | β | p | β | p |
| 19-34 weeks | 0.28 | <0.001 | 0.26 | <0.001 | 0.19 | <0.001 |
| 0-1 year | 0.44 | <0.001 | 0.44 | <0.001 | 0.33 | <0.001 |
| 1-2 years | 0.32 | <0.001 | 0.40 | <0.001 | 0.39 | <0.001 |
| 2-3 years | 0.23 | <0.001 | 0.33 | <0.001 | 0.35 | <0.001 |
| 3-4 years | 0.14 | <0.001 | 0.15 | <0.001 | 0.14 | 0.001 |
| R2 | 0.48 | 0.56 | 0.44 | |||
Length measured as femur length (19-34 weeks) and crown-heel length birth-1 year and height 2-4 years.
Tables show standardised beta coefficient (SD change in bone outcome per SD change in conditional growth) from multiple regression analyses with maternal height, parity, late pregnancy triceps, walking speed and smoking as confounders.
BA = Bone area, BMC= Bone mineral content; aBMD = Areal bone mineral density
Figure 1b.
Plot of standardised regression coefficients for whole body BA, BMC and aBMD at 4 years on conditional growth in abdominal circumference from 11-19 weeks (early) and 19-34 weeks (late). Growth variables are all independent. *=p<0.05; **=p<0.01; ***=p<0.001
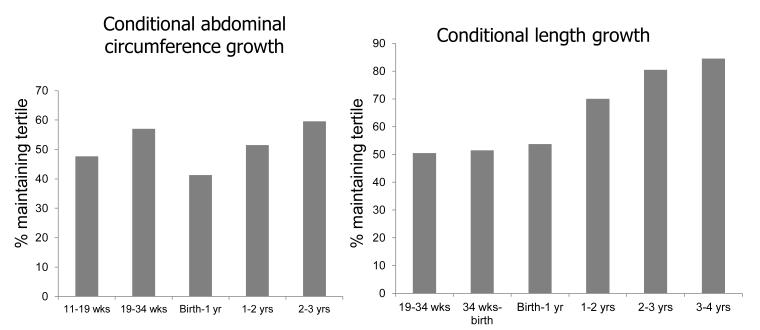
Further investigation revealed that growth from 0-6 months accounted for much of the influence of growth from birth to 1 year. Thus the regression coefficient for BMC at 4 years on AC growth 0-6 months was 0.26 (p<0.001) and for 6-12 months was 0.14 (p=0.022); the corresponding results for conditional growth in length were 0.39 (p<0.001) and 0.23 (p<0.001). Figure 3 shows the proportion of subjects who stayed in the same third of the abdominal circumference or length growth distribution at each time interval. These data demonstrate a progressive increase in the proportion of subjects who stay in the same third of the distribution with each successive postnatal time interval.
Figure 2.
Plot of standardised regression coefficients for whole body minus head BMC at 4 years on conditional growth at each time point. Growth variables are all independent.
a) Abdominal circumference
b) Length (Uses: Femur length at 19 and 34 weeks, Crown-rump length birth and 1 year, height at 2,3 and 4 years)
*=p<0.05; **=p<0.01; ***p<0.001
Correlation between rates of growth in fetal and postnatal life
Non-mutually adjusted conditional growth variables were generated to explore whether growth in early pregnancy (11-19 weeks) was related to that at 2-3 or 3-4 years, when the children appeared to be settling onto a more sustained trajectory. The correlation between conditional change in abdominal circumference 11-19 weeks and 2-3 years was very low (r=-0.1, p=0.126); that between absolute femur length at 19 weeks (equivalent to growth from 0-19 weeks) and conditional change in crown-heel length from 3-4 years also demonstrated little association (r=0.03, p=0.581). Exploring changes around birth, conditional growth in femur length from 19-34 weeks was statistically significantly associated with conditional crown-heel length growth from birth to 6 months (r=0.16,p=0.002) but not 6 months to 1 year (r=0.0,p=0.972). The pattern with abdominal circumference was contrasting, revealing stronger relationships between conditional AC growth 19-34 weeks and conditional AC growth 6 months to 1 year (r=0.19,p=0.004) than conditional AC growth from birth to 6 months (r=0.11,p=0.093).
Maternal determinants of offspring growth
We have previously demonstrated that maternal factors such as height, parity, and fat stores (triceps skinfold thickness), smoking and walking speed in late pregnancy all predict offspring bone mass at birth12. However, when these variables were added into the regression models including growth and bone indices at birth or 4 years, the observed relationships did not change appreciably.
Discussion
To the best of our knowledge, this is the first study to measure fetal and postnatal growth prospectively in relation to objectively measured bone size and density. Our results are consistent with their being at least two general phases of fetal growth, and with late intrauterine growth strongly predicting DXA-derived bone indices at birth and, in contrast, early pregnancy growth more strongly predicting DXA indices at 4 years. These two phases were defined by the available measurement times, and thus may represent a summary of a more complicated multi-phasic trajectory. The postnatal growth measurements are consistent with the notion that, after the temporary disturbance of growth patterns in late gestation, there is a time of readjustment over the first two years of postnatal life, with subsequent settling to a more stable trajectory. These observations suggest that a failure to adequately adjust the growth trajectory in postnatal life after a late pregnancy insult will have an adverse influence on skeletal development.
We studied a large, well-established cohort, with detailed characterisation of mothers before and during pregnancy; we also used DXA, a well validated technique for measuring bone mineral in the neonates. However, there are several limitations to our study: Intrauterine ultrasound measurements are prone to a certain amount of error, but we used two experienced operators following internationally agreed guidelines 16, 17 and repeatability was good. Additionally, we were only able to measure two time intervals during pregnancy, and therefore may have missed a growth pattern which compromised more than two phases. Furthermore, the differences between regression coefficients for early and late growth and offspring bone outcomes did not achieve statistical significance. Measurement of bone mineral in young children is hampered by their tendency to move and also by their low absolute BMC. However, we used specific paediatric software, and movement artefact was modest and uniform across the cohort; those few children with excessive movement were excluded from the analysis. DXA measures of bone mass have been shown to correlate well with whole body calcium content in ashing studies of piglets18. The study cohort was a subset of the SWS: mothers whose children underwent DXA scanning were on average taller, more educated and smoked less than mothers of children who did not undergo DXA. However, our results are based on internal comparisons, so will not have been biased by these differences. Additionally, there is no reason to suppose that the relationships between fetal growth and childhood bone mass would be different in these two groups. Finally, although in our earlier paper we found associations between fetal growth and estimated volumetric BMD, in this current subset, these relationships were attenuated, consistent with a stronger relationship between fetal growth and postnatal bone size than with volumetric density. However, the use of DXA does not allow measurement of true volumetric bone density, thus making it difficult to be certain about differential determinants of skeletal size and volumetric density. There are very few existing data relating to fetal growth using objective ultrasound and DXA measurement. Clinical observation suggests that the increase in fetal weight reaches a maximum velocity at around 34 weeks, when it begins to slow, probably because of physical constraint, partly as a result of maternal pelvis size2. Thus a child genetically predisposed to be large, as a result of a large father, but carried by a small mother, can be delivered. This is consistent with animal studies of crossbreeding shire horses and Shetland ponies: when the mother is the shire horse, the baby is larger than when the mother is the Shetland pony19. In this study, both foals became of similar size after a few months, midway between the size of their parents. We have previously demonstrated persisting positive relationships between fetal growth in late pregnancy (femur length and abdominal circumference) and post-natal bone size and density at 4 years old1. This earlier study compared the influence of these two different intrauterine growth parameters on post-natal skeletal indices, and did not examine the relative contribution of growth in early and late pregnancy, as it was not possible to acquire femur length measurements at 11 weeks. Thus the results of the current analysis confirm positive relationships between late pregnancy AC growth and 4 year BMC and aBMD, but additionally indicate this association is weaker than that between 4 year bone indices and AC growth measured in early pregnancy (11-19 weeks).
Much of the work on human growth and development has been carried out by Tanner, using external linear measurements rather than ultrasound, and no inclusion of bone indices as outcomes. These early studies have comprehensively mapped out the possible growth patterns in utero and childhood, suggesting a relatively early postnatal reversion to the initial intrauterine trajectory2, 3. These sorts of data have informed the development of standard child linear-growth charts and our data are consistent with the pattern of growth so documented, with stronger associations for the non-size-corrected variables. Karlberg developed mathematical models to describe phases of growth, labelling them “Infancy”, “Childhood” and “Puberty”. These models, based on analysis of a birth cohort, but without measurements of intrauterine growth or postnatal bone mass, suggested a rapid slowing of growth velocity in the first 6 months of postnatal life (infancy component) and a more slowly decelerating childhood component commencing at about 6 months, with increased adjustment of the distribution in year two and subsequent settling to a more sustained trajectory20, 21. This model, which the authors suggest matches the understanding of the endocrine determinants of growth, gives a similar overall pattern to that which we found in our study. Karlberg made the assumption that the infancy phase was a continuation of the growth trajectory in late pregnancy. Indeed our results for femur length are consistent with this notion. However, the relationships we observed for abdominal circumference suggest a stronger relationship between late pregnancy growth and that from 6 to 12 months postnatally, rather than in the first 6 months of life. This may reflect differences in the trajectories of longitudinal growth and accumulation of body fat/ liver size, both of which contribute to abdominal circumference.
We explored the correlations between non-mutually adjusted conditional measures of growth in early pregnancy and late infancy/ early childhood, to test the idea that the child eventually reverts to the early pregnancy growth trajectory. Although the growth in AC from 11-19 weeks predicted bone indices at 4 years more strongly than did growth in AC from 19-34 weeks, we found that the correlation between AC growth 11-19 weeks and 2-3 years was very weak; these data, and similar results for length, suggest that the child settles on a longer term postnatal trajectory, but that its similarity to that experienced in early gestation is limited.
Epidemiological studies suggest that growth in early life, both in utero and during infancy, predicts adult bone mineral content22, 23, as well as the risk of hip fracture24. Our results demonstrated a 0.56 SD difference in aBMD between highest and lowest quintiles of conditional AC growth from 19 to 34 weeks. If this difference were to persist into adult life, it could account for up to a 50% difference in relative risk of fracture, as a 1 SD difference in adult aBMD equates to up to a two-fold fracture risk difference 25, 26, suggesting that these differences will be clinically relevant. Our results clearly demonstrate that tracking of skeletal growth has its origins in utero, but that there is potential for change in later pregnancy and infancy. We have previously shown that maternal parity, lifestyle, body build and physical activity influence offspring bone mass 11, 12. Inclusion of these variables in our regression models did not alter the relationships found, and it is likely that these factors act on bone mass via an effect on fetal growth. Since the eventual childhood trajectory appears to be different from that experienced in early gestation, it is possible that such environmental factors might have permanent influence on the future development of the child, either through modulation of physiological systems or epigenetic mechanisms27. Further work will be needed to explore these interactions more fully, possibly to elucidate findings on which to base novel public health strategies aimed at optimising childhood skeletal development.
In conclusion, we have demonstrated objectively, with validated methodology, in a large prospective population cohort, that if fetal growth is dichotomised into early (11-19 weeks gestation) and late (19-34 weeks gestation) phases, growth velocity in the late phase appears to predict bone mass at birth more strongly than does growth in the early phase. In contrast, bone mass at 4 years appears more strongly predicted by growth in early than in late pregnancy. Our results are consistent with a reordering of individuals within the growth distribution during late pregnancy and in the early years of postnatal life serving to bring the individual to a more stable trajectory by 4 years old, but which is different to that experienced in early gestation. Further exploration of the environmental characteristics which might modify gene expression in these developmentally plastic periods should increase our understanding of possible interventions to optimise growth.
Figure 3.
Percent of subjects who stay within original third of distribution for each growth period. Left panel: Conditional change in abdominal circumference; Right panel: Conditional change in length (Femur length at 19 and 34 weeks, Crown-rump length birth and 1 year, height at 2,3 and 4 years)
Acknowledgements
We thank the mothers who gave us their time; and a team of dedicated research nurses and ancillary staff for their assistance. This work was supported by grants from the Medical Research Council, British Heart Foundation, Arthritis Research Campaign, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, NIHR Biomedical Research Unit, University of Southampton, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. Participants were drawn from a cohort study funded by the Medical Research Council and the Dunhill Medical Trust. We thank Mrs G Strange for helping prepare the manuscript.
Funding sources: Medical Research Council, British Heart Foundation, Arthritis Research Campaign, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust and NIHR Musculoskeletal Biomedical Research Unit, Oxford; participants were drawn from a cohort study funded by the Medical Research Council and the Dunhill Medical Trust.
Footnotes
Conflict of interest All authors report no conflict of interest
Reference List
- 1.Harvey NC, Mahon PA, Robinson SM, Nisbet CE, Javaid MK, Crozier SR, et al. Different indices of fetal growth predict bone size and volumetric density at 4 years of age. Journal of Bone and Mineral Research. 2010;25:920–927. doi: 10.1359/jbmr.091022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner JM. Foetus into Man: Physical growth from conception to maturity. Castlemead Publications; Ware: 1989. Growth before birth; pp. 36–50. [Google Scholar]
- 3.Tanner JM. Foetus into Man: Physical growth from conception to maturity. Castlemead Publications; Ware: 1989. The organisation of the growth process; pp. 165–177. [Google Scholar]
- 4.Kovacs CS. Skeletal physiology: fetus and neonate. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. ASBMR; Washington: 2003. pp. 65–71. [Google Scholar]
- 5.Little RE, Sing CF. Genetic and environmental influences on human birth weight. American Journal of Human Genetics. 1987;40:512–526. [PMC free article] [PubMed] [Google Scholar]
- 6.Little RE. Mother’s and father’s birthweight as predictors of infant birthweight. Paediatric and Perinatal Epidemiology. 1987;1:19–31. doi: 10.1111/j.1365-3016.1987.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 7.Hanson MA, Godfrey KM. Commentary: Maternal constraint is a pre-eminent regulator of fetal growth. International Journal of Epidemiology. 2008;37:252–254. doi: 10.1093/ije/dyn015. [DOI] [PubMed] [Google Scholar]
- 8.Schwarzler P, Bland JM, Holden D, Campbell S, Ville Y. Sex-specific antenatal reference growth charts for uncomplicated singleton pregnancies at 15-40 weeks of gestation. Ultrasound in Obstetrics and Gynecology. 2004;23:23–29. doi: 10.1002/uog.966. [DOI] [PubMed] [Google Scholar]
- 9.Altman DG, Chitty LS. New charts for ultrasound dating of pregnancy. Ultrasound in Obstetrics and Gynecology. 1997;10:174–191. doi: 10.1046/j.1469-0705.1997.10030174.x. [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godfrey K, Walker-Bone K, Robinson S, Taylor P, Shore S, Wheeler T, et al. Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. Journal of Bone and Mineral Research. 2001;16:1694–1703. doi: 10.1359/jbmr.2001.16.9.1694. [DOI] [PubMed] [Google Scholar]
- 12.Harvey NC, Javaid MK, Arden NK, Poole JR, Crozier SR, Robinson SM, et al. Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. Journal of Developmental Origins of Health and Disease. 2010;1:35–41. doi: 10.1017/S2040174409990055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey NC, Javaid MK, Poole JR, Taylor P, Robinson SM, Inskip HM, et al. Paternal skeletal size predicts intrauterine bone mineral accrual. Journal of Clinical and Endocrinological Metabolism. 2008;93:1676–1681. doi: 10.1210/jc.2007-0279. [DOI] [PubMed] [Google Scholar]
- 14.Mahon P, Harvey N, Crozier S, Inskip H, Robinson S, Arden N, et al. Low maternal vitamin D status and fetal bone development: cohort study. Journal of Bone and Mineral Research. 2010;25:14–19. doi: 10.1359/jbmr.090701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. International Journal of Epidemiology. 2006;35:42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 3. Abdominal measurements. British Journal of Obstetrics and Gynaecology. 1994;101:125–131. doi: 10.1111/j.1471-0528.1994.tb13077.x. [DOI] [PubMed] [Google Scholar]
- 17.Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 4. Femur length. British Journal of Obstetrics and Gynaecology. 1994;101:132–135. doi: 10.1111/j.1471-0528.1994.tb13078.x. [DOI] [PubMed] [Google Scholar]
- 18.Abrams SA, Schanler RJ, Sheng HP, Evans HJ, Leblanc AD, Garza C. Bone mineral content reflects total body calcium in neonatal miniature piglets. Pediatric Research. 1988;24:693–695. doi: 10.1203/00006450-198812000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Walton A, Hammond J. The maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proceedings of the Royal Society London (Biology) 1938;125:311–335. [Google Scholar]
- 20.Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatrica Scandinavica Supplement. 1989;350:70–94. doi: 10.1111/j.1651-2227.1989.tb11199.x. [DOI] [PubMed] [Google Scholar]
- 21.Karlberg J, Fryer JG, Engstrom I, Karlberg P. Analysis of linear growth using a mathematical model. II. From 3 to 21 years of age. Acta Paediatrica Scandinavica Supplement. 1987;337:12–29. doi: 10.1111/j.1651-2227.1987.tb17122.x. [DOI] [PubMed] [Google Scholar]
- 22.Cooper C, Cawley M, Bhalla A, Egger P, Ring F, Morton L, et al. Childhood growth, physical activity, and peak bone mass in women. Journal of Bone and Mineral Research. 1995;10:940–947. doi: 10.1002/jbmr.5650100615. [DOI] [PubMed] [Google Scholar]
- 23.Dennison EM, Syddall HE, Sayer AA, Gilbody HJ, Cooper C. Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the Hertfordshire cohort study. Pediatric Research. 2005;57:582–586. doi: 10.1203/01.PDR.0000155754.67821.CA. [DOI] [PubMed] [Google Scholar]
- 24.Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJ. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporosis International. 2001;12:623–629. doi: 10.1007/s001980170061. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporosis International. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]
- 26.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. British Medical Journal. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]