Abstract
The auditory portion of the inner ear, the cochlea, is an ideal organ for local gene transfection owing to its relative isolation. Various carriers have been tested for cochlear gene transfection. To date, viral vectors appear to have much higher transfection efficacy than non-viral mechanisms. Among these vectors, recombinant adeno-associated virus (rAAV) vectors have several advantages such as being non-pathogenic and the ability to produce prolonged gene expression in various cell types. However, rAAV vectors cannot pass through the intact round window membrane (RWM), otherwise a very attractive approach to access the human inner ear. In this study, performed in guinea-pigs, we describe a method to increase the permeability of RWM to rAAV vectors by partial digestion with collagenase solution. Elevated delivery of rAAV across the partially digested RWM increased transfection efficacy to a satisfactory level, even though it was still lower than that achieved by direct cochleostomy injection. Functional tests (auditory brainstem responses) showed that this enzymatic manipulation did not cause permanent hearing loss if applied appropriately. Morphological observations suggested that the damage to RWM caused by partial digestion healed within four weeks. Taken together, these findings suggest that partial digestion of the RWM is a safe and effective method for increasing the transfection of cochlear sensory cells with rAAV.
Keywords: adeno-associated viral vector, collagenase, round window membrane, cochlear gene transfection, hair cell, spiral ganglion neuron
INTRODUCTION
Sensorineural hearing loss is the most prevalent neurological disorder in human beings. It may result from aging, noise exposure, ototoxic drugs and genetic deficits, and other as-yet undefined causes. The predominant mechanism varies according to different etiologies and sensorineural hearing loss types. With recent developments in neuroscience and molecular biology, genes critical to the survival, repair and development of cells affected in various sensorineural hearing losses are being identified. These genes could possibly also be involved in the regeneration of sensory hair cells and neurons in the cochlea.1–8 Manipulating expression of these genes in vulnerable cochlear cells may serve as an avenue for promising treatments for sensorineural hearing loss. Moreover, the cochlea of mammals is an ideal organ for gene therapy because it is relatively isolated from neighboring organs, and therefore genetic manipulation in the cochlea is less likely to produce side effects and transfection of unwanted targets.
Many different vectors have been tested for delivering gene-related DNA into the cochlea. They can be divided into two major categories: viral and non-viral vectors. To date, viral vectors have proven to be much more effective than their non-viral counterparts for gene transfection in the cochlea.1,5,9 Three types of viral vectors have been evaluated to establish their suitability for gene therapy in the cochlea: (1) lentivirus, (2) adenovirus (AV) and (3) recombinant adeno-associated virus (rAAV).3 rAAV vectors are favored for cochlear gene therapy for several reasons: (1) they are not pathogenic because they cannot autonomously replicate and contain no viral genes; (2) gene(s) transferred with this group of vectors can be expressed for long periods of time, perhaps permanently in non-dividing cells; (3) the vectors in this group are not toxic to cochlear cells; and (4) they can transfect most cell types tested so far. However, these vectors have smaller packaging capacities than other alternative vectors.3
There are two major obstacles to the use of rAAV vectors in cochlear gene therapy. The first barrier arises from the fact that the round window membrane (RWM), the structure that provides the easiest and potentially safest access point to the human cochlea, is not permeable to rAAV.10 Gene transfection to cells of interest could potentially be achieved by delivering the targeted gene into either the perilymphatic or endolymphatic spaces of the cochlea. The endolymphatic space is very small, actually bathed in the perilymphatic space and cannot be accessed directly without causing hearing loss (for example, via the lateral wall of scale media). It can be indirectly accessed through the perilymphatic space by perforating the otic capsule at several possible points, such as by a cochleostomy via the lateral wall of the scala tympani or via the semicircular canals. Accessing the perilymphatic space of the cochlea is easier and much less traumatic than trying to directly access the endolymph. Two common surgical access points to the perilymphatic space are via a stapedotomy in the oval window (used in stapedotomy surgery) and via a cochleostomy or round window perforation (used for cochlear implantation). The oval window approach is familiar to otologic surgeons through stapedotomy surgery, and primarily accesses the scala vestibuli and the vestibular structures.11 However, this may not be an optimal approach for gene transfection of hair cells in the cochlea because the scala vestibuli is separated from the hair cells by Reissner’s membrane, which is relatively impermeable to vectors, when compared with the basilar membrane.12 To reach the basilar membrane from a vestibule injection (the perilymphatic space below the oval window), vectors must travel all the way up to the apex of the cochlea, around the helicotrema and then into the scala tympani to finally reach the basilar membrane, and then to reach the most commonly affected region (the basal region), the vectors much travel the length of the cochlea again. This makes the scala tympani a more attractive portal for reaching hair cells, as it is directly in contact with the more permeable basilar membrane. In animal studies using gene transfection through the perilymph compartment, the scala tympani has been accessed by three approaches: (1) penetration through the bony shell of the cochlea (cochleostomy); (2) penetration through the RWM; and (3) diffusion across the intact RWM. Access to the perilymphatic space by a direct injection into this compartment via cochleostomy may in itself cause hearing impairment.13,14 Cochleostomy requires knowledge of the anatomy of the cochlea to avoid puncture and damage of the basilar membrane, and has all the limitations described below for RWM puncture or injection. Direct injection through the RWM may also rupture this structure, causing leakage of perilymph and hearing loss.15 By contrast, applying vectors into the middle ear space to facilitate their passage across the intact RWM minimizes surgical damage to the inner ear. With this approach, there is no leakage of perilymph or accidental suctioning of perilymph that may result in acoustic, biochemical and mechanical trauma and no direct path for infection to reach the inner ear. To hold a solution for a long period of time in contact with the RWM, a piece of gelatin sponge (for example, Gelfoam or Surgifoam) is often applied onto the RWM. This is a safe, easy and non-traumatic technique for cochlea transfection, and therefore the most attractive method for clinical use. In fact, diffusion across an intact RWM has been extensively used for delivery of drugs such as steroids or gentamicin to the inner ear in conditions such as Meniere’s disease.16–18 Despite the attractiveness of the RWM diffusion approach, rAAV vectors unfortunately cannot diffuse across the intact RWM.10
Another obstacle to gene therapy arises from the fact that rAAV vectors less effectively transfect outer hair cells (OHCs) than inner hair cells (IHCs).3 This is a major limitation because OHCs are much more vulnerable to many different types of insults than IHCs.19
In this study, we established a method to increase the permeability of the RWM to rAAVs to promote their diffusion across this structure and into the inner ear. We show that damage to the RWM produced by our manipulation is reversible and that it greatly improved transfection of inner ear cells to within the range achieved by direct cochleostomy injection. Furthermore, we have improved the transfection of OHCs by using a newly developed rAAV vector that has a mutation on a surface-exposed tyrosine residual. This mutation was designed to reduced degradation of the virus in the cytoplasm so that more vector reaches the nucleus.20
RESULTS
Animals appeared normal after all surgical approaches (cochleostomy and RWM, with and without enzyme digestion). In particular, no signs of vestibular disturbance, such as circling behaviors or head tilting, were observed. The animals were also free from otitis media on visual inspection during removal of the cochlea.
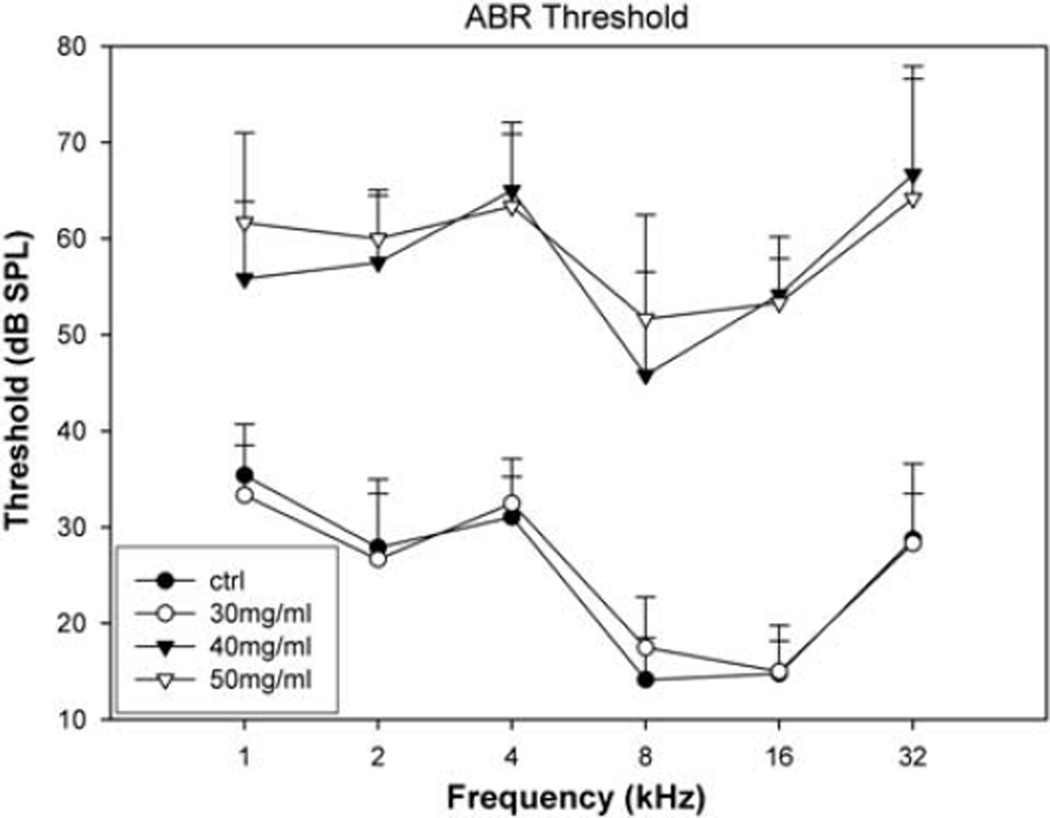
Both collagenase I and II appeared to be equally effective in digesting the RWM and inducing RWM permeability to rAAV vectors. Hence, we report only the results for the use collagenase type I. The safety of the digestion was evaluated across a range of concentrations of the enzyme solutions from 50, 40 to 30 mg ml−1 (8 or 9 ears for each). Figure 1 shows the auditory brainstem response (ABR) thresholds obtained in the control recordings (n=13 guinea-pigs, the thresholds of both ears were averaged), and those recorded 4 weeks after the surgery in animals (ears) grouped according to the concentration of digesting solution. Digestion with 30 mg ml−1 enzyme for 10 min appeared to be safe as the post-surgery ABR thresholds largely overlap with the baseline situation, whereas digestion at the two higher concentrations resulted in irreversible hearing loss. In the following experiment using rAAV vectors, the digestion was carried out using 30 mg ml−1 enzyme solution. The post-surgery ABR threshold was similar to that of ears treated with same concentration of enzyme solution: there was no significant threshold shift across all the frequencies tested as compared with the control. In those animals with permanent hearing loss, the RWM was usually found to have an unhealed perforation when the cochleae were harvested at the end of the experiment (3–4 weeks after the surgery).
Figure 1.
ABR thresholds recorded 4 weeks after RWM digestion as compared with the control ABRs (n=18, ●) recorded before the surgery for RWM treatment. Significant threshold shifts were seen in the two groups in which the RWM was treated with 40 or 50 mg ml−1 collagenase solution for 10 min (▼ and ▽, respectively). The averaged threshold from the group treated at 30 mg ml−1 (○) largely overlapped with the baseline situation (n=6 for each group of digestion, mean ± s.d.).
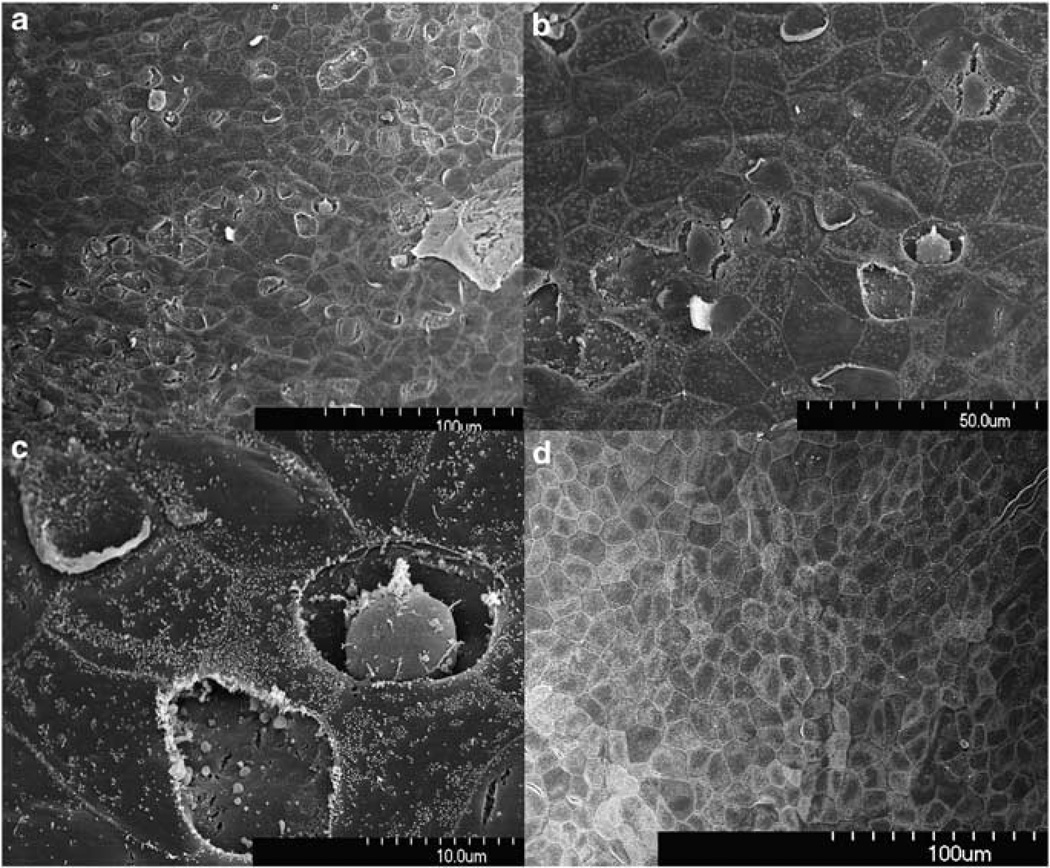
RWM structure was observed at different time points after the digestion with the collagenase solution at 30 mg ml−1 for 10 min. Damage to RWM structures was seen in samples that were taken 1 h after the treatment. Figure 2 shows scanning electronic microscope (SEM) images of the RWM surface facing the middle ear cavity. The digestive treatment caused many epithelial cells on this side to appear shrunken and partially peeled off so that the middle layer of the RWM deep to the epithelial layer was exposed. In some areas, the entire epithelial cell was missing, whereas in other areas shrinkage of the epithelial cells resulted in their separation from surrounding cells along the borders where the cells contact one another forming tight junctions.
Figure 2.
SEM view of the epithelial surface facing the tympanic cavity. Samples taken 1 h after enzyme treatment (a–c from one sample with different magnification) show damaged and lost epithelia. Shrinkage of cells resulted in separation from surrounding cells. RWM from the control animal and at 3–4 weeks after the digestion show no signs of damage (d).
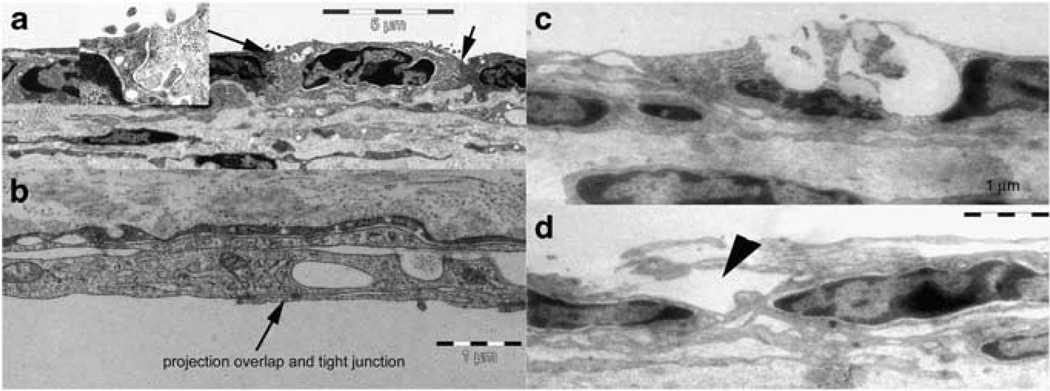
Damage to the epithelial cells is also visible in transmission electronic microscope (TEM) images, which show a cross-sectional view of the RWM (Figure 3). The RWM has three layers: the outer epithelial layer facing the middle ear cavity consists of low cubical cells that are connected to each other by tight junctions (Figure 3a arrow, and the inset). The middle layer contains mainly connective tissue; the inner epithelial layer facing the cochlear space is made up of squamous cells. The inter-cell connections in this layer are different from those seen for the outer layer as is characterized by large overlaps of the lateral projections from the cells (Figure 3b). As the enzyme solution is applied to the outer surface of the RWM, the damage is more localized to the outer epithelial cells and ranges from partial breaks in their outer membranes to total loss of the cells (Figures 3c and d). Vesicles are seen inside and outside of the epithelial cells in this layer. The tight junction becomes loose at many places. The connective layer is also disorganized and largely vacuolated. Samples taken 3 or 4 weeks after the enzyme treatment show no sign of damage and cannot be differentiated from untreated control samples.
Figure 3.
TEM view of the RWM. (a, b) Normal RWM structure with (a) and (b) showing bother outer and inner surface of the RWM. (c, d) RWM damage seen at the outer surface of the RWM 1 h after the enzyme digestion.
Vector treatment
The cochlear vector transfection was compared in a self-control design such that for each animal, the viral vector was applied to both ears differently from two of the three approaches: cochleostomy and RWM with and without enzyme digestion. The overall findings were that: (1) the transfection rate was lower via RWM diffusion than by cochleostomy; (2) transfection of IHCs was much greater than for OHCs; (3) there was a gradient of transfection from basal to apical turns with stronger transfection at the base; and (4) spiral ganglion neurons (SGNs) in Rosenthal canal were also transfected, with a similar but less pronounced basal–apical gradient.
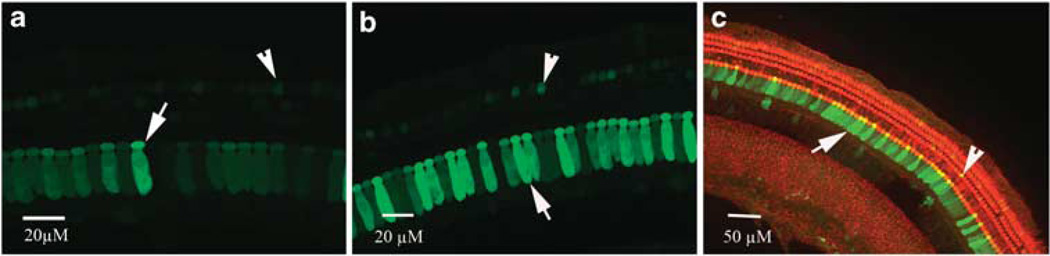
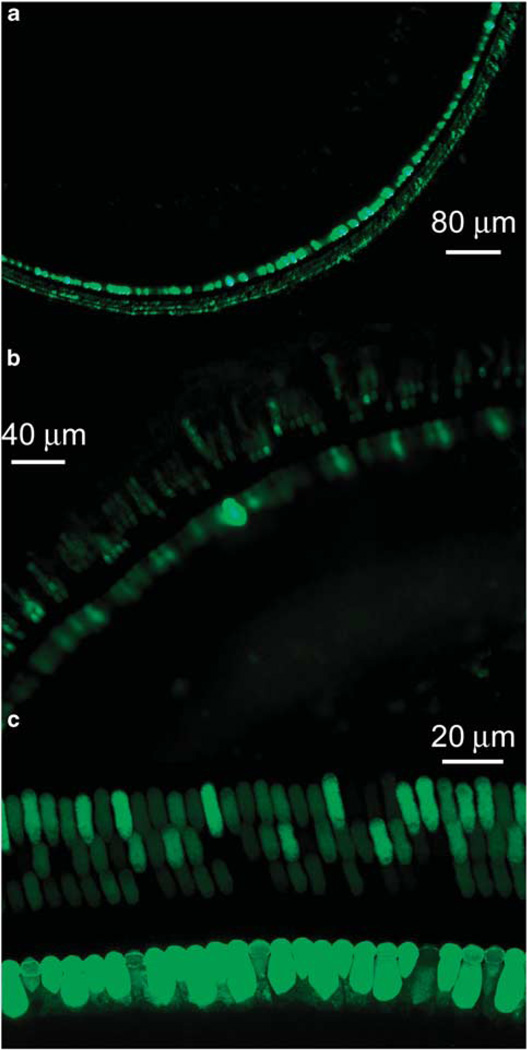
Using rAAV2/2-human cytomegalovirus-enhanced green fluorescent protein (EGFP) vector at titer of 1 × 1012 vector genome containing particles per ml, expression was compared first between diffusion through intact and digested RWM. No EGFP signal was found in ears receiving the virus on intact RWMs. Having established that the vector does not cross the intact RWM, we then compared the transfection achieved by cochleostomy or partial digestion of the RWM. Figure 4 shows typical images of EGFP expression in cochlear surface preparations transfected by partial digestion of the RWM (Figure 4a) or direct injection via cochleostomy (Figure 4b). For both methods, the transfection efficiency was significantly higher in IHCs than in OHCs. Double staining with phalloidin identified transfection in sensory hair cells (Figure 4c).
Figure 4.
Confocal images of EGFP expression in hair cells mediated by rAAV2 infection in the guinea-pig cochlea via RWM exposed to collagenase (a) and cochleostomy (b, c).
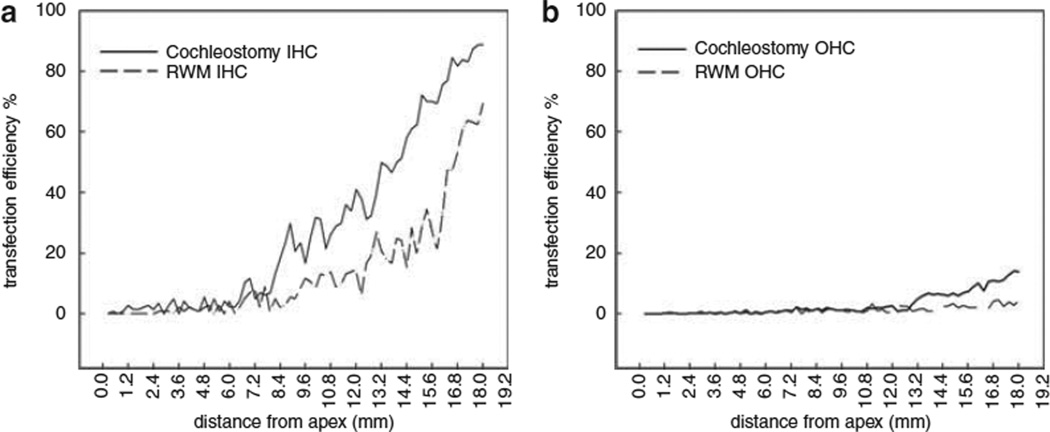
Figure 5 shows the transfection cochleograms for both IHCs (a) and OHCs (b). The transfection efficiency for IHCs at the basal turn were 63.3 ± 12.4% and 33.27 ± 7.73% by cochleostomy (n=6 ears) or partial digestion of the RWM (n=6 ears), respectively, whereas the corresponding percentages of OHCs transfected by the two approaches were 7.3 ± 3.63% and 2.33 ± 2.07%, higher in cochleostomy.
Figure 5.
The cochleogram for the percentages of IHCs (a) and OHCs (b) achieved by cochleostomy or partial digestion of the RWM.
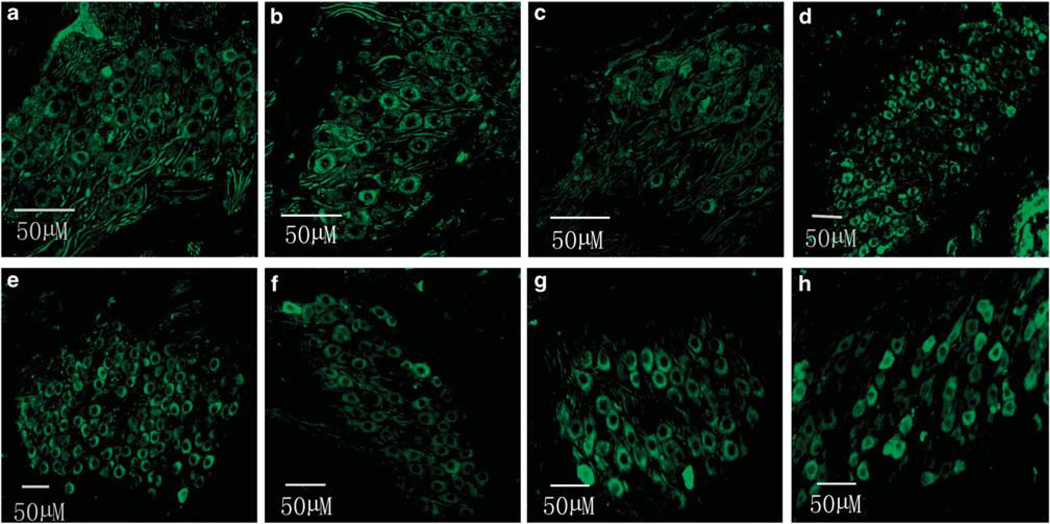
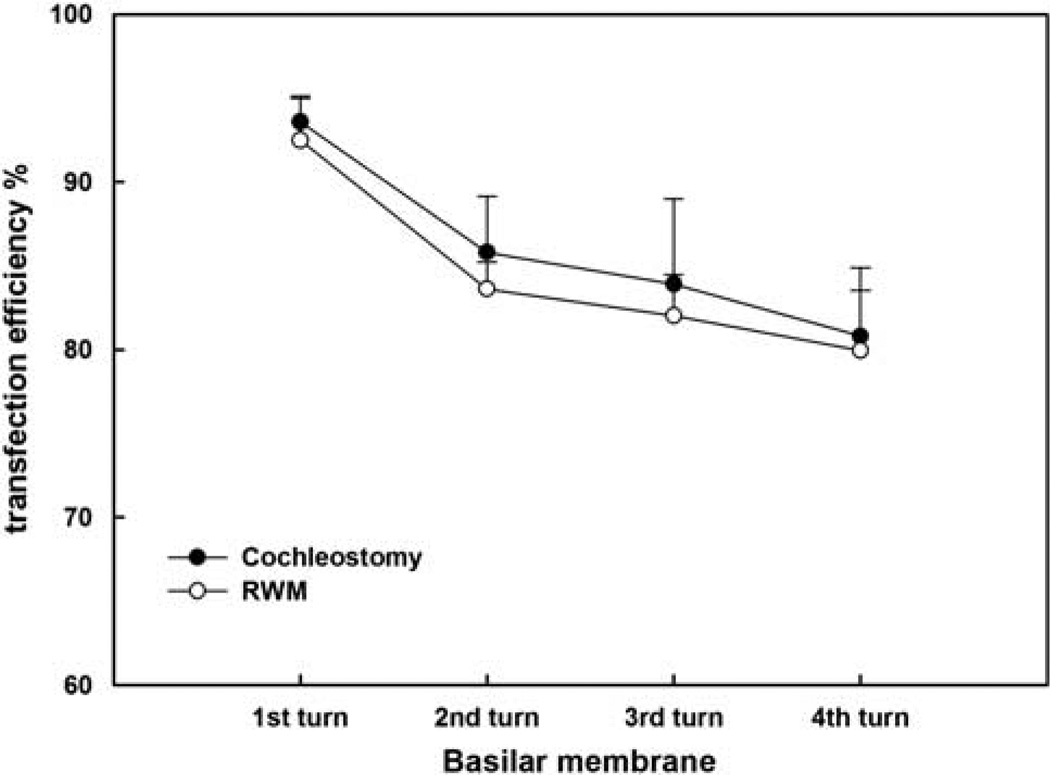
Figure 6 shows robust EGFP expression in the SGNs via cochleostomy (Figures 6a, b, c and d for 1, 2, 3 and apical turn, respectively) or via RWM after collagenase exposure (Figures 6e, f, g and h for 1, 2, 3 and apical turn, respectively) in the cochleae treated with rAAV2-EGFP. A transfection gradient from basal turn to the apex was seen for SGNs also, but it was not as marked as for HCs; transfection efficiencies of SGNs from basal to apical turns (reported in sequence) were 93.56, 85.78, 83.89, 80.79% for cochlestomy and 92.48, 83.61, 82.02, 79.96% for RWM approach (Figure 7). A two-way analysis of variance performed against the factors of the approach to rAAV delivery and location (turns) shows no significant effect of the approach (F1,46=0.434, P=0.513), that is, cochleostomy was not significantly better than partial digestion of the RWM. Similar transfection efficacy was achieved using rAAV2-GFP for both HCs and SGNs (data not shown).
Figure 6.
EGFP images of cryosectioned modiolus after transfection using two approaches: (a–d) for cochleostomy and (e–h) for the RWM approach. From left to right the images are from base to apex (n=6 for each group).
Figure 7.
Comparison of the percentage of SGNs transfected in different turns of cochleae between vector delivery by cochleostomy or by partial digestion of the RWM. The error bars are standard errors.
To improve the transfection of OHCs, we tested a newly developed rAAV vector containing a mutation in one of the surface-exposed tyrosine residues in the capsids of the viral vector (rAAV-GFP-mut733).21 The concentration of rAAV-GFP-mutant is extremely higher as compared with that of conventional rAAV (about 75-folds), which resulted in improved efficiency of transfection of mutant vector. The transfection rates of the OHCs were 45.7 ± 13.5% (n=4) via partial digestion of the RWM and 72.3 ± 16.8% (n=4) for cochleostomy at the basal turn. The transfection rates to OHCs were 25.6 ± 8.8% and 11.53 ± 5.45% for the middle and apical turns, respectively, for RWM approach, and 38.7 ± 7.9% and 19.3 ± 11.2% for cochleostomy. The transfection to IHCs reached nearly 100% at the basal turn for both approaches, and reduced to 78.9 ± 13% and 57.5 ± 15.4% in RWM approach and 88.4 ± 15.8% and 66.5 ± 11.5% in cochleostomy. Figure 8 shows typical images of hair cell transfection for both the treated RWM (a and b) and cochleostomy (c) approaches.
Figure 8.
HC transfection using rAAV-8-GFP with a mutation at tyrosine residue 733. The titer of the vector was 7.5 × 1013 (vector genome containing particles per ml). (a and b) HC transfection at both basal (a) and second turn (b) via the RWM approach. (c) Transfection of HC at second turn in a cochlea achieved by direct injection via cochleostomy.
DISCUSSION
The main aim of this study was to (1) develop a minimally invasive approach for delivering rAAV vectors into cochleae and (2) determine whether this approach improved rAAV-mediated gene transfection of OHCs. In this report, cochlear gene transfection was evaluated and compared across two approaches into the perilymphatic space of the cochlea: (1) intracochlear infusion via cochleostomy or (2) diffusion across RWM treated with collagenase. We showed that a partial digestion of the RWM increased permeability of this membrane to rAAV vectors enabling cochlear cells to be more transfected in greater number than without RWM treatment, although the transfection efficiency through this approach was significantly lower than that obtained by direct injection through cochleostomy. Furthermore, by increasing viral titer and utilizing rAAV with a single tyrosine mutation, the percentage of OHCs transfected was increased from about none to ~50% using the RWM diffusion approach.
The anatomical isolation of the inner ear makes it an ideal organ for local gene therapy in that manipulation directed to this organ should have minimal impact on surrounding tissues and structures. However, this isolation also makes drug or viral vector delivery difficult. Hearing impairment resulting from localized surgical trauma, inflammation and infection may occur after invasive surgical approaches such as cochleostomy or microinjections through the RWM.13 With the exception of the mouse, the semicircular canal is difficult to access surgically in many species, including human beings.11 By contrast, our RWM approach does not disrupt cochlear integrity and can be performed in a shorter period of time via a myringotomy; hence, it represents a less invasive, faster and less traumatic method than the alternatives, and may be the only approach that is clinically acceptable by human subjects who are not already deaf. Moreover, the RWM approach has been used for the delivery of steroids or gentamicin to the inner ear in the treatment of sudden hearing loss and vertigo associated with Meniere’s disease, respectively.22
Although many drugs can be delivered successfully into the cochlea via RWM diffusion, rAAV vectors cannot pass this membrane.10 This is unfortunate because the AAV vector represents the best available viral vector for cochlear gene transfection owing to its non-pathogenesis, and abilities to transfect most cell types and generate long-term expression of the transgene.9,23 A variety of experiments have shown that expression of genes carried by rAAV vector are stable and long lasting,9,20,24–28 while showing minimal vector-related cytotoxicity compared with other vector systems. In contrast to rAAV, AV can successfully traverse the intact RWM. A popular explanation for the disparate results between rAAV and AV is the presence or absence of the necessary viral receptors on the RWM.10 It has been shown that the entry of AV is facilitated by the presence of the Coxsackie’s virus and AV receptor, as well as integrins avb5 and avb3.29,30 Likewise, heparin sulfate and integrin α5β1 have been proposed as the host cell receptors for rAAV2.31 Sialic acid is a co-receptor for rAAV-4 and rAAV-5.32 The presence or absence of these receptors likely modulates the ability of these viruses to successfully traverse the RWM and may explain why rAAV cannot enter the fluid spaces of the cochlea through the RWM.
Although AV vectors can pass through the RWM easily, significant disadvantages are associated with this class of vectors, such as transient expression due to failure of the virus to integrate into the host cell chromosomes as well as the potent immune response to AV that may result in local toxicity.23 Although wild-type AAV integrates into a 2-kb region on the long arm of human chromosome 19 (19q13.3-qter),33,34 recombinant AAV vectors do not contain the AAV viral REP gene necessary for chromosomal integration, and therefore do not efficiently integrate their passenger DNA. They maintain persistence of their passenger DNA within the host cell by converting their single-stranded DNA genome into a circular, double-stranded DNA that is stably maintained in non-dividing cells. As cochlear hair cells do not divide, it is expected, like that seen for retinal neurons, that a rAAV delivered transgene would persist for the life of the target cell.
Although the RWM varies in thickness across different species, in mammals this structure is composed of three layers.35 The outer epithelium of the RWM consists of light and dark cells containing dense cytoplasm, as well as numerous microvilli on their free surface facing the middle ear cavity. In this layer, cells are bounded by tight junctions with extensive interdigitations. This layer is likely the major barrier to AAV vector diffusion. The connective tissue layer contains fibroblasts, collagen and elastic fibers, as well as blood and lymph vessels. Collagen fibers have a characteristic periodicity. The epithelial cells lining the inner ear are squamous, with areas of discontinuity and loose junctions between long lateral extensions. These structural features suggest that substances may pass this layer relatively easily.35 Substances can traverse the RWM through different pathways: (1) diffusing through the cytoplasm (for example, exotoxin); (2) carried in pinocytotic vesicles (for example, cationic ferritin); and (3) through the channels between cells.32 The permeability of a substance across the RWM is determined by factors such as size, concentration, liposolubility, electrical charge as well as the thickness of RWM. Mechanical or chemical stimulation and inflammation of the RWM increases the permeable of viral vectors and thus transgene expression in the inner ear.
We employed collagenase to increase RWM permeability to promote rAAV delivery to the cochlea and determined the optimal conditions for this application. The collagenases used in this study (type I and II) are mixtures of enzymes (mostly proteases) secreted by Clostridium histolyticum, which contains collagenase, nonspecific proteases and clostripain. Collagenases in nature have an important role in collagen degradation.36 In a laboratory setting, they are usually used to separate cells, or more specifically epithelial cells. As the RWM contains mainly type I collagen,37 we hypothesized that type I collagenase may be more effective in digesting the RWM. However, we have found that at appropriate concentrations, both type I and II collagenase are equally effective. Damage to the RWM by digestion at the concentration of 30 mg ml−1 for 10 min is limited to the outer epithelia. The membrane of epithelial cells facing the tympanic cavity was partly disrupted, and tight junctions near the surface of the membrane became ‘leaky’, and we therefore hypothesize that this is the main factor responsible for increasing rAAV transfection across the RWM. Microvilli on the surface of cells were rare or absent after digestion, even in RWMs that had healed 3–4 weeks after protease treatment. In the acute stage soon after collagenase treatment, numerous vesicles were present between and within the epithelial cells, as well as within the connective tissue. The epithelial cells lining the inner ear are squamous in nature and irregularly arranged with long lateral extensions, and this was not affected by protease treatment. Digestion was therefore limited primarily to the outer RWM layer, and appeared to extend at most to the middle connective layer. No inflammatory cells were found to infiltrate this middle layer. Because of the high regenerative capacity of epithelial cells and connective tissues, lesions produced by collagenase digestion soon healed, as suggested by morphological recovery in RWMs observed using SEM and TEM. However, overdigestion may cause frank disruption of the RWM, or make it too fragile to tolerate gelfoam placement. This was apparently evident in cases in which permanent hearing loss was seen in ears treated with high concentration of enzyme (40 and 50 mg ml−1).
IHCs and SGNs are transfected in greater number than OHCs by rAAV vectors.24,25,38–40 The mechanism underlying this difference between IHCs and OHCs is not understood. A possible explanation is that the transfection pathway for rAAV to the hair cells in the organ of Corti is from perilymph in the scala tympani to the SGNs via the modiolus, and then out to the HCs via the habenula perforate.41 It is proposed that these small openings located below the IHCs provide easy access for vectors to IHCs, whereas transfection to OHCs may rely upon the diffusion of the vector across the basilar membrane. However, this idea is in conflict with other studies that have shown the converse of ours, that is, IHCs could be transfected when SGNs were not.24 Another explanation for the lack of transfection of OHCs by rAAVs is that the two types of hair cells show different tropism for these viral vectors. However, this hypothesis is negated to some extent by the fact that AAV-mediated gene expression in cochlear explants is comparable in OHCs and IHCs.28
Various methods have been explored to promote the transfection of OHCs with limited success so far. One approach that has been intensively explored is the use of OHC specific promoters.38 Although AAV vectors generated with the cytomegalovirus and/or chicken β-actin promoters have been shown to transfect OHCs as well as IHCs in previous studies,9,24 the transfection rate is low for OHCs. Many other promoters including myosin 7A promoter have been tested, and none of them have so far favored transfection of OHCs.42,43 Another approach is to design or select vectors based on the availability of entry systems in host cells. Although the mechanisms are not clear, the entry of viral vectors into cells is considered to be via a receptor/co-receptor system. Depending on the AAV serotype, several receptors and co-receptors have been identified.32,44 Ideally, vector design for transfection to targeted cells should utilize these receptors. However, as none has been detected in the organ of Corti, it is not clear whether differential distribution of any one of the known entry systems is responsible for the predominant transfection of IHCs over OHCs by AAV vectors.23,24
To date, increasing the titer of the viral stock appears to be the most practical way to increase OHC transfection. Similar to previous reports,28 we observed a tendency towards increased transfection efficiency with higher titers (from 1 × 1012 to 7.5 × 1013 particles per ml). However, interpretation of the effects of increased vector concentration was complicated in our experiments because the tyrosine mutant vector was generated at highest titer. These point mutations of surface tyrosine residues reduces ubiquitination and proteosomal degradation of AAV, resulting in increased vector entry into the cell nuclei.20 The successful transfection of OHCs that we report using this mutant AAV8-Y733F vector could have been due to either the high titer and/or increased stability. Nevertheless, AAV vectors applied at such high titers are safe and do not cause sensory hair cell or hearing loss.
In summary, we have demonstrated the feasibility of increasing the efficiency of gene delivery into the inner ear by partial digestion of the RWM with collagenase. This technique is easy to perform, non-traumatic and showed neither signs nor symptoms of systemic or inner ear inflammation. After treatment with the appropriate protease concentration, rAAV transfects sensory hair cells and SGNs with an efficacy comparable to that produced by direct injection into the cochlea (cochleostomy). OHC transfection can also be improved using vectors at high titer that have point mutations in surface tyrosines that prevent phosphorylation and subsequent ubiquitination of the rAAV.21 This technique may be easily transferred to human cochlear gene therapy. In vitro models using human RWMs will enable delineation of the optimal conditions for AAV vector transduction of cells in the cochlea.
MATERIALS AND METHODS
Subjects and planning
A total of 33 adult albino male guinea-pigs were used in this multi-lab study. They were either provided by Sheng-Wang Co. Limited (Shanghai, China) or by Charles River Co. (Senneville, QC, Canada). All experimental procedures were approved by the ethics committees for laboratory animals at Shanghai Jiao Tong University in China and at Dalhousie University in Canada. They also conformed to the NIH guidelines for laboratory animals. Otological evaluation in each animal ruled out any abnormalities of the external and middle ears, and normal hearing was verified with ABRs before the animal was used in the experiments. Animals were randomly assigned to one of three different experimental arms: (1) evaluation of the safety of collagenase digestion (n=13); (2) comparison of the vector transfection efficiency through the RWM by diffusion with and without enzyme digestion, and comparison between cochleostomy and RWM diffusion after enzyme digestion (eight guinea-pigs using rAAV2-EGFP, two ears for transfection via intact RWM and six for cochleostomy, eight ears for transfection via digested RWM; and four guinea-pigs using rAAV8-GFP-mut733, four ears for cochleostomy and four for RWM after digestion); and (3) damage and recovery of RWM after enzyme digestion (n=8). In arms (1) and (2), each animal was subjected to the following procedures in sequence: (1) a control baseline ABR recording; (2) gene transfection surgery; and (3) post-surgery ABR recording.
After the final ABR was recorded, animals were euthanized and the cochleae harvested to examine their morphology and perform immunohistology. In task (3), four GPs each were used to determine the morphology of the RWM 1 h or 3–4 weeks after treatment with the digestive solution. No ABR recordings were performed on this group. Surgery was performed to apply the digestive enzyme solution, and then the animals were killed at specific time points (four animals at each time point). In each animal, one ear was evaluated using a SEM, whereas the other was examined with a TEM.
ABR recording
All stimuli were generated and all responses were recorded and processed using the Tucker–Davis Technologies hardware and software (TDT System III, Alachua, FL, USA). The ABR tests were performed in a sound-attenuating audiometric chamber. Hearing sensitivity was evaluated using tone-burst-evoked ABR threshold searches. Guinea-pigs were anesthetized with a mixture of ketamine and xylazine (40 and 8 mg kg−1, intraperitoneally, respectively). Body temperature was maintained at approximately 38 °C using a thermostatic heating pad. Evoked responses were collected using three subdermal electrodes positioned at the vertex and both mastoid regions. Tone bursts at 1, 2, 4, 8, 16 and 32 kHz were generated digitally with duration of 10 ms and rise–fall time of 1 ms through an electrostatic transducer (EC1; TDT). The acoustic stimuli were presented at a rate of 21.1 per second via a plastic tube that was inserted into the external ear canal of the animal. This closed field stimulation ensured separate evaluations of hearing in each ear. The evoked potentials were filtered with a bandpass filter between 100 and 3000 Hz, and averaged 1000 times. The ABR threshold was approached in a downward sequence of 5-dB steps and was determined as the lowest sound pressure level to which a visible, repeatable wave III could be identified.
Surgery for gene transfection or RWM treatment
The subjects were anesthetized in a similar method to that described above for ABR testing. The animal’s head was fixed with a stereotaxic restraint, and surgery performed under sterile conditions. A post-auricular approach was used to expose the tympanic bony bulla. After local analgesia with lidocaine, a retro-auricular incision was made to expose the mastoid. A hole of 2–3 mm in diameter was drilled into the mastoid to expose the RW niche and the bony wall of the cochlea immediately inferior to the RW niche. A micropump (Micro4; WPI, Kissimmee, FL, USA) was used to drive a microsyringe to apply the solution. For the digestive treatment arm, a solution of collagenase (either type I or II, C0130 or C6885, respectively; Sigma, St Louis, MO, USA) was freshly prepared in distilled water just before the surgery. The duration of exposure for the digestive treatment varied from 5 to 10 min and the quantity of the digestive solution from 5 to 10 µl. The first 1.5 µl solution was injected at a high rate of 200 nl s−1 and observed under a surgical microscope to ensure that the RW niche was filled with the solution. The injection was then continued at a low rate (15–30 nl s−1) to ensure the desired volume was applied during the appropriate amount of time. Next, residual solution inside the RW niche and in the surrounding area was removed using fine paper wicking. A piece of gelfoam (5–10 mm3) was then inserted gently to make contact with the RWM. In safety evaluation (n=13 guinea-pigs), the digestion was carried out with the concentration of the enzyme at 50, 40 and 30 mg ml−1, respectively (each for 8 or 9 ears). All other ears receiving virus via RWM after digestion were treated with the enzyme at 30 mg ml−1.
In those animals enrolled in the safety evaluation arm, surgery stopped here and the bulla opening and skin incision were closed. For the gene transfection arm, 5–10 µl of viral vector was applied to the RWM via gelfoam before closing the incision. In the cochleostomy arm, a small hole of 0.3 mm in diameter was drilled through the bony shell at the basal turn of the cochlea to access the scala tympani. Vectors of two types were tested: rAAV2 vectors were obtained from Vector Gene Technology Company Limited (Beijing, China) (rAAV2-EGFP vector promoted by human cytomegalovirus) and rAAV8 vectors were generated according to our previously described methods for rAAV8-GFP-mut733.21
Tissue preparation for morphology and immunostaining
Under deep anesthesia with an overdose of pentobarbital (100 mg kg−1), the animal was decapitated and the temporal bone was rapidly removed and the cochlea extracted. To fix the RWM, the cochlea was immersed into a solution containing 2.5% glutaraldehyde overnight at 4 °C and post-fixed in 2% OsO4 for 2 h. The attachment of the RWM to the bone ring of the niche was cut in a half-circle to reduce the tension in the sample before dehydration with a graded series of ethanol concentrations, concluding with 100%. For the SEM observations, the RWM samples were critical-point dried with liquid CO2 and sputter-coated with gold. Photographic images were taken with a Hitachi S-4700 SEM system (Hitachi, Tokyo, Japan). For the TEM observations, the RWM specimens were dehydrated in 100% ethanol, embedded in Epon 812, and then 70-nm-thick sections were cut and examined under the TEM microscope (JEM-1230, JEOL Ltd, Akishima, Tokyo, Japan).
To prepare the cochlea prepared for GFP fluorescence detection, the apex of the cochlea and the round window were punctured and then the cochlea was perfused through the apical hole with fixative (phosphate-buffered saline containing 2.5% paraformaldehyde) that exited through the round window. The cochlea was then immersed in fixative overnight at 4 °C. The basilar membrane and the organs of Corti were dissected under a microscope. The remaining portion of the cochlea containing the modiolus was decalcified for 4 days or longer as needed in 0.1 m EDTA, immersed in 15 and 30% sucrose overnight at 4 °C, and then embedded in OCT for 2 h. To examine the morphology and transfection of SGNs, 8-µm-thick sections were cut parallel to the midline of the modiolus to visualize Rosenthal’s canal. The samples taken from the cochleae that were transfected using the rAAV8-GFP-mut733 were first treated with 0.25% Triton X-100 for 1 h, and then blocked by 10% goat serum for 1 h, followed by primary antibody against GFP (Polyclonal from rabbit, NB600-308; Novus Biologicals, Littleton, CO, USA) at a concentration of 1:200 overnight at 4 °C. After washing three times in phosphate-buffered saline, the samples was treated with the second antibody (goat anti-rabbit, 70243-Cy2; Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) at a dilution of 1:200 for 2 h at 4 °C, and then washed again three times in the phosphate-buffered saline in a dark room. For the samples from the cochleae that were transfected with rAAV2-EGFP, no immunostaining was necessary. To distinguish transfection of hair cells versus supporting cells, some samples were double stained with phalloidin-TRITC (P1951; Sigma) at a concentration of 50 µg ml−1 for 10 min at room temperature. This was followed by a final three washes in phosphate-buffered saline. After all staining procedures, samples were mounted in glycerin on glass slides and examined by fluorescent microscopy (Axioplan2, Zeiss, Thornwood, NY, USA) or confocal microscopy (Laser Scanning, Zeiss LSM510 META). In the cochleae that were transfected with rAAV2-EGFP, the signal from EGFP was visible without immunostaining. Only cells that clearly displayed green fluorescence were counted as GFP positive (Figure 5). The transfection cochleogram for HCs was then established by counting the GFP-positive cells with the microscope focusing on the level of HCs in the organ of Corti.
ACKNOWLEDGEMENTS
This study was supported by grants from Natural Outstanding Youth Foundation of China (Grant No. 30925035), Special Program for Key Basic Research of the Ministry of Science and Technology, China (Grant No. 2009CB526504) and National Natural Science Foundation of China (Grant No. 30901669).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Duan M, Venail F, Spencer N, Mezzina M. Treatment of peripheral sensorineural hearing loss: gene therapy. Gene Therapy. 2004;11(Suppl 1):S51–S56. doi: 10.1038/sj.gt.3302369. [DOI] [PubMed] [Google Scholar]
- 2.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 3.Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, et al. Advances in molecular and cellular therapies for hearing loss. Mol Ther. 2008;16:224–236. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- 4.Brigande JV, Heller S. Quo vadis, hair cell regeneration? Nat Neurosci. 2009;12:679–685. doi: 10.1038/nn.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kesser BW, Lalwani AK. Gene therapy and stem cell transplantation: strategies for hearing restoration. Adv Otorhinolaryngol. 2009;66:64–86. doi: 10.1159/000218208. [DOI] [PubMed] [Google Scholar]
- 6.Maeda Y, Sheffield AM, Smith RJ. Therapeutic regulation of gene expression in the inner ear using RNA interference. Adv Otorhinolaryngol. 2009;66:13–36. doi: 10.1159/000218205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei D, Yamoah EN. Regeneration of the mammalian inner ear sensory epithelium. Curr Opin Otolaryngol Head Neck Surg. 2009;17:373–380. doi: 10.1097/MOO.0b013e328330345b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotanche DA. Genetic and pharmacological intervention for treatment/prevention of hearing loss. J Commun Disord. 2008;41:421–443. doi: 10.1016/j.jcomdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luebke AE, Rova C, Von Doersten PG, Poulsen DJ. Adenoviral and AAV-mediated gene transfer to the inner ear: role of serotype, promoter, and viral load on in vivo and in vitro infection efficiencies. Adv Otorhinolaryngol. 2009;66:87–98. doi: 10.1159/000218209. [DOI] [PubMed] [Google Scholar]
- 10.Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Therapy. 2000;7:1046–1054. doi: 10.1038/sj.gt.3301180. [DOI] [PubMed] [Google Scholar]
- 12.Konishi T, Salt AN, Hamrick PE. Effects of exposure to noise on permeability to potassium of the endolymph–perilymph barrier in guinea pigs. Acta Otolaryngol. 1982;94:395–401. doi: 10.3109/00016488209128927. [DOI] [PubMed] [Google Scholar]
- 13.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- 15.Salt AN, Sirjani DB, Hartsock JJ, Gill RM, Plontke SK. Marker retention in the cochlea following injections through the round window membrane. Hear Res. 2007;232:78–86. doi: 10.1016/j.heares.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan DM, Hehar SS, Bance ML, Rutka JA. Intentional ablation of vestibular function using commercially available topical gentamicin–betamethasone eardrops in patients with Meniere’s disease: further evidence for topical eardrop ototoxicity. Laryngoscope. 2002;112:689–695. doi: 10.1097/00005537-200204000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Bath AP, Walsh RM, Bance ML. Presumed reduction of vestibular function in unilateral Meniere’s disease with aminoglycoside eardrops. J Laryngol Otol. 1999;113:916–918. doi: 10.1017/s002221510014558x. [DOI] [PubMed] [Google Scholar]
- 18.Bath AP, Walsh RM, Bance ML, Rutka JA. Ototoxicity of topical gentamicin preparations. Laryngoscope. 1999;109:1088–1093. doi: 10.1097/00005537-199907000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverstein H, Arruda J, Rosenberg SI, Deems D, Hester TO. Direct round window membrane application of gentamicin in the treatment of Meniere’s disease. Otolaryngol Head Neck Surg. 1999;120:649–655. doi: 10.1053/hn.1999.v120.a91763. [DOI] [PubMed] [Google Scholar]
- 23.Luebke AE, Foster PK, Muller CD, Peel AL. Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Hum Gene Ther. 2001;12:773–781. doi: 10.1089/104303401750148702. [DOI] [PubMed] [Google Scholar]
- 24.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- 25.Liu YH, Ke XM, Qin Y, Gu ZP, Xiao SF. Adeno-associated virus-mediated Bcl-xL prevents aminoglycoside-induced hearing loss in mice. Chin Med J (Engl) 2007;120:1236–1240. [PubMed] [Google Scholar]
- 26.Cooper LB, Chan DK, Roediger FC, Shaffer BR, Fraser JF, Musatov S, et al. AAV-mediated delivery of the caspase inhibitor XIAP protects against cisplatin ototoxicity. Otol Neurotol. 2006;27:484–490. doi: 10.1097/01.mao.0000202647.19355.6a. [DOI] [PubMed] [Google Scholar]
- 27.Romano G. Current development of adeno-associated viral vectors. Drug News Perspect. 2005;18:311–316. doi: 10.1358/dnp.2005.18.5.917326. [DOI] [PubMed] [Google Scholar]
- 28.Stone IM, Lurie DI, Kelley MW, Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–848. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Carson SD. Limited proteolysis of the coxsackievirus and adenovirus receptor (CAR) on HeLa cells exposed to trypsin. FEBS Lett. 2000;484:149–152. doi: 10.1016/s0014-5793(00)02144-x. [DOI] [PubMed] [Google Scholar]
- 30.Soudais C, Boutin S, Hong SS, Chillon M, Danos O, Bergelson JM, et al. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J Virol. 2000;74:10639–10649. doi: 10.1128/jvi.74.22.10639-10649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu J, Handa A, Kirby M, Brown KE. The interaction of heparin sulfate and adeno-associated virus 2. Virology. 2000;269:137–147. doi: 10.1006/viro.2000.0205. [DOI] [PubMed] [Google Scholar]
- 32.Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samulski RJ, Zhu X, Xiao X, Brook JD, Housman DE, Epstein N, et al. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kotin RM, Siniscalco M, Samulski RJ, Zhu XD, Hunter L, Laughlin CA, et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goycoolea MV, Lundman L. Round window membrane. Structure function and permeability: a review. Microsc Res Technol. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 36.Shi L, Ermis R, Garcia A, Telgenhoff D, Aust D. Degradation of human collagen isoforms by Clostridium collagenase and the effects of degradation products on cell migration. Int Wound J. 2010;7:87–95. doi: 10.1111/j.1742-481X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshihara T, Kaname H, Ishii T, Igarashi M. Subepithelial fiber components of the round window membrane of the guinea pig: an ultrastructural and immunohistochemical study. ORL J Otorhinolaryngol Relat Spec. 1995;57:115–121. doi: 10.1159/000276723. [DOI] [PubMed] [Google Scholar]
- 38.Lalwani A, Walsh B, Reilly P, Carvalho G, Zolotukhin S, Muzyczka N, et al. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Therapy. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Okada T, Sheykholeslami K, Shimazaki K, Nomoto T, Muramatsu S, et al. Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol Ther. 2005;12:725–733. doi: 10.1016/j.ymthe.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Okada T, Nomoto T, Ke X, Kume A, Ozawa K, et al. Promoter effects of adeno-associated viral vector for transgene expression in the cochlea in vivo. Exp Mol Med. 2007;39:170–175. doi: 10.1038/emm.2007.19. [DOI] [PubMed] [Google Scholar]
- 43.Boeda B, Weil D, Petit C. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum Mol Genet. 2001;10:1581–1589. doi: 10.1093/hmg/10.15.1581. [DOI] [PubMed] [Google Scholar]
- 44.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]