Abstract
Treatment for limb lymphedema is challenging. The recent development of the super-microsurgical technique has made lymphaticovenular (LV) anastomosis an easier and more accurate surgical method for lymphedema. A summary of our experience as well as recent developments in surgical treatments for lymphedema are described.
Methods and Results: Ultra-microstructural analysis demonstrated that dysfunction of the lymphatics in lymphedema was caused by the degeneration and incomplete regeneration of smooth muscle cells and valve insufficiency in the lymphatic channel. ICG and infrared ray examinations have been proposed as new means of assessment of lymphatic function. LV anastomosis is suitable for genital edema, arm edema with severe phlegmone with leg edema, and early stage leg edema. Although pre- and postoperative compression therapy is generally required for limb edema, some cases do not require postoperative compression due to remaining or regenerated smooth muscle cells. As new methods of treatment, the vascularized lymphadiposal flap has been effective for progressive cases with LV anastomosis. LV anastomosis is also effective for congenital chyloabdomen. (*English Translation of J Jpn Coll Angiol 2008; 48: 173–178.)
Keywords: lymphedema, lymphatic channel, lymphaticovenous anastomosis, microsurgery, supra-microsurgery
Introduction
Lymphedema has been treated conservatively or surgically (such as tissue resection, direct or indirect lymphatic drainage). Conservative therapy is aimed to suppress the progression of edema, and surgical treatments are performed for radical cure by returning retained lymph to the venous system through an artificially prepared bypass. However, marked control of edema has been considered difficult to maintain over a long period by either method.1,2) Nevertheless, a surgical technique to treat very small vessels has recently been established (super-microsurgery: anastomosis of vessels 0.8–0.5 mm in diameter) in the field of plastic and reconstructive surgery, making anastomosis of extremely small blood vessels possible.3–6) Using this microscopic anastomosis technique, a new method for lymphaticovenular anastomosis with outcomes exceeding those of conventional gross lymphaticovenous drainage1,2,7) or lymphaticovenous anastomosis8–10) has been developed. Presently, cases showing marked alleviation of severe edema over 10 years or more after surgery are beginning to be reported.3–6) This has dispelled the notion that lymphedema has no cure, and prompted the shift to early or prophylactic anastomosis or combination therapy of anastomosis and compression. In this article, treatments that we are performing now are described.
Fate of Lymphatic Channels in Lymphedema
Lymphedema is considered to occur in individuals with congenital hypoplasia of the lymphatic and venous collaterals. If even a small number of lymph nodes on the proximal side of the limb are resected, related lymphatic channels are rapidly dilated. As a result, the diffuse degeneration of lymphatic channel smooth muscle cells occurs from the proximal side early after lymphadenectomy. Thereafter, while smooth muscle regenerates to some extent, regeneration is limited to small muscle cells.3) As a result, the powerful contractile function of the lymphatic channel is not restored, and the lymphatic return function remains impaired. During this period, lymphatic channels are obstructed and lost in patients having repeated episodes of lymphangitis, and collagen fibers proliferate in the subcutaneous adipose tissue and dermis due to the activation of fibroblasts. From a review of biopsy findings of lymphatic channels during surgery in clinical cases obtained to date, the degeneration and regeneration of smooth muscle cells are considered to vary morphologically among individuals and according to the site in the limb. There is no correlation between the duration of edema and degree of regeneration.3) Also, the degree of smooth muscle cell regeneration is considered to be correlated at least to some extent with the degree of alleviation of postoperative edema and cellulitis. Moreover, even if lymphatic channels are destroyed in the entire area, the lymphatic channel function is often intact in distal parts of the limbs. Consequently, improvements restricted to peripheral regions are often observed after bypass surgery in many patients. In addition, as edema of the femoral region is mitigated in some patients by anastomosis at the ankle, lymphatic channels in patients with edema are considered to have valve insufficiency and back flow of lymph.
New Diagnostic Method for Lymphatic Channel Function
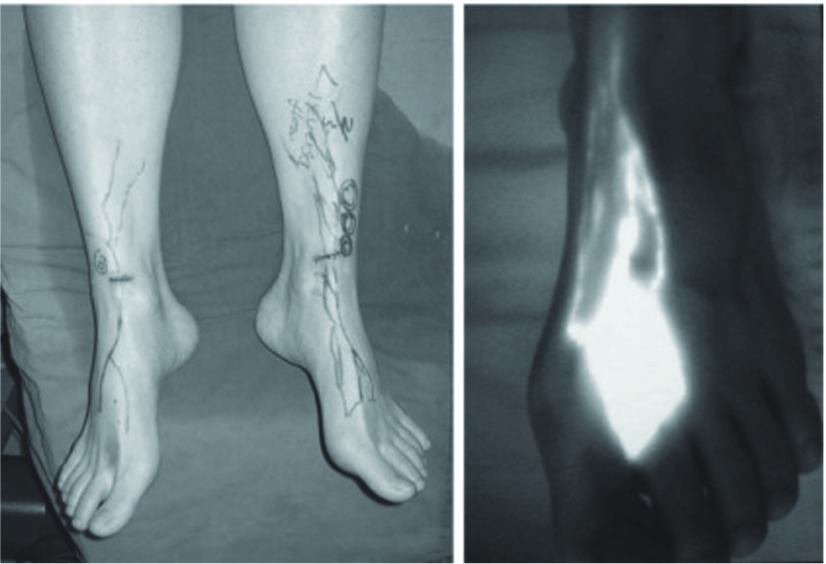
Since the lymphatic channels were not directly examined by the conventional method, the site of lymphatic channel obstruction and lymphatic channel function were unclear, and they were only indirectly estimated. Fluorescence lymphography is a recently developed examination of lymphatic channel function and is expected to be useful. When infrared rays are applied after the subcutaneous injection of indocyanine green (ICG), fluorescence emitted by ICG is seen to be rapidly drained through lymphatic channels in the normal limbs. In edematous areas, the severity of impairment of lymph drainage can be evaluated readily by direct observation11,12) (Fig. 1). In patients with mild lymphedema, only a few lymphatic channels are fluorescently stained in the peripheral region, and none are stained up to the proximal region. Also, in many patients with unilateral lower limb lymphedema, the other lower limb also remains unstained. This suggests that, in patients with lower limb lymphedema, the lymph draining function may be considerably lost in the non-edematous as well as edematous limb already at the onset of edema.
Fig. 1.
Infrared lymphography
A: Fluorescent lymphatics were traced with infrared lymphography. Only the distal lymphatics are detected on this bilateral legs with mild lymphedema.
B: After subdermal injection of indocyanin green, several fluorescent lymphatics can be detected with irradiation of infrared ray.
Treatments
(1) Sustained compression using an elastic stocking (bandage)
An elastic stocking (bandage) is used to prevent the exacerbation of edema by applying external force and increasing the tissue pressure. It is an essential treatment even for mild edema and is applied daily from an early stage. It must always be worn particularly when the patient remains standing during the day time. Since the stocking is washed frequently, it loosens in about 6 months, so it should be replaced with a new one at appropriate intervals. It should be modified individually to fit the limb, if necessary, by attaching a zipper or rubber string. For large patients whom a standard size elastic stocking does not fit, compression should be made using an order-made stocking or a bandage. Recently, the Faeldi method,3) which is a combination of compression and massage requiring hospitalization, has been reported, but rebound is considered to occur in many patients after discharge.
(2) Lymphaticovenular (LV) anastomosis
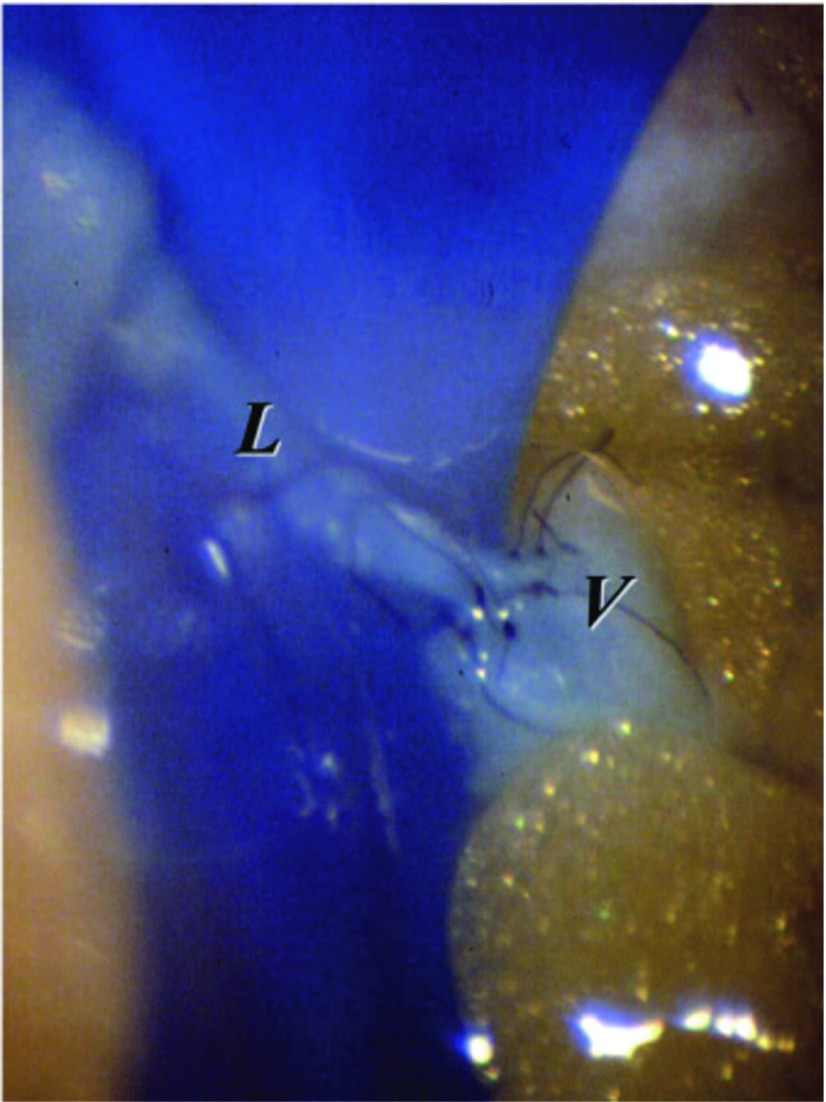
This is a radical treatment aimed to drain lymph to the venous system and eventually to the heart by creating a bypass and to make compression therapy unnecessary. In an early period, anastomosis was performed grossly. We used to perform LV anastomosis under a microscope at a magnification of × 20–30 by the super-microvascular anastomotic technique using a 50-micron (1/20 mm) needle (Fig. 2). This technique differs from conventional microscopic LV anastomosis (O’Brien, 1977)8) in that the cutaneous veins to be drained are anastomosed end-toend to small veins immediately below the dermis or in the superficial adipose layer.3–6) In principle, LV anastomosis must be carried out after thorough conservative treatment by sustained compression (primarily on an outpatient basis) over at least 6 months. Compression therapy must also be continued after surgery. If fluorescent lymphography, which has been developed recently, is used, lymphatics retaining the drainage function can be identified percutaneously, and those that are certain to have the drainage function can be readily exposed during surgery by a small incision under local anesthesia. As a result, this procedure, which used to be possible only by experienced surgeons, is spreading rapidly.
Fig. 2.
Microsurgical lymphaticovenular (LV) anastomosis. Under an operating microscope, a lymphatic channel (L) of 0.5 mm was anastomosed to a venule (V) of the same size. Flow from lymphatic channel to venule was established. Six to eight stitches were placed using 50-micron needle.
Comparison with Compression Therapy
Twelve of the 35 patients with unilateral or bilateral lower limb lymphedema were conservatively treated as outpatients primarily by compression therapy. In these patients, the mean duration of history after the onset of edema was 5.2 years, and the preoperative mean excessive crural (10 cm distal to the knee) girth was 7.1 cm, but a mean decrease of 0.6 cm was observed after conservative treatment for a mean of 1.5 years. The girth decreased markedly (≥4 cm) in 2 patients (16.7%). Sixteen patients in whom compression therapy was ineffective at other facilities or our department underwent surgery. In these patients, the mean preoperative excessive girth was 9.8 cm, and the mean history of edema after the onset was 8.9 years. The girth decreased by a mean of 2.7 cm after a mean postoperative course of 3.3 years. Marked decreases (≥4 cm) were observed in 8 patients (50%).3) From these results, compression therapy is considered to be more effective when combined with anastomotic surgery than alone. Also, this combination is considered to be indicated in most cases of edema.
Patients Early after the Onset of Edema
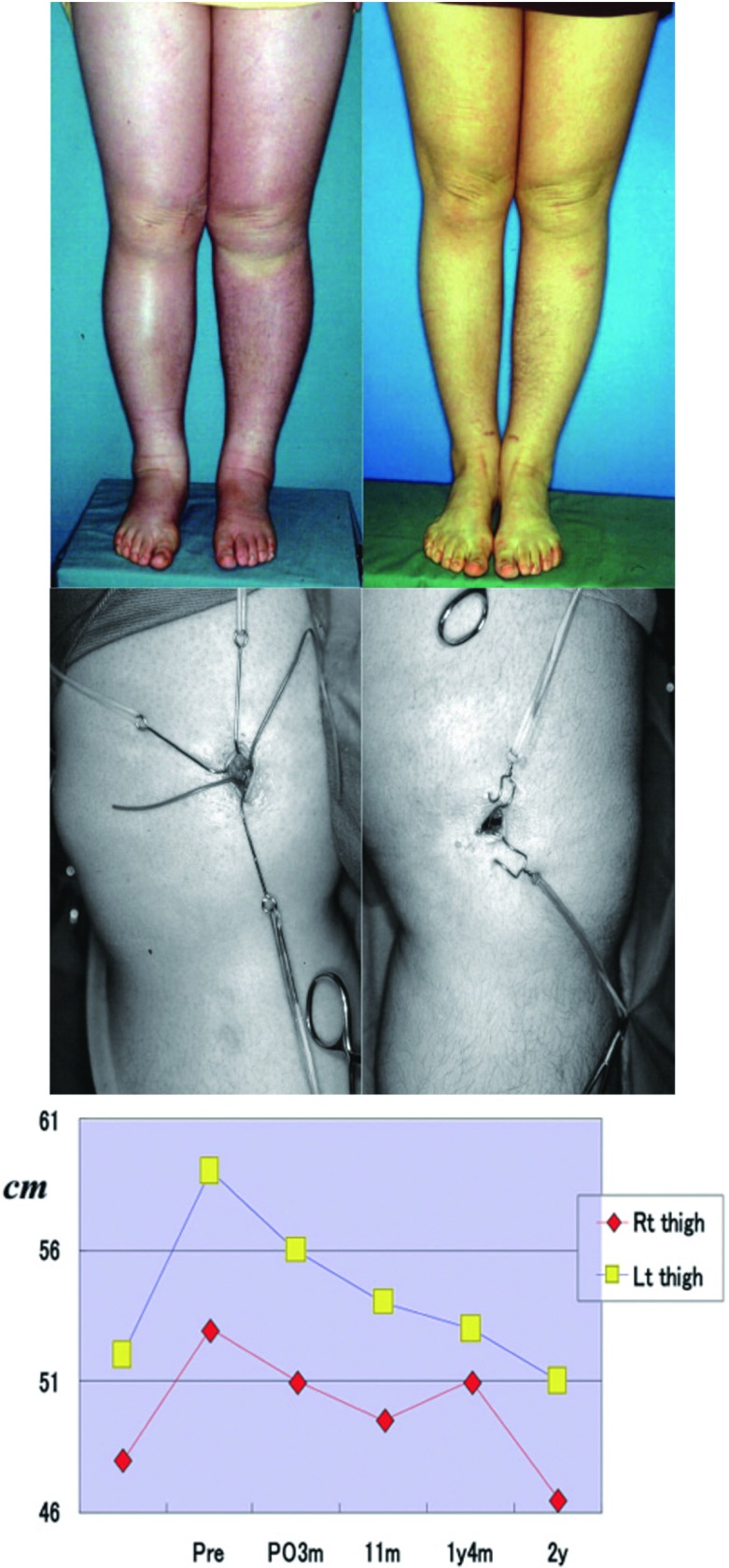
This procedure is expected to be considerably effective before or early after the onset of edema. Lymphedema can be prevented by anastomosis of only 1 vessel in many patients while lymphatic smooth muscle cells are still functioning before or early after its onset. In our recent study, we performed anastomosis of 1–3 vessels (mean, 1.4 vessels) under local anesthesia in 35 patients with early edema of the lower limbs within 9 months (mean, 2.3 months) after the onset and followed up the patients over a maximum of 6 years (mean, 31.7 months) after surgery. The treatment was effective in 88.5% of the patients, and no improvement was observed in 11.5% (Fig. 3).
Fig. 3.
Postoperative change of leg circumferential lengths.
A: Case 1. A 42-year-old woman with early-stage lymphedema of the bilateral legs following hysterectomy.
Left: Preoperative compression could not stop the progression of edema. LV anastomoses.
Right: Two years after anastomoses. Remarkable decrease with conventional compression.
B: Early stage LV anastomoses under local anesthesia at 3 and 4 months after the appearance of edema.
Left: 3 months after edema
Right: 4 months after edema
C: Periodic decrease in lower leg size up to two years after surgery.
© 2008 Lippincott Williams & Wilkins. All rights reserved. Koshima I, Nanba Y, Tsutsui T et al.: Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for Stage III and IV? Ann Plast Surg 2004; 53: 261-266.
Evaluation of Patients Who Require No Compression Therapy after Anastomosis
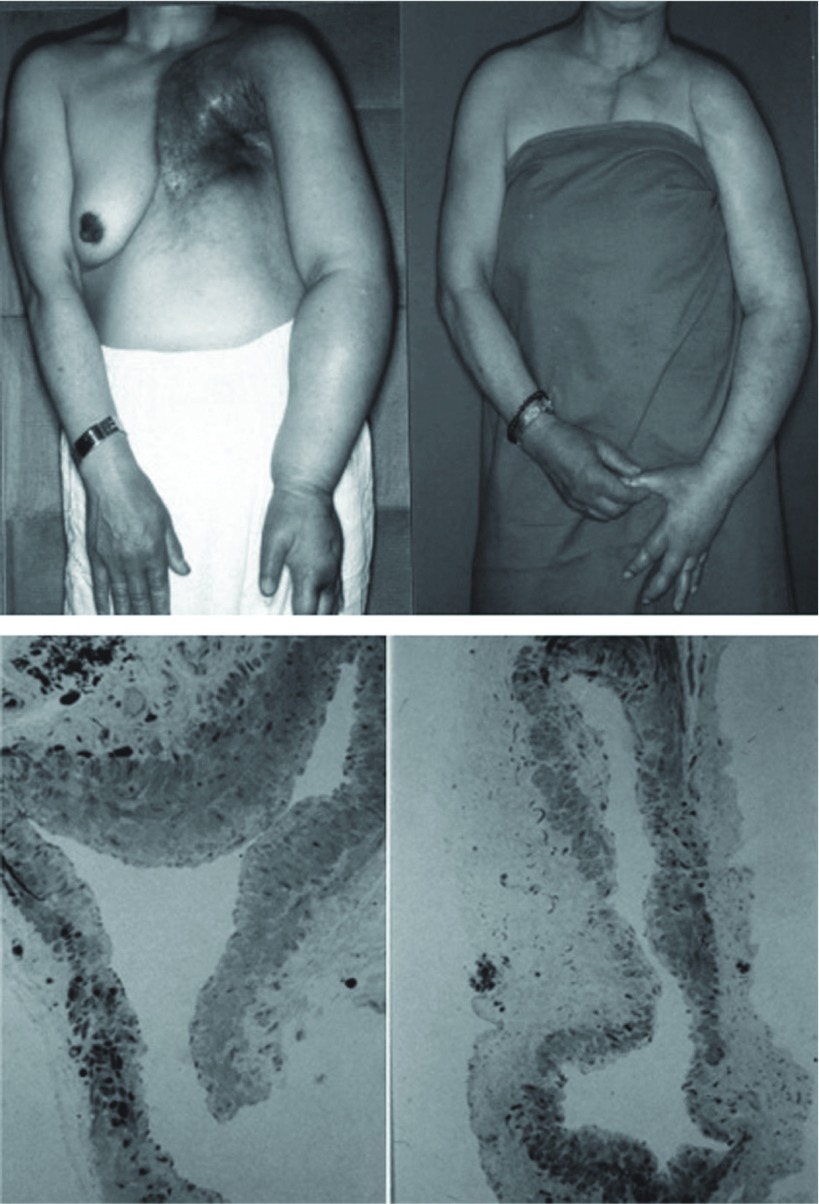
Patients showing recovery to a nearly normal state on receiving compression alone continued for several months to a few years after surgery have begun to be reported. In these patients, the lymph drainage function is considered to have recovered in the anastomosed lymphatics. Of the about 200 upper or lower limbs with edema treated by LV anastomosis, postoperative compression became unnecessary in 4 upper limbs and 6 lower limbs. Edema was primary in 1 and secondary in 9 of these limbs. The duration of postoperative follow-up was 2 months to 15 years. Concerning the upper limbs, anastomosis was effective in mild and severe edema within 1 year after the onset, but it was also effective in severe edema 11 years after the onset. Concerning the lower limbs, the treatment was effective in mild and severe edema within 1 year after the onset, but it was also effective in mild edema 11 years after the onset. From these results, regeneration of lymphatic channel smooth muscle is considered to vary individually depending on the site and symptoms, and, in patients in whom postoperative compression becomes unnecessary, regenerated smooth muscle is considered to differentiate, and the lymph drainage function to recover to a nearly normal level (Fig. 4).
Fig. 4.
Effect of lymphaticovenous anastomosis for severe arm edema patient.
A: Case 2. A 64-year-old woman with longstanding (11 years) progressive lymphedema following breast resection and irradiation. Left: The patient had complete brachial nerve palsy of the arm. Two LV anastomoses at the elbow fossa.
Right: Eight years after anastomoses without any compression.
B: Light microscopic findings of biopsied lymphatics (toluidine blue stain). The lymphatics show dilatation and have small regenerated smooth muscle cells, which means loss of drainage function of lymphatics.
Left: Elbow fossa.
Right: Wrist ulnar side.
© 2007 American Society of Plastic Surgeons. All rights reserved. Koshima I, Kawada S, Moriguchi T et al: Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg, 1996, 97: 397-405.
Patients Long after the Onset of Edema
Even patients with a long history of edema showing marked lymphatic sclerosis and subcutaneous fibrosis under surgical microscope often exhibit some positive response to postoperative compression therapy. This is probably due to the passive drainage of lymph. This combination therapy is extremely effective compared with conservative therapy alone, particularly in the upper limbs. In the lower limbs, also, it is effective in about half of patients. Particularly, in patients who frequently developed cellulitis, the incidence of anastomosis-induced cellulitis was markedly reduced, and the treatment was very effective.3–6) Since lymph is drained by compression, although not so thoroughly as in the normal limb, compression occasionally causes a decrease in the frequency of cellulitis, a marked decrease in the limb volume compared with the preoperative level, and a marked decrease in the limb girth (Fig. 4).
Edema Not Responding to Anastomotic Surgery and Its Treatment
Anastomosis was ineffective in about 10% of even early cases of edema. In many of these cases, edema was observed in the lower limb, postoperative compression therapy was abandoned, marked fibrosis was noted, or lymphatic channels were intraoperatively shown to be sclerosed or obstructed or were not found. In such cases, lymph may not be returned to the venous system sufficiently even after successful anastomosis. Also, even patients who have shown postoperative improvements and been treated continuously by compression may exhibit sudden exacerbation. For their management, compression must be continued, and supplementary anastomosis must be performed to establish additional drainage channels and promote regeneration of the drainage function.
Frequency of Bilateral Lower Limb Lymphedema and Its prevention
In our 356 cases of lower limb lymphedema, primary edema was relatively infrequent, and secondary lymphedema after the resection of uterine cancer or urological malignant tumors was predominant (87.4%). Primary edema was bilateral in 44% of the patients, and had been bilateral since its onset in more than half of these patients, but unilateral edema became bilateral in some patients often about 9 years after the onset. Secondary edema was bilateral from immediately after surgery in only about 26% of the patients and was initially unilateral but subsequently became bilateral in about 69%. Edema occurred unilaterally a mean of about 4 and a half years after surgery and became bilateral as it also occurred in the other limb about 7 years after surgery. This means that unilateral lymphedema is expected to become bilateral at a probability of 26%. We also encountered a case in which unilateral lower limb edema developed into bilateral edema and was rapidly exacerbated during preoperative compression therapy. Therefore, the intact lower limb in patients with unilateral edema does not necessarily remain free of edema, and unilateral edema may have an indication of prophylactic anastomosis before it becomes bilateral. Presently, we are attempting simultaneous prophylactic anastomosis of the intact as well as affected limb early after the onset, being confident about its effectiveness.5,6)
Future Therapeutic Strategy: Necessity of More Effective Anastomotic Surgery
It is becoming clear that edema can be prevented by prophylactic anastomosis before its onset. Recently, Fujiwara (former Associate Professor at Kawasaki Medical School) reported that the incidence of edema could be reduced by a combination of the resection of uterine cancer and transfer of the greater omentum (personal correspondence). Similarly, it is becoming clear that LV anastomosis simultaneous with lymphadenectomy or before the onset of lymphedema is effective. Moreover, for severe lymphedema with a long history, treatment by collecting normal lymphatics with a vascular pedicle from a healthy region, grafting them to the affected limb, and draining lymph into the venous system through this vascularized lymphatic graft with a normal lymph drainage function has been developed. While the long-term results of this treatment have not been reported, it is expected to be effective. For the future, we are sure that the incidence of lymphedema will be markedly reduced by a multidisciplinary team approach participated in by many professionals, such as a plastic surgeon, breast surgeon, obstetrician/gynecologist, and urologist.
Conclusion
Concerning lymphedema of the limbs, serial structural changes and dysfunction of the lymphatics, the current state and effectiveness of early or prophylactic LV anastomosis under local anesthesia using super-microsurgical techniques were discussed. Presently, the importance of the selection of various treatments according to the degree of lymphatic dysfunction is being increasingly recognized.
References
- Smith JW, Conway H. Selection of appropriate surgical procedures in lymphedema. Introduction of the hinged pedicle. Plast Reconstr Surg Transplant Bull 1962; 30: 10-31 [DOI] [PubMed] [Google Scholar]
- Thompson N. Buried dermal flap operation for chronic lymphedema of the extremities. Ten-year survey of results in 79 cases. Plast Reconstr Surg 1970; 45: 541-8 [DOI] [PubMed] [Google Scholar]
- Koshima I, Kawada S, Moriguchi T, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg 1996; 97: 397–405, discussion 406-7 [DOI] [PubMed] [Google Scholar]
- Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 2000; 16: 437-42 [DOI] [PubMed] [Google Scholar]
- Koshima I, Nanba Y, Tsutsui T, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. J Reconstr Microsurg 2003; 19: 209-15 [DOI] [PubMed] [Google Scholar]
- Koshima I, Nanba Y, Tsutsui T, et al. Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for stage III and IV? Ann Plast Surg 2004; 53: 261-6 [DOI] [PubMed] [Google Scholar]
- Degni M. New technique of lymphatic-venous anastomosis (buried type) for the treatment of lymphedema. VASA 1974; 3: 479-83 [PubMed] [Google Scholar]
- O’Brien BM, Sykes P, Threlfall GN, et al. Microlymphaticovenous anastomoses for obstructive lymphedema. Plast Reconstr Surg 1977; 60: 197-211 [DOI] [PubMed] [Google Scholar]
- Degni M. New technique of lymphatic-venous anastomosis for the treatment of lymphedema. J Cardiovasc Surg (Torino) 1978; 19: 577-80 [PubMed] [Google Scholar]
- Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg 1990; 85: 64-74, discussion 75-6 [DOI] [PubMed] [Google Scholar]
- Ogata F, Azuma R, Kikuchi M, et al. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg 2007; 58: 652-5 [DOI] [PubMed] [Google Scholar]
- Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg 2007; 59: 180-4 [DOI] [PubMed] [Google Scholar]