Abstract
A 61-year-old woman with multiple splanchnic arterial aneurysms (SAAs) was transferred to our hospital in a state of shock. She underwent coil embolization under the diagnosis of ruptured pancreaticoduodenal artery aneurysm. Follow-up computed tomography performed 2 weeks later showed rapid enlargement of a gastric artery aneurysm, and she underwent an additional embolization. Atherosclerotic, inflammatory or hereditary causes were excluded, and the patient was clinically diagnosed with segmental arterial mediolysis accompanied by multiple SAAs, one of which showed acute remodeling after endovascular treatment.
Keywords: segmental arterial mediolysis, splanchnic arterial aneurysms, endovascular repair
Introduction
Splanchnic arterial aneurysm (SAA) sometimes causes fatal shock after rupture.1,2) Surgical and radiological interventions are performed on SAA patients to prevent aneurysm rupture or to achieve emergency hemostasis on rupture. To prevent disease progression, adjuvant therapies, e.g., anti-inflammatory therapy with steroid for arteritis, and antibiotic therapy for infection, are sometimes indispensable for patients who have SAAs of systemic disease origin. Therefore, it is important to infer the pathogenesis of SAAs mainly from the patients’ clinical course, laboratory data, and radiological images, in the absence of pathological specimens for diagnosis.
Segmental arterial mediolysis (SAM) was first described by Slavin et al. in 1976 and has been focused on recently owing to its unique pathological characteristics of lysed smooth muscle cells within the arterial media accompanied by surrounding fibrosis.3–5) Thirty-three percent to 44% of patients with SAM have multiple SAAs.5,6) In addition, the angiographic findings of some patients show the presence of bead-like appearance other than aneurysms. Although histological examination is the gold standard for the diagnosis of SAM,4) and the criteria for the clinical diagnosis of SAM are yet to be established, radiological findings sometimes contribute to the clinical diagnosis of SAM after excluding other suspected systemic diseases.6–9)
Herein, we report the case of a patient who had multiple SAAs without any atherosclerotic, inflammatory, or hereditary causes; one of the SAAs showed intra-abdominal hemorrhage. After coil embolization of the responsible arteries, an adjacent SAA showed unexpected rapid enlargement and was successfully treated with endovascular therapy. We diagnosed our patient with SAM on the basis of the presence of multiple SAAs and “acute” remodeling,4,5) and we have also discussed the feasibility of the clinical diagnostic steps.
Case Report
A 61-year-old woman, who had been diagnosed with multiple SAAs and dissection of the superior mesenteric artery (SMA), visited our hospital with the complaint of dull lower abdominal pain. Although she was initially diagnosed with cystitis on the basis of the physical and urine examination findings, she was transferred to the emergency department of our hospital several hours later owing to the sudden onset of severe abdominal pain and circulatory collapse. Although the patient was in a state of shock, she was conscious and complained of severe right upper abdominal pain. After fluid resuscitation, the result of laboratory examination showed severe anemia with a hemoglobin concentration of 4.2 g/dL. The patient underwent rapid blood transfusion, causing her blood pressure to recover to approximately 100 mmHg, after which she underwent computed tomography (CT). The CT scan showed retroperitoneal hematoma and extravasation of contrast agents around the anterior superior pancreaticoduodenal artery (ASPDA) (Fig. 1), along with aneurysms in the splenic artery (SA) and the right gastric artery (RGA), irregular dilatation and stenosis of the SA, and dissection of the SMA.
Fig. 1.
CT scan showed intraperitoneal fluid collection and massive hematoma around the pancreatic head. Multiple splanchnic arterial aneurysms of the splenic artery (A: white arrow), of the right gastic artery (B: black arrow), of the ASPDA (C: white arrow), extravasation (D: white arrow), and irregular vascular dilatation and stenosis of the splenic artery (B: white arrow).
The patient had never smoked or consumed alcohol: she did not have any family history of aneurysms. She had mild hypertension, which did not require medication, but no other comorbid risk factors such as hyperlipidemia, diabetes mellitus, ischemic heart disease, cerebrovascular disease, and chronic kidney disease.
The patient was treated via emergency endovascular coil embolization. Although the patient’s blood pressure was temporarily stabilized by embolization of the ASPDA, additional embolization of the posterior superior pancreaticoduodenal artery (PSPDA) and the lower trunk of the gastroduodenal artery (GDA) was required for achieving successful hemostasis 10 hours later (Fig. 2). Two weeks later, a follow-up contrast-enhanced CT scan showed rapid enlargement of the maximum diameter of the RGA aneurysm from 11 mm to 21 mm which indicated acute remodeling due to hemodynamic change of pancreaticoduodenal arcade distribution (Fig. 3). A third intervention involving coil embolization of the RGA aneurysm was successfully performed. The patient was discharged on the thirty eighth day after the initial intervention. A CT scan performed 2 months later showed reduction in the retroperitoneal hematoma with neither any enlargement of nor new appearance of an SAA.
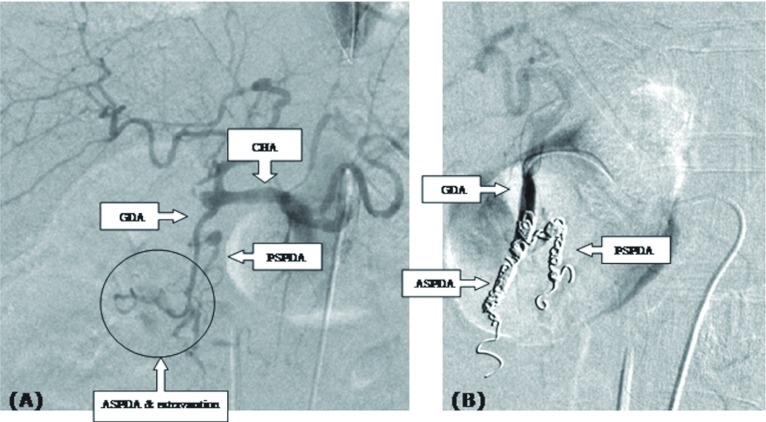
Fig. 2.
Extravasation was seen around the ASPDA; however, the dissected site was difficult to detect (A). First, coil embolization was performed in the ASPDA, and then additional coil embolization was performed both in the PSPDA and the lower part of the GDA trunk (B).
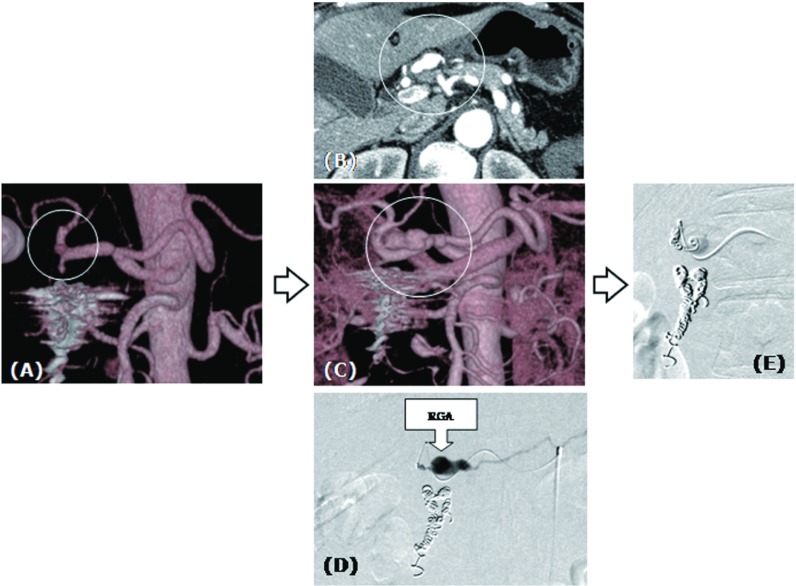
Fig. 3.
Rapid enlargement of the right gastric aneurysm was seen. Small right gastric artery aneurysm (A) showed rapid enlargement 2 weeks after the second intervention (B, C, D). Additional coil embolization was performed successfully (E).
Discussion
Although the incidental findings of SAA have increased owing to the advent of CT and other radiological imaging technologies,10) the prevalence of SAA is still relatively rare,11,12) and cases of multiple SAAs are ever rarer (3.6%–15% of patients with SAA). For the patients with multiple SAAs and irregular splanchnic arterial dilatation as observed in our patient, the presence of following peculiar systemic backgrounds should be suspected, such as: SAM, fibromuscular dysplasia (FMD), polyarteritis nodosa, ANCA-associated vasculitis, giant cell arteritis, Takayasu arteritis, Behcet’s disease, Kawasaki disease, infection, Ehlers-Danlos syndrome type IV, Marfan’s syndrome, neurofibromatosis, pseudoxanthoma elasticum, and atherosclerosis.7) From these diseases, we excluded vasculitis, infection, and atherosclerosis from the differential diagnosis in our case. Furthermore, diseases of genetic origin were also excluded in our case owing to the lack of specific clinical features and family history. FMD was the last and the most difficult condition to differentiate from SAM in the absence of histopathologic findings. FMD is observed mainly in the young female population, usually affects the renal arteries, and occasionally causes renal hypertension13); these clinical features helped us differentiate between FMD and SAM.
The pathological phases of SAM range from an injurious phase to a reparative one, i.e., (1) mediolysis which begins in the outer media and results in vasculization; (2) separation of the media from the adventitia, resulting in a dissecting hematoma; (3) arterial gaps created by trans-medial mediolysis; and (4) replacement of granulation tissue by reparative fibrosis.3–5,14) Slavin concluded that SAM is an “acute” disease in his paper which reviewed 25 specimens of the SAM.4) The evidence of this supposition is that a patient of SAM who was incidentally discovered did not show radiological abnormality 1 month previously.15) As the histopathologic observations showed rapid transformation of the SAM from the injurious phase to the reparative one, Slavin supposed that both the injurious and reparative changes occurring in different arteries or even in the same artery in the first week after the initial bleeding.4) In several studies, SAM patients have shown remodeling within a short duration, which was in accordance with the histopathological findings described above.6,8,9,16) We think that rapid remodeling in the adjacent or the same artery after rupture and subsequent endovascular treatment that were observed in our case were key to the diagnosis of SAM. Hemodynamic change in blood-flow distribution within the splanchnic arterial arcades could contribute to rapid aneurysmal enlargement or emergence of a new lesion. Considering that SAM is an “acute” disease,4,5) close follow-up is necessary after the rupture and intervention for SAAs, once SAM is suspected. However, the hypothesis that a healed SAM lesion might transform into an FMD-like lesion and the variation of clinical features such as aneurysm reduction and recanalization in the delayed phase could explain the good outcomes of SAM that were reported in previous studies, and we can expect SAM to progress to the state of a stable and benign disease.4,5,14,17)
The treatment of SAM depends on its presentation, site and the aneurysm size. Both surgical and endovascular interventions have been reported to be safe and effective,16,17) and a hybrid therapy combining both would be a good option for SAM involving multiple diseased lesions.9) For cases involving ruptured aneurysms, detecting the site of rupture in a massive hematoma is sometimes difficult via open surgery, especially when the site adjoins the pancreatic head, as in our case.
We suspect that many more cases of SAM have not been detected in the cases of patients diagnosed with multiple SAA, especially among patients with acute morphological changes in the aneurysms, because of the absence of histological specimens.5,18) We clinically diagnosed our patient with SAM on the basis of the following features: (1) multiple SAAs; (2) middle to old age; (3) non-inflammatory, non-atherosclerosis and non-genetic backgrounds; (4) radiological features involving the presence of bead-like appearance, irregular dilatation and stenosis and (5) acute vessel remodeling. The clinical diagnosis of SAM is important because it greatly affects the treatment strategy involving interventions and follow-ups.
Disclosure Statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication on this article. The authors received no financial support for the research, author-ship, and/or publication of this article.
Acknowledgement
The authors wish to thank Dr. Jiro Sato and Dr. Takeyuki Watadani for excellent radiological intervention and Dr. Masaaki Akahane for reviewing the radiological findings.
References
- Carr SC, Mahvi DM, Hoch JR, et al. Visceral artery aneurysm rupture. J Vasc Surg 2001; 33: 806-11 [DOI] [PubMed] [Google Scholar]
- Pasha SF, Gloviczki P, Stanson AW, et al. Splanchnic artery aneurysms. Mayo Clin Proc 2007; 82: 472-9 [DOI] [PubMed] [Google Scholar]
- Slavin RE, Gonzalez-Vitale JC. Segmental mediolytic arteritis: a clinical pathologic study. Lab Invest 1976; 35: 23-9 [PubMed] [Google Scholar]
- Slavin RE. Segmental arterial mediolysis: course, sequelae, prognosis, and pathologic-radiologic correlation. Cardiovasc Pathol 2009; 18: 352-60 [DOI] [PubMed] [Google Scholar]
- Inada K, Maeda M, Ikeda T. Segmental arterial mediolysis: unrecognized cases culled from cases of ruptured aneurysm of abdominal visceral arteries reported in the Japanese literature. Pathol Res Pract 2007; 203: 771-8 [DOI] [PubMed] [Google Scholar]
- Sakano T, Morita K, Imaki M, et al. Segmental arterial mediolysis studied by repeated angiography. Br J Radiol 1997; 70: 656-8 [DOI] [PubMed] [Google Scholar]
- Baker-LePain JC, Stone DH, Mattis AN, et al. Clinical diagnosis of segmental arterial mediolysis: differentiation from vasculitis and other mimics. Arthritis Care Res (Hoboken) 2010; 62: 1655-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulen MC, Cohen DL, Itkin M, et al. Segmental arterial mediolysis: angioplasty of bilateral renal artery stenoses with 2-year imaging follow-up. J Vasc Interv Radiol 2004; 15: 763-7 [DOI] [PubMed] [Google Scholar]
- Ryan JM, Suhocki PV, Smith TP. Coil embolization of segmental arterial mediolysis of the hepatic artery. J Vasc Interv Radiol 2000; 11: 865-8 [DOI] [PubMed] [Google Scholar]
- Pulli R, Dorigo W, Troisi N, et al. Surgical treatment of visceral artery aneurysms: A 25-year experience. J Vasc Surg 2008; 48: 334-42 [DOI] [PubMed] [Google Scholar]
- Sachdev-Ost U. Visceral artery aneurysms: review of current management options. Mt Sinai J Med 2010; 77: 296-303 [DOI] [PubMed] [Google Scholar]
- Panayiotopoulos YP, Assadourian R, Taylor PR. Aneurysms of the visceral and renal arteries. Ann R Coll Surg Engl 1996; 78: 412-9 [PMC free article] [PubMed] [Google Scholar]
- Kojima A, Shindo S, Kubota K, et al. Successful surgical treatment of a patient with multiple visceral artery aneurysms due to fibromuscular dysplasia. Cardiovasc Surg 2002; 10: 157-60 [DOI] [PubMed] [Google Scholar]
- Slavin RE, Saeki K, Bhagavan B, et al. Segmental arterial mediolysis: a precursor to fibromuscular dysplasia? Mod Pathol 1995; 8: 287-94 [PubMed] [Google Scholar]
- Michael M, Widmer U, Wildermuth S, et al. Segmental arterial mediolyis: CTA findings at presentation and follow-up. Am J Roentgenol 2006; 187: 1463-9 [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Deguchi J, Endo H, et al. Successful treatment tailored to each splanchnic arterial lesion due to segmental arterial mediolysis (SAM): report of a case. J Vasc Surg 2008; 48: 1338-41 [DOI] [PubMed] [Google Scholar]
- Obara H, Matsumoto K, Narimatsu Y, et al. Reconstructive surgery for segmental arterial mediolysis involving both the internal carotid artery and visceral arteries. J Vasc Surg 2006; 43: 623-6 [DOI] [PubMed] [Google Scholar]
- Sakpal SV, McCarthy CS, Addis MD, et al. Acute progression of multiple visceral artery aneurysms. Clin Imaging 2009; 33: 137-9 [DOI] [PubMed] [Google Scholar]