Abstract
The baculovirus-insect cell expression system is widely used to produce recombinant glycoproteins for many different biomedical applications. However, due to the fundamental nature of insect glycoprotein processing pathways, this system is typically unable to produce recombinant mammalian glycoproteins with authentic oligosaccharide side chains. This minireview summarizes our current understanding of insect protein glycosylation pathways and our recent efforts to address this problem. These efforts have yielded new insect cell lines and baculoviral vectors that can produce recombinant glycoproteins with humanized oligosaccharide side chains.
Overview
Baculovirus expression vectors (reviewed by Jarvis, 1997; Luckow and Summers, 1988; O’Reilly et al., 1992) are widely used to produce recombinant proteins during productive infection of insect larvae or established insect cell lines. Historically, one of the most appealing features of baculovirus-insect expression systems has been the eucaryotic protein processing capabilities of the host. Accordingly, these systems are widely considered to be excellent tools for recombinant glycoprotein production. However, this perception is inconsistent with the fact that there are fundamental differences in the glycoprotein processing pathways of insects and higher eucaryotes (reviewed by Marchal et al., 2001; Marz et al., 1995). The molecular analysis of the glycoprotein processing pathways in insect systems is the central topic of research in our laboratory. One of our goals is to use the knowledge gained through this research to direct metabolic engineering efforts to “humanize” insect glycoprotein processing pathways. These efforts will lead to the development of new baculovirus-insect systems that can be used to produce more authentic recombinant human glycoproteins for various biomedical applications (reviewed by Jarvis et al., 1998). This minireview will summarize our current understanding of insect glycoprotein processing pathways and describe our recent efforts to engineer these pathways, with a specific focus on the N-glycosylation pathway.
The insect cell protein N-glycosylation pathway
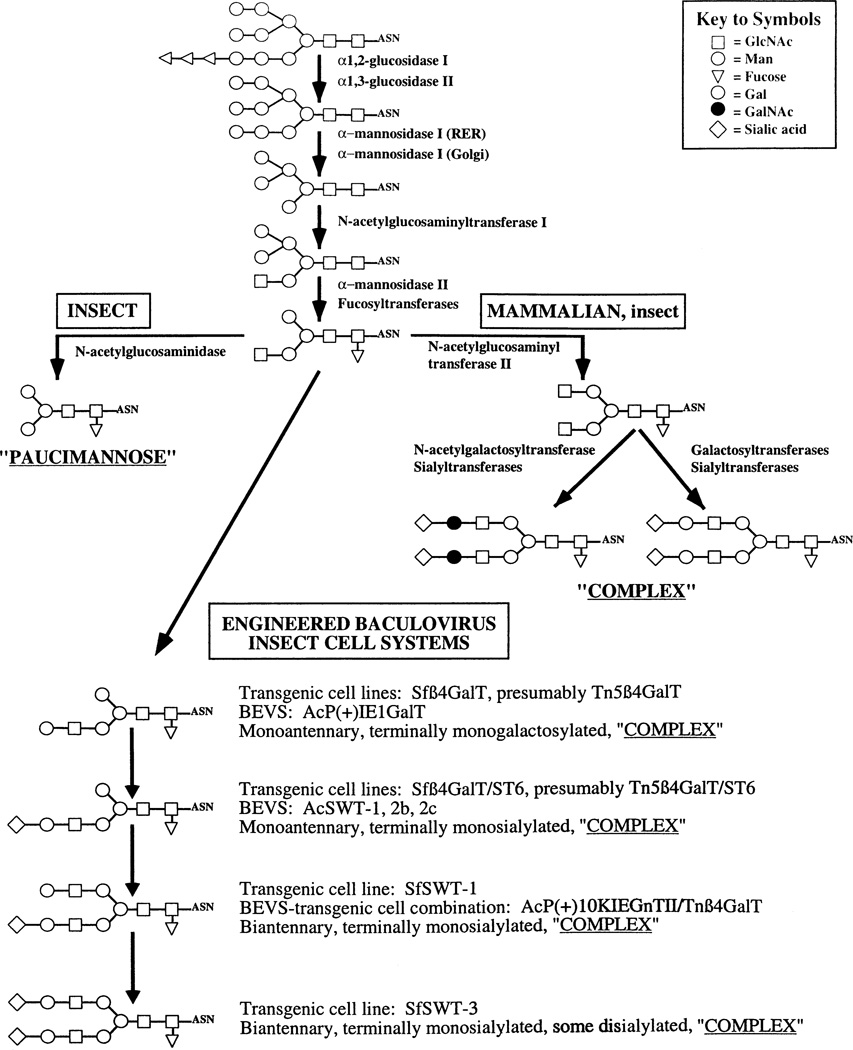
Studies on the N-glycan structures produced by mosquito cells provided our earliest views of the insect protein N-glycosylation pathway (reviewed by Marchal et al., 2001; Marz et al., 1995). For virologists, it is relevant to note that one factor driving some of these studies was an interest in examining potential differences in the structures and functions of Sindbis virus glycoproteins produced during its replication in mammalian, as compared to insect hosts (Hsieh and Robbins, 1984; Stollar et al., 1976). The results showed that there were indeed some striking differences, as the structures of the N-glycans on the viral glycoproteins produced by insect cells were much less complex than those produced by mammalian cells. These observations suggested that the insect cell N-glycan processing pathway is truncated relative to the mammalian pathway. More specifically, the insect pathway appeared to include all the enzymes involved in N-glycan trimming, but few of the enzymes involved in N-glycan elongation in mammalian cells (Fig. 1). This general conclusion was supported and extended in many subsequent structural studies of the N-glycans from various recombinant glycoproteins produced using baculovirus-insect expression systems (reviewed by Altmann et al., 1999; Marchal et al., 2001). Additional support came from the findings that insect cells have no detectable sialyltransferase activity (Hollister and Jarvis, submitted for publication; Hooker et al., 1999; Jarvis et al., 2001; Seo et al., 2001; Stollar et al., 1976) or CMP-sialic acids (Hooker et al., 1999; Tomiya et al., 2001). These and other studies established that the major processed N-glycans produced by insect cells are highly trimmed, but minimally elongated structures known as paucimannose structures (Man3-GlcNAc2-N-Asn), either with or without fucose residues, linked to the chitobiose (GlcNAc-GlcNAc-N-Asn) core (Fig. 1).
Fig. 1.
Protein N-glycosylation pathways in insect and mammalian cells. Monosaccharides are indicated by their standard symbolic representations, as defined in the key. The insect and mammalian N-glycan processing pathways share a common intermediate, as shown. The major products derived from this intermediate are paucimannose and complex N-glycans in insect and mammalian cells, respectively. It is generally recognized that insect cells have only a limited capacity, at best, to produce complex N-glycans. However, this model accommodates the possibility that some insect cells can produce complex N-glycans under certain circumstances. Complex N-glycans are extremely diverse and only representative examples are shown in the figure. The structures of the N-glycans produced by transgenic lepidopteran insect cell lines, modified baculovirus expression vectors (BEVS), and BEVS-transgenic insect cell combinations are shown as well.
In practical terms, this conclusion may be accepted, without further discussion, by any investigator who wants or needs to predict the structures of the glycans linked to a recombinant N-glycoprotein produced in the baculovirus-insect cell expression system. However, a more thorough analysis of the basic science demands a brief consideration of other data, which suggest that insect cells might have more extensive N-glycan processing capabilities. These data indicate that, under some conditions, some insect cells can express these additional functions and produce complex, even terminally sialylated N-glycans identical to those produced by mammalian cells.
A structural analysis of recombinant human plasminogen provided the first direct and convincing evidence that the baculovirus-insect cell system might be able to produce complex, terminally sialylated N-glycans (Davidson et al., 1990). Considering its high impact, an independent confirmation of this observation would be important, but this has not yet appeared in the literature. During the 1990s, there were several other reports of recombinant glycoprotein sialylation in the baculovirus-insect cell system (e.g., Davis and Wood, 1995; Sridhar et al., 1993), but these provided only indirect and relatively unconvincing support for this conclusion. More recently, it was reported that a new Trichoplusia ni cell line could produce a sialylated recombinant glycoprotein when cultured in the presence of the sialic acid precursor, N-acetylmannosamine (Joshi et al., 2001). Another recent report indicated that treatment of several established insect cell lines with a β-N-acetylglucosaminidase (Fig. 1) inhibitor allowed these cells to produce recombinant glycoproteins with terminally sialylated N-glycans (Watanabe et al., 2001). Similar to the plasminogen study, these studies provided convincing evidence for recombinant glycoprotein sialylation in baculovirus-infected insect cells, but neither has been independently confirmed in the literature at this time. If these studies are correct, they indicate that at least some insect cells encode the machinery needed to produce complex, terminally sialylated N-glycans. If this is true, the established insect N-glycosylation paradigm must be modified to accommodate this finding. As we proposed long ago, a simple way to do this is to envision a branched insect cell pathway (Fig. 1). One branch could provide trimming with little elongation to produce the major processed N-glycan subpopulation, while the other could provide trimming and elongation to produce the minor, more highly processed N-glycan subpopulations (Jarvis and Finn, 1995; Jarvis et al., 1998).
The best way to unequivocally test this model and determine if lepidopteran insect cells have the genetic potential to produce complex, sialylated N-glycans is to isolate and characterize the genes and gene products involved in protein N-glycosylation in these cells. Our group has been using this approach since the mid-1990s as part of an overall effort to try to elucidate the N-glycan processing pathway in lepidopteran insect cells at the molecular level (Francis et al., 2002; Jarvis et al., 1997; Kawar et al., 1997, 2000, 2001; Kawar and Jarvis, 2001). In addition, a consortium of investigators recently initiated a lepidopteran insect genome project, which will facilitate molecular genetic analyses of the protein N-glycosylation pathway in these insects. Meanwhile, several labs, including our own, have turned to the relative wealth of information already available in a more widely studied insect, Drosophila melanogaster.
There is convincing evidence that sialic acids exist in D. melanogaster tissues (Roth et al., 1992) and bioinformatic analyses have identified seven Drosophila genes predicted to encode enzymes involved in the production of terminally sialylated, complex N-glycans (Altmann et al., 2001; Aumiller and Jarvis, 2002; Farkas et al., 1999; Kim et al., 2002; Segawa et al., 2002; Vadaie et al., 2002). On the other hand, only one of these genes has actually been shown to encode a product with the predicted function (Kim et al., 2002). Two encode products with related, but distinct functions (Aumiller and Jarvis, 2002; Segawa et al., 2002; Vadaie et al., 2002) and the others have yet to be experimentally tested. Therefore, it remains to be determined whether the fruitfly truly has the genetic potential to produce complex, sialylated N-glycans.
In the final analysis, it should be appreciated that there is some tantalizing evidence indicating that at least some insect cells, under some conditions, can produce complex, terminally sialylated N-glycans, identical to those produced by mammalian cells. However, it also should be recognized that the lepidopteran insect cell lines routinely used as hosts for baculovirus expression vectors typically fail to produce these highly processed N-glycans. Therefore, as stated above, investigators using the baculovirus-insect cell system may confidently presume that their recombinant glycoprotein end products will have highly trimmed, but not elongated paucimannose N-glycans in place of the complex N-glycans found on the native mammalian products (reviewed by Marchal et al., 2001).
Genetically engineering insect N-glycosylation pathways
The fundamental difference between the major N-glycan processing pathways of lepidopteran insect and mammalian cells imposes a serious limitation on the utility of the baculovirus-insect cell system for recombinant glycoprotein production. In particular, one should expect the absence of terminal sialic acids on therapeutic recombinant glycoproteins produced with this system to be problematic because glycoproteins lacking sialic acids have extremely short half-lives in vivo (reviewed by Raju et al., 1996; Varki, 1993). In fact, it has been directly demonstrated that at least two different recombinant glycoproteins produced in the baculovirus system have short half-lives in vivo (Grossmann et al., 1997; Sareneva et al., 1993). A few years ago, our group began to address this problem.
Our basic approach was to use mammalian glycosyltransferase genes to create new baculovirus vectors and transgenic insect cell lines with extended N-glycan processing capabilities. The platform for this work was established by our earlier efforts to genetically transform lepidopteran insect cell lines. Methods developed in mammalian cell systems and a promoter from the immediate early baculovirus gene, ie1 (Guarino and Summers, 1987), were used to produce the first stably transformed lepidopteran insect cell lines (Jarvis et al., 1990). Later, additional plasmids containing the ie1 promoter and a baculovirus enhancer element, hr5, which stimulates ie1-mediated transcription (Guarino et al., 1986), were created to facilitate this work (Jarvis et al., 1996). These “immediate-early” plasmids could be used to create either transgenic insect cells, which would express foreign genes constitutively in the absence of viral infection, or immediate-early baculovirus expression vectors, which would express foreign genes beginning immediately after infection. Finally, we began to insert cDNAs encoding mammalian N-glycan processing enzymes, which were generously provided by the glycobiology community, into these expression plasmids. The resulting constructs were used, together with various selectable markers, either to transform established lepidopteran insect cell lines, such as Sf9 (Summers and Smith, 1987) and Tn-5B1-4 (High Five; Wickham et al., 1992), or to create novel recombinant baculoviruses.
The first example of an effort to modify the insect N-glycosylation pathway using these tools involved the production of an immediate-early baculovirus expression vector, AcP (+) IE1GalT, which encoded a bovine β1,4-galactosyl-transferase (β4GalT) cDNA under hr5-ie1 control (Jarvis and Finn, 1996). This vector induced high levels of β4GalT activity, beginning immediately after infection, and host cells infected with this vector, unlike controls infected with a wild-type baculovirus, produced a terminally galactosylated form of the major virion glycoprotein, gp64. Later, a similar β4GalT construct was used to genetically transform Sf9 cells to extend their N-glycan processing pathway independently of baculovirus infection (Hollister et al., 1998). This resulted in the production of a transgenic insect cell line, Sfβ4GalT, which contained stably integrated genomic copies of the hr5-ie1-driven mammalian β4GalT gene, had normal growth properties, supported baculovirus infection, and constitutively expressed the integrated β4GalT gene. Expression of this gene induced high levels of β4GalT activity, which allowed Sfβ4GalT cells, unlike the parental Sf9 cells, to produce terminally galactosylated foreign glycoproteins during infection with a conventional baculovirus expression vector. Subsequently, additional constructs were prepared and used to incorporate two mammalian genes, β4GalT and β2,6-sialyltransferase (ST6GalI) into the Sf9 (Hollister and Jarvis, 2001), Tn-5B1-4 (Breitbach and Jarvis, 2001), and baculovirus (Jarvis et al., 2001) genomes. These efforts yielded the first transgenic insect cell lines (Sfβ4GalT/ST6 and Tn5β4GalT/ST6) and baculovirus expression vectors (AcSWT series) that could routinely sialylate recombinant glycoproteins. In essence, the new transgenic cell lines represented improved hosts for conventional baculovirus expression vectors and the new viruses represented improved baculoviral vectors for recombinant glycoprotein production in conventional insect cell lines or larvae.
Structural analyses lead to more engineering
These extremely exciting results led us to perform detailed mass spectroscopic analyses to more precisely determine the structures of the N-glycans produced by Sfβ4GalT/ST6 cells (Hollister et al., 2002). The results revealed that these cells actually produced monoantennary structures in which only the lower (β3) branch was elongated (Fig. 1). The same results were obtained when we analyzed the N-glycans on another recombinant glycoprotein produced by Tn-5B1-4 cells infected with AcP (+)IE1GalT (Ailor et al., 2000) and on total N-glycans isolated from Sf9 cells infected with this same virus (Wolff et al., 1999). In the context of our efforts to humanize glycoprotein processing pathways in the baculovirus-insect cell system, these results were extremely disappointing because there are no monoantennary N-glycans on native mammalian glycoproteins. Fortunately, a previous study had shown that lepidopteran insect cell lines have extremely low levels of endogenous N-acetylglucosaminyltransferase II (GlcNAc-TII) activity (Altmann et al., 1993), which is the enzyme responsible for initiating elongation of the upper (β6) branch (Bendiak and Schachter, 1987). Thus, there appeared to be an obvious explanation for the inability of our cell lines to produce biantennary N-glycans.
We recently tested this hypothesis by genetically transforming Sf9 cells with expression plasmids encoding five mammalian glycosyltransferases, including GlcNAc-TII, to produce a transgenic insect cell line designated SfSWT-1 (Hollister et al., 2002). Similar to their progenitors, SfSWT-1 cells have normal growth properties and can support baculovirus infection. These cells also constitutively express RNAs from all five glycosyltransferase genes and have high levels of β4GalT, ST6GalI, and GlcNAc-TII activities. We have not yet measured the activities encoded by the two other mammalian transgenes, N-acetylglucosaminyltransferase I (GlcNAc-TI) and β2,3-sialyltransferase (ST3GalIV), expressed by these cells. However, we have used conventional baculovirus vectors to produce two different recombinant glycoproteins and have analyzed their N-glycans by HPLC and mass spectroscopy (Hollister et al., 2002). These detailed structural analyses revealed that both products had biantennary, terminally monosialylated N-glycans. These results demonstrated that induction of GlcNAc-TII, β4GalT, and ST6Gall activities is necessary and sufficient for the production of humanized N-glycans by baculovirus-infected Sf9 cells. A parallel study done in collaboration with Y.-C. Lee and M. Betenbaugh at Johns Hopkins University demonstrated that induction of GlcNAc-TII activity is necessary and sufficient for biantennary N-glycan production in T. ni cells, as well (Tomiya et al., 2003).
Where does the sialic acid come from?
The ability of our transgenic insect cell lines to produce sialylated glycoproteins was surprising because it has been shown that Sf9 cells have no detectable CMP-sialic acid, which is the donor substrate required by mammalian sialyltransferases (Hooker et al., 1999; Tomiya et al., 2001). We had engineered these new cell lines to produce mammalian glycosyltransferases, but had made no attempt to engineer these cells to produce CMP-sialic acid or transport it into the Golgi apparatus. Thus, our results raised a compelling question: how can transgenic insect cells, with no obvious source of CMP-sialic acid, sialylate newly synthesized glycoproteins? We knew that one requirement was the intracellular sialyltransferase activity encoded by a mammalian transgene in these cells because Sfβ4GalT cells could not produce sialylated N-glycans. Recently, we learned that another requirement for glycoprotein sialylation by Sfβ4GalT/ST6 and SfSWT-1 cells is that they have to be cultured in a growth medium containing fetal bovine serum (Hollister et al., in press). Interestingly, serum-free media supplemented with extensively dialyzed fetal bovine serum (50,000 MWCO) also supported glycoprotein sialylation by these cell lines. Furthermore, we found that the serum requirement could be met by culturing these cells in a serum-free medium supplemented with a purified mammalian sialoglycoprotein, but not its desialylated counterpart. These new results indicated that lepidopteran insect cells can salvage terminal sialic acids from extracellular sialoglycoproteins and convert them to a form, presumably CMP-sialic acid, that can be utilized by the intracellular sialyltransferase in our transgenic cell lines.
Engineering insect cells with bits of two mammalian biosynthetic pathways
The salvaging mechanism proposed above would be an unusual and interesting way for insect cells to acquire sialic acids from extracellular sources and we are working to elucidate the details of this pathway and determine if this working hypothesis is correct. Meanwhile, we began working to address the practical impact of the exogenous sialoglycoprotein requirement, which was that our existing transgenic insect cell lines could not sialylate recombinant glycoproteins when cultured in serum-free media. This imposed a significant limitation on the use of these cells for recombinant glycoprotein production because the use of fetal bovine serum in cell growth media raises safety and regulatory issues and complicates efforts to recover and purify the end products. In addition, we considered that a putative salvaging pathway might be a relatively inefficient way for these cells to obtain sialic acids, which might limit their efficiency of recombinant glycoprotein sialylation. It was recently shown that Sf9 cells can produce large amounts of CMP-sialic acid when infected with conventional baculovirus vectors encoding human sialic acid synthase (SAS) and CMP-sialic acid synthetase (CMP-SAS) and cultured in a serum-free growth medium supplemented with N-acetylmannosamine (Lawrence et al., 2001). These results were no surprise, as virtually all recombinant enzymes produced in the baculovirus-insect cell system have had the expected activities (reviewed by Jarvis, 1997; Luckow and Summers, 1988; O’Reilly et al., 1992). In addition, these results supported the idea that transformation of SfSWT-1 cells with mammalian genes encoding these two enzymes would yield a new transgenic insect cell line that can produce sialylated recombinant glycoproteins when cultured in serum-free media supplemented with N-acetylmannosamine.
This prediction was upheld in our most recent genetic engineering efforts, which yielded a new transgenic insect cell line designated SfSWT-3 (Aumiller et al., in press). SfSWT-3 cells encode and constitutively express all five of the mammalian glycosyltransferase genes found in Sf-SWT-1 cells, plus murine SAS (Nakata et al., 2000) and CMP-SAS (Munster et al., 1998) genes, under hr5-ie1 control. SfSWT-3 cells have normal morphology and normal growth properties and support baculovirus replication as well as Sf9 cells. However, unlike any other transgenic insect cell line, SfSWT-3 cells can produce CMP-sialic acid and sialylate a recombinant glycoprotein when cultured in a serum-free medium supplemented with N-acetylmannosamine. Furthermore, SfSWT-3 cells grown under these conditions can sialylate this recombinant glycoprotein, a glutathione-S-transferase-tagged soluble Sf9 β1,2-mannosidase (GST-SfManI; Kawar et al., 1997, 2000), more efficiently and extensively than SfSWT-1 cells grown in serum. GST-SfManI acquires roughly equal amounts of two processed N-glycans when produced in SfSWT-1 cells cultured in the presence of serum. One is an unsialylated, terminally galactosylated biantennary structure and the other is its monosialylated counterpart. However, this same glycoprotein acquires a single major processed N-glycan, which is the monosialylated, biantennary structure, and very minor amounts of an N-glycan that appears to be a disialylated, biantennary structure, when produced in SfSWT-3 cells cultured in a serum-free medium supplemented with N-acetylmannosamine. Thus, SfSWT-3 cells have the most efficient and extensive N-glycan processing pathway of any transgenic insect cell line described to date and should be widely useful as an improved host for baculovirus-mediated recombinant glycoprotein production.
Summary
Historically, the inability to produce authentic mammalian glycans has been one of the most significant limitations of baculovirus-insect expression systems. However, we have successfully addressed this limitation by genetically transforming established lepidopteran insect cell lines with constitutively expressible mammalian genes. This approach has yielded transgenic insect cell lines with normal growth properties that can support baculovirus infection, have new N-glycan processing enzyme activities, and can produce humanized recombinant glycoproteins. These cells require an extracellular sialoglycoprotein for de novo glycoprotein sialylation, which provided the first evidence that these cells have an interesting sialic acid salvaging pathway. This requirement prompted us to introduce genes for de novo CMP-sialic acid production into a transgenic insect cell line with a large repertoire of mammalian glycosyltransferase genes. This led to the creation of a transgenic insect cell line that can very efficiently sialylate recombinant glycoproteins in the absence of exogenous sialoglycoproteins. The development of transgenic insect cell lines for use as improved hosts for conventional recombinant baculovirus expression vectors also has been accompanied by the development of novel recombinant baculoviruses, which can be used as improved vectors with conventional insect or insect cell hosts. Efforts to improve the host cells have clearly outpaced efforts to improve the viral vectors. However, these efforts have revealed the requirements for recombinant glycoprotein sialylation by insect cells cultured under various conditions, which will expedite future efforts to develop baculoviral vectors for humanized glycoprotein production.
Acknowledgments
I gratefully acknowledge the financial support of the National Institutes of Health (GM49734), the National Science Foundation (BES-9814157 and BES-9818001), and the support of colleagues who have generously provided cDNA clones for our work (Jim Paulson, Harry Schachter, Joel Shaper, Nancy Shaper, Pamela Stanley, and Shuichi Tsuji). I also thank Invitrogen Corporation and HyClone Corporation for providing supplies and media and for supporting professional travel for a graduate student in our group, Jason Hollister.
Note added in proof. Two recently published studies on the endogenous N-glycosylation capabilities of lepidopteran insect cells are highly relevant to this minireview. In one of these studies, it was reported that Tn-4s, a variant of the Tn-4h (Trichoplusia ni) cell line originally described by Joshi et al., (2001), terminally sialylated about 20% of the N-glyans on human secreted alkaline phosphatase expressed during infection with a baculovirus vector (Joosten and Shuler, 2003). These results support and extend the previous report that Tn-4h cells sialylated human secreted alkaline phosphatase when cultured under certain conditions, as discussed in the minireview. In the other recently published study, it was reported that another new lepidopteran insect cell line, DpN1, which is derived from the monarch butterfly, terminally sialylated about 13% of the N-glycans on human secreted alkaline phosphatase produced during infection with a baculovirus vector (Palomares et al., 2003). Together, these studies add to the growing body of evidence that at least some insect cells encode the machinery needed to produce complex, terminally sialylated N-glycans and that this machinery functions under at least some conditions. Thus, while this minireview focused on genetically engineered systems, it seems increasingly likely that some native, non-engineered baculovirus–insect cell systems could be useful for humanized recombinant glycoprotein production, as well.
References
- Ailor E, Takahashi N, Tsukamoto Y, Masuda K, Rahman BA, Jarvis DL, Lee YC, Betenbaugh MJ. N-glycan patterns of human transferrin produced in trichoplusia ni insect cells: effects of mammalian galactosyltransferase. Glycobiology. 2000;10:837–847. doi: 10.1093/glycob/10.8.837. [DOI] [PubMed] [Google Scholar]
- Altmann F, Fabini G, Ahorn H, Wilson IB. Genetic model organisms in the study of N-glycans. Biochimie. 2001;83:703–712. doi: 10.1016/s0300-9084(01)01297-4. [DOI] [PubMed] [Google Scholar]
- Altmann F, Kornfeld G, Dalik T, Staudacher E, Glossl J. Processing of asparagine-linked oligosaccharides in insect cells. N-acetylglucosaminyltransferase I and II activities in cultured lepidopteran cells. Glycobiology. 1993;3:619–625. doi: 10.1093/glycob/3.6.619. [DOI] [PubMed] [Google Scholar]
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj. J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Jarvis DL. Expression and functional characterization of a nucleotide sugar transporter from Drosophila melanogaster: relevance to protein glycosylation in insect cell expression systems. Protein Expr. Purif. 2002;26:438–448. doi: 10.1016/s1046-5928(02)00550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumiller JJ, Hollister JR, Jarvis DL. A transgenic insect cell line engineered to produce CMP-sialic acid and sialylated glycoproteins. Glycobiology. 2003 doi: 10.1093/glycob/cwg051. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiak B, Schachter H. Control of glycoprotein synthesis. Kinetic mechanism, substrate specificity, and inhibition characteristics of UDP-N-acetylglucosamine: alpha-d-mannoside beta 1–2-N-acetylglucosaminyltransferase II from rat liver. J. Biol. Chem. 1987;262:5784–5790. [PubMed] [Google Scholar]
- Breitbach K, Jarvis DL. Improved glycosylation of a foreign protein by Tn-5B1-4 cells engineered to express mammalian glycosyltransferases. Biotechnol. Bioeng. 2001;74:230–239. doi: 10.1002/bit.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson DJ, Fraser MJ, Castellino FJ. Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry. 1990;29:5584–5590. doi: 10.1021/bi00475a024. [DOI] [PubMed] [Google Scholar]
- Davis TR, Wood HA. Intrinsic glycosylation potentials of insect cell cultures and insect larvae. In Vitro Cell. Dev. Biol. Anim. 1995;31:659–663. doi: 10.1007/BF02634086. [DOI] [PubMed] [Google Scholar]
- Farkas R, Medvedova L, Mechler BM. Cloning of Drosophila beta-galactoside alpha-2,6-sialyltransferase. GenBank. 1999 Accession No. AF218237. [Google Scholar]
- Francis BR, Paquin L, Weinkauf C, Jarvis DL. Biosynthesis and processing of Spodoptera frugiperda alpha-mannosidase III. Glycobiology. 2002;12:369–377. doi: 10.1093/glycob/12.6.369. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Wong R, Teh NG, Tropea JE, East-Palmer J, Weintraub BD, Szkudlinski MW. Expression of biologically active human thyrotropin (hTSH) in a baculovirus system: effect of insect cell glycosylation on hTSH activity in vitro and in vivo. Endocrinology. 1997;138:92–100. doi: 10.1210/endo.138.1.4897. [DOI] [PubMed] [Google Scholar]
- Guarino LA, Gonzalez MA, Summers MD. Complete sequence and enhancer function of the homologous DNA regions of Autographa californica nuclear polyhedrosis virus. J. Virol. 1986;60:224–229. doi: 10.1128/jvi.60.1.224-229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino LA, Summers MD. Nucleotide sequence and temporal expression of a baculovirus regulatory gene. J. Virol. 1987;61:2091–2099. doi: 10.1128/jvi.61.7.2091-2099.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister J, Conradt H, Jarvis DL. Evidence for a sialic acid salvaging pathway in lepidopteran insect cells. Glycobiology. 2003 doi: 10.1093/glycob/cwg053. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister J, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian β1,4-galactosyltransferase and alpha 2,6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Grabenhorst E, Nimtz M, Conradt HO, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister JR, Shaper JH, Jarvis DL. Stable expression of mammalian beta 1,4-galactosyltransferase extends the N-glycosylation pathway in insect cells. Glycobiology. 1998;8:473–480. doi: 10.1093/glycob/8.5.473. [DOI] [PubMed] [Google Scholar]
- Hooker AD, Green NH, Baines AJ, Bull AT, Jenkins N, Strange PG, James DC. Constraints on the transport and glycosylation of recombinant IFN-gamma in Chinese hamster ovary and insect cells. Biotechnol. Bioeng. 1999;63:559–572. doi: 10.1002/(sici)1097-0290(19990605)63:5<559::aid-bit6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Hsieh P, Robbins PW. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J. Biol. Chem. 1984;259:2375–2382. [PubMed] [Google Scholar]
- Jarvis DL. Baculovirus expression vectors. In: Miller LK, editor. The Baculoviruses. New York: Plenum Press; 1997. pp. 389–431. [Google Scholar]
- Jarvis DL, Bohlmeyer DA, Liao YF, Lomax KK, Merkle RK, Weinkauf C, Moremen KW. Isolation and characterization of a class II alpha-mannosidase cDNA from lepidopteran insect cells. Glycobiology. 1997;7:113–127. doi: 10.1093/glycob/7.1.113. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Finn EE. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–511. doi: 10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Finn EE. Modifying the insect cell N-glycosylation pathway with immediate early baculovirus expression vectors. Nat. Biotech. 1996;14:1288–1292. doi: 10.1038/nbt1096-1288. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Fleming JA, Kovacs GR, Summers MD, Guarino LA. Use of early baculovirus promoters for continuous expression and efficient processing of foreign gene products in stably transformed lepidopteran cells. Biotechnology. 1990;8:950–955. doi: 10.1038/nbt1090-950. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Howe D, Aumiller JJ. Novel baculovirus expression vectors that provide sialylation of recombinant glycoproteins in lepidopteran insect cells. J. Virol. 2001;75:6223–6227. doi: 10.1128/JVI.75.13.6223-6227.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis DL, Kawar ZS, Hollister JR. Engineering N-glycosylation pathways in the baculovirus-insect cell system. Curr. Opin. Biotech. 1998;9:528–533. doi: 10.1016/s0958-1669(98)80041-4. [DOI] [PubMed] [Google Scholar]
- Jarvis DL, Weinkauf C, Guarino LA. Immediate early baculovirus vectors for foreign gene expression in transformed or infected insect cells. Protein Expr. Purif. 1996;8:191–203. doi: 10.1006/prep.1996.0092. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Production of a sialylated N-linked glycoprotein in insect cells: Role of glycosidases and effect of harvest time on glycosylation. Biotechnol. Progr. 2003;19:193–201. doi: 10.1021/bp025695h. [DOI] [PubMed] [Google Scholar]
- Joshi L, Shuler ML, Wood HA. Production of a sialylated N-linked glycoprotein in insect cells. Biotech. Progr. 2001;17:822–827. doi: 10.1021/bp010071h. [DOI] [PubMed] [Google Scholar]
- Kawar Z, Herscovics A, Jarvis DL. Isolation and characterization of an alpha 1,2-mannosidase cDNA from the lepidopteran insect cell line Sf9. Glycobiology. 1997;7:433–443. doi: 10.1093/glycob/7.3.433. [DOI] [PubMed] [Google Scholar]
- Kawar Z, Romero PA, Herscovics A, Jarvis DL. N-glycan processing by a lepidopteran insect alpha 1,2-mannosidase. Glycobiology. 2000;10:347–355. doi: 10.1093/glycob/10.4.347. [DOI] [PubMed] [Google Scholar]
- Kawar ZS, Jarvis DL. Biosynthesis and intracellular localization of a lepidopteran insect alpha 1,2-mannosidase. Insect Biochem. Mol. Biol. 2001;31:289–297. doi: 10.1016/s0965-1748(00)00121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawar ZS, Moremen KW, Jarvis DL. Insect cells encode a class II alpha-mannosidase with unique properties. J. Biol. Chem. 2001;276:16335–16340. doi: 10.1074/jbc.M100119200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lawrence SM, Park J, Pitts L, Vann WF, Betenbaugh MJ, Palter KB. Expression of a functional Drosophila melanogaster N-acetylneuraminic acid (Neu5Ac) phosphate synthase gene: evidence for endogenous sialic acid biosynthetic ability in insects. Glycobiology. 2002;12:73–83. doi: 10.1093/glycob/12.2.73. [DOI] [PubMed] [Google Scholar]
- Lawrence SM, Huddleston KA, Tomiya N, Nguyen N, Lee YC, Vann WF, Coleman TA, Betenbaugh MJ. Cloning and expression of human sialic acid pathway genes to generate CMP-sialic acids in insect cells. Glycoconj. J. 2001;18:205–213. doi: 10.1023/a:1012452705349. [DOI] [PubMed] [Google Scholar]
- Luckow VL, Summers MD. Trends in the development of baculovirus expression vectors. Biotechnology. 1988;6:47–55. [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol. Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, Schachter H, editors. Glycoproteins. Vol. 29a. Amsterdam: Elsevier; 1995. pp. 543–563. [Google Scholar]
- Munster AK, Eckhardt M, Potvin B, Muhlenhoff M, Stanley P, Gerardy-Schahn R. Mammalian cytidine 5′-monophosphate N-acetylneuraminic acid synthetase: a nuclear protein with evolutionarily conserved structural motifs. Proc. Natl. Acad. Sci. USA. 1998;95:9140–9145. doi: 10.1073/pnas.95.16.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata D, Close BE, Colley KJ, Matsuda T, Kitajima K. Molecular cloning and expression of the mouse N-acetylneuraminic acid 9-phosphate synthase which does not have deaminoneuraminic acid (KDN) 9-phosphate synthase activity. Biochem. Biophys. Res. Commun. 2000;273:642–648. doi: 10.1006/bbrc.2000.2983. [DOI] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, Luckow VA. Baculovirus Expression Vectors. New York: W.H. Freeman and Company; 1992. [Google Scholar]
- Palomares LA, Joosten CE, Hughes PR, Granados RR, Shuler ML. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol. Progr. 2003;19:185–192. doi: 10.1021/bp025598o. [DOI] [PubMed] [Google Scholar]
- Raju TS, Lerner L, O’Connor JV. Glycopinion: biological significance and methods for the analysis of complex carbohydrates of recombinant glycoproteins. Biotechnol. Appl. Biochem. 1996;24:191–194. [PubMed] [Google Scholar]
- Roth J, Kempf A, Reuter G, Schauer R, Gehring W. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Sareneva T, Cantell K, Pyhala L, Pirhonen J, Julkunen I. Effect of carbohydrates on the pharmacokinetics of human interferon-gamma. J. Interferon Cytokine Res. 1993;13:267–269. doi: 10.1089/jir.1993.13.267. [DOI] [PubMed] [Google Scholar]
- Segawa H, Kawakita M, Ishida N. Human and Drosophila UDP-galactose transporters transport UDP-N-acetylgalactosamine in addition to UDP-galactose. Eur. J. Biochem. 2002;269:128–138. doi: 10.1046/j.0014-2956.2001.02632.x. [DOI] [PubMed] [Google Scholar]
- Seo NS, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Protein Expr. Purif. 2001;22:234–241. doi: 10.1006/prep.2001.1432. [DOI] [PubMed] [Google Scholar]
- Sridhar P, Panda AK, Pal R, Talwar GP, Hasnain SE. Temporal nature of the promoter and not relative strength determines the expression of an extensively processed protein in a baculovirus system. FEBS Lett. 1993;315:282–286. doi: 10.1016/0014-5793(93)81179-4. [DOI] [PubMed] [Google Scholar]
- Stollar V, Stollar BD, Koo R, Harrap KA, Schlesinger RW. Sialic acid contents of sindbis virus from vertebrate and mosquito cells. Equivalence of biological and immunological viral properties. Virology. 1976;69:104–115. doi: 10.1016/0042-6822(76)90198-7. [DOI] [PubMed] [Google Scholar]
- Summers MD, Smith GE. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex. Agric. Exp. Stn. Bull. No. 1555. 1987 [Google Scholar]
- Tomiya N, Ailor E, Lawrence SM, Betenbaugh MJ, Lee YC. Determination of nucleotides and sugar nucleotides involved in protein glycosylation by high-performance anion-exchange chromatography: sugar nucleotide contents in cultured insect cells and mammalian cells. Anal Biochem. 2001;293:129–137. doi: 10.1006/abio.2001.5091. [DOI] [PubMed] [Google Scholar]
- Tomiya N, Howe D, Aumiller JJ, Pathak M, Park J, Palter K, Jarvis DL, Betenbaugh MJ, Lee YC. Complex-type biantennary N-glycans of recombinant human transferrin from Trichoplusia ni insect cells expressing mammalian β1,4-galactosyltransferase and β1,2-N-acetylglucosaminyltransferase II. Glycobiology. 2003;13:23–34. doi: 10.1093/glycob/cwg012. [DOI] [PubMed] [Google Scholar]
- Vadaie N, Hulinsky R, Jarvis DL. Expression and functional characterization of Drosophila β4-galactosyltransferase VII. Glycobiology. 2002;12:589–597. doi: 10.1093/glycob/cwf074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kokuho T, Takahashi H, Takahashi M, Kubota T, Inumaru S. Sialylation of N-glycans on the recombinant proteins expressed by a baculovirus-insect cell system under β-N-acetylglucosaminidase inhibition. J. Biol. Chem. 2001;277:5090–5093. doi: 10.1074/jbc.M110548200. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Davis T, Granados RR, Shuler ML, Wood HA. Screening of insect cell lines for the production of recombinant proteins and infectious virus in the baculovirus expression system. Biotech. Progr. 1992;8:391–396. doi: 10.1021/bp00017a003. [DOI] [PubMed] [Google Scholar]
- Wolff MW, Murhammer DW, Jarvis DL, Linhardt RJ. Electrophoretic analysis of glycoprotein glycans produced by lepidopteran insect cells infected with an immediate early recombinant baculovirus encoding mammalian β1,4-galactosyltransferase. Glycoconj. J. 1999;16:753–756. doi: 10.1023/a:1007131611378. [DOI] [PubMed] [Google Scholar]