Abstract
Background
Based on the observation of topoisomerase-1, upregulation by mitomycin C (MMC), and the phase I antitumor activity of sequential MMC/irinotecan in esophageal cancer, we conducted a phase II evaluation of two schedules of this combination in previously untreated stage III/IV esophageal/gastroesophageal junction adenocarcinomas.
Patients and Methods
Patients (n = 76) were randomized to either 6 mg/m2 MMC on day 1 and 125 mg/m2 irinotecan on days 2 and 9 (arm A) or 3 mg/m2 MMC on days 1 and 8 and 125 mg/m2 irinotecan on days 2 and 9 (arm B). Each cycle was repeated every 28 days. Restaging was planned after two cycles, and resections were performed whenever possible. A two-stage Simon minimax design was used for each arm, with a “pick-the-winner” approach based on efficacy.
Results
The response rate (complete response + partial response) in 73 evaluable patients was 52% (21 of 40 patients) for arm A and 33% (11/33) for arm B. Moderate or severe toxicity was similar. Twenty-seven patients were resected (20:7, arm A:B). There was one complete pathologic response; five others were node negative.
Conclusion
Irinotecan/MMC is feasible in esophageal/gastro-esophageal junction adenocarcinoma. MMC (6 mg/m2) every 28 days for up to six cycles is the recommended modulatory dose for irinotecan in future trials.
Keywords: Gastroesophageal adenocarcinoma, Irinotecan, Mitomycin C, Topoisomerase I
Irinotecan has antitumor activity in many malignancies. Because topoisomerase I (Topo I) is the cellular target of irinotecan, its cellular level and activity may be proportional to the cytotoxic effects of irinotecan.1,2 Solid tumors have increased Topo I when compared with corresponding non-malignant tissue, and increased Topo I activity corresponds to increased sensitivity to Topo I interactive agents.3,4
The mechanisms of tumor resistance to irinotecan5 include mutations of the Topo I gene and stable decrements in Topo I activity.6,7 The exposure to irinotecan produces a rapid, transient decrease in Topo I concentration, which in cell cultures correlates with a decrease in irinotecan cytotoxicity.6,7
Because the increases in Topo I activity were reported in vitro after treatment with mitomycin C (MMC) with synergistic irinotecan cytoxicity,8 we previously conducted a phase I clinical trial evaluating MMC as a modulating agent at doses of 6 mg/m2 24 hours before irinotecan.9 Patients received MMC every 4 weeks with a cumulative dose-cap of 36 mg/m2. At these doses, we not only showed an acceptable toxicity profile at full doses of irinotecan and lack of pharmacokinetic interactions but also substantial antitumor activity in patients with treatment refractory malignancies, including esophageal and gastric cancers. An induction of Topo I expression in mononuclear cells was associated with response to the regimen.9
Because we previously demonstrated the upregulation of Topo I by MMC, we designed a randomized phase II clinical trial in patients with previously untreated advanced esophageal and gastroesophageal junction (GE) adenocarcinomas. To discern whether MMC at the modulatory dose (6 mg/m2 24 hours before irinotecan) has an effect on clinical activity or toxicity, patients were randomized to two dose schedules of MMC in combination with irinotecan, with the goal of selecting one of two schedules for further efficacy evaluation studies using a 2-stage pick the winner approach. The two dose schedules compared modulatory dose versus nonmodulatory dose of MMC, while keeping the total cumulative monthly dose of both MMC and irinotecan the same.
PATIENTS AND METHODS
Eligibility Criteria
Patients had histologically confirmed advanced esophageal or GE adenocarcinoma and were not candidates for surgical intervention at presentation, as assessed by a surgical oncologist. Squamous histology was excluded. Eligibility included (a) age ≥18 years, no pregnancy, or lactation; (b) Eastern Cooperative Oncology Group performance status of ≤2; (c) life expectancy ≥12 weeks; (d) no previous chemotherapy; (e) prior radiation was allowed if <20% of the bone marrow was irradiated and the target lesions were not in the radiation field; (f) no major surgeries within 28 days; (h) adequate organ function including absolute neutrophil count ≥1500/mm3, hemoglobin ≥9 g/dl, platelet count ≥100,000/mm3, serum creatinine <1.5 mg/dl or creatinine clearance >60 ml/min, serum bilirubin <1.5 mg/dl, transaminases <3 times upper normal limit; (i) no active neoplastic involvement of the nervous system; (j) no uncontrolled diabetes mellitus; (k) no history of myocardial infarction within 6 months, congestive heart failure requiring therapy, and unstable angina; (l) no coumadin treatment; (m) no coexisting medical or psychiatric disorders interfering with consent or follow-up; (n) quantifiable disease by either computed tomography (CT) or positron emission tomography (PET) was required. All patients provided informed consent.
Treatment Plan
A cycle consisted of 28 days. The MMC dose was either 6 mg/m2 (arm A) on day 1 or 3 mg/m2 on days 1 and 8 (arm B). The MMC dose was limited to a total of 36 mg/m2 (six cycles) and 125 mg/m2 irinotecan on days 2 and 9 (24 hours after MMC, when the MMC was required). Two weeks of rest completed the cycle. Ondansetron or granisetron and dexamethasone premedication were used. Loperamide was recommended at the earliest onset of diarrhea. Prophylactic use of colony-stimulating factors was not permitted. Use of erythropoietin for hemoglobin <10 g/dl was permitted.
Dose Modifications
Weekly cell counts, chemistries, and electrolytes were obtained. Within a cycle, doses were held for neutropenic fever and grade 3/4 hematologic and nonhematologic toxicities. For subsequent cycles, MMC was reduced by 25% for grade 4 hematologic toxicity, neutropenic fever, and grade 3/4 nonhematologic toxicities. Irinotecan was reduced by 25% for grade 4 nausea and grade 3 nonhematologic toxicities, and 50% for grades 4 nonhematologic toxicities.
Clinical Benefit Evaluation
The measurement of tumor size or tumor metabolic activity was performed before treatment and after every two cycles. Patients had baseline CT measurements, and 2-fluoro-2-deoxy-D glucose (FDG)-PET standard uptake values (SUV) evaluations were obtained in patients with GE tumors that were not measurable by CT at baseline. CT and PET responses were assessed based on RECIST 1.0 criteria10 and European Organization for Research and Treatment of Cancer (EORTC) criteria,11 respectively. The latter defines complete metabolic response as complete resolution of SUV within the tumor volume and progressive metabolic disease as an increase in SUV of >25% in target lesions or the appearance of new lesions. Partial metabolic response is an SUV reduction of >25% in target lesions after two cycles, and stable metabolic disease is an increase or decrease of <25%. Surgical resections were offered to patients who were suitable surgical candidates. Resected patients were referred for chemotherapy and radiation when appropriate. Criteria for study removal included documented or symptomatic disease progression, unacceptable toxicity that did not respond to dosage modifications, pregnancy, withdrawal of consent, treatment delay of >3 weeks, dose reduction of irinotecan to <25 mg/m2, hemolytic uremic syndrome, or evidence of pulmonary interstitial fibrosis.
Statistical Considerations
The primary objective was to select one of two regimens based on objective responses for evaluation in future efficacy trials. Patients were randomized using a stratified fixed block design, with stratification by locally advanced or metastatic disease. Each arm was treated as a parallel phase II study with an open-label, minimax two-stage design.12 The primary endpoint was objective response using RECIST or PET EORTC response criteria. Either regimen was considered ineffective if the true response probability was <30% (p0), because response rates in previously reported phase II trials of single agent irinotecan in stomach cancer approached this level. A regimen was worthy of further study if the true response probability was ≥50% (p1). A two-stage design of 28 and 39 patients, with an alpha error of 0.10 and beta error of 0.10 was obtained. If ≤7 patients demonstrated responses in the first 28 evaluable patients, the regimen was terminated early and deemed ineffective. If ≥8 patients showed responses in the first 28 patients, 11 additional patients were treated for a total of 39. If ≤15 of the 39 patients showed responses, the regimen was not recommended for further study.
RESULTS
Patient Population
Between July 2002 and May 2006, 82 patients were consented. Among them 77 were treated (Table 1). The reasons for no treatment included consent withdrawal before treatment, screening eligibility failure (one in each), and three patients with declining performance status or rapidly progressive disease (PD) to whom treatment was not offered. Forty-three patients had lower esophageal and 33 GE adenocarcinomas; one patient had fundus stomach cancer. The distribution of stages at baseline was 24 stage III, 24 stage IV-A, and 29 stage IV-B. CT scan was completed for baseline staging in all patients, whereas baseline PET was obtained in 50 patients (65%) and endoscopic ultrasound in 11 patients (14%).
TABLE 1.
Patient Characteristics
| Arm A (n = 42) | Arm B (n = 35a) | |
|---|---|---|
| Age (yr), mean ± SE | 58.9 ± 1.6 | 61.7 ± 2.2 |
| Gender, male:female | 39:3 | 33:2 |
| ECOG Score, N (%) | ||
| 0 | 21 (50) | 15 (43) |
| 1 | 17 (40) | 19 (54) |
| 2 | 4 (10) | 1 (3) |
| Stage, N (%) | ||
| III | 17 (40) | 7 (20) |
| IV-A | 14 (33) | 10 (29) |
| IV-B | 11 (26) | 18 (51) |
| Metastatic sites, N (%) | ||
| Lung | 3 (7) | 2 (6) |
| Liver | 8 (19) | 15 (43) |
| Bone | 1 (2) | 1 (3) |
| Lymph nodes | 20 (48) | 26 (75) |
One patient in arm B was ruled ineligible because of lack of quantifiable disease. ECOG, Eastern Cooperative Oncology Group.
The Data Safety Monitoring Board recommended stopping enrollment to arm B when it became clear that the prespecified response rate would not be met. Patients consented, but not treated, before halting arm B were allowed treatment on arm A. Overall, 42 patients received treatment on arm A and 35 on arm B. One patient on arm B was later determined to be ineligible because of lack of quantifiable disease by CT or PET.
Treatment Summary
The distribution of the number of cycles was as follows: 10 one cycle, 47 two cycles, 5 three cycles, 6 four cycles, 3 five cycles, and 5 six cycles. There were no significant differences in the number of cycles by treatment group. The reasons for discontinuing treatment after one cycle included PD (three patients), toxicity (four patients), death (two patients), and one early surgery. The reasons for discontinuation after two cycles included surgery (22 patients), tumor progression (17 patients), referral for concurrent chemotherapy and radiation (5 patients), or radiation (2 patients), and toxicity (1 patient).
Toxicity
Cumulative incidence of toxicities was similar for both arms (Table 2). A total of 22 patients (52%) in arm A and 16 patients (47%) in arm B needed dose reductions. Six patients in arm A and five patients in arm B needed reductions for both drugs. There was no evidence of chronic myelosuppression, hemolytic uremic syndrome, or pulmonary interstitial fibrosis. Six patients died because of reasons other than tumor progression. The reasons for fatality included: thrombosis in two patients, sudden cardiac arrest of unknown etiology in two patients, and neutropenic sepsis in one patient. One patient was transitioned to hospice after complications of gastrointestinal bleeding. There were no cases of intraoperative mortality.
TABLE 2.
Adverse Events
| Arm A
|
Arm B
|
|||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hematologic toxicity, n (%) | ||||
| Leukopenia | 3 (7) | 4 (10) | 4 (12) | 1 (3) |
| Anemia | 5 (12) | 0 | 1 (3) | 0 |
| Thrombocytopenia | 1 (2) | 0 | 0 | 0 |
| Neutropenia | 3 (7) | 6 (14) | 3 (9) | 5 (15) |
| Neutropenic fever | 4 (10) | 2 (5) | 3 (9) | 2 (6) |
| Nonhematologic toxicity, n (%) | ||||
| Fatigue | 2 (5) | 0 | 5 (15) | 0 |
| Nausea/vomiting | 7 (17) | 3 (7) | 6 (18) | 0 |
| Diarrhea | 9 (21) | 6 (14) | 4 (12) | 5 (15) |
| Dehydration | 8 (19) | 3 (7) | 6 (18) | 4 (12) |
| Sepsis | 0 | 1 (2)a | 0 | 0 |
| Anorexia/weight loss | 2 (5) | 0 | 3 (9) | 1 (3) |
| Thrombosis | 0 | 3 (7)b | 1 (3) | 1 (3) |
| Cardiac | 1 (2) | 1 (2)c | 0 | 1 (3)c |
| Acute renal failure | 5 (12) | 0 | 0 | 1 (3) |
| Bleeding | 0 | 1 (2)a | 0 | 0 |
Includes one patient each with grade 5 toxicity.
Includes two patients with grade 5 toxicity.
Includes one patient with sudden death in each arm.
Efficacy
Antitumor activity is depicted in Table 3. Of the 76 eligible patients receiving at least one cycle of the combination treatment, 70 patients (92%) had repeat tumor measurements after two cycles. Three patients died before repeat imaging and were assigned as PD. Two patients were considered response nonevaluable because of removal from the study after one cycle because of toxicity without progression. An additional patient was considered to be nonevaluable as he withdrew consent and went to surgery after the first dose of irinotecan.
TABLE 3.
Antitumor Activitya
| Arm A, n (%) | Arm B, n (%) | |
|---|---|---|
| Overall response rates (n = 76)b | ||
| CR | 2/40 (5) | 1/33 (3) |
| PR | 19/40 (47)c | 10/33 (30)c |
| SD | 12/40 (30) | 9/33 (27) |
| PD | 7/40 (18) | 13/33 (39) |
| Not evaluable | 2 | 1 |
Antitumor activity assessed by PET EORTC criteria when available and by CT RECIST criteria 1.0 when no PET was available.
Six patients did not get repeat imaging: Three patients (one in Arm A and two in Arm B) died before repeat imaging and were assigned as PD. One patient in Arm A went to surgery during cycle 1, and one patient was taken off trial for toxicity without progression. In Arm B, one patient was removed for toxicity without progression.
95% confidence interval for overall response (CR + PR) 36–68% and 18–52% for Arm A and B, respectively.
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; PET, positron emission tomography; EORTC, European Organization for Research and Treatment of Cancer.
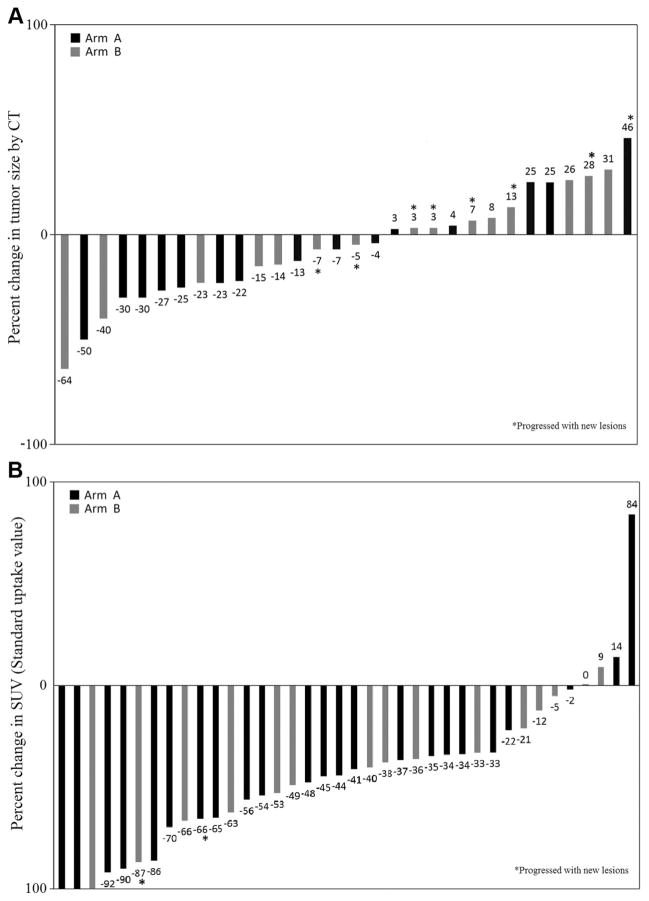
Forty patients had baseline and follow-up PET imaging to assess best overall response (complete response [CR] and partial response), whereas 30 patients had response assessment only by CT. Figures 1A, B depict waterfall plots of changes in the tumor size and metabolic activity, respectively. The best overall response rates were 52% (95% confidence interval 36–68) for arm A and 33% (95% confidence interval 18–52) for arm B (respectively, 50% and 33% by intent-to-treat). Overall median progression free survival was 155 days for arm A and 95 days for arm B.
FIGURE 1.
A, Plot of percentage change in computed tomography (CT) tumor size by arm. Individual percentage change in tumor size by CT scan according to the treatment arm (n = 30). B, Plot of percentage change in standard uptake values (SUV) by arm. Individual percentage change in tumor SUV by positron emission tomography according to the treatment arm (n = 38). Two additional patients with progressive disease because of new metabolic lesions were not included.
Twenty-eight patients (37%) were deemed surgical candidates at reassessment, and 27 of them were successfully resected (20 in arm A and 7 in arm B). One patient in arm B went to surgery and was not resectable because of peritoneal implants. The median number of cycles before surgery was 2 (range 1–6). Pathologic staging in these patients is depicted in Table 4. One patient (arm A) had a complete pathologic response. Five other patients had node negative disease. Among the patients with PET CR, two went to surgery and both had pathologic stage T3N1. The other was not a surgical candidate because of medical comorbidities. Fifteen patients were referred for chemoradiation, and two patients were referred for radiation alone.
TABLE 4.
Pathologic Responsesa
| Arm A | Arm B | |
|---|---|---|
| Total N | 20 | 8 |
| Complete pathologic response—T0N0 | 1 (5) | 0 |
| Stage I—T1N0 | 2 (10) | 0 |
| Stage IIA | ||
| T2N0 | 0 | 2 (25) |
| T3N0 | 1 (5) | 0 |
| Stage IIB | ||
| T1N1 | 1 (5) | 0 |
| T2N1 | 4 (20) | 0 |
| Stage III | ||
| T3N1 | 11 (55) | 5 (62) |
| Stage IV | 0 | 1 (13) |
Surgical approach: thoracotomy, 13 patients; nonthoracotomy, 14 patients. The median number of nodes resected was 12. One patient was unresectable.
DISCUSSION
On the basis of preclinical rationale that upregulation of Topo I by MMC would increase the antitumor activity of irinotecan, and demonstration of encouraging antitumor activity in refractory esophageal/gastric cancer patients in a phase I trial,9 we conducted this phase II trial in advanced esophageal and GE adenocarcinoma patients. The activity of modulatory doses of MMC in combination with standard doses of irinotecan in two schedules was evaluated. The randomized nature of the study permitted selection of the most suitable schedule for future trials.
The incidence of esophageal cancer is increasing in the United States and Europe with most of the increase because of adenocarcinomas, which now accounts for more than half of newly diagnosed cases.13 Surgical resection is the optimal treatment option but the majority of patients are not candidates for resection at the time of presentation because of locally advanced or metastatic disease. Neoadjuvant chemotherapy and radiation have been investigated to increase the number of patients amenable to resection and ultimately improve overall survival.
The combination of cisplatin (Cis) and 5-fluoruracil (5-FU) has been the most commonly investigated neoadjuvant regimen with variable results across trials. The North American Intergroup Trial failed to see a survival advantage in the preoperative group with Cis/5-FU.14 In the Medical Research Counsel trial, however, the chemotherapy-treated group had improved survival and increased R0 resections.15 The differences in outcomes have been attributed to larger sample size and higher proportion of adenocarcinomas in the Medical Research Counsel trial compared with the Intergroup trial. Additional trials have shown promising results with Cis/5-FU16 and with epirubicin combined with Cis/5Fu.17
The rates of pathologic CR remain low with existing neoadjuvant chemotherapy regimens. The combination of radiation with Cis/5-FU-based chemotherapy has proven survival advantage to radiation therapy alone in unresectable disease.18 When preoperative chemoradiation is compared with surgery alone, improved local control is reported but improvements in survival have not been consistently demonstrated.19–21 Investigation of additional systemic strategies is clearly needed. To that regard, Radiation Therapy Oncology Group (RTOG) 0113 explored 2 taxane-containing regimens followed by conventional chemoradiation.22 However, both regimens failed to meet the prospectively set 1-year median survival endpoint.
In this study, patients with previously untreated advanced esophageal and GE-junction adenocarcinomas were randomized to two schedules of the combination of MMC and irinotecan. Although caution must be exercised to not perform inferential statistical comparisons in a randomized phase II trial,23 toxicities were similar between the two arms, and only arm A met the prespecified response criteria (≥50%). Treatment did not interfere with resection and possibly increased the number of resectable patients. In contrast to many neoadjuvant trials, patients were ineligible for resection before therapy. Of the 28 patients taken to surgery, 19 (68%) were down staged, including all patients with stage IVa disease. One patient had a complete pathologic response. Patients who had node positive disease at surgery were referred for chemotherapy and radiation.
The toxicity of the combination regimen of MMC and irinotecan is comparable with other neoadjuvant regimens. The most common ≥3 toxicities reported in the Intergroup trial with Cis/5-FU were neutropenia (29%) and mucositis (25%).14 Similarly, in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial, ≥3 neutropenia occurred in 24% of patients.17
We can only speculate why one schedule (arm A) performed better than the other (arm B). The randomization prevented investigators’ bias in patient selection as a factor, but an imbalance occurred between the arms in terms of stage IVB disease. This imbalance could account for differences in survival and even progression-free survival, but less so for antitumor response after two cycles. It is possible that MMC may have independent antitumor activity, instead of modulatory activity. However, a dose-dependent effect for a single-agent treatment would be expected to be higher than 3 mg/m2, as the total dose per cycle was the same.
A significant challenge in GE tumors is the ability to assess response in locally advanced disease. The assessment of metabolic activity by PET has been increasingly used by surgeons and medical oncologists. This study was specifically designed to use PET scans for response assessments in those patient whose tumors could not be measured by CT scan alone, expanding patient eligibility for response evaluation. The arbitrary cutoff of 25% change in metabolic activity to define response or progression was chosen based on the EORTC criteria although other cutoff values have been used in other studies.11 As shown by others,24,25 FDG PET was a reasonable tool for assessment of response and respectability but did not have complete correlation with surgical pathology. That is, the two patients with complete metabolic responses had node positive disease at the time of surgery. Both progression-free and overall survival were associated with PET SUV changes (Figure 2).
FIGURE 2.
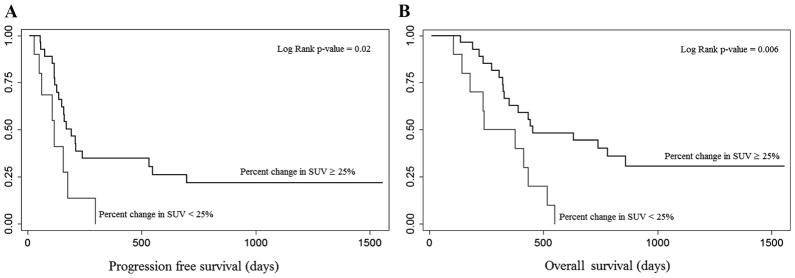
A, Kaplan-Meier plots of progression free survival by percentage change in standard uptake values (SUV). Kaplan-Meier plots showing progression-free survival according to percent decreases in SUV >25% and <25%. There is a statistically significant difference between patients with and without a metabolic response (P = 0.02). B, Kaplan-Meier plots of overall survival by percentage change in SUV. Kaplan-Meier plots showing overall survival according to percent decreases in SUV >25% and <25%. There is a statistically significant difference between patients with and without a metabolic response (P = 0.006).
To define this regimen’s role in esophageal/GE tumors, combination with radiation or evaluation in a sequential fashion with platinum based therapy and radiation will be necessary. In fact, most patients with node positive disease at surgery went on to receive Cis/5FU with radiation in the adjuvant setting with good tolerability. It would be of interest to see whether a greater local control can be achieved before surgery with either strategy.
In conclusion, the results of this trial show that low dose MMC in combination with irinotecan can be safely given to patients with esophageal and GE adenocarcinomas. Single dose of MMC was more efficacious than split dosing. The antitumor activity observed encourages future clinical trials using the recommended schedule of 6 mg/m2 MMC on day 1 and irinotecan 125 mg/m2 on days 2 and 9, every 4 weeks. However, in the setting of locally advanced disease, based on low number of complete pathologic responses, the combination with radiation or a sequential use of this regimen with Cis/5FU and radiation would be a more reasonable schedule for future exploration of this regimen.
Acknowledgments
Supported by NCI R21CA92956 to MAV and P30 CA16059 to The Ohio State University Comprehensive Cancer Center.
Footnotes
Disclosure: Tanios Bekaii-Saab, MD, is a paid consultant and is on the speaker bureau for Pfizer, the manufacturer of Irinotecan. The other authors declare no conflicts of interest.
References
- 1.Kanzawa F, Sugimoto Y, Minato K, et al. Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res. 1990;50:5919–5924. [PubMed] [Google Scholar]
- 2.Reid RJ, Benedetti P, Bjornsti MA. Yeast as a model organism for studying the actions of DNA topoisomerase-targeted drugs. Biochim Biophys Acta. 1998;1400:289–300. doi: 10.1016/s0167-4781(98)00142-0. [DOI] [PubMed] [Google Scholar]
- 3.Giovanella BC, Stehlin JS, Wall ME, et al. DNA topoisomerase I-targeted chemotherapy of human colon cancer in xenografts. Science. 1989;246:1046–1048. doi: 10.1126/science.2555920. [DOI] [PubMed] [Google Scholar]
- 4.Rubin EH. DNA topoisomerase expression in tumors—a novel target for chemotherapy. Hum Pathol. 2000;31:631–632. doi: 10.1053/hupa.2000.8629. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. 2002;13:1841–1851. doi: 10.1093/annonc/mdf337. [DOI] [PubMed] [Google Scholar]
- 6.Beidler DR, Cheng YC. Camptothecin induction of a time- and concentration-dependent decrease of topoisomerase I and its implication in camptothecin activity. Mol Pharmacol. 1995;47:907–914. [PubMed] [Google Scholar]
- 7.Murren JR, Beidler DR, Cheng YC. Camptothecin resistance related to drug-induced down-regulation of topoisomerase I and to steps occurring after the formation of protein-linked DNA breaks. Ann N Y Acad Sci. 1996;803:74–92. doi: 10.1111/j.1749-6632.1996.tb26378.x. [DOI] [PubMed] [Google Scholar]
- 8.Gobert C, Bracco L, Rossi F, et al. Modulation of DNA topoisomerase I activity by p53. Biochemistry. 1996;35:5778–5786. doi: 10.1021/bi952327w. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Kolesar JM, Schaaf LJ, et al. Phase I and pharmokinetic study of mitomycin C and celecoxib as potential modulators of tumor resistance to irinotecan in patients with solid malignancies. Cancer Chemother Phamacol. 2009;63:1073–1082. doi: 10.1007/s00280-008-0826-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 12.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 13.Devesa S, Blot W, Fraumeni J. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 14.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 15.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 16.Boige V, Pignon B, Saint-Aubert P, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil/cisplating to surgery alone in adeno-carcinoma of stomach and lower esophagus: FNLCC ACORD07-FFCD 9703 trial. J Clin Oncol, ASCO Annual Meeting Proc Part I. 2007;25:4510. [Google Scholar]
- 17.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 18.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 19.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–167. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 20.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 21.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajani JA, Winter K, Komaki R, et al. Phase II randomized trial of two nonoperative regimens of induction chemotherapy followed by chemo-radiation in patients with localized carcinoma of the esophagus: RTOG 0113. J Clin Oncol. 2008;26:4551–4556. doi: 10.1200/JCO.2008.16.6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu PY, LeBlanc M, Desai M. False positive rates of randomized phase II designs. Control Clin Trials. 1999;20:343–352. doi: 10.1016/s0197-2456(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 24.Downey RJ, Akhurst T, Ilson D, et al. Whole body 18FDG-PET and the response of esophageal cancer to induction therapy: results of a prospective trial. J Clin Oncol. 2003;21:428–432. doi: 10.1200/JCO.2003.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Rizk N, Downey RJ, Akhurst T, et al. Preoperative 18[F]-fluorodeoxy-glucose positron emission tomography standardized uptake values predict survival after esophageal adenocarcinoma resection. Ann Thorac Surg. 2006;81:1076–1081. doi: 10.1016/j.athoracsur.2005.09.063. [DOI] [PubMed] [Google Scholar]