Summary
High order chromatin was reconstituted in vitro. This species reflects the criteria associated with transcriptional regulation in vivo. Histone H1 was determinant to formation of condensed structures, with deacetylated histones giving rise to highly compacted chromatin that approximated 30-nm fibers as evidenced by electron microscopy. Using the model PEPCK promoter, we validated the integrity of these templates that were refractory to transcription by attaining transcription through the progressive action of the pertinent factors. The retinoic acid receptor binds to highly compacted chromatin, but the NF1 transcription factor binds only after histone acetylation by p300 and SWI/SNF-mediated nucleosome mobilization, reflecting the in vivo case, as evidenced by ChIP analyses. Mapping studies revealed the same pattern of nucleosomal repositioning on the PEPCK promoter in vitro and in vivo, correlating with NF1 binding and transcription. The reconstitution of such highly compacted “30-nm” chromatin that mimics in vivo characteristics should advance studies of its conversion to a transcriptionally active form, as well as the relevant function(s) of histone posttranslational modifications.
Introduction
The genomic DNA in eukaryotic organisms is hierarchically packaged into chromatin (Kornberg, 1977). The fundamental repeating unit of chromatin is the nucleosome comprising 147 base pairs of DNA wrapped 1.7 superhelical turns around an octamer of histone proteins (H2A, H2B, H3 and H4) (Richmond et al., 1984). The nucleosomes are connected by about 20-60 base pairs (bp) of linker DNA to form a “beads-on-a-string” fiber with a diameter of ∼11-nm (Luger, et al., 1997). Subsequently, the binding of the linker histone (H1 or H5) organizes the nucleosomal arrays into a more condensed fiber, usually referred to as the 30-nm chromatin fiber (Finch and Klug, 1976; Robinson et al., 2006; Thoma et al., 1979). The X-ray crystal structure of the nucleosome core particles was resolved at 2.8-Å, showing the precise path of the 147 bp of DNA and the localization of individual core histones (Luger et al., 1997). However, the structure of the higher order chromatin fiber is controversial (Robinson and Rhodes, 2006).
It is now clear that chromatin structure exhibits a highly dynamic equilibrium between an open conformation exemplified by the 11-nm beads-on-a-string and a compacted 30-nm fiber. Two main processes have been shown to modulate these dynamic structural changes. One involves ATP-dependent nucleosome remodeling complexes and the other, posttranslational modifications of histones (Varga-Weisz and Becker, 2006). ATP-dependent nucleosome-remodeling factors are regarded as key facilitators of chromatin dynamics, acting at the level of the nucleosome proper through the following mechanisms: nucleosome repositioning (Flaus and Owen-Hughes, 2003; Maier et al., 2008), histone-DNA interactions (Narlikar et al., 2001), nucleosome disassembly (Bruno et al., 2003; Vicent et al., 2004), and canonical and variant histone exchange (Mizuguchi et al., 2004). Yet much less is known about the functional interactions of nucleosome-remodeling factors with folded chromatin fibers. Peterson and colleagues reported that acetylation of histone H4 on lysine 16 (H4K16ac), a mark with a functional role in transcription activation, can disrupt higher order chromatin folding that had been induced by Mg2+ in the absence of histone H1 (Shogren-Knaak et al., 2006). Histone acetylation and ATP-dependent nucleosome remodeling activities are frequently coordinated with each other in the regulation of chromatin dynamics during gene expression (Narlikar et al, 2002; Vaquero et al., 2003). Also, both H4K16 acetylation and linker histone eviction are required for unfolding the 30-nm chromatin fiber in vitro (Robinson et al., 2008), suggesting that the decompaction of the 30-nm chromatin fiber is not a simple single-step process but at least a two-step mechanism.
The dynamic nature of chromatin and its folding allows genes to be switched on or off rapidly after an inducing stimulus (Eberharter et al., 2005). A very useful example of how chromatin dynamics influences gene expression is provided by the mouse mammary tumor virus (MMTV) promoter, one of the best studied in mammals. The MMTV promoter is organized into an array with six well-positioned nucleosomes in vivo (Fragoso et al., 1995), with the second nucleosome (NucB) covering the hormone responsive regions containing several hormone responsive elements (HREs) and a binding site for Nuclear Factor 1 (NF1) (Pina et al., 1990). In vitro binding experiments demonstrated that the glucocorticord receptor (GR) can bind to the reconstituted nucleosomal array on the MMTV promoter, but that NF1 was not able to do so (Venditti et al., 1998). In the absence of hormone, the binding site for NF1 was not accessible on this positioned nucleosome (Archer et al., 1992). Upon hormone binding, hormone receptors such as GR bind to the exposed HREs and recruit the ATP-dependent chromatin remodeling complexes (BRG1 or BRM) (Johnson et al., 2008) and histone acetyltransferase (p300 or pCAF) (Li et al., 2003) that reorganize the nucleosome structure underlying the promoter (Belikov et al., 2000; Fragoso et al., 1995; Richard-Foy and Hager, 1987). This chromatin-remodeling event now enables NF1 to bind (Belikov et al., 2004; Truss et al., 1995) as well as the assembly of a transcription initiation complex (Johnson et al., 2008).
In its modus operandi, GR is regarded as a pioneer transcription factor, capable of binding to its sites on chromatin and triggering gene activation via its recruitment of various coactivators that form stable multi-factor complexes and remodel the chromatin structure of the MMTV promoter. Yet in contrast to this presumably fixed binding of GR, FRAP experiments demonstrated that GR and its interacting coactivators undergo highly dynamic interactions with the MMTV promoter, measured in the order of seconds in living cells (McNally et al., 2000). Subsequently, UV laser cross-linking experiments revealed that the interaction between GR and the chromatinized MMTV promoter is cyclical and highly dynamic with peaks occurring approximately every 5 min (Nagaich et al., 2004). This interaction is facilitated by the SWI/SNF chromatin-remodeling factor in an ATP-dependent manner involving sequential reorganization of histone H2A and H2B within the nucleosome (Nagaich et al., 2004). Futhermore, time-resolved chromatin immunoprecipitation (ChIP) assays were employed to analyze the dynamic binding of nuclear receptors (NR), interacting cofactors and histone modifications upon hormone activation (Metivier et al., 2003). These studies revealed that rapid dynamic changes are a common theme for NR-regulated gene expression (Aoyagi and Archer, 2008).
Most studies addressing the dynamic changes in chromatin organization during gene expression have thus far been focused at the level of the nucleosome. Here we investigated how the ordered recruitment and exchange of factors within the chromatin substrate occurs at the level of higher order chromatin. We developed an in vitro system to reconstitute “30-nm” chromatin fiber. The data shows that the performance of this in vitro assembled higher order chromatin containing the model RAR/RXR-inducible PEPCK promoter was in accordance with regard to the chromatin dynamics observed at the in vivo PEPCK promoter and the processes required to attain transcription activation in vivo.
Results
Conditions to attain different types of chromatin
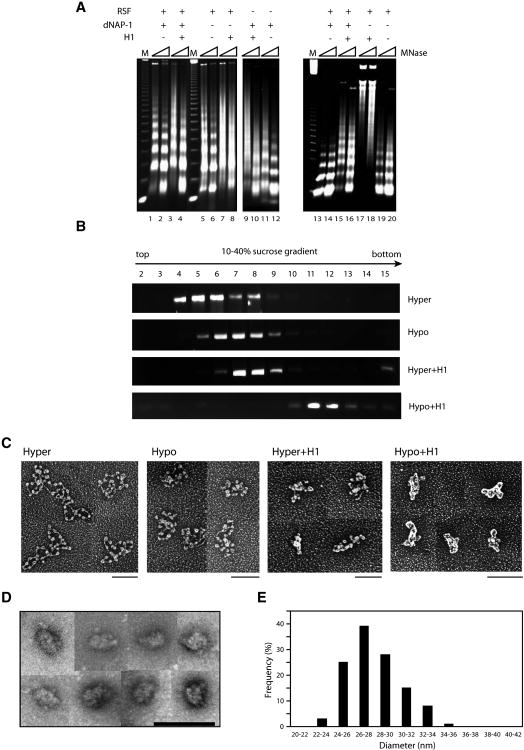
We set out to define the process whereby highly compacted chromatin is relieved of its constraints to transcription. To this end we tested conditions to recapitulate in vitro the formation of compacted chromatin, the intermediates of and the resultant “30”-nm fiber observed in vivo. Deacetylated histones and the linker histone H1 have been recognized as features associated with compacted chromatin, therefore we tested for chromatin compaction as a function of the presence of either or both. Supercoiled 5 kb DNA was assembled into chromatin using native core histones isolated from HeLa cells. This was preformed as a function of the presence of each of the following: the core histone chaperone RSF, linker histone H1, and the histone H1 chaperone NAP-1. The successful formation and alignment of nucleosomes was initially analyzed by micrococcal nuclease (MNase) digestion (Figure 1A, left panel). While each independently could assemble histones onto correctly spaced nucleosomes, a tighter alignment of nucleosome arrays was observed when both RSF and NAP-1 were present (Fig. 1A, left panel). The addition of histone H1 to core histones during the assembly reaction resulted in correctly spaced nucleosomes in an RSF- and NAP-1-dependent manner, as evidenced by the 20-bp increase in the nucleosome repeat length (NRL) after micrococcal digestion. In the absence of RSF or NAP-1, histone H1 appeared to bind randomly.
Figure 1.
The reconstitution of 30-nm chromatin fiber in vitro: requirements for H1 incorporation and deacetylation of core histones.
(A) Microccocal nuclease digestion analysis of chromatin assembled with supercoiled 5 kb template (pG5MLP 5S) using RSF and/or NAP-1 as a function of the presence of histone H1 either during (lane1-12) or after (lane 13-20) the chromatin assembly reaction. (B) Purification of reconstituted chromatin by sucrose gradient sedimentation. The fractions were analyzed by agarose gel electrophoresis and visualized using ethidium bromide staining. (C) Sucrose gradient peak fractions were subjected to analysis by electron microscopy (EM) using tungsten shadowing. Scale bar: 100 nm. (D) Gallery of side views of negatively stained compacted chromatin fibers in the presence of 0.5 mM MgCl2. Scale bar: 100 nm. Negative stain images are more appropriate for establishing the diameter of filaments as metal buildup around the particles in rotary shadowed images significantly increases their apparent diameter. (E) Bar graph representation of the diameters of chromatin fibers formed with hypoacetylated histones and histone H1 in the presence of 0.5 mM MgCl2 shown in Figure 1D. See also Figure S1.
Since histone H1 is deposited subsequent to core histones in vivo, we tested for histone H1 incorporation as a function of the presence of NAP-1 after chromatin was preassembled with core histones (Figure 1A, right panel). Indeed, the subsequent addition of histone H1 resulted in correctly spaced nucleosomes and the digestion pattern appeared to be sharper with the nucleosomes exhibiting a more rigorous pattern of alignment at higher regions of migration. NAP-1 was required for histone H1 incorporation after core histones were assembled, as expected. These results indicate that NAP1-dependent histone H1 incorporation subsequent to RSF-mediated core histone assembly is optimal for giving rise to a pattern of nucleosomes that more accurately reflects that found in vivo.
We next examined the characteristics of chromatin assembled in the absence and presence of histone H1 as a function of the state of core histone acetylation. Native histones were deacetylated by SirT1 (Supplementary Figure 1A). Chromatin was pre-assembled using either untreated (acetylated) or SirT1-treated (deacetylated) native histones, yet the acetylation status of these core histones showed little effect on RSF/NAP-1 chromatin assembly (Supplementary Figure 1B). Histone H1 was then either added or not, subsequent to this pre-assembly reaction. Sucrose gradient analysis of these chromatinized templates revealed the sedimentation profiles for chromatin assembled with core histones whether (hyper) or not (hypo) they were acetylated (Figure 1B). The addition of histone H1 after this preassembly did affect somewhat the sedimentation of chromatin containing acetylated histones, and in the case of deacetylated histones a faster migration was observed (Figure 1B). The presence of the appropriate histone components in the peak fractions in each case was verified using silver staining (Supplementary Figure 1C).
Aliquots of these peak fractions were examined next by EM (Figure 1C). While acetylated histones alone gave rise to the typical beads on a string pattern of nucleosome placement, the incorporation of histone H1 resulted in a clearly discernible further compaction similar to that obtained with deacetylated core histones alone, but the disparate images suggested different extents of compaction. Thus even chromatin composed of acetylated histones can be compacted upon histone H1 incorporation. The highest degree of compaction was attained upon assembling chromatin with deaceylated core histones and the linker histone H1 (Figure 1C). The chromatin appeared to be homogenous as evidenced by negative staining (Figure 1D; Supplementary Figure 1D, left and middle panels) and cryo-EM (Supplementary Figure 1D, right panel). A large fraction of these species exhibited a diameter reflective of highly condensed fibers with a peak at 26-28 nm (Figure 1E). Of note, the diameter of the particles observed by cryo-EM is consistent with this observation.
We next examined this set of parameters for the extent of compaction obtained when the length and/or topology of the DNA template was varied. Similar results were obtained using linearized 5 kb DNA (not shown) or DNA of 20 kb either linearized (see below) or supercoiled (not shown) with the highest degree of compaction necessitating deacetylated histones and linker histone H1 in all cases. The EM results of chromatin assembled with linearized, 20 kb DNA exhibited longer filaments relative to those obtained with 5 kb DNA (Supplementary Figure 1E). The species examined in all subsequent experiments were composed of supercoiled 5 kb DNA as shown in Figure 1C.
Reconstituted highly compacted chromatin is refractory to transcription, and impedes some, but not all activator binding
In general, compacted chromatin is considered as being repressive to transcription as the nucleosomes present an obstacle to RNA polymerase II (RNAP II) with respect to its accessing the template and/or translocating along the transcription unit. Given the electrostatic charge imparted by the acetyl groups, chromatin composed of acetylated histones is recognized as being open and accessible, yet chromatin remodeling factors are requisite for transcription in this case as well (Pavri et al., 2006). We next compared each of the four assembled chromatin templates, those with acetylated (hyper) versus deacetylated (hypo) histones, with or without incorporated linker histone H1, for their ability to support transcription in nuclear extracts and also in a highly purified reconstituted transcription system. The chromatin templates contained Gal4 DNA binding sites and transcription levels in nuclear extracts were gauged in the absence or presence of Gal4-VP16 (Supplementary Figure 2A). As expected, the basal and activated levels of transcription were clearly detectable using acetylated chromatin as template, however when histone H1 was also incorporated, these levels were markedly reduced. Deacetylated chromatin templates gave rise to undetectable levels of transcription regardless of histone H1 incorporation.
None of the chromatin samples exhibited transcription in a minimally reconstituted transcription assay, as previously reported (Supplementary Figure 2B) (Orphanides et al., 1998). The addition of increasing levels of purified FACT that facilitates transcription from chromatin templates enabled detectable levels of transcription in all cases. However, deacetylated chromatin was less competent in supporting transcription relative to acetylated chromatin and importantly, the incorporation of histone H1 in both cases resulted in markedly lower transcript levels. Thus the reconstituted chromatin templates exhibited the expected affects on transcription in both nuclear extracts and in a reconstituted system and this reflected the extent of their compaction observed in the EM analyses shown above.
As our goal was to identify conditions that would convert highly compacted chromatin to one conducive to transcription, we sought to identify the impediment(s) to transcription using the four different chromatin templates described thus far. One such likely impediment that is requisite to transcription is transcription factor binding. We focused on retinoic acid (RA) inducible promoters targeted by the RAR and RXR members of the nuclear receptor family that activate transcription of targeted genes upon ligand (tRA) induction. We examined RAR/RXR binding to the RA response elements (RAREs) in the context of the different states of chromatin composition obtained above. We started with an artificial gene containing RAREs and then extended our analyses to a natural, more complex RA-inducible promoter, that of the PEPCK gene.
Supplementary Figure 2C shows a schematic representation of an artificial template containing the RARβ2 promoter with five RAREs directing transcription of the β-globin gene. This template was assembled into chromatin containing either acetylated or deacetylated histones, in the absence or presence of histone H1. Increasing amounts of RAR/RXR exhibited binding to all chromatin templates whether tRA ligand was added (Supplementary Figure 2D) or not (not shown). Although binding to highly compacted chromatin containing deacetylated histones and histone H1 was relatively sub-optimal, this would likely not be an impediment that would completely thwart transcription.
Characteristics of compacted chromatin containing the natural PEPCK promoter
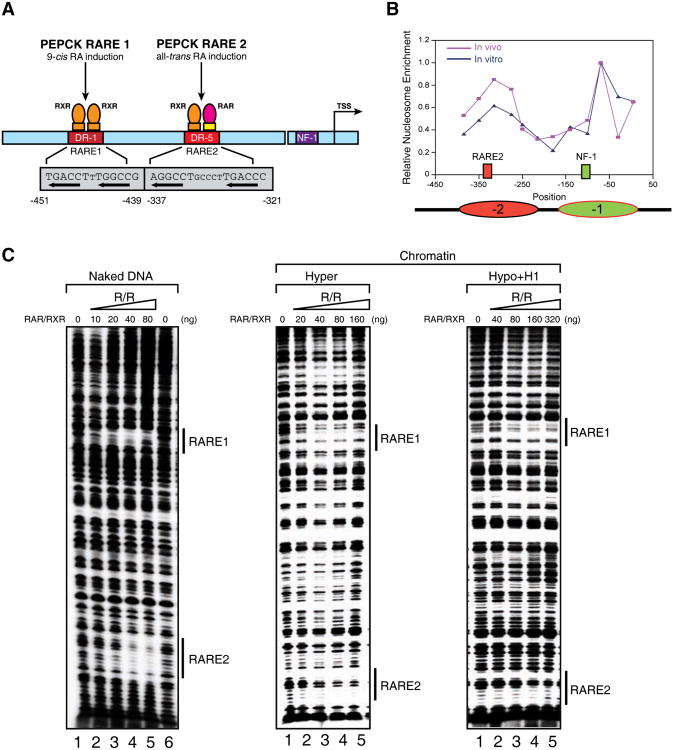
Our next goal was to test systematically the characteristics of the highly compacted chromatin generated in vitro for the parameters required to render these templates amenable to activated transcription, and then contrast these parameters to those identified in vivo. To this end we sought a well-studied and well-characterized promoter that is natural, inducible, and relatively complex in its factor requirements for activation. We chose the PEPCK promoter that contains an NF1 binding site in addition to two RAREs, the cognate binding sites for RAR/RXR (Figure 2A). Once again the same criteria were employed to assemble the different chromatin species whose integrity was verified by MNase digestion and EM (not shown). Before examining the ability of RAR/RXR to access this natural promoter compacted by nucleosomes in vitro, we compared the positions of these nucleosomes with those of the in vivo case with respect to the promoter region on the PEPCK gene. We observed two distinct peaks of nucleosome occupancy in both cases with the first peak overlapping RARE2 and the second overlapping the binding site for transcription factor NF1 (Figure 2B). In the latter case, the nucleosome position observed in vitro was slightly broader.
Figure 2.
The effects of chromatin structure on RAR/RXR binding to chromatin templates in vitro.
(A) Diagram of the p(PEPCK-500)G template containing the PEPCK promoter used for in vitro DNase I footprinting and transcription assays. (B) Line graph of nucleosomal positioning at the PEPCK promoter in vivo (red) and in vitro (blue). The nucleosomes covering RARE2 and the NF1 binding site are represented as ovals in red and green, respectively. The mapping of nucleosomal positioning at the PEPCK promoter in vitro and in vivo is described in Experimental Procedures. Nucleosomal positioning in vivo shown here represents the nucleosome structure after tRA treatment (165 min). (C) DNase I footprinting analysis of RAR/RXR bound to the PEPCK promoter on naked DNA (left panel), or chromatin fiber assembled with either hyperacetylated histones (middle panel), or hypoacetylated histones and histone H1 (right panel). The binding sites for RAR/RXR (RARE1 and -2) are indicated by bond bars. See also Figure S2.
Similar to the case of the artificial RA-inducible promoter, increasing amounts of RAR/RXR exhibited binding to all chromatin states including those that are most disparate with respect to their degree of compaction and their ability to support transcription: acetylated histones without histone H1 versus deacetylated histones with histone H1 (Figure 2C). Similar to the results obtained with the artificial promoter however, the binding was less efficient in the latter case. Interestingly, given the profile of nucleosome positioning, both RARE1 and -2 were accessible to RAR/RXR binding reflecting the case of naked DNA (Figure 2C). We next tested for NF1 binding. Previous studies indicated that the NF1 transcription factor is completely blocked from binding to chromatin in general (Venditti et al., 1998). Indeed, NF1 binding to any of the four chromatinized templates containing the PEPCK promoter was undetectable, while its binding to naked DNA was clearly apparent (Supplementary Figure 3). Given that RAR/RXR can bind while NF1 cannot, we next sought conditions that would enable NF1 binding to the PEPCK promoter when present within highly compacted, transcriptionally inert chromatin composed of deacetylated histones and histone H1.
Conditions to enable compacted chromatin to open for NF1 binding in vitro
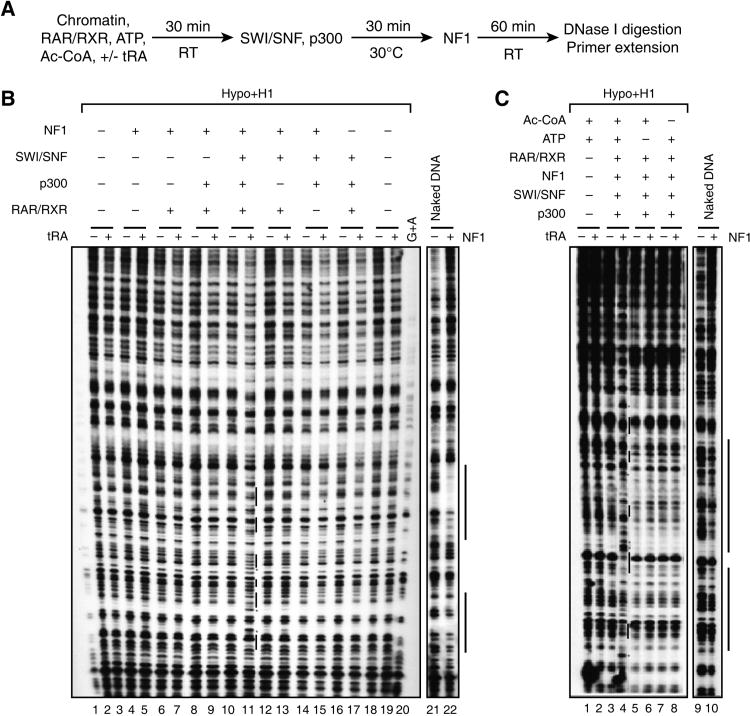
Previous studies have shown that all-trans retinoic acid (tRA) induces a conformational change in the RAR/RXR heterodimer such that its pre-associated co-repressor complexes that include histone deacetylases (HDACs) are dislodged and can be replaced with co-activator complexes that include histone acetyltransferases (HATs) and chromatin remodeling complexes such as SWI/SNF (Dilworth and Chambon, 2001). Therefore we tested if the p300 HAT in combination with SWI/SNF can provide the activities critical to relieve repressive chromatin structure and thereby enable NF1 to bind its cognate site in the PEPCK promoter. If this were to be the case, then such binding would be expected to be ligand (tRA)-dependent.
Figure 3A shows the protocol used with maximally compacted DNA containing deacetylated histones, histone H1, and the PEPCK promoter. Chromatin was incubated with RAR/RXR transcription factor, ATP, and the co-factor acetyl-CoA, in the absence or presence of ligand (tRA) after which SWI/SNF and p300 were added. The resultant chromatin was then exposed to NF1 and its binding was analyzed by DNase I digestion and primer extension (Figure 3B). The results show that only when the chromatin was incubated with RAR/RXR, SWI/SNF, and p300, was detectable NF1 binding achieved in the presence of ligand (Figure 3B, compare lanes 10 and 11). Also, the presence of acetyl-CoA and ATP were required for this binding (Figure 3C). Thus highly compacted chromatin generated in vitro is accessible to RAR/RXR binding as shown above and its impediment to NF1 binding can be overcome by histone acetylation along with nucleosome mobilization, reflected by the requirement for acetyl-CoA and ATP, respectively (see below). It is likely that RAR recruits p300 (Dowell, et al., 1997). As RAR and NF1 exhibit interaction in vitro (data not shown), it is also likely that NF1 can bind cooperatively with RAR after the chromatin is altered sufficiently such that NF1 can access the DNA.
Figure 3.
Remodeling/opening of 30-nm chromatin fiber in vitro: requirements for SWI/SNF and p300.
(A) Reaction scheme for analyzing NF1 binding to compacted chromatin template p(PEPCK-500)G. (B, C) DNase I footprinting analysis for NF1 binding using chromatinized template assembled with hypoacetylated histones and histone H1, as a function of the presence of the factors indicated at the top and also shown in the reaction scheme in panel (A). (B) The binding site of NF1 is indicated by a bond bar, and hypersensitive sites are indicated by red stars. Naked DNA is shown as control. (C) ATP and Ac-CoA are required for the remodeling/opening of compacted chromatin mediated by SWI/SNF and p300 to facilitate NF1 binding. See also Figure S3.
Mobilization of nucleosomes within chromatin compacted in vitro
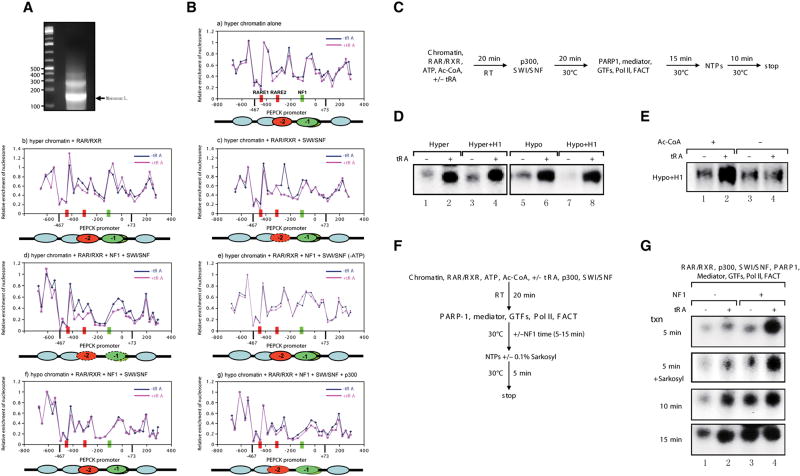
Having established conditions that permit NF1 to access chromatin compacted in vitro and containing the natural PEPCK promoter, we next examined if the profile of nucleosome occupancy is accordingly altered. We isolated the mononucleosomal DNA (Figure 4A) composing the different types of chromatin and scored for their coverage of the RAREs and the NF1 binding site as a function of the addition of the various activities identified above. In the reconstituted system using highly purified factors, the pattern of nucleosome coverage was unaltered as a function of addition of ligand (Figure 4B, panel a), or ligand plus RAR/RXR (panel b) as shown in the case of hyperacetylated chromatin. However, the nucleosome positioned at RARE2 was dislodged upon addition of SWI/SNF and this required ligand addition (panel c). Further dislodgement of the nucleosome covering the NF1 binding site immediately downstream was observed upon the addition of NF1 (panel d) suggesting that the vacancy of the first nucleosome enabled contact between RAR/RXR and NF1 to facilitate binding of the latter. Importantly this mobilization was dependent on ATP (panel e). p300 was not required (not shown), as expected given that the histones were hyperacetylated. In contrast, compacted chromatin formed with hypoacetylated histones did require the presence of p300 to achieve a similar pattern of nucleosome mobility (compare panels f and g). Thus the determinants to nucleosome mobility are the same as those to attain NF1 binding to chromatin as shown above, such that the physical removal of occluding nucleosomes can allow NF1 access, putatively through its interaction with RAR/RXR. Importantly the nucleosomes can be dislodged only when their composite histones are acetylated.
Figure 4.
Retinoid acid receptor (RAR/RXR)-induced chromatin transitions allow NF1 to facilitate ligand-dependent transcription at the PEPCK promoter in vitro.
(A) Mononucleosomes derived from microccocal nuclease digestion of chromatinized template p(PEPCK-500)G assembled with hyperacetylated histone octamers using the RSF/NAP-1 system in vitro. (B) Nucleosomal mapping of the PEPCK promoter region within chromatinized p(PEPCK-500)G template assembled with hyperacetylated histone octamers (a-e) or hypoacetylated histone octamers (f, g) as a function of the presence of the factors indicated at the top of each panel. (C) The reaction scheme of the reconstituted transcription assays for (D) and (E). (D)In vitro reconstituted ligand-dependent transcription assays using the different chromatin templates indicated. (E) Optimal transcription from 30-nm chromatin fiber by RNAPII requires acetylation of core histones. (F) The reaction scheme of the reconstituted transcription assay for (G). (G) NF1 stimulates ligand-dependent transcription by RNAPII from compacted 30-nm chromatin fiber in the absence and presence of 0.1% Sarkosyl.
NF1 stimulates transcription at the PEPCK promoter
Given our findings of the minimal requirements to attain NF1 binding to compacted chromatin in vitro and the importance of this factor for achieving optimal PEPCK transcription in vivo (Crawford et al., 1998), we next assayed the levels of PEPCK transcription from compacted chromatin in vitro under defined conditions. We used the minimal, chromatin-dependent reconstituted system that we employed earlier to study the dynamics of transcription at the RARβ2 promoter (Pavri et al., 2006). We first compared the levels of PEPCK transcription obtained from compacted chromatin templates in the absence of NF1. Figure 4C shows the protocol used with chromatin compacted as a function of its acetylation state and incorporation of histone H1. Regardless of the state of core histone acetylation, or of the presence of histone H1, during the generation of the compacted chromatin templates, transcription activation was attained in the presence of ligand, and in the absence of NF1 (Figure 4D). This process was however dependent on p300-mediated acetylation in the case of highly compacted chromatin derived from hypoacetylated core histones and histone H1, as evidenced by the requirement for acetyl-CoA (Figure 4E). This result is consistent with our previous report that p300 is required for ligand-dependent transcription from chromatin templates devoid of histone H1 (Pavri et al., 2006).
We next assayed for the level of transcription obtained as a function of time after NF1 addition. Of note, NF1 was added after the chromatin was subjected to conditions shown above that facilitate its binding (Figure 4F). While transcription activation was evident in its absence, NF1 addition led to markedly increased levels of transcription at considerably earlier times consistent with its important role in PEPCK gene expression in vivo (Figure 4G). This NF1-mediated increase in transcription was independent of transcription re-initiation as the addition of Sarkosyl was ineffectual (Figure 4G). It is likely that NF1 stimulates the rate of formation of the transcription initiation complex, although an effect on transcription elongation cannot be ruled out.
Cycling of factors at the PEPCK promoter upon ligand induction in vivo
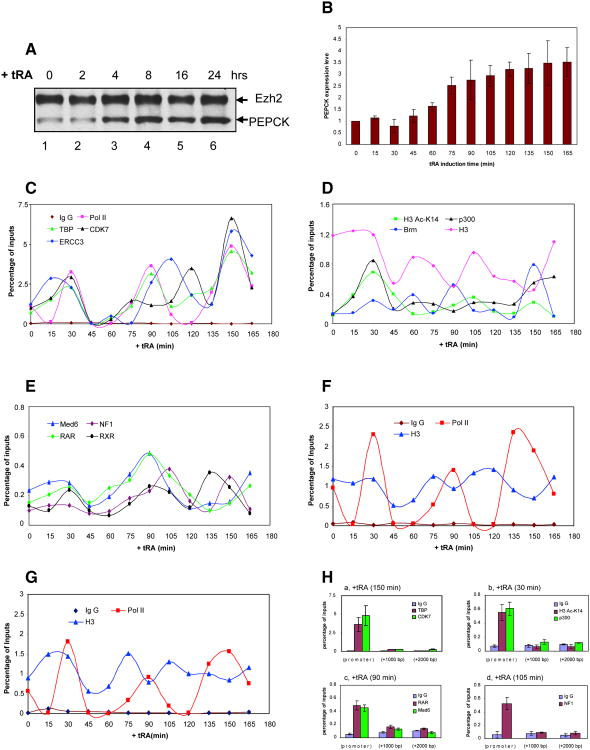
We next probed for any possible pattern of recruitment by which these pertinent factors, RAR/RXR, p300, SWI/SNF, and NF1, as well as the general transcription factors and RNAPII can access the PEPCK gene in vivo. This was performed using qChIP experiments as a function of time after ligand addition to cells and in the context of PEPCK gene expression, using primers that cover −400 to +2000 nt relative to the start site of PEPCK transcription. Ligand addition resulted in activation of PEPCK gene expression as evidenced at the protein level by western blot and at the mRNA level by q-PCR (Figures 5A and 5B, respectively). Interestingly, factor recruitment to the PEPCK promoter appeared for the most part to be a highly dynamic process (Figures 5C-E).
Figure 5.
In vivo ChIP on the endogenous PEPCK promoter: cyclical and ordered recruitment of factors.
(A) Western blot analysis of PEPCK expression upon tRA induction. (B) RT-qPCR analysis of PEPCK mRNA levels upon tRA induction. Values are the mean ± SD (standard deviation) of three independent experiments. (C, D, E)In vivo kinetic ChIP analyses of RNAPII and general transcriptional factors (C), coactivators and modified histones (D), and transcriptional activators and Mediator (E) at the PEPCK promoter. The amount of immunoprecipitated PEPCK promoter was quantified by q-PCR and normalized to the input DNA. (F-G)In vivo ChIP analyses of RNAPII and histone H3 at the PEPCK coding regions: +1000 nt (F) and +2000 nt (G) from the transcription start site. (H) Enrichment of general transcriptional factors (a), coactivators and modified histones (b), transcriptional activators and Mediator (c-d) at the PEPCK promoter, but not at the coding regions. For simplicity, a representative time point is shown in each case, but similar results were obtained at all other 15 min increments of time. Percent of input are the mean ± SD (standard deviation) of three independent experiments.
In the absence of ligand, RNAPII and the general transcription factors TBP and TFIIH (CDK7 and ERCC3) were present at the promoter (Figure 5C), thus we suspect that in the absence of ligand a transcription competent complex is formed, but maintained in a repressive state. After 30 min of ligand addition, we observed a first wave of factor recruitment at the PEPCK promoter as evidenced with RAR/RXR and Mediator (Med6) (Figure 5E), Brm and p300 (Figure 5D), along with the general transcription machinery components (Figure 5C). Concomitantly, H3K14ac was robustly increased (Figure 5D). Of note, NF1 was not significantly enriched at the PEPCK promoter during this first wave of nuclear receptor and cofactor recruitment (Figure 5E). However, a second wave of RAR/RXR and cofactor recruitment was apparent after 60 min of ligand stimulation and peaked at 90 min, during which NF1 was now recruited with a slight delay relative to that of RAR (Figures 5C-E). This profile is in accordance with the order of addition experiments performed in vitro using purified RAR, p300, SWI/SNF, and NF1, as shown above. As well, this second wave of recruitment that incorporated NF1 corresponded with increased levels in PEPCK mRNA (Figure 5B). Yet another wave of factor recruitment at the PEPCK promoter was evident between 135 to 165 min post-ligand addition (Figures 5C-E).
With regard to the PEPCK coding region (+1000 and +2000 nt from the transcription start site), RNAPII and histone H3 were found along all regions of the gene examined after ligand induction (Figures 5F and 5G). In contrast, the general transcription factors TBP and TFIIH (CDK7) (Figure 5H, panel a), p300 (panel b), nuclear receptor RAR and Mediator (Med6) (panel c), and NF1 (panel d) were not detectable at any appreciable level. Of note, H3K14ac was also detected only at the promoter (Figure 5H, panel b).
Importantly, all the factors exhibited ebb and flow at the promoter as a function of time, reflecting a dynamic fluctuation rather than a static all or none phenomenon, being inherent to factor occupancy. This apparent dynamic nature of the transcription complex is most apparent when analyzing RNAPII occupancy within the transcribed region. Rather than a full occupancy at +1000 and +2000 nucleotides, RNAPII appears in waves (Figures 5F and 5G), consistent with there being a periodic reassembly of factors at the promoter that function to attain an open chromatin conformation resulting in RNAPII recruitment.
Transcription factor cycling reflects nucleosome repositioning at the PEPCK promoter
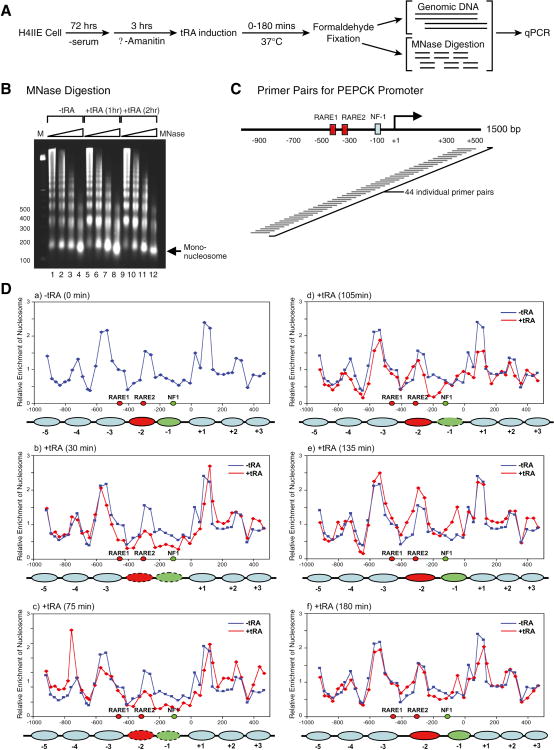
Thus far, we demonstrated that SWI/SNF was requisite to attain NF1 binding to the PEPCK promoter when contained within highly compacted chromatin in vitro and that its Brm subunit was recruited before NF1 upon ligand induction of the PEPCK gene in vivo. We also observed that most factors that occupy the PEPCK promoter upon ligand induction do so with fluidity. To explain these observations at the level of the basic chromatin unit, we systematically examined the positions of individual mononucleosomes at the PEPCK gene (from −1000 to +400 nt relative to the start site of transcription) as a function of time of ligand exposure.
H4IIE rat hepatoma cells were prepared for these analyses as shown in Figure 6A. The cells were first arrested for growth under conditions of serum starvation, and then cellular transcription was inhibited by treatment with α-amanitin, after which tRA was added for the same periods of time used in the ChIP experiments shown above. The cells were then fixed with formaldehyde and genomic DNA and chromatin were isolated for each time point. Mononucleosomal DNA was isolated after MNase digestion (Figure 6B) and subjected to quantitative PCR (q-PCR), using primer pairs that cover 1500 nt of the PEPCK promoter as shown in Figure 6C. The results were normalized against those obtained using genomic DNA, and as well against results obtained for mononucleosome positioning at the 3′-end of the β-globin gene that is not expected to alter as a function of time of hormone treatment (Flavin et al., 2004). The relative enrichment of mononucleosomes at specific regions of the PEPCK gene was plotted for the time periods of tRA exposure indicated (Figure 6D). The data shows that the level of mononucleosomes that are positioned at the downstream RARE (RARE2) and the NF1 binding site before ligand addition (Figure 6D, panel a) have been markedly reduced after 30 min of tRA treatment (panel b) and stay as such at 75 min (panel c). This coincides with the steady increase in PEPCK transcription (Figure 5B). By 105 min (Figure 6D, panel d), the mononucleosomes in this specific area of the PEPCK promoter now exhibited a renewed occupancy at RARE2 and at the NF1 site, but in the latter case there was a slight shifting downstream.. This slight shifting of the mononucleosome associated with the NF1 site relative to its position pre-ligand addition becomes clearly evident by 135 min of tRA treatment (panel e) and is maintained at 180 min (panel f).
Figure 6.
Dynamic nucleosome repositioning at the PEPCK promoter during transcription activation in vivo.
(A) Protocol used for nucleosomal mapping in vivo. (B) Microccocal nuclease digestion of chromatin isolated from H4IIE cells as a function of treatment with tRA. (C) A schematic of the tiling primers covering the PEPCK promoter used for identifying in vivo nucleosomal positions are shown. (D) The dynamic change in nucleosomal positions at the PEPCK promoter in the absence (blue graph) and in the presence (red graph) of tRA induction (30-180 min). The filled ovals represent the nucleosomes covering RARE2 (red) and NF1 (green) that are repositioned upon tRA exposure.
These in vivo analyses were initiated to test the validity of the results we obtained in vitro using reconstituted highly compacted chromatin fiber. The in vivo results corroborate the integrity of the in vitro template and the information it provided with respect to 1) nucleosome and factor residencies at the uninduced PEPCK promoter, 2) the progressive recruitment of factors, and 3) resultant modulation of repressive chromatin as evidenced by changes in nucleosome occupancy at the induced PEPCK promoter. Such dynamics for achieving PEPCK gene expression are in accordance in vitro and in vivo.
Discussion
In this report, we demonstrated a novel in vitro system that results in the reconstitution of highly compacted chromatin fibers using purified chromatin assembly factor RSF and histone H1 chaperone NAP-1. The measurable characteristics of this compacted chromatin were consistent with those of the in vivo case. In particular, by incorporating the well-studied PEPCK promoter in highly compacted chromatin generated in vitro, we found that these templates exhibited the appropriate requirements for PEPCK activation as previously established in vivo. Moreover, the nucleosome positioning at the PEPCK promoter in vitro reflected well the endogenous case, highlighting that proper nucleosomal patterning was attained in vitro under both uninduced and induced conditions.
Mechanistic model: Opening compacted higher order chromatin for RNAPII transcription
We found that RAR/RXR can bind to highly compacted “30-nm” chromatin fiber containing the model PEPCK promoter although at lower affinity compared to that of extended nucleosomal arrays (Figure 2B). Chambon and colleagues showed that histone H1 alone is ineffectual with respect to RAR/RXR binding to the RARβ2 promoter assembled into chromatin (Dilworth et al., 2000), and this is consistent with our results showing that incorporation of histone H1 within hyperacetylated chromatin in vitro does not inhibit RAR/RXR access to chromatin.
The second transcription factor NF1 is required for both hormone-dependent chromatin remodeling and transcriptional activation (Hebbar and Archer, 2003). NF1 binds proximal to the PEPCK promoter and its interaction with other transcription factors or activators, such as CBP or p300 is critical to PEPCK gene expression (Leahy et al., 1999). We found that RAR/RXR can recruit p300 and SWI/SNF to the PEPCK promoter and the subsequent rearrangement in nucleosomal structure allowed NF1 to bind to its cognate site. Importantly, in the absence of NF1, our in vitro nucleosomal mapping experiments showed that while RAR/RXR can target the ATP-dependent chromatin remodeling complex SWI/SNF to the RARE2-specific nucleosome, the chromatin remodeling reaction occurred only at this position and not at the nucleosome overlapping the NF1 binding site (Figure 4B). It was only when NF1 was present that we observed a significant remodeling of the nucleosome covering its binding site, indicating that NF1 and RAR/RXR cooperate in chromatin remodeling. Of note, these remodeling events did not include nucleosome coverage of RARE1 and this might be a reflection of the ligand we employed, that being all-trans retinoid acid (tRA) that specifically activates RAR/RXR through the RARE2 (DR5) element on the PEPCK promoter.
Our results support the two-step model proposed in the case of MMTV activation (Vicent et al., 2002), in which binding of the initial factor, that being hormone receptor PR/GR, to chromatin triggers an ATP-driven chromatin remodeling event that facilitates access of NF1 to nucleosomal DNA. This in turn maintains an open nucleosome conformation allowing additional nuclear receptor binding and full transcription initiation (Di Croce et al., 1999).
In the case of the PEPCK promoter, the highly compacted chromatin generated in this study displayed in vitro the cogent features of its regulated expression in vivo (Figure 7). In this model, RAR/RXR and NF1 synergize during activation of PEPCK transcription. RAR/RXR binds to compacted higher order chromatin and triggers the recruitment of histone modifying enzymes (p300) and ATP-dependent chromatin remodeling activities (SWI/SNF) resulting in nucleosomal rearrangement upon hormone treatment. This rearrangement displaces histone H1 and facilitates NF1 binding to nucleosomal DNA that in turn stabilizes the open nucleosome conformation at the PEPCK promoter such that other transcription factors (general transcription factors and RNAPII) can bind and establish transcription.
Figure 7.
Model for the dynamic folding and opening of 30 nm chromatin fiber for RNAPII transcription. See text for details.
Chromatin dynamics and the ordered cycling of transcription factor recruitment on the PEPCK promoter
Our ChIP results revealed that the pertinent transcription factors, cofactors, and the transcriptional apparatus are recruited to the PEPCK promoter in a specific sequential, but also cyclical manner (Figure 5). Gannon and colleagues demonstrated that following estradiol treatment, there is a cyclical (20-40 min cycles) and ordered recruitment of combinations of coactivators, changes in histone modifications and nucleosome organization, and assembly of the transcriptional machinery on the estrogen responsive pS2 promoter (Metivier et al., 2003). Moreover, similar to our findings with the PEPCK promoter, Gannon and colleagues also showed that the nucleosome organization over the pS2 promoter was also dynamically remodeled by synergism between SWI/SNF (or NuRD) and p300 (Direnzo et al., 2000; Metivier et al., 2003).
The dynamic binding of transcription factors and interacting cofactors to various nuclear receptor (NR)-regulated promoters (Burakov et al., 2002; Gévry et al., 2009; Metivier et al., 2003; Shang et al., 2000) indicates that these rapid yet orderly changes are a common theme for NR-regulated gene expression (Aoyagi and Archer, 2008; Kang et al., 2002; Sharma and Fondell, 2002). Of note, our ChIP results for another RAR/RXR-regulated promoter, that of Cyp26A1, exhibited very different kinetics even though several of the factors involved are common to the PEPCK case (data not shown, see Pavri et al., 2006), suggesting that the exact timing of factor recruitment may be promoter specific.
Formation of 30-nm chromatin fibers: incorporation of histone H1 and deacetylation of core histones
We close with a discussion of the goal we sought to achieve: the generation of compacted chromatin fibers that reflect the natural case. The linker histone H1 is known to act as a general transcriptional repressor (Laybourn and Kodanaga, 1991; Weintraub, 1985) and functions in the dynamics of higher order chromatin folding and unfolding (Robinson and Rhodes, 2006). Yet, little is known as to just how the linker histone H1 is deposited onto chromatin. Kadanoga and colleagues showed that ACF could assemble chromatin in the absence or presence of linker histone H1 in a NAP-1-dependent manner (Bulger et al., 1995). Our previous study showed that RSF could assemble regularly spaced chromatin independent of NAP-1 and in the absence of linker histone H1 (Loyola et al., 2001). We demonstrated here that NAP-1 functions as a chaperone for linker histone H1 and assists RSF in the assembly of regularly spaced chromatin in the presence of linker histone H1 in vitro. These results are supported by two studies in which NAP-1 was characterized as a linker histone H1 chaperone using the dinucleosome system in vitro (Saeki et al., 2005; Shintomi et al., 2005). In addition, our results show that both the incorporation of linker histone H1 and the deacetylation of core histones are required for full folding of chromatin fiber into a compacted higher order “30-nm” like-chromatin fiber. Importantly, nucleosome organization on the PEPCK promoter in the chromatin template assembled by RSF/NAP-1 in vitro exhibited a very similar pattern as that observed in vivo (Figure 2C), indicating that the assembled chromatin reflects the compaction behavior of native chromatin.
An important and remaining question is exactly how the linker histone H1 dissociates from highly compacted higher order chromatin. Our ChIP results showed that linker histone H1 dissociates from chromatin at 45 min after tRA treatment (data not shown), and this is preceded by histone acetylation and chromatin remodeling events, suggesting that these processes are prerequisite for histone H1 dissociation. However, it is still not clear whether an additional activity is required. Two important studies shed light on this question. Firstly, Chandraratna and colleagues demonstrated that HMGA protein can interact with various transcription factors including RAR/RXR and displace linker histone H1 from compacted chromatin (Nagpal et al., 1999). Secondly, Zaret and colleagues showed that the early developmental transcription factor FoxA could open compacted chromatin containing histone H1 without chromatin remodeling or histone acetylation (Cirillo et al., 2002), by competing with histone H1 (Cirillo et al., 1998). In addition, NAP-1 was shown to modulate the binding of linker histone H1 to chromatin in vitro (Kepert et al., 2005), suggesting that NAP-1 may dissociate histone H1 via its histone H1 chaperone activity. As NAP-1 was employed in our in vitro chromatin assembly system, it is likely that it plays a role in dissociating histone H1 in cooperation with p300 and SWI/SNF in our in vitro chromatin opening/remodeling (Figure 3) and chromatin transcription (Figure 4) assays.
The generation of the “30-nm” chromatin species in vitro described here offers a solid and promising platform to investigate at the molecular level other pivotal candidates that might infringe upon or promote either the formation or the extension of highly compacted chromatin, including histone modification marks.
Experimental Procedures
Templates, constructs for transcription and footprinting
The plasmid p(PEPCK-500)G used for footprinting, transcription, and nucleosomal mapping assays was derived from p(DR5)5β2G by replacing the 5xDR5 elements and RARβ2 promoter (Kpn I and Hind III fragment) with the rat PEPCK promoter (−467 to +73 fragment).
Purifications of proteins
See Supplemental Data.
Chromatin assembly and Sucrose gradient sedimentation
Hyperacetylated histones were deacetylated in vitro by SirT1 as described previously (Vaquero et al., 2004). Chromatin assembly was performed as previously described (Loyola et al., 2001), with some modifications. Briefly, a standard chromatin assembly reaction containing 2.0 μg of DNA template, 2.0 μg of hyper- or hypo-acetylated histone octamers, 0.4 μg of RSF and/or 6.0 μg of NAP1 was performed in 150 μl of 10 mM Hepes, pH 7.5, at 30°C overnight. For histone H1 incorporation, an equal molar amount of histone H1 (relative to mononucleosomes) was added prior to or after the chromatin assembly reaction.
After chromatin assembly, the samples were dialyzed against Buffer I (10 mM Hepes-KOH, pH 7.5, 1 mM EDTA) at 4°C for 24 hours, then changed to Buffer II (10 mM Hepes-KOH, pH 7.5, 0.2 mM EDTA, and 25 mM KCl) for another 24 hours at 4°C. After dialysis, the chromatin samples were loaded onto a sucrose gradient (10%-40%) in Buffer II and centrifuged at 40,000 rpm (SW60Ti rotor, Beckman) for 4 hours at 4°C. Fractions from the gradient were analyzed by agarose gel electrophoresis. The peak fractions of chromatin were analyzed by SDS-PAGE followed by silver staining and also by EM.
Electron Microscopy
Rotary shadow cast with tungsten was essentially performed as previously described (Trojer et al., 2007). The negative staining and electron cryo-microscopy experiments were performed as described in the Supplemental Data.
In vitro transcription assays
Ligand-dependent transcription assays were performed as described previously (Pavri et al., 2006) and as indicated in Figure 4C. The transcription products were analyzed by primer extension with the sequence of the [32P]-labeled primer (5′-CACAGGGCAGTGACCGCAGACTT-3′) being 200 bp downstream of the transcription start site of the PEPCK promoter.
Mapping of nucleosomal positioning in vivo and in vitro
Preparation of mononucleosomes by MNase digestion and analysis of nucleosomal positioning in vivo and in vitro are described in Supplemental Data.
ChIP assays and RT-PCR
ChIPs and RT-PCR were performed essentially as previously described (Margueron et al., 2008). Cells were treated with all-trans RA as described in Supplemental Data. Primers sequences for q-PCR are provided in Supplemental Data.
DNase I footprinting
Binding of RAR/RXR and NF1 to chromatins were analyzed by DNase I footprinting (see Supplemental Data).
Supplementary Material
Acknowledgments
We are grateful to Dr. Lynne Vales for critical reading of our manuscript. We would like to thank Drs. Richard M. Gronostajski, Daryl K. Granner, Lynne Vales, and Naoko Tanese for providing constructs for NF1, PEPCK promoter, pBR322 Ad59-100, and antibody to NF1, respectively. We also thank the colleagues in Danny Reinberg's lab for their helpful comments and suggestions. This work was supported by grants from National Institutes of Health 4R37GM037120-24 (D.R.) and partially by NIH grant 5R01GM064844 (DR) and the Howard Hughes Medical Institute (D.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyagi S, Archer TK. Dynamics of coactivator recruitment and chromatin modifications during nuclear receptor mediated transcription. Mol Cell Endocrinol. 2008;280:1–5. doi: 10.1016/j.mce.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer TK, Lefebvre P, Wolford RG, Hager GL. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992;255:1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- Belikov S, Astrand C, Holmqvist PH, Wrange O. Chromatin-mediated restriction of nuclear factor 1/CTF binding in a repressed and hormone-activated promoter in vivo. Mol Cell Biol. 2004;24:3036–3047. doi: 10.1128/MCB.24.7.3036-3047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikov S, Gelius B, Almouzni G, Wrange O. Hormone activation induces nucleosome positioning in vivo. EMBO J. 2000;19:1023–1033. doi: 10.1093/emboj/19.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M, Flaus A, Stockdale C, Rencurel C, Ferreira H, Owen-Hughes T. Histone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activities. Mol Cell. 2003;12:1599–1606. doi: 10.1016/s1097-2765(03)00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger M, Ito T, Kamakaka RT, Kadonaga JT. Assembly of regularly spaced nucleosome arrays by Drosophila chromatin assembly factor 1 and a 56-kDa histone-binding protein. Proc Natl Acad Sci USA. 1995;92:11726–11730. doi: 10.1073/pnas.92.25.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakov D, Crofts LA, Chang CP, Freedman LP. Reciprocal recruitment of DRIP/mediator and p160 coactivator complexes in vivo by estrogen receptor. J Biol Chem. 2002;277:14359–14362. doi: 10.1074/jbc.C200099200. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, McPherson CE, Bossard P, Stevens K, Cherian S, Shim EY, Clark KL, Burley SK, Zaret KS. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 1998;17:244–254. doi: 10.1093/emboj/17.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DR, Leahy P, Hu CY, Chaudhry A, Gronostajski R, Grossman G, Woods J, Hakimi P, Roesler WJ, Hanson RW. Nuclear factor I regulates expression of the gene for phosphoenolpyruvate carboxykinase (GTP) J Biol Chem. 1998;273:13387–13390. doi: 10.1074/jbc.273.22.13387. [DOI] [PubMed] [Google Scholar]
- Di Croce L, Koop R, Venditti P, Westphal HM, Nightingale KP, Corona DF, Becker PB, Beato M. Two-step synergism between the progesterone receptor and the DNA-binding domain of nuclear factor 1 on MMTV minichromosomes. Mol Cell. 1999;4:45–54. doi: 10.1016/s1097-2765(00)80186-0. [DOI] [PubMed] [Google Scholar]
- Dilworth FJ, Chambon P. Nuclear receptors coordinate the activities of chromatin remodeling complexes and coactivators to facilitate initiation of transcription. Oncogene. 2001;20:3047–3054. doi: 10.1038/sj.onc.1204329. [DOI] [PubMed] [Google Scholar]
- Dilworth FJ, Fromental-Ramain C, Yamamoto K, Chambon P. ATP-driven chromatin remodeling activity and histone acetyltransferases act sequentially during transactivation by RAR/RXR In vitro. Mol Cell. 2000;6:1049–1058. doi: 10.1016/s1097-2765(00)00103-9. [DOI] [PubMed] [Google Scholar]
- DiRenzo J, Shang Y, Phelan M, Sif S, Myers M, Kingston R, Brown M. BRG-1 is recruited to estrogen-responsive promoters and cooperates with factors involved in histone acetylation. Mol Cell Biol. 2000;20:7541–7549. doi: 10.1128/mcb.20.20.7541-7549.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell P, Ishmael JE, Avram D, Peterson VJ, Nevrivy DJ, Leid M. p300 functions as a coactivator for the peroxisome proliferator-activated receptor alpha. J Biol Chem. 1997;272:33435–33443. doi: 10.1074/jbc.272.52.33435. [DOI] [PubMed] [Google Scholar]
- Eberharter A, Ferreira R, Becker PB. Dynamic chromatin: concerted nucleosome remodelling and acetylation. Biol Chem. 2005;386:745–751. doi: 10.1515/BC.2005.087. [DOI] [PubMed] [Google Scholar]
- Finch JT, Klug A. Solenoidal model for superstructure in chromatin. Proc Natl Acad Sci USA. 1976;73:1897–1901. doi: 10.1073/pnas.73.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaus A, Owen-Hughes T. Dynamic properties of nucleosomes during thermal and ATP-driven mobilization. Mol Cell Biol. 2003;23:7767–7779. doi: 10.1128/MCB.23.21.7767-7779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin M, Cappabianca L, Kress C, Thomassin H, Grange T. Nature of the accessible chromatin at a glucocorticoid-responsive enhancer. Mol Cell Biol. 2004;24:7891–7901. doi: 10.1128/MCB.24.18.7891-7901.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G, John S, Roberts MS, Hager GL. Nucleosome positioning on the MMTV LTR results from the frequency-biased occupancy of multiple frames. Genes Dev. 1995;9:1933–1947. doi: 10.1101/gad.9.15.1933. [DOI] [PubMed] [Google Scholar]
- Gévry N, Hardy S, Jacques PE, Laflamme L, Svotelis A, Robert F, Gaudreau L. Histone H2A.Z is essential for estrogen receptor signaling. Genes Dev. 2009;23:1522–1533. doi: 10.1101/gad.1787109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar PB, Archer TK. Nuclear factor 1 is required for both hormone-dependent chromatin remodeling and transcriptional activation of the mouse mammary tumor virus promoter. Mol Cell Biol. 2003;23:887–898. doi: 10.1128/MCB.23.3.887-898.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TA, Elbi C, Parekh BS, Hager GL, John S. Chromatin remodeling complexes interact dynamically with a glucocorticoid receptor-regulated promoter. Mol Biol Cell. 2008;19:3308–3322. doi: 10.1091/mbc.E08-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Pirskanen A, Jänne OA, Palvimo JJ. Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem. 2002;277:48366–48371. doi: 10.1074/jbc.M209074200. [DOI] [PubMed] [Google Scholar]
- Kepert JF, Mazurkiewicz J, Heuvelman GL, Tóth KF, Rippe K. NAP1 modulates binding of linker histone H1 to chromatin and induces an extended chromatin fiber conformation. J Biol Chem. 2005;280:34063–34072. doi: 10.1074/jbc.M507322200. [DOI] [PubMed] [Google Scholar]
- Kornberg RD. Strcuture of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Laybourn PJ, Kadonaga JT. Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science. 1991;254:238–245. doi: 10.1126/science.254.5029.238. [DOI] [PubMed] [Google Scholar]
- Leahy P, Crawford DR, Grossman G, Gronostajski RM, Hanson RW. CREB binding protein coordinates the function of multiple transcription factors including nuclear factor I to regulate phosphoenolpyruvate carboxykinase (GTP) gene transcription. J Biol Chem. 1999;274:8813–8822. doi: 10.1074/jbc.274.13.8813. [DOI] [PubMed] [Google Scholar]
- Li X, Wong J, Tsai SY, Tsai MJ, O'Malley BW. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol Cell Biol. 2003;23:3763–3773. doi: 10.1128/MCB.23.11.3763-3773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola A, LeRoy G, Wang YH, Reinberg D. Reconstitution of recombinant chromatin establishes a requirement for histone-tail modifications during chromatin assembly and transcription. Genes Dev. 2001;15:2837–2851. doi: 10.1101/gad.937401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Maeder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Maier VK, Chioda M, Rhodes D, Becker PB. ACF catalyses chromatosome movements in chromatin fibres. EMBO J. 2008;27:817–826. doi: 10.1038/sj.emboj.7601902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally JG, Müller WG, Walker D, Wolford R, Hager GL. The glucocorticoid receptor: rapid exchange with regulatory sites in living cells. Science. 2000;287:1262–1265. doi: 10.1126/science.287.5456.1262. [DOI] [PubMed] [Google Scholar]
- Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell. 2003;115:751–763. doi: 10.1016/s0092-8674(03)00934-6. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G, Shen X, Landry J, Wu WH, Sen S, Wu C. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- Nagaich AK, Walker DA, Wolford R, Hager GL. Rapid periodic binding and displacement of the glucocorticoid receptor during chromatin remodeling. Mol Cell. 2004;14:163–174. doi: 10.1016/s1097-2765(04)00178-9. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Ghosn C, DiSepio D, Molina Y, Sutter M, Klein ES, Chandraratna RA. Retinoid-dependent recruitment of a histone H1 displacement activity by retinoic acid receptor. J Biol Chem. 1999;274:22563–22568. doi: 10.1074/jbc.274.32.22563. [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- Narlikar GJ, Phelan ML, Kingston RE. Generation and interconversion of multiple distinct nucleosomal states as a mechanism for catalyzing chromatin fluidity. Mol Cell. 2001;8:1219–1230. doi: 10.1016/s1097-2765(01)00412-9. [DOI] [PubMed] [Google Scholar]
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Pavri R, Zhu B, Li G, Trojer P, Mandal S, Shilatifard A, Reinberg D. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125:703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Piña B, Brüggemeier U, Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990;60:719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Richard-Foy H, Hager GL. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987;6:2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond TJ, Finch JT, Rushton B, Rhodes D, Klug A. Structure of the nucleosome core particle at 7 Å resolution. Nature. 1984;311:532–537. doi: 10.1038/311532a0. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, An W, Routh A, Martino F, Chapman L, Roeder RG, Rhodes D. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J Mol Biol. 2008;381:816–825. doi: 10.1016/j.jmb.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Fairall L, Huynh VA, Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fiber: evidence for a compact, interdigitated structure. Proc Natl Acad Sci USA. 2006;103:6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Rhodes D. Structure of the ‘30 nm’ chromatin fibre: a key role for the linker histone. Curr Opin Struct Biol. 2006;16:336–343. doi: 10.1016/j.sbi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Saeki H, Ohsumi K, Aihara H, Ito T, Hirose S, Ura K, Kaneda Y. Linker histone variants control chromatin dynamics during early embryogenesis. Proc Natl Acad Sci USA. 2005;102:5697–5702. doi: 10.1073/pnas.0409824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- Sharma D, Fondell JD. Ordered recruitment of histone acetyltransferases and the TRAP/Mediator complex to thyroid hormone-responsive promoters in vivo. Proc Natl Acad Sci USA. 2002;99:7934–7939. doi: 10.1073/pnas.122004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintomi K, Iwabuchi M, Saeki H, Ura K, Kishimoto T, Ohsumi K. Nucleosome assembly protein-1 is a linker histone chaperone in Xenopus eggs. Proc Natl Acad Sci USA. 2005;102:8210–8215. doi: 10.1073/pnas.0500822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- Thoma F, Koller T, Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979;83:403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojer P, Li G, Sims RJ, 3rd, Vaquero A, Kalakonda N, Boccuni P, Lee D, Erdjument-Bromage H, Tempst P, Nimer SD, Wang YH, Reinberg D. L3MBTL1, a histone-methylation-dependent chromatin lock. Cell. 2007;129:915–928. doi: 10.1016/j.cell.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Truss M, Bartsch J, Schelbert A, Haché RJ, Beato M. Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J. 1995;14:1737–1751. doi: 10.1002/j.1460-2075.1995.tb07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero A, Loyola A, Reinberg D. The constantly changing face of chromatin. Sci Aging Knowledge Environ. 2003;2003:1–16. doi: 10.1126/sageke.2003.14.re4. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Varga-Weisz PD, Becker PB. Regulation of higher-order chromatin structures by nucleosome-remodelling factors. Curr Opin Genet Dev. 2006;16:151–156. doi: 10.1016/j.gde.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Venditti P, Di Croce L, Kauer M, Blank T, Becker PB, Beato M. Assembly of MMTV promoter minichromosomes with positioned nucleosomes precludes NF1 access but not restriction enzyme cleavage. Nucleic Acids Res. 1998;26:3657–3666. doi: 10.1093/nar/26.16.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent GP, Koop R, Beato M. Complex role of histone H1 in transactivation of MMTV promoter chromatin by progesterone receptor. J Steroid Biochem Mol Biol. 2002;83:15–23. doi: 10.1016/s0960-0760(02)00253-4. [DOI] [PubMed] [Google Scholar]
- Vicent GP, Nacht AS, Smith CL, Peterson CL, Dimitrov S, Beato M. DNA instructed displacement of histones H2A and H2B at an inducible promoter. Mol Cell. 2004;16:439–452. doi: 10.1016/j.molcel.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985;42:705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.