Abstract
Background
Phytochemicals are bioactive nutrients that help reduce disease risk. High intake of these compounds is important for optimal health and prevention of disease, but quantification of these nutrients in vivo is costly and time consuming. It was examined whether an alternative, simple “phytochemical index” (PI) ratio calculation (PI=the ratio of the energy from high-nutrient phytochemical-rich foods to overall daily energy consumed [kJ phytochemical rich foods/total kJ consumed]) was related to several precursors of future disease: annual weight gain, adiposity, oxidative stress and inflammation.
Methods
This was a cross-sectional, quantitative, descriptive study (N=54, age range 18–30 years). Participants were stratified into normal weight and overweight groups. Three-day dietary records were analysed for food items, food groups, energy and the PI score at repeated time points. Blood plasma samples were analysed by colorimetric or ELISA method for cholesterol subfractions, glycated hemoglobin, total antioxidant status, lipid hydroperoxides, cytokines (interleukins 1β and 6) and C-reactive protein).
Results
PI values were higher in the overweight-obese group. Correlation values between the PI score and BMI, waist circumference, waist-to-hip ratio and plasma oxidative stress were significant. The PI score did not correlate with any cytokine levels. The PI score was a significant contributor to yearly weight gain.
Conclusions
The PI is inversely related to adiposity and oxidative stress in healthy young adults, is responsive to body weight changes. This simple, easy to administer index might be useful as a dietary target for appropriate proportion consumption of nutrient-rich foods in weight reduction or management programs.
Keywords: obesity, phytochemical, lipid peroxidation, cytokine, inflammation
Introduction
Diets containing a variety of fresh fruits and vegetables, whole grains, nuts, legumes and plant-based foods such as olive oil and wine are rich in phytochemicals, fiber, and antioxidants. (Rajaram, 2003) Phytochemicals are bioactive compounds (Liu R.H., 2004) that are linked with chronic disease risk reduction. Antioxidant imbalance is implicated in the development of cardiovascular diseases and diabetes. (Keaney et al., 2003) Potential mechanisms of protection by a phytochemical rich diet includes lowering of cardiovascular disease precursors, lowering of oxidative stress and inflammation, (Sanchez-Moreno et al., 2004) and preservation of vascular function (Esposito et al., 2004; Liu R. H., 2004) and a lower incidence of obesity.
Obesity is considered a state of chronic oxidative stress and inflammation. (Davi et al., 2002; Olusi, 2002; Keaney et al., 2003; Esposito et al., 2004; Vincent et al., 2004) Obese persons consume fewer fruits, vegetables and other nutrient-rich foods than normal weight counterparts. (Neuhouser et al., 2001) Dietary patterns low in fruit and vegetables, whole grains, beans and lean meats are associated with weight gain and larger waist circumferences (He et al., 2004; Ledikwe et al., 2004) and inflammation. Inflammation in obesity is reflected by elevations in several blood interleukins. (Davi et al., 2003; Saito et al., 2003) Low phytochemical intake is likely associated with weight gain and increased oxidative stress and inflammation, though these relationships have not yet been clarified.
Monitoring phytochemical intake in the clinical setting could have great utility in assisting patients or clients with optimizing dietary intake for optimal health and disease prevention. Quantification of phytochemicals in consumed foods or in human tissue samples is expensive, laborious and impractical for large patient or client bases, however. An alternative, simple way of monitoring phytochemical intake could be through a concept of a “phytochemical index” (PI) score originally proposed by McCarty in 2004. The premise of the PI measure is to divide the energy supplied by foods high in phytochemicals by the total energy consumed from all foods as a ratio score. A vegan diet (excluding potato products, hard liquors and refined sugars) could have a score of 100, with less optimal dietary patterns, such as those in Western diets, may range below 20. (McCarty, 2004) While there are inherent limitations to this estimate, there are highly practical clinical uses of the PI. For example, the PI could be used by clinical nutritionists to estimate the quality of diets, and for quantifying improvement in the dietary intake of phytochemical rich foods during a dietary modification program (McCarty, 2004) without expensive equipment or trained technicians. While other scores such as the prudent diet score and the Mediterranean scores (Schroder et al., 2004) exist, these encompass a score inclusive of meats and fish. Evidence suggests that incorporation of plant-based foods/drinks into Western style diets can attenuate the oxidative mechanisms related to disease onset (Perez et al. 2002), thereby stressing the importance of monitoring plant-based phytochemical rich foods. Compared with other scores which might require separate scoring for individual foods, the PI is a simple ratio value between the energy consumed as phytochemical-rich foods compared with the overall energy intake. The PI index might be an ideal, simple assessment that could provide important feedback to encourage intake of these protective foods. It is presently unknown whether the PI score is related to disease precursors of adiposity, weight gain, oxidative stress and inflammation. The purpose of this study was to determine whether the PI score is related to adiposity, weight gain, oxidative stress and inflammation.
Materials and Methods
Participants
Fifty four men and women (18–30 years of age; 19 men, 35 women) volunteered as participants for this study. All participants had to meet the following criteria prior to enrollment in the study: no participation in regular physical activity, no chronic health problems or smoking, no history of cardiovascular, metabolic or respiratory disease, and had not consumed antioxidant supplements within the past six months. Subjects were not enrolled into the study if they were using any anti-inflammatory medications, birth control medications, statins or obesity-related medications. All volunteers read, understood and signed a written informed consent statement consistent with the university policy on protection of human subjects. The protocol of the study was approved by the Institutional Review Board (IRB) for Studies Involving Human Subjects at the University of Virginia (UVa) and the Review Board for the UVa General Clinical Research Center.
Participants were placed into one of two groups based on body mass index (BMI) values: normal weight (BMI< 25 kg/m2) and overweight (≥25 kg/m2). (Brown et al., 2000) Among the participants, 26 were normal weight and 28 were overweight/obese (range of BMI values 25.7–52.5 kg/m2). The overweight/obese participants were named the “overweight group” for this study.
Anthropometrics
Height and weight were measured using a standard medical grade scale. Waist and hip girths were measured using a soft, cloth measuring tape at anatomical landmarks described by ACSM. (Medicine, 2000) Body mass index (BMI) values were determined by the following: BMI = weight (kg)/height (m)2. Body volume was estimated using air displacement plethysmography in a BodPod® device (Bod-Pod, Life Measurement Instruments, Concord, CA) corrected for thoracic gas volume as described previously; (Dempster et al., 1995) body density was calculated and the Siri equation was used to predict body fat. (Siri, 1961) Self-reported weight gain within the last year was recorded from each participant.
Dietary Assessment
Three-day dietary records were provided to each participant with standard instructions on how to complete it. The same investigator reviewed diet records with each participant and analysed the food records using Nutritionist Pro® Software (First DataBank, SanBruno CA). All food records were analysed for macronutrient, antioxidant and caloric intake. To ensure the stability of the habitual diet of the subjects, all subjects completed a second three-day dietary record (Freese et al., 2002) eight weeks later. Correlation values (r) between specific macro- and micronutrient intake between the first and second dietary records ranged from 0.7–0.93, indicating a stable intrasubject dietary pattern.
Participants were instructed to maintain their respective general eating patterns during the course of the eight week period to avoid changes in body weight. Participants were instructed to estimate servings of foods using household measurements (volume) as described in national dietary guidance documents. (USDA, 1995) Each participant received individual training sessions with the same investigator with regard to measuring technique and volume estimation using measurement cups and spoons. Picture books of portion sizes were also provided after the dietary estimation training session. (Freese et al., 2002) A vegetable serving was ½ cup of cut-up vegetables, 1 cup (240 ml) of raw leafy green vegetables or 4 oz (120 mL) of vegetable juice. A fruit serving was ½ cup (120 mL) of cut-up fruit, ¼ cup (60 mL) of dried fruit, 1 medium piece of fresh fruit, or 4 oz of (4 oz) of fruit juice. A legume serving was defined as ½ cup (120 mL) of cooked legumes, and whole grains serving was defined as 1 slice (309 g) of whole grain bread or ½ cup (120 mL) cooked whole grain product or equivalent. We applied a form of dietary assessment that has been previously used to capture fruit and vegetable consumption. (Pierce et al., 2004) For each mixed dish recipe, the participant either brought in the empty packages/cans or food inserts, recorded each component consumed with salads or sandwiches, or provided the meal selection to the nutritionist and the recipe was obtained from comparative recipes in a dietary databank.
Calculation of the Phytochemical Index (PI)
As proposed by McCarty, (McCarty, 2004) the PI was calculated as the percentage of the daily energy derived from phytochemical-rich foods divided by the total daily caloric intake (PI = (phytochemical kJ/total kJ) X 100). Foods that were included in the phytochemical-rich category included: fruits and vegetables (and prepared foods derived from these), legumes, whole grains, seeds, nuts, fruit or vegetable juices, olive oil, soy sources, wine, beer and cider. (McCarty, 2004) Once the dietary analysis was performed as described above, the diet records were re-analysed for phytochemical rich foods and the PI was determined.
To determine whether the sources of phytochemicals were different between the normal weight and overweight-obese groups, each dietary record was analysed for specific food distributions (Pierce et al., 2004) within the phytochemical-rich classification. Specifically, food groupings were identified on each dietary record as green leafy vegetables (e.g., spinach, kale), lettuce, green vegetables (e.g., broccoli, green beans), carrots, orange squashes, onions, olive oil, alcohol (e.g., red wine, cider or beer), seeds, nuts, whole grain breads, brown rice, cold cereals, hot cereals (e.g., oatmeal), fresh garlic, dried fruit, legumes (e.g., chickpeas, lentils), soy (e.g., milk or bean curd) and tea.
Biochemical Analysis
Following a 12 hour fast, blood samples were collected from the antecubital fossa of a forearm vein into EDTA-treated vacutainer tubes (Becton Dickinson; Franklin Lakes NJ). Blood samples were analysed for oxidative stress (lipid peroxidation; PEROX), total antioxidant status (TAS), cholesterol and cholesterol subfractions. Another portion of whole blood was analysed for glucose and glycated hemoglobin (HbA1c) levels. A final portion was used to measure inflammatory cytokines IL-1β and IL-6, and hsCRP. A portion of the blood was immediately centrifuged at 1500 X g for 5 min to separate plasma from red blood cell pellets. Plasma samples were immediately frozen and stored at −70°C until analysis.
Cholesterol samples were analysed by the UVa Health System Clinical Core and Toxicology Laboratories using standard laboratory procedures. Plasma cholesterol subfractions (total cholesterol, high density lipoproteins (HDL-C), triglycerides) were assessed using an Olympus AU640 Chemistry Analyser. Briefly, cholesterol esters were hydrolysed by cholesterol esterase, and the free cholesterol produced was oxidised by cholesterol oxidase to yield hydrogen peroxide as a by-product. Hydrogen peroxide was coupled with 4-aminoantipyrine and phenol in the presence of peroxidase to generate a chromophore that was measured spectrophotometrically at 600nm (Olympus cholesterol calibrator, Cat#DR0040). HDL-C was isolated by removal of low density lipoproteins and chylomicrons from the plasma samples by a mixture of polymers and polyanions. The hydrogen peroxide chromophore was then measured in the same method described for the cholesterol (Genzyme HDL-C calibrator, Cat# 80-4529-00). Low density lipoprotein (LDL-C) concentrations were estimated from the formula: LDL-C = total cholesterol − HDL-C − (triglycerides/5).
Blood glucose was assessed using an Olympus AU640 procedure, in which glucose was phosphorylated by hexokinase in the presence of adenosine triphosphate and magnesium. The resultant glucose-6-phosphonate (G-6-P) dehydrogenase oxidised G-6-P to 6-phosphogluconate and reduced nicotinamide adenine dinucleotide (NAD+) to NADH. The change in absorbance at 340/380 was proportional to the amount of glucose in the sample (Olympus Glucose Reagent, Calibrator Cat# DR0040). HbA1c was analysed using automated high performance liquid chromatography (Tosoh G7 Automated HPLC Analyser, using TSKgel G& HSi elution columns). All samples were performed in duplicate.
Two measures were used to represent the state of oxidative stress, a biomarker of oxidative damage (lipid hydroperoxides) and an overall level of plasma antioxidants (total antioxidant status, [including phytochemicals such as carotenoids, phenolics, alkaloids, organosulfur compounds and nitrogen containing compounds]). Lipid hydroperoxides (PEROX) were quantified using the colorimetric, spectrophotometric ferrous oxidation/xylenol orange technique previously reported, where cumene hydroperoxide was used as the standard for this assay. (Hermes-Lima et al., 1995) Samples were read spectrophotometrically at 580nm. All samples were performed in triplicate. The coefficient of variation for this assay was 4.5%. PEROX values across the age spectrum using this technique vary depending on the obesity status of the participant. For example, PEROX values range from 0.32–0.8 nmol/ml for younger adults at rest to post-exercise, (Vincent et al., 2004) and from 2.3–3 nmol/ml in older non-obese and obese adults. (Vincent et al., 2005)
Oxidative stress is influenced in part by the total available antioxidant pool in the plasma. As an estimate of the total antioxidant capacity of the plasma, a colorimetric commercial kit was used for total antioxidant status (Randox Laboratories, TAS Cat#NX2332). In brief, 2,2-azion-di-[3-ethylbenzenthiazoline sulphonate])(ABTS) was incubated with a peroxidase and hydrogen peroxide to produce a radical cation ABTS. The suppression of the ABTS radical in vitro was proportional to the antioxidant level in the plasma samples. Samples were read in two batches at 600nm on a spectrophotometer. Samples were expressed in antioxidant capacity in mmol/l plasma. All samples were performed in duplicate. The interassay coefficient of variation for this analysis was 4%.
Interleukins 1 and 6 (IL-1β, IL-6) and high sensitivity C-reactive protein (hs-CRP) were measured by the UVa General Clinical Research Core Laboratory. IL-1β and IL-6 were measured using commercial enzyme-linked immunosorbent assays (ELISA, Quantikine R&D Systems, Minneapolis MN). Measurements of hs-CRP were measured using an Immulite 2000 ELISA (Siemens Medical Solutions Diagnostics, Los Angeles, CA). All samples were performed in duplicate.
Statistical Anlaysis
All data are expressed as means ± standard deviation (SD). Data were analysed using SPSS software (v. 17.0, Chicago IL). For the two group comparisons, descriptive, physiologic, dietary and biochemical variables were analysed using t-tests. Given that this was an exploratory study, Pearson correlations were performed among PI values, adiposity (BMI, body fat, waist circumference), weight gain within the last year, oxidative stress measures (PEROX, TAS) and blood cholesterol subfractions, glucose and HbA1c. Hierarchal regression models were generated to determine the contribution of the PI score to weight gain within the past year. The level of significance was set at 0.05.
Results
Characteristics of the subject groups are shown in Table 1. Body mass, BMI, waist circumference, waist-to-hip ratio, body fat percentage, fat and fat free mass values were significantly greater in the overweight/obese group (p<0.05). BMI did not change significantly from the first to the week 8 dietary assessment period for the normal weight participants (22.0 ± 0.3 to 22.1 ± 1.7 kg/m2) or the overweight participants (34.0 ± 1.5 to 33.0 ± 1.4 kg/m2). Resting blood glucose and weight gain accrued within the last year were 9% and an average of 2.2kg higher, respectively, in the overweight group compared with the normal weight group (p<0.05). A total of 7.6% of the normal weight group experienced either minor weight loss or weight gain over the past year (under 4.5 kg); a total of 46% of the overweight group experienced a weight gain or loss (2.2–22.7 kg weight gain range and 4.5–9.0 kg weight loss range) over the last year. HDL-C values were lower in the overweight group than the normal weight group (p<0.05).
Table 1.
Baseline characteristics of normal weight and overweight groups. Means ± SD are shown.
| Variable | Normal weight (N=26) | Overweight (N=28) |
|---|---|---|
| Age (yr) | 23.3 ± 2.7 | 24.1 ± 3.7 |
| Height (cm) | 171.4 ± 8.7 | 171.8 ± 8.4 |
| Weight (kg) | 64.9 ± 10.8 | 100.4 ± 24.2* |
| BMI (kg/m2) | 22.0 ± 2.3 | 34.0 ± 7.4 * |
| Waist circumference (cm) | 73.1 ± 7.1 | 101.0 ± 15.5 * |
| WHR | 0.76 ± 0.05 | 0.85 ± 0.09 * |
| Body fat (%) | 21.1 ± 6.3 | 39.2 ± 7.6 * |
| Body fat mass (kg) | 13.6 ± 12.1 | 43.5 ± 17.1 * |
| Body fat free mass (kg) | 51.1 ± 12.2 | 56.1 ± 10.4 * |
| Total cholesterol (mmol/L) | 4.1 ± 0.6 | 4.2 ± 1.0 |
| HDL-C (mmol/L) | 1.5 ± 0.3 | 1.2 ± 0.3 * |
| LDL-C (mmol/L) | 2.3 ± 0.6 | 2.5 ± 0.8 |
| Glucose (mmol/L) | 4.4 ± 0.4 | 4.8 ± 0.9 * |
| HbA1c (%) | 5.0 ± 0.2 | 5.0 ± 0.3 |
| Weight gain within 1 year (kg) | 0.2 ± 4.7 | 2.4 ± 12.2 * |
different from non-obese group at p<0.05
BMI = body mass index, WHR = waist to hip ratio, HDL-C = high density lipoprotein, LDL = low density lipoprotein, HbA1c = glycated hemoglobin
Daily energy intake (kJ), and carbohydrate and protein intakes were not different between the groups as shown in Table 2 (p>0.05). However, the overweight group consumed 60% more fat (kJ/day) compared with the normal weight group (p<0.05). In addition, manganese and β-cryptoxanthin intakes were 42% and 45% lower in the overweight group compared with the normal weight group (p<0.05). The obese group had a PI value 10.3% lower than that of the normal weight group (p<0.05). When a subanalysis was performed to separate out BMI quartiles (≤20, 20–25, 25.1–29.9, ≥30 kg/m2), PI values were progressively lower from 28.6, 21.6, 18.8 and 9.5 for each BMI quartile increase, respectively (p=0.007). The PI score was also positively correlated with the total carotenoid intake (a sum of all carotenoids carotenes, lutien, lycopene, lutein (+ zeaxanthin), β-cryptoxanthin) (r = 0.303; p = 0.026).
Table 2.
Average three day dietary intakes of normal weight and overweight young adults. Values are means ± SD.
| Normal weight (N=26) | Overweight (N=28) | |
|---|---|---|
| Total energy (kJ/day) | 8827 ± 2704 | 9365 ± 3219 |
| Protein (kJ/day) | 1293 ± 514 | 1469 ± 648 |
| Carbohydrates (kJ/day) | 4654 ± 1331 | 4818 ± 1687 |
| Fat (kJ/day) | 1364 ± 695 | 2189 ± 1553 * |
| Fiber (g/day) | 17.6 ± 7.9 | 15.5 ± 5.9 |
| Vitamin E (RE) | 891.5 ± 760.1 | 883.1 ± 441.5 |
| Vitamin E (mg) | 7.8 ± 7.2 | 6.0 ± 2.5 |
| Vitamin C (mg) | 117.7 ± 111.4 | 92.1 ± 53.9 |
| α-tocopherol (mg) | 3.1 ± 3.1 | 2.6 ± 1.7 |
| Zinc (mg) | 8.1 ± 5.2 | 9.3 ± 4.7 |
| Copper (mg) | 0.9 ± 0.4 | 0.8 ± 0.3 |
| Manganese (mg) | 2.6 ± 1.8 | 1.5 ± 0.7 * |
| Selenium (μg) | 73.7 ± 49.8 | 73.9 ± 42.7 |
| Chromium (mg) | 0.03 ± 0.03 | 0.03 ± 0.02 |
| Thiamine (mg) | 1.6 ± 0.8 | 1.3 ± 0.7 |
| Niacin (mg) | 19.1 ± 9.2 | 19.3 ± 9.4 |
| Riboflavin (mg) | 1.6 ± 0.7 | 1.5 ± 0.7 |
| α–carotene (μg) | 561 ± 117 | 199 ± 98 * |
| β-carotene (μg) | 1824 ± 2800 | 896 ± 837 * |
| Lutein (+ Zeaxanthin)(μg) | 1283 ± 653 | 1428 ± 243 |
| β-cryptoxanthin (μg) | 157 ± 246 | 87 ± 112 * |
| Lycopene (μg) | 899 ± 136 | 1443 ± 184 |
| kJ from Phytochemical Rich foods | 1946 ± 1264 | 1171 ± 1042 * |
| Phytochemical Index | 23.5 ± 13.8 | 13.2 ± 11.2 * |
different than the non-obese group at p<0.05.
The average daily intakes of various phytochemical-rich foods are shown in Table 3. Among the food groups, overweight-obese persons consumed fewer servings of green leafy vegetables, green vegetables, fruits, dried fruits and whole grain breads than their normal weight counterparts (all p<0.05). When a subanalysis was performed to separate out BMI quartiles (≤20, 20–25, 25.1–29.9, ≥30 kg/m2), sequentially lower intakes of these same food groups were found.
Table 3.
Average daily food patterns between normal weight and overweight young adults. Values are means ± SD.
| Normal weight | Overweight | |
|---|---|---|
| Green leafy vegetables (servings) | 0.61 ± 0.9 | 0.16 ± 0.3 * |
| Lettuce (servings) | 0.76 ± 0.7 | 0.47 ± 0.8 |
| Green vegetables (servings) | 1.42 ± 1.2 | 0.75 ± 1.2 * |
| Carrots (servings) | 0.37 ± 0.7 | 0.24 ± 0.4 |
| Orange Squashes (servings) | 0.04 ± 0.2 | 0.23 ± 1.3 |
| Onions (Tbsp) | 1.24 ± 2.1 | 2.01 ± 3.2 |
| Other vegetables (servings) | 0.86 ± 1.0 | 0.87 ± 0.9 |
| Fresh tomatoes (servings) | 0.85 ± 0.9 | 0.62 ± 0.1.7 |
| Tomato sauces, ketchup (Tbsp) | 2.42 ± 3.7 | 3.13 ± 0.3.5 |
| Fruit (servings) | 2.66 ± 2.3 | 1.16 ± 1.0 * |
| Fruit juice (fl os.) | 10.08 ± 14.4 | 6.21 ± 8.6 |
| Whole grain breads (servings) | 2.28 ± 2.0 | 0.58 ± 0.6 * |
| Brown rice (servings) | 0.16 ± 0.5 | 0.16 ± 0.4 |
| Cold cereals (servings) | 0.70 ± 1.3 | 0.30 ± 0.4 |
| Hot cereals (servings) | 0.02 ± 0.08 | 0.06 ± 0.23 |
| Seeds (Tbsp) | 0.18 ± 0.56 | 0.1 ± 0.42 |
| Nuts (servings) | 0.70 ± 1.51 | 0.11 ± 0.43 |
| Dried fruit (servings) | 0.63 ± 0.86 | 0.02 ± 0.11 * |
| Olive oil (Tbsp) | 0.73 ± 1.52 | 0.58 ± 0.40 |
| Red wine (fl os.) | 0.17 ± 0.43 | 0.11 ± 0.63 |
| Beer (fl os.) | 0.96 ± 4.31 | 0.42 ± 2.56 |
| Beans (servings) | 0.26 ± 0.42 | 0.21 ± 0.66 |
| Legumes (servings) | 0.28 ± 0.52 | 0.1 ± 0.31 |
| Fresh garlic (cloves) | 0.26 ± 0.56 | 0.21 ± 0.59 |
| Soy (servings) | 0.52 ± 1.03 | 0.33 ± 0.67 |
| Tea (fl.os.) | 0.8 ± 3.7 | 1.5 ± 2.1 |
different from non-obese at p<0.05.
Fasting plasma PEROX values were nearly threefold higher in the overweight group compared with the normal weight group (Table 4; p<0.05). When a subanalysis was performed to separate out BMI quartiles (≤20, 20–25, 25.1–29.9, ≥30 kg/m2), PEROX values were 2.4, 2.16, 2.7 and 2.8 nmol/ml (p = 0.044). Basal fasting TAS concentrations were not significantly different between the groups (p>0.05). IL-6 and hs-CRP values were 105–223% higher in the overweight group compared with the normal weight group (p<0.05). When a subanalysis was performed to separate out BMI quartiles (≤20, 20–25, 25.1–29.9, ≥30 kg/m2), IL-6 values were 1.2, 1.4, 2.4, 3.1 pg/ml, respectively (p=0.01). Hs-CRP values were 0.23, 0.08, 0.18, 0.79 mg/dl for the same BMI quartiles (p = 0.0001). The group difference between IL-1β values was not significant (p=0.15).
Table 4.
Oxidative stress and inflammatory markers in normal weight and overweight groups. Values are means ± SD.
| Normal weight | Overweight | |
|---|---|---|
| PEROX (nmol/ml) | 0.042 ± 0.11 | 0.16 ± 0.25 * |
| TAS (nmol/ml) | 1.40 ± 0.32 | 1.27 ± 0.33 |
| IL-1β (pg/ml) | 0.04 ± 0.10 | 0.15 ± 0.37 |
| IL-6 (pg/ml) | 1.33 ± 0.91 | 2.72 ± 1.22 * |
| hs-CRP (mg/dL) | 0.13 ± 0.20 | 0.42 ± 0.45 * |
different from normal weight at p<0.05.
PEROX = lipid hydroperoxide, TAS = total antioxidant status, IL = interleukin, hs-CRP = high sensitivity C-reactive protein
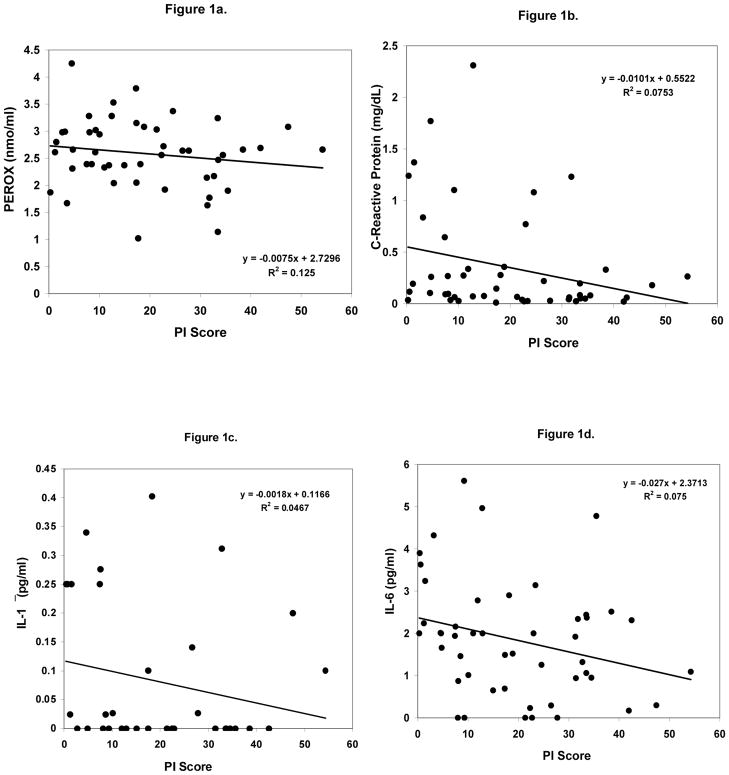
Among all participants, significant inverse correlations were found between the PI value and BMI (r = −0.421), body weight (r = −0.417), waist circumference (r = −0.418), waist-to-hip ratio (r = −0.358) and body fat percent (r = −0.323) (all p<0.05). PEROX values were found to be correlated with the PI value (p<0.05) (Figure 1a). However, the correlations between the inflammatory cytokines (IL-1β, IL-6 and hs-CRP), blood cholesterol subfractions, HbA1c, blood glucose or TAS and the PI were not significant (p>0.05) (Figures 1b–d). An inverse association between the PI index and weight gain over the last year was significant (r = −0.369, p<0.05).
Figure 1.
Scatter plots of the PI score and plasma markers of oxidative stress and inflammation. The closed circles represent the normal weight group and the open triangles represent the overweight/obese group. 1a). The relationship between PEROX and the PI score (p = −0.346; p<0.05); 1b). The relationship between hs-CRP and the PI score; 1c). The relationship between IL-1β and the PI score; and 1d). The relationship between IL-6 and the PI score.
Hierarchal regression modeling showed that after controlling for age, gender and BMI values in this cohort (R2 = 0.202), the PI was found to be a significant contributor to the model predicting weight gain within the past year (p<0.05) (Table 5). The PI score explained an additional 8% of the variance in the yearly weight gain (p=0.034). Thus, the overall model with all variables explained a total of 28.2% of the yearly weight gain (p<0.002). These data suggest that the change in body weight is in part influenced by phytochemical-rich food intake.
Table 5.
Hierarchal regression analysis for the yearly weight gain in normal weight and overweight young adults.
| Step | variable | r | R2 | p-value Overall model |
|---|---|---|---|---|
| 1 | Gender | .244 | .059 | .076 |
| 2 | Age | .290 | .084 | .106 |
| 3 | BMI | .461 | .202 | .007* |
| 4 | PI score | .531 | .282 | .002* |
denotes a significant contributor to the model
Each step includes the listed variable with the addition of the previous factor(s).
Discussion
The PI score was inversely related with several indices of adiposity, weight gain and oxidative stress, but not with plasma total antioxidant status or inflammation. These findings are similar to previous data showing that prudent dietary patterns are associated with low BMI and waist circumference. (Ledikwe et al., 2004; Schroder et al., 2004) Despite similar caloric intakes between the study groups, the overweight group consumed fewer daily servings of green leafy vegetables, green vegetables, fresh fruit, dried fruit and whole grain breads than the normal weight group. Consequently, β-cryptoxanthin, manganese and other carotenoid intakes were lower in the overweight than the normal weight group. There was variance with the PI scores for both groups, and this variance might be due to error with self-reporting, and personal variations in dietary preferences (including phytochemical-rich foods with fruit, vegetable, nut, legume content). McCarty (McCarty, 2004) predicted that the average PI scores for adults on the typical American diet may not even exceed 20. In the present study, the PI scores for the normal weight and overweight groups were 23.5 and 13.2, respectively. All these young adults could benefit from increased consumption of phytochemical rich foods (emphasizing the overweight group).
In a Spanish cohort of 4709 participants, (Schroder et al., 2004) higher “Mediterranean diet” scores (vegetables, fruits, nuts, fish, meat, cereals, olive oil, and wine) were associated with lower BMIs. In the Nurses’ Health Study cohort (N=74,063), participants with the largest increase in fruit and vegetable intake over time had a lesser risk of becoming obese compared with those who had the largest decrease in fruit-vegetable intake over time. (He et al., 2004) Ledikwe et al. (Ledikwe et al., 2004) reported that nutrient-dense dietary patterns (i.e. cereals, dark green/yellow vegetables, other vegetables, citrus/melons/berries, fruit juices, other fruits, milk, poultry, fish, and beans) are associated with a lower waist circumference and higher plasma vitamin levels compared with low nutrient dense diets in rural older adults (N=179).
In this study, PI score differences were not due to alcohol, tea, ketchup, garlic, onions or other foods which contain many phytochemicals but do not contribute much to the energy intake. Hence, PI differences are related to the overall numbers of servings of major food groups. Despite the inherent limitations, the PI was a significant contributor to weight gain reported within the past year. Further studies are required to corroborate these findings. The PI may be a useful dietary target for weight loss, where there is less focus on energy, but increased intake of these high-nutrient lower energy foods over time. Monitoring the PI score over time might motivate some overweight individuals. While there are no comparative data for an ideal PI score, replacement of popular Western diet components (fried potatoes, red meat, refined sugars, pre-packed foods and snacks, processed fats) with plant-based whole food could shift the typical PI scores closer to 0.7. While it is not realistic to expect that all overweight individuals will adopt an entirely plant-based phytochemical rich diet and attain a PI score of 1.0, overweight individuals can improve this score with experience, and reduce disease risk.
Fruit and vegetable intake and corresponding serum concentrations of tocopherol and phytochemicals are lower in obese than normal weight adults. (Neuhouser et al., 2001) In one study, wine and fruit vegetable intake were associated with lower antioxidant capacity and lower levels of oxidative damage to cholesterol and DNA. (Pérez et al., 2002) Even when obese children consume similar levels of fruits and vegetables as non-obese children, their serum levels of β-carotene and α-tocopherol are lower. (Strauss, 1999) In this sample, there were lower intakes of β-cryptoxanthin and α and β carotene in the overweight group, and oxidative stress was inversely related to the PI. Estimated dietary intakes of phytochemicals/antioxidant micronutrients are positively correlated with serum levels of various antioxidant nutrients and phytochemicals. (Neuhouser et al., 2001; Pierce et al., 2004) These data show that plasma TAS values were not correlated with PI scores, however. This finding contrasts previous findings that show obese individuals have relatively low TAS (Fenkci et al., 2003; Molnar et al., 2004) Because TAS represents a composite measure of numerous antioxidants in the plasma, other plasma antioxidants may have been elevated in the healthy overweight participants of this study to compensate for specific lowered dietary antioxidants and phytochemicals in the overweight group, such as glutathione (Faber et al., 2002). Also, the sources of phytochemicals may vary even among persons with the same PI score. For example, if two persons with identical PI values were compared, one person may consume more nuts, fruits and vegetables, whereas the second person may consume more tomato sauce, carrots and soy. The actual phytochemicals consumed would vary and may not contribute equally to the antioxidant TAS defense. Further investigation of the PI index in relation to specific plasma phytochemical concentrations is warranted to clarify these issues. Finally, increased dietary fat intake can induce inflammation and oxidative stress, (Carroll et al., 2003) processes that tax the TAS. Variations in inflammatory cytokines and fat intake among participants may independently affect compounds that comprise TAS.
Despite higher IL-6 and hs-CRP levels in the overweight compared to the healthy weight participants, these cytokines were not related to the PI score. The phytochemical intake per se might not strongly influence systemic inflammation in overweight persons. Similarly, Freese et al. (Freese et al., 2004) reported that six weeks of low fruit, vegetable, berry and apple intake did not adversely affect CRP levels. Gao et al. (Gao et al., 2004), however, showed an inverse dose-response association between fruit and vegetable intake and plasma CRP in Hispanic and non-Hispanic white elders. Other studies (Nurses Health Study, Multi-Ethnic Study of Atherosclerosis) also showed inverse relationships between prudent dietary intake and C-reactive protein, (Lopez-Garcia et al., 2004) and between fruit-whole grains-fruit, legumes and vegetables-fish and IL-6 and C-reactive protein (Nettleton et al., 2006). In another large cross sectional study (N=521), prudent eating patterns were associated with lower systemic levels of C-reactive protein but not IL-6. (Esmaillzadeh et al., 2007) The discrepancies among these studies may be attributed to the assessment of food intake (PI versus specific foods, food frequency questionnaires, recall), endogenous antioxidant defenses and co-morbidities, participant ages and variations due to our smaller sample size compared with previous studies.
The new PI analysis is inversely related with adiposity, weight gain and oxidative stress in healthy young adults. The PI method was not significantly related with markers of inflammation or total antioxidant status, however. This index might have clinical utility as a dietary target for appropriate proportion consumption of nutrient-rich foods in weight reduction or management programs, and is easy to perform and inexpensive.
Acknowledgments
Funding: The project described was supported in part by Grant Numbers T32-AT00052 and K30-AT-00060 from the National Center for Complementary and Alternative Medicine (NCCAM), and from the UVa General Clinical Center Grant Number 5 M01 RR000847 and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
Footnotes
There is no conflict of interest to declare.
Authorship details:
Dr Heather Vincent was the study PI, and was involved in the study design, all data collection and entry, data, database generation, some statistical analysis and interpretation and prepared the primary draft of the manuscript.
Dr. Cheryl Bourguignon was involved with study design, statistical analysis and review, statistical interpretation and manuscript preparation.
Dr. Ann Gill Taylor was involved with study design, data collection oversight and data entry, statistical interpretation and manuscript preparation.
References
- Rajaram S. The effect of vegetarian diet, plant foods, and phytochemicals on hemostasis and thrombosis. American Journal of Clinical Nutrition. 2003;78(3 Suppl):552S–558S. doi: 10.1093/ajcn/78.3.552S. [DOI] [PubMed] [Google Scholar]
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. Journal of Nutrition. 2004;134(12 Suppl):347S–348S. doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, Framingham S. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23(3):434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Cano MP, deAncos B, Plaza L, Olmedilla B, Granado F, Martin A. Consumption of high-pressurized vegetable soup increases plasma vitamin C and decreases oxidative stress and inflammatory biomarkers in healthy humans. Journal of Nutrition. 2004;134(11):3021–3025. doi: 10.1093/jn/134.11.3021. [DOI] [PubMed] [Google Scholar]
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. Journal of Nutrition. 2004;134(12 Suppl) doi: 10.1093/jn/134.12.3479S. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, DiPalo C, Giugliano D, Giugliano F, D’Armiento M, D’Andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Journal of the American Medical Association. 2004;292(12):1490–1492. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Davi G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–2014. doi: 10.1001/jama.288.16.2008. [DOI] [PubMed] [Google Scholar]
- Olusi SO. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. International Journal of Obesity & Related Metabolic Disorders. 2002;26(9):1159–1164. doi: 10.1038/sj.ijo.0802066. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Morgan JW, Vincent KR. Obesity exacerbates oxidative stress levels after acute exercise. Medicine & Science in Sports & Exercise. 2004;36(5):772–779. doi: 10.1249/01.mss.0000126576.53038.e9. [DOI] [PubMed] [Google Scholar]
- Saito I, Yonemasu K, Inami F. Association of body mass index, body fat, and weight gain with inflammation markers among rural residents in Japan. Circulation Journal. 2003;67(4):323–329. doi: 10.1253/circj.67.323. [DOI] [PubMed] [Google Scholar]
- Davi G, Chiarelli F, Santilli F, Pomilio M, Vigneri S, Falco A, Basili S, Ciabattoni G, Patrono C. Enhanced lipid peroxidation and platelet activation in the early phase of type 1 diabetes mellitus: role of interleukin-6 and disease duration. Circulation. 2003;107(25):3199–3203. doi: 10.1161/01.CIR.0000074205.17807.D0. [DOI] [PubMed] [Google Scholar]
- Neuhouser ML, Rock CL, Eldridge AL, Kristal AR, Patterson RE, Cooper DA, Neumark-Sztainer D, Cheskin LJ, Thornquist MD. Serum concentrations of retinol, alpha-tocopherol and the carotenoids are influenced by diet, race and obesity in a sample of healthy adolescents. Journal of Nutrition. 2001;131(8):2184–2191. doi: 10.1093/jn/131.8.2184. [DOI] [PubMed] [Google Scholar]
- He K, Hu FB, Colditz GA, Manson JE, Willett WC, Liu S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28(12):1569–1574. doi: 10.1038/sj.ijo.0802795. [DOI] [PubMed] [Google Scholar]
- Ledikwe JH, Smiciklas-Wright H, Mitchell DC, Miller CK, Jensen GL. Dietary patterns of rural older adults are associated with weight and nutritional status. Journal of the American Geriatrics Society. 2004;52(4):589–595. doi: 10.1111/j.1532-5415.2004.52167.x. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Proposal for a dietary “phytochemical index”. Medical Hypotheses. 2004;63(5):813–817. doi: 10.1016/j.mehy.2002.11.004. [DOI] [PubMed] [Google Scholar]
- Schroder H, Marrugat J, Vila J, Covas MI, Elosua R. Adherence to the traditional mediterranean diet is inversely associated with body mass index and obesity in a spanish population. Journal of Nutrition. 2004;134(12):3355–3361. doi: 10.1093/jn/134.12.3355. [DOI] [PubMed] [Google Scholar]
- Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, Obarzanek E, Ernst ND, Horan M. Body mass index and the prevalence of hypertension and dyslipidemia.[see comment] Obesity Research. 2000;8(9):605–619. doi: 10.1038/oby.2000.79. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 6. Philadelphia: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- Dempster P, Aitkins S. A new air displacement method for the determination of human body composition. Medicine & Science in Sports & Exercise. 1995;27:1692–1697. [PubMed] [Google Scholar]
- Siri WE. Body composition from fluid spaces and density: analysis of methods. In: BJ, HA, editors. Techniques for measuring body composition. Washington DC: National Academy of Sciences; 1961. pp. 223–244. [Google Scholar]
- Freese R, Alfthan G, Jauhiainen M, Basu S, Erlund I, Salminen I, Aro A, Mutanen M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. American Journal of Clinical Nutrition. 2002;76(5):950–960. doi: 10.1093/ajcn/76.5.950. [DOI] [PubMed] [Google Scholar]
- USDA, United States Department of Health and Human Services. Home Health and Garden Bulletin no 232. Washington, DC: 1995. Dietary guidelines for Americans. [Google Scholar]
- Pierce JP, Newman VA, Flatt SW, Faerber S, Rock CL, Natarajan L, Caan BJ, Gold EB, Hollenbach KA, Wasserman L, Jones L, Ritenbaugh C, Stefanick ML, Thomson CA, Kealey S Women’s Healthy E, Living Study G. Telephone counseling intervention increases intakes of micronutrient- and phytochemical-rich vegetables, fruit and fiber in breast cancer survivors. Journal of Nutrition. 2004;134(2):452–458. doi: 10.1093/jn/134.2.452. [DOI] [PubMed] [Google Scholar]
- Hermes-Lima M, Willmore WG, Storey KB. Quantification of lipid peroxidation in tissue extracts based on Fe(III)xylenol orange complex formation. Free Radical Biology & Medicine. 1995;19(3):271–280. doi: 10.1016/0891-5849(95)00020-x. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Vincent KR, Bourguignon C, Braith RW. Obesity and postexercise oxidative stress in older women. Medicine & Science in Sports & Exercise. 2005;37(2):213–219. doi: 10.1249/01.mss.0000152705.77073.b3. [DOI] [PubMed] [Google Scholar]
- Russell AP, Gastaldi G, Bobbioni-Harsch E, Arboit P, Gobelet C, Deriaz O, Golay A, Witztum JL, Giacobino JP. Lipid peroxidation in skeletal muscle of obese as compared to endurance-trained humans: a case of good vs. bad lipids? FEBS Letters. 2003;551(1–3):104–106. doi: 10.1016/s0014-5793(03)00875-5. [DOI] [PubMed] [Google Scholar]
- Pérez DD, Strobel P, Foncea R, Díez MS, Vásquez L, Urquiaga I, Castillo O, Cuevas A, San Martín A, Leighton F. Wine, diet, antioxidant defenses, and oxidative damage. Annals of the New York Academy of Sciences. 2002;957:136–145. doi: 10.1111/j.1749-6632.2002.tb02912.x. [DOI] [PubMed] [Google Scholar]
- Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. Journal of Pediatrics. 1999;134(2):160–165. doi: 10.1016/s0022-3476(99)70409-9. [DOI] [PubMed] [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertility & Sterility. 2003;80(1):123–127. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- Molnar D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. International Journal of Obesity & Related Metabolic Disorders. 2004;28(10):1197–1202. doi: 10.1038/sj.ijo.0802719. [DOI] [PubMed] [Google Scholar]
- Faber P, Johnstone AM, Gibney ER, Elia M, Stubbs RJ, Duthie GG, Calder AG, Lobley GE. The effect of rate of weight loss on erythrocyte glutathione concentration and synthesis in healthy obese men. Clinical Science. 2002;102(5):569–577. doi: 10.1042/cs1020569. [DOI] [PubMed] [Google Scholar]
- Carroll MF, Schade DS. Timing of antioxidant vitamin ingestion alters postprandial proatherogenic serum markers. Circulation. 2003;108(1):24–31. doi: 10.1161/01.CIR.0000074221.68903.77. [DOI] [PubMed] [Google Scholar]
- Freese R, Vaarla O, Turpeinen AM, Mutanen M. No difference in platelet activation or inflammation markers after diets rich or poor in vegetables, berries and apple in healthy subjects. European Journal of Nutrition. 2004;43(3):175–182. doi: 10.1007/s00394-004-0456-4. [DOI] [PubMed] [Google Scholar]
- Gao X, Bermudez OI, Tucker KL. Plasma C-reactive protein and homocysteine concentrations are related to frequent fruit and vegetable intake in Hispanic and non-Hispanic white elders. Journal of Nutrition. 2004;134(4):913–918. doi: 10.1093/jn/134.4.913. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. American Journal of Clinical Nutrition. 2004;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, Jacobs DRJ. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) American Journal of Clinical Nutrition. 2006;83(6):1369–1379. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. Journal of Nutrition. 2007;137(4):992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]