Abstract
Hypothalamic obesity is a potential sequela of craniopharyngioma, arising from hypothalamic damage inflicted by either the tumor and/or its treatment. The marked weight gain that characterizes this disorder appears to result from impaired sympathoadrenal activation, parasympathetic dysregulation, and other hormonal and hypothalamic disturbances that upset the balance between energy intake and expenditure. Given hypopituitarism is commonly present, careful management of hormonal deficits is important for weight control in these patients. In addition, diet, exercise, and pharmacotherapy aimed at augmenting sympathetic output, controlling hyperinsulinism, and promoting weight loss have been used to treat this disease, but these measures rarely lead to sustained weight loss. While surgical interventions have not routinely been pursued, emerging data suggests that surgical weight loss interventions including Roux-en-Y gastric bypass can be safely and effectively used for the management of hypothalamic obesity in patients with craniopharyngioma.
Keywords: Craniopharyngioma, Hypopituitarism, Hypothalamic obesity, Gastric bypass
Introduction
A link between hypothalamic injury and obesity was first described over a century ago when Dr. Fröhlich reported a case of a 14-year-old boy with rapid weight gain, hypogonadism, and vision loss in the setting of a hypophyseal tumor destroying the body of the sphenoid and dorsum of the sella [1]. Since then hypothalamic obesity (HO) has been associated with a number of disease processes that structurally impact the hypothalamus including trauma, aneurysms, infiltrative diseases, and tumors as well as with an array of genetic syndromes like Prader-Willi syndrome (PWS), leptin and leptin receptor deficiency, proopiomelanocortin mutations, and melanocortin four receptor (MC4R) mutations, which result in hypothalamic dysfunction. Of the tumors, craniopharyngioma (and/or its treatment) is now regarded as the most common cause of HO in humans. Craniopharyngiomas comprise between 1.2 and 4% of all brain tumors [2]. While these histologically benign tumors arising from the remnants of Rathke's pouch are associated with high overall survival rates, they are often the source of significant morbidity. Up to 52% of pediatric craniopharyngioma (CP) patients develop obesity following tumor resection and many suffer from recalcitrant hyperphagia and weight related reductions in quality of life [3]. The development of obesity in these patients stems from hypothalamic damage arising from the tumor itself, or from surgical and radiotherapeutic interventions. The hypothalamic arcuate nucleus is a critical mediator of energy homeostasis, housing several neuronal populations including proopiomelanocortin (POMC) and Agouti-related peptide (AGRP)/Neuropeptide Y (NPY) neurons that are key regulators of feeding and energy expenditure [4, 5]. These neurons express receptors for both insulin and leptin, and respond to peripheral changes in energy balance [6]. Disruption of this complex circuitry by hypothalamic damage results in hyperphagia, decreased energy expenditure, and significant weight gain—the manifestations varying with the location and extent of structural changes. Lesions in the ventromedial hypothalamus in particular, are proposed to increase vagal tone and pancreatic beta-cell insulin secretion with resultant anabolism and weight gain [7]. Hormonal deficiencies, which are also common in HO, can independently contribute to weight gain if not properly managed.
While our understanding of the mechanisms underlying the development of obesity in patients with CP has progressed, therapeutic advances for treating HO remain limited. Several medical treatment options have been explored in patients with HO, but the weight control achieved with lifestyle modification and pharmacotherapy is typically modest and often transient. Given the benefits of bariatric surgery for the treatment of conventional morbid obesity there has been recent interest in the use of surgical weight loss techniques in patients with HO. In this review we will discuss the medical and neuroendocrine management of HO and will describe a case of successful weight loss following gastric bypass surgery in a patient with HO after CP treatment. We will use the case to illustrate key ideas involved in the clinical treatment of HO and to highlight the emerging role of surgical weight loss interventions in this patient population.
Clinical case
An 18-year-old Caucasian female with a history of a CP status post resection, presented to our neuroendocrine unit with rapid weight gain. Historically, the patient had normal growth and development until age 10 years when she developed migraines and stopped growing. Menarche occurred the following year but menses stopped at age 13 years, in the setting of anorexia nervosa. She was followed by a pediatrician and nutritionist from age 13 to 16 for her eating disorder; during that period her nadir weight was 45.4 kg (BMI 18.3) but increased to 52.2 kg (BMI 21.0) with treatment. In spite of weight gain, menses did not return and at 16.5 years she developed frequent headaches with associated polyuria, polydipsia, and intermittent enuresis, paired with poor appetite, lethargy, and a 6.8 kg unintentional weight loss. She developed worsening vision and elected to stop driving. Visual field defects were detected and brain MRI was notable for a complex bilobed enhancing mass lesion arising in the suprasellar region, separate from the pituitary gland, which appeared normal in size. The mass lesion distorted the anatomy of the anterior third ventricle including its infundibular and optic recesses, and extended into the interpeduncular cistern, compressing and superiorly displacing the optic chiasm (Fig. 1a, b). The patient underwent craniotomy and decompression with improvement in her vision and symptoms of polyuria, however, 3 months later she developed recurrent headaches and vision loss and a ventricular drain was placed. One month later, repeat MRI demonstrated tumor growth and she underwent a second craniotomy at another medical center with complete resection of the tumor and modest improvement in her vision. Post-operatively she developed panhypopituitarism and diabetes insipidus (DI) and was started on hormone replacement therapy.
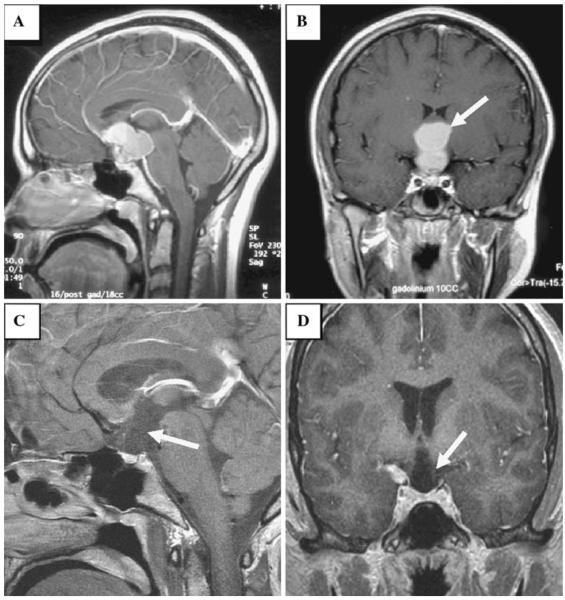
Fig. 1.
T1 weighted post-contrast sagittal (a) and coronal (b) image demonstrates a complex bilobed enhancing mass (arrow in b) at the level of the anterior third ventricle filling the suprasellar cistern; the mass is separate from the normal appearing pituitary gland. T1 weighted post contrast sagittal (c) and coronal (d) image obtained post-operatively shows resection of the suprasellar lesion, now with a deformed expanded appearance to the anterior inferior third ventricle and the hypothalmic region (arrow in d) as well as a deficiency along the floor of the third ventricle and an absent pituitary stalk (arrow in c)
Following the operation she noted an increase in appetite followed by rapid weight gain. Eighteen months postoperatively she presented to our neuroendocrine unit, endorsing a 52 kg weight gain. Her physical exam was notable for a morbidly obese female, ambulating with a white cane for visual assistance. Her weight was 101 kg, height 62 inches (mid-parental height 66 inches) and BMI 40.8 kg/m2. Dense bilateral visual field cuts involving both the temporal fields and part of the nasal fields, with preserved acuity, were noted. The exam was otherwise normal. Medications included hydrocortisone 10 mg twice daily, levothyroxine 100 mcg daily, desmopressin tablets 0.2 mg three times a day and conjugated estrogen 0.3 mg daily. Laboratory evaluation revealed a TSH <0.03 mU/l, FT4 0.9 ng/dl (0.9–1.4), T4 6.5 ng/dl, T3 72 ng/dl, prolactin 17 ng/ml, IGF-1 57 ng/ml (182–780), GH 2 ng/ml, sodium 141 mM/l. Post-operative MRI demonstrated resection of the large suprasellar mass lesion seen on the pre-operative study and distortion of the region of the anterior third ventricle and the paraventricular hypothalamic nuclei. The optic and the infundibular recesses of the anterior third ventricle were no longer seen and were replaced by a CSF filled cavity. The hypothalamic nuclei, which make up the margins of the anterior third ventricle, were deficient. The tuber cinereum or the floor of the third ventricle, and the pituitary/infundibular stalk were also absent on the scan (Fig. 1c, d).
The levothyroxine dose was up-titrated to 175 mcg daily for goal FT4 in the upper range of normal, GH replacement was initiated at 0.2 mg SC daily, and the hydrocortisone dose was decreased to 5 mg twice daily and then changed to prednisone 2.5 mg daily for compliance reasons. Conjugated estrogen was increased to 0.6 mg along with the cyclic addition of medroxyprogesterone acetate. Diet and exercise were encouraged but her weight continued to increase and 31 months after presentation she weighed 108.8 kg (Fig. 2). Sibutramine 10 mg daily was started and then up-titrated to 15 mg, and an aggressive daily exercise routine was initiated. Eight months later the patient's weight decreased to 90.3 kg (BMI 36.4 kg/m2), however, due to expense, sibutramine was discontinued. The patient was briefly lost to follow-up but when she re-presented after 1.5 years her weight had increased to 115 kg. She resumed regular exercise and nutrition visits but her weight continued to increase over the subsequent 10 months, peaking at 128.2 kg (BMI 51.6 kg/m2). Concomitantly she developed cholecystitis requiring cholecystectomy and osteoarthritis of the knees, limiting her mobility. RYGB was recommended. Oral desmopressin was discontinued and the patient was transitioned to desmopressin nasal spray with better control of her DI in preparation for surgery. The patient underwent an uncomplicated RYGB with a 150 cm Roux limb. Nine months post-bypass her weight had decreased to 93.9 kg (BMI 39.0), reflecting a 26.6% weight loss and remained relatively stable 19 months post-bypass.
Fig. 2.
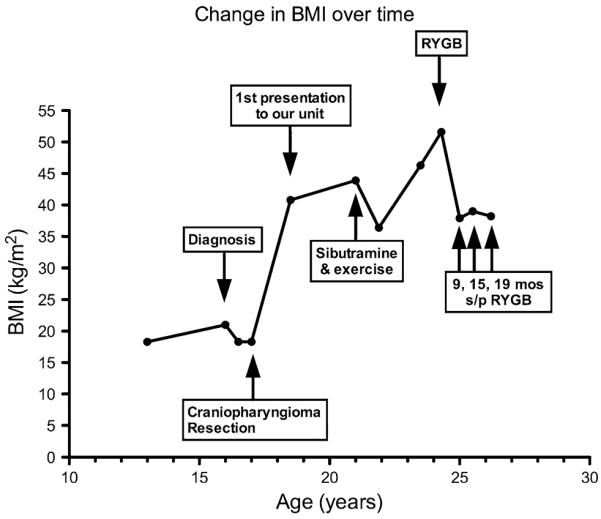
Change in patient's BMI and clinical events over time
To further examine the impact of RYGB on the patient's metabolic profile, we evaluated resting energy expenditure (REE), and gut hormone secretion in response to a mixed meal, both pre and 9 and 15 months post-operatively. REE was measured in the fasted state by indirect calorimetry using the ParvoMedics TrueOne 2400 Metabolic Cart. Prior to surgery, indirect calorimetry revealed a resting energy expenditure of 1,877 kcal/24 h, 9% lower than predicted REE calculated by established formulas (Table 1) [8, 9]. Nine months post-operatively REE decreased to 1,142 kcal/24 h (31–34% less than predicted), and 15 months post-operatively REE further decreased to 1,079 kcal/24 h (35–39% less than predicted). Leptin, insulin, ghrelin, acyl-ghrelin, peptide YY (PYY), and glucagon-like peptide (GLP-1) were measured in the fasted state (Table 1). Fasting leptin and insulin levels decreased from baseline. Conversely, there was a 64% increase in circulating ghrelin levels at 9 months and a 107% increase from baseline at 15 months. Acyl-ghrelin levels also increased post-operatively. Fasting PYY and GLP-1 did not change following RYGB.
Table 1.
Resting energy expenditure and hormone levels at baseline and 9 and 15 months following gastric bypass
| Baseline | 9 mos | 15 mos | |
|---|---|---|---|
| Body weight (kg) | 128 | 94 | 97 |
| REE (kcal/day)a | |||
| Measured | 1,877 | 1,142 | 1,079 |
| Harris-Benedict | 2,065 | 1,737 | 1,764 |
| Mifflin-St. Jeor | 1,986 | 1,645 | 1,672 |
| REE (kcal/kg)a | |||
| Measured | 14.7 | 12.2 | 11.2 |
| Harris-Benedict | 16.1 | 18.4 | 18.2 |
| Mifflin-St. Jeor | 15.5 | 17.5 | 17.3 |
| Hormones | |||
| Leptin ng/mlb | 87.6 | 34.0 | 41.4 |
| Insulin μIU/mlc | 22.5 | 2.18 | 3.62 |
| Ghrelin pg/mld | 407 | 668 | 843 |
| Acyl-Ghrelin pg/mle | 2.8 | 7.6 | 13.0 |
| PYY pg/mlf | 199 | 208 | 197 |
| GLP-1 pmol/1 | 13.5 | 10.8 | 10.4 |
Measured energy expenditure obtained via indirect calorimetry is reported as is calculated resting energy expenditure using Harris-Benedict and Mifflin-St. Jeor equations
To convert REE from kcal to kJ, multiply by 4.184
To convert leptin from ng/ml to μg/l, multiply by 1
To convert insulin from μIU/ml to pmol/l, multiply by 6
To convert ghrelin from pg/ml to pmol/l, multiply by 0.296
To convert acyl-ghrelin from pg/ml to pmol/l, multiply by 3.37
To convert PYY from pg/ml to pmol/l, multiply by 0.27
Discussion
Craniopharyngiomas are histologically benign tumors with high overall survival rates. Nonetheless, they are associated with considerable morbidity secondary to the sequelae of HO. Longitudinal data from 90 patients with childhood CP suggests that only 10% of patients with pre-operative hypothalamic tumor involvement maintain their baseline weight [10]. In some series over 50% of patients develop frank obesity after treatment of CP, likely as a function of several mechanisms including impaired sympathoadrenal activation, parasympathetic dysregulation, and other hypothalamic disturbances that upset the balance between energy intake and expenditure [3, 10, 11]. In the presented case, although the patient's tumor appeared to involve the hypothalamus at diagnosis, she had no evidence of pre-operative HO. In fact prior to CP resection she experienced unintentional weight loss, which may have been a consequence of undiagnosed secondary adrenal insufficiency. However, in spite of her history of anorexia nervosa, following CP resection and appropriate hormone replacement therapy, weight gain was profound, with doubling of her pre-operative body weight over an 18 months period. While the degree of weight gain in this patient was marked, the extent of weight gain following CP resection has been associated with the severity of hypothalamic damage on MRI. Patients with bilateral hypothalamic injury, like ours, have been reported to have increases in median BMI standard deviation (SD) scores of +5.44 SD [12]. Treating such weight gain must be prioritized in the management of these patients.
Medical treatment of hypothalamic obesity
Lifestyle management
Treatment of patients with HO should begin with a focus on lifestyle measures, which are the cornerstone of weight management. While severe hyperphagia is experienced by up to 25% of CP patients following tumor resection, attention should still be given to the tenets of healthy eating and caloric restriction and patients with HO should be seen regularly by a nutritionist [3]. Additionally, the importance of exercise should be emphasized. Disruption of hypothalamic thermoregulatory sites, including the pre-optic anterior hypothalamus and the ventromedial nuclei, by CP and the surgical procedures used to treat them impacts REE. Patients with CP and HO have been shown to have lower basal metabolic rates than matched controls and it has been suggested that REE has a greater role in weight gain in these patients than energy intake [13, 14]. Accordingly, our patient's REE following tumor resection was noted to be lower than predicted by Harris-Benedict and Mifflin-St. Jeor equations and this may have contributed to her initial weight gain following tumor resection. While the effect of exercise on weight loss in HO has not been systematically evaluated in humans, rats with monosodium l-glutamate induced HO that are exercise trained demonstrate reduced adiposity compared to sedentary controls [15]. It is reasonable to postulate that by increasing total daily energy expenditure, exercise may help counteract some of the observed decrements in REE in these patients. Indeed in our patient intensive supervised exercise in combination with sibutramine was associated with the greatest degree of non-surgical weight loss.
Management of impaired sympathoadrenal activation—drug treatment with sympathomimetics
In addition to decreased REE, patients with HO exhibit impaired sympathoadrenal activation, illustrated by reduced urinary catecholamines [16, 17]. Sympathomimetic pharmacotherapy has subsequently been used to attenuate weight gain and promote weight loss in patients with HO. Mason et al. treated five pediatric patients who developed HO following CP resection with dextroamphetamine for 24 months. On doses ranging from 12.5 to 20 mg per day, the velocity of weight gain decreased from 2 ± 0.3 kg/month to 0.4 ± 0.2 kg/month and weight stabilization was achieved after 1 month and remained stable at 24 months follow-up. While caloric intake did not differ before and after treatment, exercise logs revealed increased physical activity on dextroamphetamine [18]. Similarly, weight stabilization or modest weight loss (−0.7 SDS in males, −0.44 SDS in females) was observed in ten out of 12 patients with HO treated with 10 mg daily of dexamphetamine [19].
The sympathomimetic sibutramine, which inhibits norepinephrine and serotonin reuptake, has also been evaluated for the treatment HO. In a double-blind placebo-controlled 20 × 20 week cross-over study treatment with 10–15 mg sibutramine resulted in a significant decrease in BMI compared to placebo, although notably weight loss was more pronounced in those with non-hypothalamic obesity [20]. This differential response may reflect the fact that this medication likely targets regions of body weight regulation that are potentially damaged in HO. Nonetheless, our patient's weight loss on sibutramine was pronounced, although it should be noted that she was highly committed to intensive exercise at the time. While sibutramine has clearly demonstrated benefits in patients with HO, it was withdrawn from the market in October, 2010 due to data from the Sibutramine Cardiovascular Outcomes (SCOUT) trial, that suggested an increased risk of stroke and myocardial infarction [21].
Phentermine, which stimulates the hypothalamic release of norepinephrine, is approved for the short-term treatment of obesity. It has been shown to result in modest weight loss as monotherapy and in combination with topiramate, but it has not been studied in patients with HO [22, 23]. The use of the sympathomimetics caffeine and ephedrine in combination can alsobe considered.Inthree case studiesof CP patientswithHO, caffeine and ephedrine were associated with a mean weight loss of 13.9% at 6 months, with a maintained weight loss of 8.5 and 9.5% in 2/3 cases at 2 and 6 years respectively [24].
Management of parasympathetic dysregulation and hyperinsulinemia
In addition to suffering from impaired sympathoadrenal activation, patients with HO are thought to have increased parasympathetic activity. Destruction of the ventromedial hypothalamic area results in parasympathetic disinhibition, with increased vagal tone promoting insulin hypersecretion, increased energy storage, and weight gain. Suppression of insulin secretion with the somatostatin analog octreotide, which binds to the somatostatin receptor-5 on the beta-cell membrane, has subsequently been investigated as a potential treatment option for HO. In an open-label study of eight patients with HO, 6 months of subcutaneous octreotide (15 mcg/kg/day) in comparison to a 6 months pre-study observation period, resulted in −4.8 ± 1.8 kg weight loss v. +6.0 ± 0.7 kg and a significant reduction in BMI of −2.0 ± 0.7 kg/m2 v. +2.1 ± 0.3 kg/m2. It should be noted that the interpretation of this observed attenuation of weight gain is limited by the fact that the natural history of HO may involve a greater velocity of weight gain following the initial hypothalamic insult. While hemoglobin A1c levels did not change with treatment, insulin secretion in response to an oral glucose tolerance test (OGTT) was significantly reduced [25]. Similarly, in a 6 month randomized double-blind placebo-controlled trial of octreotide therapy (5–15 mcg/kg/day) in 18 patients with HO, insulin response to OGTT declined significantly with octreotide use. While weight loss was not observed in this study, weight gain was significantly attenuated with the octreotide treated group demonstrating a 1.6 ± 0.6 kg weight gain compared to a 9.2 ± 1.7 kg weight gain in the placebo group [26]. In our patient we were dissuaded from using this medication due to the fact that it is not FDA approved for this indication and is subsequently quite expensive. It should also be noted that octreotide must be administered subcutaneously, and is commonly associated with several side effects including gastrointestinal discomfort, flatulence, loose stools and asymptomatic gallstones, all of which were noted in both studies [25, 26].
Hamilton et al., studied a novel oral treatment regimen aimed at decreasing insulin secretion using diazoxide (2 mg/kg, max dose 200 mg/day) and enhancing insulin action using metformin (1,000 mg BID) in patients with HO [27]. In this prospective open-label 6-month pilot treatment trial, mean weight gain was significantly attenuated. Weight gain after 6 months of therapy was +1.2 ± 5.9 kg v. +9.5 ± 2.7 kg during the 6 months prior to the study among the seven subjects who completed the study. Two of the nine participating subjects were withdrawn—one due to transaminitis and vomiting and the other due to pedal edema. While metformin alone has not been studied in HO, it can be considered as adjunctive therapy in patients with evidence of insulin resistance, glucose intolerance, or diabetes, given its association with weight loss and decreased visceral adiposity [28].
Management of weight loss with other agents
Melatonin replacement has also been studied in obese CP patients who suffer from daytime somnolence, as in these patients there appears to be a negative correlation between melatonin concentrations and BMI. Daytime sleepiness may contribute to the observed decrease in physical activity generally observed in patients with CP and HO compared to obese controls. In the 10 CP with increased daytime sleepiness and decreased melatonin secretion who were evaluated, melatonin substitution was found to improve daytime sleepiness and physical activity, although it did not clearly impact on BMI [29]. Orlistat, an inhibitor of pancreatic and gastrointestinal lipases that blocks the absorption of approximately 30% of dietary fat, is one of the two FDA approved medications for weight loss. Although it also has not been formally studied in patients with HO, its use can be considered. Additionally, while little is known about the impact of HO on appetitive gut hormones such as ghrelin, GLP-1, and PYY it has been hypothesized that GLP-1 agonists may benefit patients with HO by slowing gastric emptying and reducing food intake, particularly since much of this action may be mediated by intact receptors within the hindbrain. However, it is unclear how the central disruption that characterizes HO will impact the ability of GLP-1 to decrease food intake via hypothalamic pathways.
Management of hypopituitarism
Given HO is commonly associated with panhypopituitarism, as in the presented case, the neuroendocrine management of hormonal deficiencies is central to the care of these patients and can have important effects on weight. Relative increases in body weight can occur in the setting of secondary hypothyroidism and hypogonadism, and can be exacerbated by overly aggressive cortisol replacement. There is evidence that patients with HO have significantly higher levels of 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) activity relative to controls and the ratios of free and conjugated cortisol to cortisone and their metabolites are reported to be higher in the urine of patients with HO [30, 31]. This increase in 11β-HSD1 activity, resulting in enhanced conversion of inactive cortisone to cortisol, may explain why lower doses of cortisol replacement are generally well tolerated in patients with HO. With regard to thyroid hormone replacement our practice is to aim for thyroxine levels in the upper limit of the normal range. Fernandes et al. reported three patients with HO who were adequately replaced with thyroxine who then lost additional weight (4.3–15.2 kg) with triiodothyronine replacement (T3) when supraphysiologic T3 levels were achieved. T3 replacement is subsequently considered by some practioners [32]. Growth hormone (GH) replacement may also be important in patients with HO secondary to CP because when used to treat adults with GH deficiency it leads to reductions in fat mass and improvements in body composition and exercise capacity [33, 34]. Adults with CP from the Pfizer International Metabolic Database (KIMS) demonstrated improvements in fat free mass and quality of life after 2 years of GH therapy. While, the CP patients had no significant decrements in body fat with GH treatment in contrast to those with non-secreting pituitary adenomas, comparisons to CP patients with untreated GH deficiency were not available [35]. In our patient, we decreased cortisol replacement to a relatively low dose that was well tolerated while maximizing thyroxine (T4) levels. Although we did not treat our patient with T3 we did initiate GH replacement, which was well received, resulting in a reported increase in strength and exercise capacity and an overall improvement in well-being which may have influenced her early weight loss. In patients with HO and DI, it should be noted that if surgical weight loss interventions are considered DI must be well-controlled prior to proceeding, as the presence of poorly controlled DI following bypass could be dangerous given fluid intake limitations. Nasal desmopressin is preferable in that it avoids problems with delayed onset of action that can occur with oral desmopressin. Our patient was successfully transitioned from oral to inhaled desmopressin and had no pre or post-operative issues with DI and her sodium levels have remained stable.
Surgical treatment of hypothalamic obesity
Given the overwhelming shortcomings of available medical therapies for the management of HO and the efficacy of bariatric surgery for the treatment of conventional obesity, surgical weight loss techniques are now being considered in patients with CP and HO when other interventions fail. Although enthusiasm for bariatric surgery in patients with HO has been tempered by concerns that appetite dysregulation may prevent compliance with post-operative dietary restrictions, the benefits of surgical interventions appear promising [36, 37]. Gastric bypass surgery has been shown to facilitate weight reduction not only by restricting stomach volume but by altering secretion of appetitive gut hormones such as ghrelin, GLP-1 and PYY in a manner that favors appetite reduction and weight loss [38, 39]. In rodents, these gastrointestinal peptide hormones have been shown to exert their metabolic effects by interacting with hypothalamic neurons that express anorexigenic and orexigenic factors [40, 41]. It is not known if the beneficial central effects of the changes in gut hormone secretion seen with gastric bypass are preserved or negated by hypothalamic damage present in HO, nonetheless there is data to suggest that surgical weight loss can be achieved in this population.
Bariatric surgery for the treatment of HO was first pursued in Prader-Willi syndrome (PWS), a genetic disorder associated with hypothalamic dysfunction, hyperphagia and obesity. Biliopancreatic diversion and RYGB in these patients have been met with some success [42–44]. Based on these outcomes, Muller et al. performed laparascopic adjustable gastric banding (LAGB) in a small cohort of pediatric patients with CP and HO complicated by persistent morbid obesity that was refractory to multiple therapeutic interventions including lifestyle measures and weight loss medications. The BMI-SDS prior to LAGB in these patients was +13.9, +10.3, +11.4, +7.3 SD. LAGB was well-tolerated and after a follow-up of 4.5, 1.5, 3.0, and 2.5 years BMI had decreased continuously (BMI SDS at follow-up: +9.9, +9.7, +9.5, +5.9 SD respectively) and self-assessment scales confirmed a profound improvement in pre-existing addiction to food and sweets in all subjects [45]. In addition to the case presented here, to date there are two other published case reports of gastric bypass for the treatment of HO in adult patients with CP. The first case by Inge et al. describes a RYGB with truncal anterior vagotomy [46]. The patient was originally diagnosed with a CP at the age of 14 years and was treated with tumor resection and cranial irradiation. Post-operatively he developed panhypopituitarism (which was successfully managed) and extreme weight gain with an increase in BMI from 25 to >60 kg/m2. He was treated with inpatient and outpatient lifestyle interventions and with 19 months of octreotide therapy without evidence of weight loss before undergoing bariatric surgery at age 18 years. Post-operatively weight gain ceased, food cravings markedly decreased and the patient lost 49 kg (22% of initial weight, 33% of initial excess weight), maintaining this weight loss at 2.5 years. Truncal anterior vagotomy was pursued in this case in an attempt to overcome the weight gain associated with the potential increase in vagal tone and resultant hyperinsulinemia that can be seen in HO. Both fasting and post-prandial hyperinsulinemia did resolve in this patient at 14 months of follow-up. However, we were able to achieve a comparable weight loss of 25% of initial weight (v. 22% of initial weight) and a normalization of fasting insulin levels without the addition of truncal anterior vagotomy.
Changes in the gut hormone ghrelin were also evaluated in the case presented by Inge et al., and fasting levels were almost halved 14 months post-operatively. While ghrelin levels rise with diet-induced weight loss, the effect of RYGB on ghrelin levels in patients without HO has been inconsistent [47–51]. In a longitudinal study of 28 patients undergoing RYGB at our center, mean weight loss was 30% 1 year post-operatively, but mean ghrelin levels were unchanged from baseline [39]. In our patient post-operative baseline ghrelin levels doubled at 15 months. Given the increase in vagal tone that characterizes HO, an autonomically mediated mechanism may underlie the increase in fasting ghrelin levels observed post-operatively and may explain the post-operative decrease in fasting ghrelin levels exhibited by Inge's patient given the anterior truncal vagotomy [46]. Initially, we were concerned that the observed post-operative rise in fasting ghrelin levels would promote weight regain in our patient, given ghrelin has been shown to promote feeding and weight gain by stimulating AGRP/NPY neurons in the hypothalamus [40]. However, while our patient reported an increase in her sensation of hunger and her craving for sweets, visual analogue scales in the fasted state, revealed a post-operative decline in perceived capacity for energy intake and following RYGB she was unable to complete a 320 kcal mixed meal test. It is possible that the profound hypothalamic injury present in this case limited the extent to which ghrelin was able to exert its normal central impact on the AGRP/NPY neurons.
Concerned that the gut hormone alterations that typically accompany proximal gastric bypass surgery would be less effective in a patient with hypothalamic damage, Schultes et al. pursued a distal gastric bypass in their 29-year-old CP patient with HO and refractory morbid obesity [52]. His surgery included the creation of an 80 cm common channel, a biliopancreatic limb of 80 cm, and an alimentary limb of 780 cm designed to increase malabsorption especially of nutritional fat. As in the other described cases, the surgery was performed laparascopically and there were no postoperative complications. Eighteen months after gastric bypass the patient's BMI had decreased from 52 to 31.9 kg/m2. While this 35% decrease in initial body weight may have been due to the strong malabsorptive effects of distal bypass, it is interesting to note that 18 months post-operatively although REE had declined in this patient, it was 28–29% higher than expected values. In contrast, while our patient's REE declined 39–43% from baseline (Table 1), at both post-operative time points her REE remained less than predicted and there was a progressive decline in energy expenditure per kilogram of body weight, suggesting an increase in energy efficiency that has not generally been observed in long-term follow-up of RYGB patients [53, 54]. While diet-induced weight loss is typically characterized by a decline in REE secondary to a loss of free fat mass and or fat mass, there is disagreement in the literature regarding the overall impact of RYGB on REE [53, 55–59]. Several authors have demonstrated a decline in REE averaging from 21 to 25% with surgical weight loss while others describe post-operative increases in REE in patients who are hypo-metabolic at baseline [54, 55, 60]. The reason for the discrepancy in post-RYGB REE in our case report and Schultes' remains unclear, but may reflect differences in the extent and location of hypothalamic damage. Given there is data to suggest that low REE may contribute to weight regain post-operatively, the declining REE observed in our patient was of some concern although 19 months post-RYGB her weight appears to have stabilized [61].
Although there is no available data comparing REE following RYGB in patients with HO to that of matched controls, our patient's relatively low baseline and post-operative REE may indicate that some patients with HO are at higher risk of weight regain. Skeletal muscle mass has been shown to decline following bypass, which likely contributes to post-bypass decrements in REE [62]. Subsequently, the importance of exercise post-operatively should be stressed as it can enhance dynamic muscle strength, REE and functional capacity and may result in greater weight loss and improvements in health-related quality of life [63, 64].
Conclusions
Ultimately, although several potential medical therapies have been explored for the treatment of HO, the success of these measures has been limited. As our understanding of the pathogenesis of this disease evolves we may be able to develop more effective pharmacotherapy. At present, the published cases of bariatric surgery for the treatment of HO secondary to CP suggest that surgical weight loss interventions can be safely and effectively used for the management of this disease. The weight loss achieved with both LAGB and RYGB appears to exceed that attained through available non-surgical treatment modalities. While more extensive exploration of surgical weight loss interventions in this unique patient population is needed, bariatric surgery may prove to be the most useful therapy for long-term weight reduction in patients with HO, as it appears to not only decrease body mass but to attenuate appetite even in the presence of hypothalamic damage. Importantly, these benefits are also accompanied by improvements in reported quality of life. While non-hypothalamic mechanisms play a role in the weight reduction observed with bariatric surgery, it is important to note there is the potential for significant variability in weight reduction based on the extent and location of hypothalamic damage. Identifying which patients are likely to respond best to surgical interventions should be evaluated in future research. Lastly, it should be noted that like our patient, the patients described in the available literature were noted to be both motivated and compliant. In addition, they were supported both pre and post-operatively by committed multidisciplinary teams. While, we believe that it is reasonable to consider bariatric surgery as a potential treatment option in patients with HO secondary to CP, careful patient selection may be paramount to the ultimate success of such interventions.
Acknowledgments
The authors would like to acknowledge the participation of our patient in this study and the expert technical assistance of Gerardo Febres and Irene M. Conwell. This project was supported by NIH/NIDDK RO1 DK072011 and NIH/NCRR UL1RR024156. JK has research support from Covidien.
References
- 1.Bruch H. The Fröhlich syndrome: report of the original case. 1939. Obes Res. 1993;1:329–331. doi: 10.1002/j.1550-8528.1993.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 2.Albright AL, Einshaus SL, editors. Craniopharyngioma. Thieme; New York: 1999. Principles and practice of pediatric neurosurgery; pp. 545–562. [Google Scholar]
- 3.Muller HL, et al. Obesity after childhood craniopharyngioma–German multicenter study on pre-operative risk factors and quality of life. Klin Padiatr. 2001;213(4):244–249. doi: 10.1055/s-2001-16855. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010. doi: 10.2741/2366. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz MW, et al. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 6.Seeley RJ, et al. Melanocortin receptors in leptin effects. Nature. 1997;390(6658):349. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S. An autonomic hypothesis for hypothalamic obesity. Life Sci. 1979;25:561–566. doi: 10.1016/0024-3205(79)90549-6. [DOI] [PubMed] [Google Scholar]
- 8.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci USA. 1918;4(12):370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mifflin MD, et al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 10.Muller HL, et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J Clin Endocrinol Metab. 2004;89(7):3298–3305. doi: 10.1210/jc.2003-031751. [DOI] [PubMed] [Google Scholar]
- 11.Sorva R. Children with craniopharyngioma. Early growth failure and rapid postoperative weight gain. Acta Paediatr Scand. 1988;77(4):587–592. doi: 10.1111/j.1651-2227.1988.tb10705.x. [DOI] [PubMed] [Google Scholar]
- 12.de Vile CJ, et al. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J Clin Endocrinol Metab. 1996;81(7):2734–2737. doi: 10.1210/jcem.81.7.8675604. [DOI] [PubMed] [Google Scholar]
- 13.Holmer H, et al. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010;95(12):5395–5402. doi: 10.1210/jc.2010-0993. [DOI] [PubMed] [Google Scholar]
- 14.Shaikh MG, Grundy RG, Kirk JM. Reductions in basal metabolic rate and physical activity contribute to hypothalamic obesity. J Clin Endocrinol Metab. 2008;93(7):2588–2593. doi: 10.1210/jc.2007-2672. [DOI] [PubMed] [Google Scholar]
- 15.Gobatto CA, et al. The monosodium glutamate (MSG) obese rat as a model for the study of exercise in obesity. Res Commun Mol Pathol Pharmacol. 2002;111(1–4):89–101. [PubMed] [Google Scholar]
- 16.Coutant R, et al. Defect in epinephrine production in children with craniopharyngioma: functional or organic origin? J Clin Endocrinol Metab. 2003;88(12):5969–5975. doi: 10.1210/jc.2003-030552. [DOI] [PubMed] [Google Scholar]
- 17.Roth CL, et al. Reduced sympathetic metabolites in urine of obese patients with craniopharyngioma. Pediatr Res. 2007;61(4):496–501. doi: 10.1203/pdr.0b013e3180332cd6. [DOI] [PubMed] [Google Scholar]
- 18.Mason PW, Krawiecki N, Meacham LR. The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch Pediatr Adolesc Med. 2002;156(9):887–892. doi: 10.1001/archpedi.156.9.887. [DOI] [PubMed] [Google Scholar]
- 19.Ismail D, O'Connell MA, Zacharin MR. Dexamphetamine use for management of obesity and hypersomnolence following hypothalamic injury. J Pediatr Endocrinol Metab. 2006;19(2):129–134. doi: 10.1515/jpem.2006.19.2.129. [DOI] [PubMed] [Google Scholar]
- 20.Danielsson P, et al. Impact sibutramine therapy in children with hypothalamic obesity or obesity with aggravating syndromes. J Clin Endocrinol Metab. 2007;92(11):4101–4106. doi: 10.1210/jc.2007-0826. [DOI] [PubMed] [Google Scholar]
- 21.James WP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363(10):905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 22.Gadde KM, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub M, et al. A double-blind clinical trial in weight control. Use of fenfluramine and phentermine alone and in combination. Arch Intern Med. 1984;144(6):1143–1148. [PubMed] [Google Scholar]
- 24.Greenway FL, Bray GA. Treatment of hypothalamic obesity with caffeine and ephedrine. Endocr Pract. 2008;14(6):697–703. doi: 10.4158/EP.14.6.697. [DOI] [PubMed] [Google Scholar]
- 25.Lustig RH, et al. Hypothalamic obesity caused by cranial insult in children: altered glucose and insulin dynamics and reversal by a somatostatin agonist. J Pediatr. 1999;135(2 Pt 1):162–168. doi: 10.1016/s0022-3476(99)70017-x. [DOI] [PubMed] [Google Scholar]
- 26.Lustig RH, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586–2592. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton JK, et al. Hypothalamic obesity following craniopharyngioma surgery: results of a pilot trial of combined diazoxide and metformin therapy. Int J Pediatr Endocrinol. 2011;2011:417949. doi: 10.1155/2011/417949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korner J, Aronne LJ. Pharmacological approaches to weight reduction: therapeutic targets. J Clin Endocrinol Metab. 2004;89(6):2616–2621. doi: 10.1210/jc.2004-0341. [DOI] [PubMed] [Google Scholar]
- 29.Muller HL, et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control. 2006;17(4):583–589. doi: 10.1007/s10552-005-9012-7. [DOI] [PubMed] [Google Scholar]
- 30.Hochberg Z, et al. Hypothalamic regulation of adiposity: the role of 11beta-hydroxysteroid dehydrogenase type 1. Horm Metab Res. 2004;36(6):365–369. doi: 10.1055/s-2004-814570. [DOI] [PubMed] [Google Scholar]
- 31.Tiosano D, et al. 11 beta-Hydroxysteroid dehydrogenase activity in hypothalamic obesity. J Clin Endocrinol Metab. 2003;88(1):379–384. doi: 10.1210/jc.2002-020511. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes JK, et al. Triiodothyronine supplementation for hypothalamic obesity. Metabolism. 2002;51(11):1381–1383. doi: 10.1053/meta.2002.35591. [DOI] [PubMed] [Google Scholar]
- 33.Chrisoulidou A, et al. Effects of 7 years of growth hormone replacement therapy in hypopituitary adults. J Clin Endocrinol Metab. 2000;85(10):3762–3769. doi: 10.1210/jcem.85.10.6910. [DOI] [PubMed] [Google Scholar]
- 34.Hoffman AR, et al. Growth hormone (GH) replacement therapy in adult-onset gh deficiency: effects on body composition in men and women in a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(5):2048–2056. doi: 10.1210/jc.2003-030346. [DOI] [PubMed] [Google Scholar]
- 35.Verhelst J, et al. Baseline characteristics and response to 2 years of growth hormone (GH) replacement of hypopituitary patients with GH deficiency due to adult-onset craniopharyngioma in comparison with patients with nonfunctioning pituitary adenoma: data from KIMS (Pfizer International Metabolic Database) J Clin Endocrinol Metab. 2005;90(8):4636–4643. doi: 10.1210/jc.2005-0185. [DOI] [PubMed] [Google Scholar]
- 36.Buchwald H, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 37.Sjöstrom L, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 38.Korner J, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90(1):359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 39.Korner J, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33(7):786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 41.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407(6806):908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 42.Antal S, Levin H. Biliopancreatic Diversion in Prader-Willi Syndrome Associated with Obesity. Obes Surg. 1996;6(1):58–62. doi: 10.1381/096089296765557286. [DOI] [PubMed] [Google Scholar]
- 43.Laurent-Jaccard A, et al. Long-term result of treatment of Prader-Willi syndrome by Scopinaro's Bilio-pancreatic diversion. Study of three cases and the effect of Dextrofenfluramine on the postoperative evolution. Obes Surg. 1991;1(1):83–87. doi: 10.1381/096089291765561529. [DOI] [PubMed] [Google Scholar]
- 44.Soper RT, et al. Gastric bypass for morbid obesity in children and adolescents. J Pediatr Surg. 1975;10(1):51–58. doi: 10.1016/s0022-3468(75)80008-x. [DOI] [PubMed] [Google Scholar]
- 45.Muller HL, et al. First experiences with laparoscopic adjustable gastric banding (LAGB) in the treatment of patients with childhood craniopharyngioma and morbid obesity. Klin Padiatr. 2007;219(6):323–325. doi: 10.1055/s-2007-985848. [DOI] [PubMed] [Google Scholar]
- 46.Inge TH, et al. Gastric bypass surgery for treatment of hypothalamic obesity after craniopharyngioma therapy. Nat Clin Pract Endocrinol Metab. 2007;3(8):606–609. doi: 10.1038/ncpendmet0579. [DOI] [PubMed] [Google Scholar]
- 47.Cummings DE, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 48.Geloneze B, et al. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13(1):17–22. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 49.Faraj M, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88(4):1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 50.Holdstock C, et al. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88(7):3177–3183. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 51.Kotidis EV, et al. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16(11):1425–1432. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 52.Schultes B, et al. Distal gastric bypass surgery for the treatment of hypothalamic obesity after childhood craniopharyngioma. Eur J Endocrinol. 2009;161(1):201–206. doi: 10.1530/EJE-09-0079. [DOI] [PubMed] [Google Scholar]
- 53.Carey DG, et al. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate. Obes Surg. 2006;16(4):469–477. doi: 10.1381/096089206776327378. [DOI] [PubMed] [Google Scholar]
- 54.Flancbaum L, et al. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery. 1997;122(5):943–949. doi: 10.1016/s0039-6060(97)90336-6. [DOI] [PubMed] [Google Scholar]
- 55.Das SK, et al. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr. 2003;78(1):22–30. doi: 10.1093/ajcn/78.1.22. [DOI] [PubMed] [Google Scholar]
- 56.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 57.Ravussin E, et al. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985;41(4):753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- 58.Leibel RL, Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33(2):164–170. doi: 10.1016/0026-0495(84)90130-6. [DOI] [PubMed] [Google Scholar]
- 59.Elliot DL, et al. Sustained depression of the resting metabolic rate after massive weight loss. Am J Clin Nutr. 1989;49(1):93–96. doi: 10.1093/ajcn/49.1.93. [DOI] [PubMed] [Google Scholar]
- 60.Carrasco F, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17(5):608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 61.Faria SL, Kelly E, Faria OP. Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes Surg. 2009;19(7):856–859. doi: 10.1007/s11695-009-9842-6. [DOI] [PubMed] [Google Scholar]
- 62.Korner J, et al. Sex differences in visceral adipose tissue post-bariatric surgery compared to matched non-surgical controls. Int J Body Composit Res. 2008;6(3):93–99. [PMC free article] [PubMed] [Google Scholar]
- 63.Stegen S, et al. Physical fitness in morbidly obese patients: effect of gastric bypass surgery and exercise training. Obes Surg. 2011;21(1):61–70. doi: 10.1007/s11695-009-0045-y. [DOI] [PubMed] [Google Scholar]
- 64.Bond DS, et al. Becoming physically active after bariatric surgery is associated with improved weight loss and health-related quality of life. Obesity. 2009;17(1):78–83. doi: 10.1038/oby.2008.501. [DOI] [PubMed] [Google Scholar]