Abstract
Aims
The Michigan Neuropathy Screening Instrument (MNSI) is used to assess distal symmetrical peripheral neuropathy in diabetes. It includes two separate assessments: a 15-item self-administered questionnaire and a lower extremity examination that includes inspection and assessment of vibratory sensation and ankle reflexes. The purpose of this study was to evaluate the performance of the MNSI in detecting distal symmetrical peripheral neuropathy in patients with Type 1 diabetes and to develop new scoring algorithms.
Methods
The MNSI was performed by trained personnel at each of the 28 Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications clinical sites. Neurologic examinations and nerve conduction studies were performed during the same year. Confirmed clinical neuropathy was defined by symptoms and signs of distal symmetrical peripheral neuropathy based on the examination of a neurologist and abnormal nerve conduction findings in ≥ 2 anatomically distinct nerves among the sural, peroneal and median nerves.
Results
We studied 1184 subjects with Type 1 diabetes. Mean age was 47 years and duration of diabetes was 26 years. Thirty per cent of participants had confirmed clinical neuropathy, 18% had ≥ 4 and 5% had ≥ 7 abnormal responses on the MNSI questionnaire, and 33% had abnormal scores (≥ 2.5) on the MNSI examination. New scoring algorithms were developed and cut points defined to improve the performance of the MNSI questionnaire, examination and the combination of the two.
Conclusions
Altering the cut point to define an abnormal test from ≥ 7 abnormal to ≥ 4 abnormal items improves the performance of the MNSI questionnaire. The MNSI is a simple, non-invasive and valid measure of distal symmetrical peripheral neuropathy in Type 1 diabetes.
Keywords: measurement, peripheral neuropathy
Introduction
The Michigan Neuropathy Screening Instrument (MNSI) is used widely for the evaluation of distal symmetrical peripheral neuropathy in diabetes. The MNSI includes two separate assessments, a 15-item self-administered questionnaire that is scored by summing abnormal responses, and a lower extremity examination that includes inspection and assessment of vibratory sensation and ankle reflexes and is scored by assigning points for abnormal findings [1]. The purpose of this study was to evaluate the sensitivity, specificity, positive and negative predictive values of the MNSI in detecting distal symmetrical peripheral neuropathy in patients with Type 1 diabetes and to develop new scoring algorithms to improve the performance of the MNSI questionnaire, the examination and the combination of the two.
Patients and methods
Study sample
At baseline, 1441 men and women between 13 and 39 years of age with Type 1 diabetes for 1–15 years were enrolled in the Diabetes Control and Complications Trial (DCCT). Subjects were randomly assigned to intensive or conventional therapy and were followed for a mean of 6.5 years [2]. In 1994, 1375 of the surviving DCCT subjects were enrolled in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (687 intensive therapy, 688 conventional therapy) and have been followed to the present time.
Scoring the Michigan Neuropathy Screening Instrument
The MNSI questionnaire is self-administered. Responses are added to obtain a total score. ’Yes‘ responses to questions 1–3, 5–6, 8–9, 11–12, 14–15 are each counted as one point. ’No‘ responses to questions 7 and 13 each count as one point. Question 4 was considered to be a measure of impaired circulation and question 10 a measure of general asthenia and were not included in the published scoring algorithm [1]. A score of ≥ 7 was considered abnormal [1]. All 15 questions were included in the new scoring algorithms.
During the MNSI examination, a health professional inspects each foot for deformities, dry skin, calluses, infections and fissures. Each foot with any abnormality receives a score of 1. Each foot is also inspected for ulcers and each foot with an ulcer receives a score of 1. The ankle reflexes are also elicited. If the reflex is absent, the patient is asked to perform the Jendrassic manoeuver and, if present, the reflex is designated as present with reinforcement and is scored as 0.5. If the reflex is absent with the Jendrassic manoeuver, the reflex is designated as absent and is scored as 1. Vibration sensation is then tested in the great toe using a 128-Hz tuning fork. In general, the examiner should be able to feel vibration in his or her hand for 5 s longer than a normal subject can at the great toe. Vibration is scored as present if the examiner senses the vibration on his or her finger for < 10 s longer than the subject feels it in the great toe, decreased if sensed for ≥ 10 s (scored as 0.5) or absent (scored as 1). The total possible score is 8 points and, in the published scoring algorithm, a score ≥ 2.5 is considered abnormal [1].
Definition of distal symmetrical peripheral neuropathy
Board-certified neurologists and electromyographers were identified, trained and certified by DCCT/EDIC to conduct neurological evaluations and electrodiagnostic studies [3,4,5]. Confirmed clinical neuropathy was defined as the presence of symptoms and signs consistent with distal symmetrical peripheral neuropathy based on the examination of a board-certified neurologist and nerve conduction abnormalities in one or more attribute(s) in a least two anatomically distinct nerves among the sural, peroneal or median nerves [5].
Statistical analysis
The performance of the MNSI questionnaire and examination in predicting confirmed clinical neuropathy was assessed by determining sensitivity, specificity, positive and negative predictive values in the full cohort. Sensitivity is the probability of having a positive questionnaire or examination in the presence of confirmed clinical neuropathy. Specificity is the probability of having normal (not positive) MNSI tests in the absence of confirmed clinical neuropathy. Positive predictive value is the proportion of subjects with neuropathy among those with positive MNSI questionnaires or examinations. Negative predictive value is the proportion of subjects without neuropathy among those with normal (not positive) MNSI tests.
All items on the questionnaire were coded as 0 for a negative response and 1 for a positive response (negative responses on items 7 and 13 counted as 1 point). For the examination, responses for the left and right feet were combined. For each measure of the examination (appearance, ulcer, reflex and vibration), a combined score ≥ 1.0 was classified as abnormal. The sensitivity and specificity of each item in predicting confirmed clinical neuropathy was evaluated. Chi-square values were used to determine the maximum discriminatory capability of each question.
Beta-coefficients from multiple logistic regression models [6] were used to develop indices for predicting confirmed clinical neuropathy based on the 15-item questionnaire alone, the 4-item examination alone and a combination of the two. Stepwise logistic regression was used to develop a parsimonious model and Madalla’s R2 based on the model likelihood ratio was used as a measure of explained variation [7].
Receiver operating characteristic (ROC) curves were used to illustrate the relationship between the true positive ratio (sensitivity) and the false positive ratio (1-specificity) of a test [8]. Optimal cut points to define abnormal tests were determined using Bayes decision rule [9], which classifies each subject into whichever population has the greatest posterior probability. This is equivalent to selecting a cut-off that maximizes the proportion correctly classified (PCC) and is computed as the {(prevalence × sensitivity) + [(1-prevalence) × specificity]}, where the prevalence was estimated from that observed in EDIC. Areas under the receiver operating characteristic curve (AUC) are a measure of the performance of a test in predicting the outcome of interest. An AUC value of 0.5 indicates that a test performs no better than chance. AUC values between 0.70 and 0.79 indicate fair test performance, values between 0.80 and 0.89 indicate good performance and values ≥ 0.9 indicate excellent performance.
Results
During EDIC years 13–14, 1184 subjects with Type 1 diabetes (89% of eligible subjects) underwent MNSI assessment, a neurologist’s examination and nerve conduction studies. Mean age (± SD) was 47 ± 7 years. Fifty-three per cent of subjects were men. Average duration of diabetes was 26 ± 5 years. Mean height was 172 ± 9 cm, mean BMI was 28.2 ± 5.0 kg/m2 and mean HbA1c was 62 ± 13 mmol/mol (7.8 ± 1.2%). Mean MNSI questionnaire score was 1.83 ± 2.25 and mean MNSI examination score was 1.84 ± 1.60. Fifty-six subjects (5%) had positive MNSI questionnaire scores (≥ 7) and 392 (33%) subjects had positive examination scores (≥ 2.5) with the published scoring system [1]. Thirty per cent of subjects had confirmed clinical neuropathy.
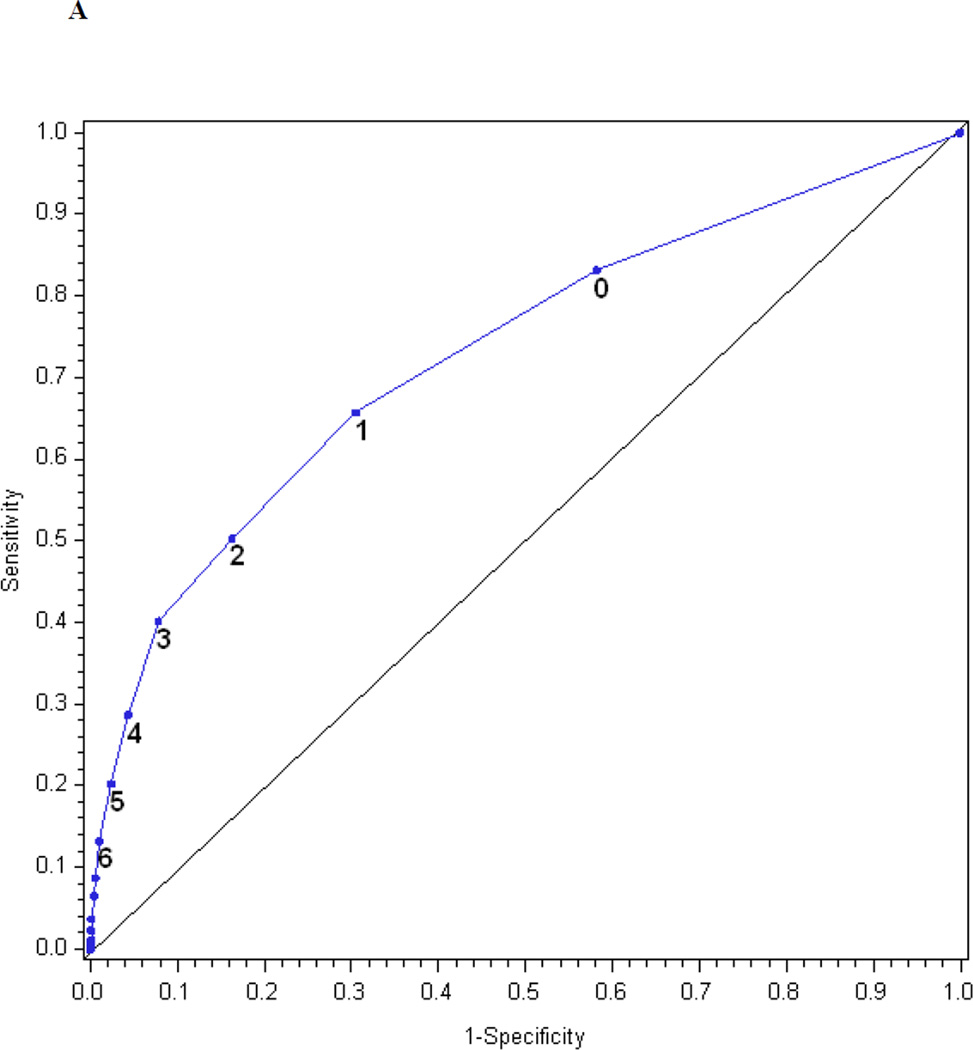
Figures 1a and 1b show the receiver operating characteristic curves for the performance of the MNSI questionnaire and the MNSI examination in predicting confirmed clinical neuropathy. The AUC for the MNSI questionnaire and examination were similar, 0.73 and 0.76, respectively. When the threshold to define an abnormal test for the MNSI questionnaire was ≥ 7, the questionnaire was 13% sensitive and 99% specific in identifying confirmed clinical neuropathy. Positive and negative predictive values were 84 and 73%, respectively. When the threshold to define an abnormal test was set at ≥ 4, the questionnaire was 40% sensitive and 92% specific and had a positive predictive value of 69% and a negative predictive value of 78%. When the threshold to define an abnormal MNSI examination was set at ≥ 2.5, the MNSI examination was 61% sensitive and 79% specific in defining confirmed clinical neuropathy and had a positive predictive value of 56% and a negative predictive value of 83%.
Figure 1.
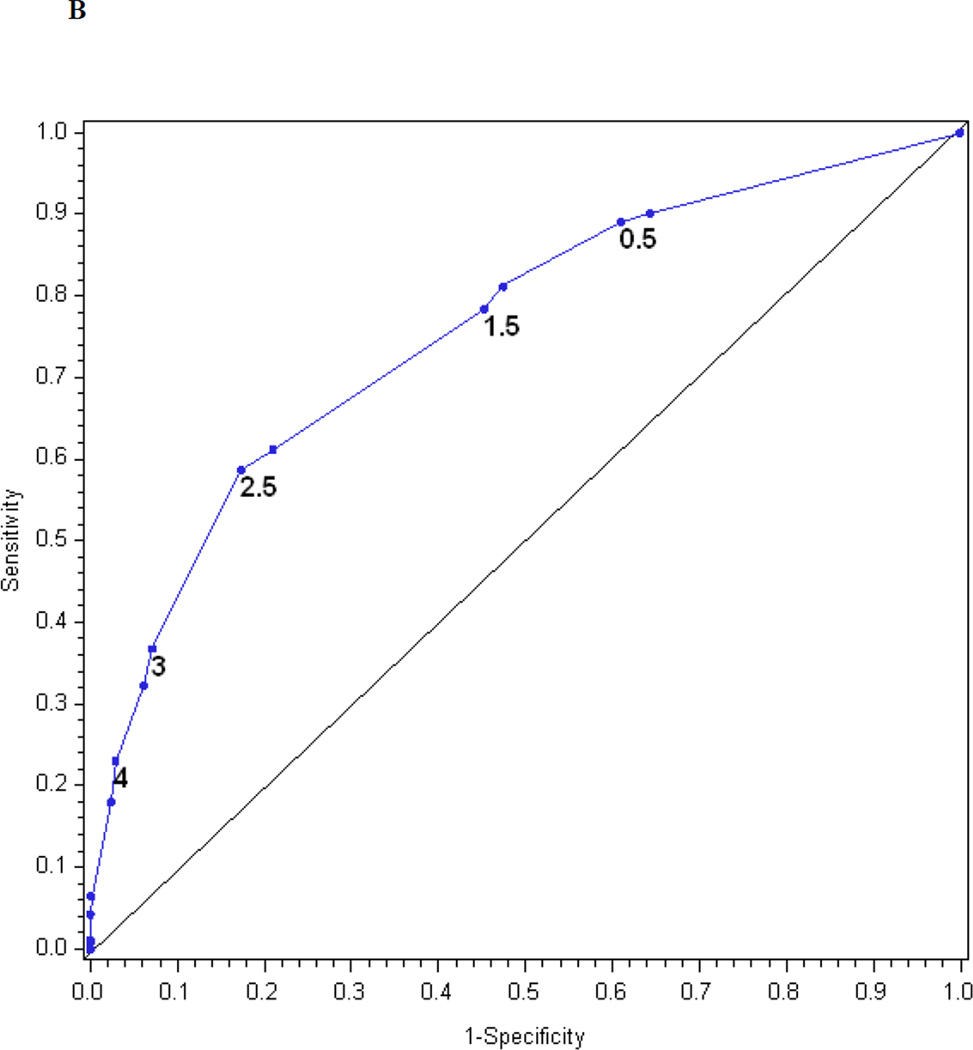
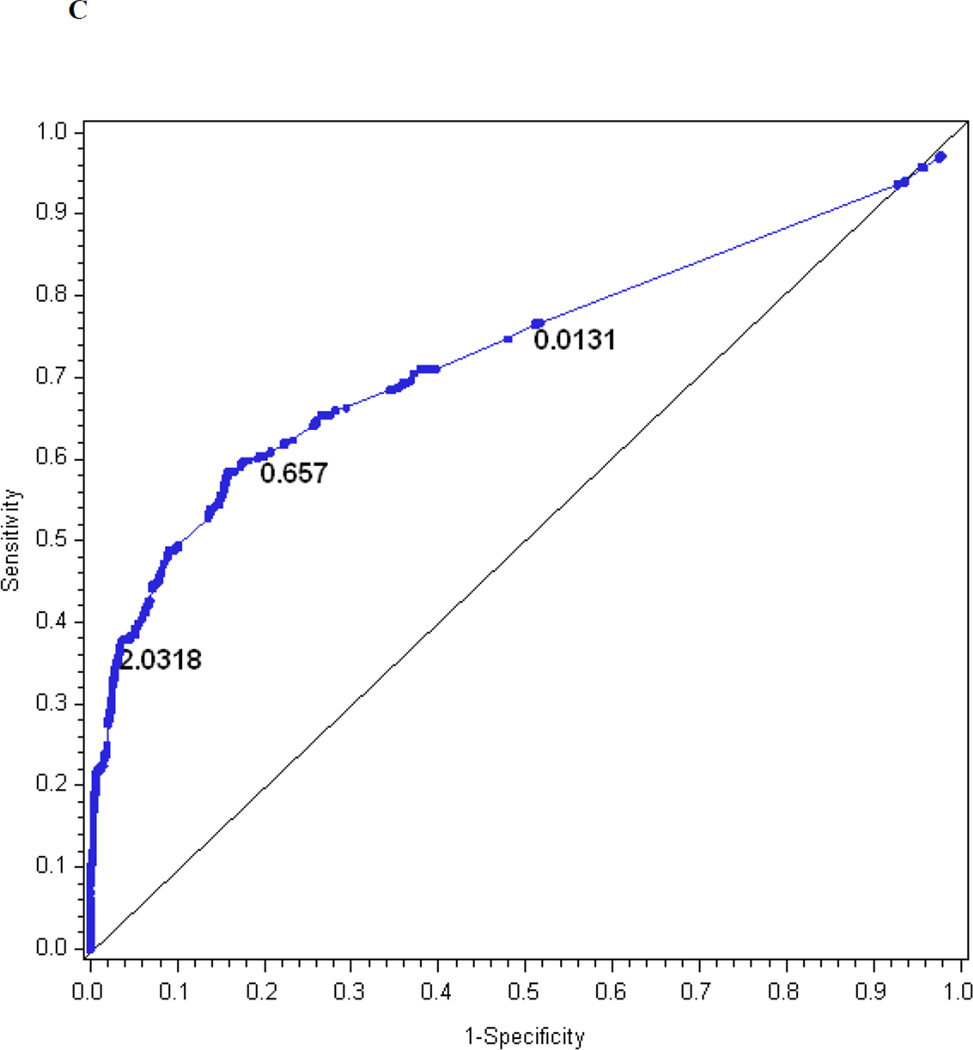
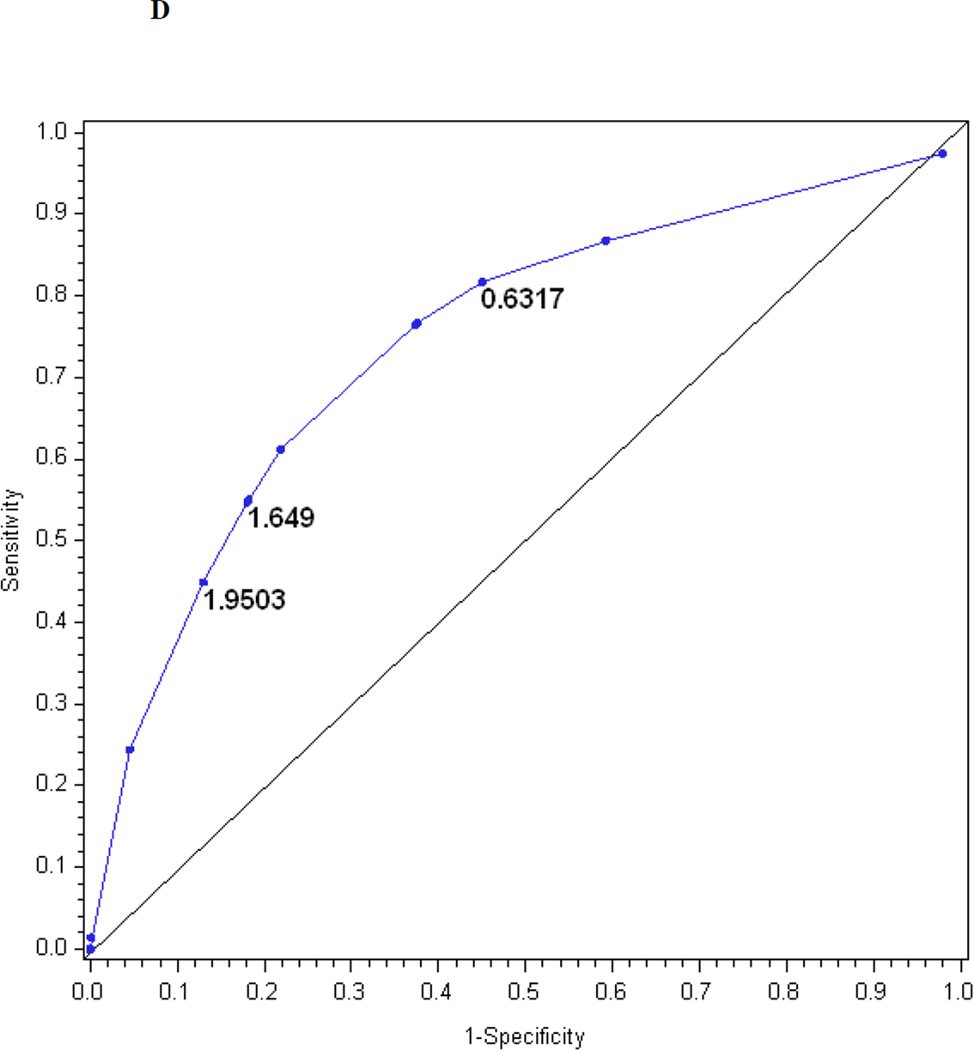
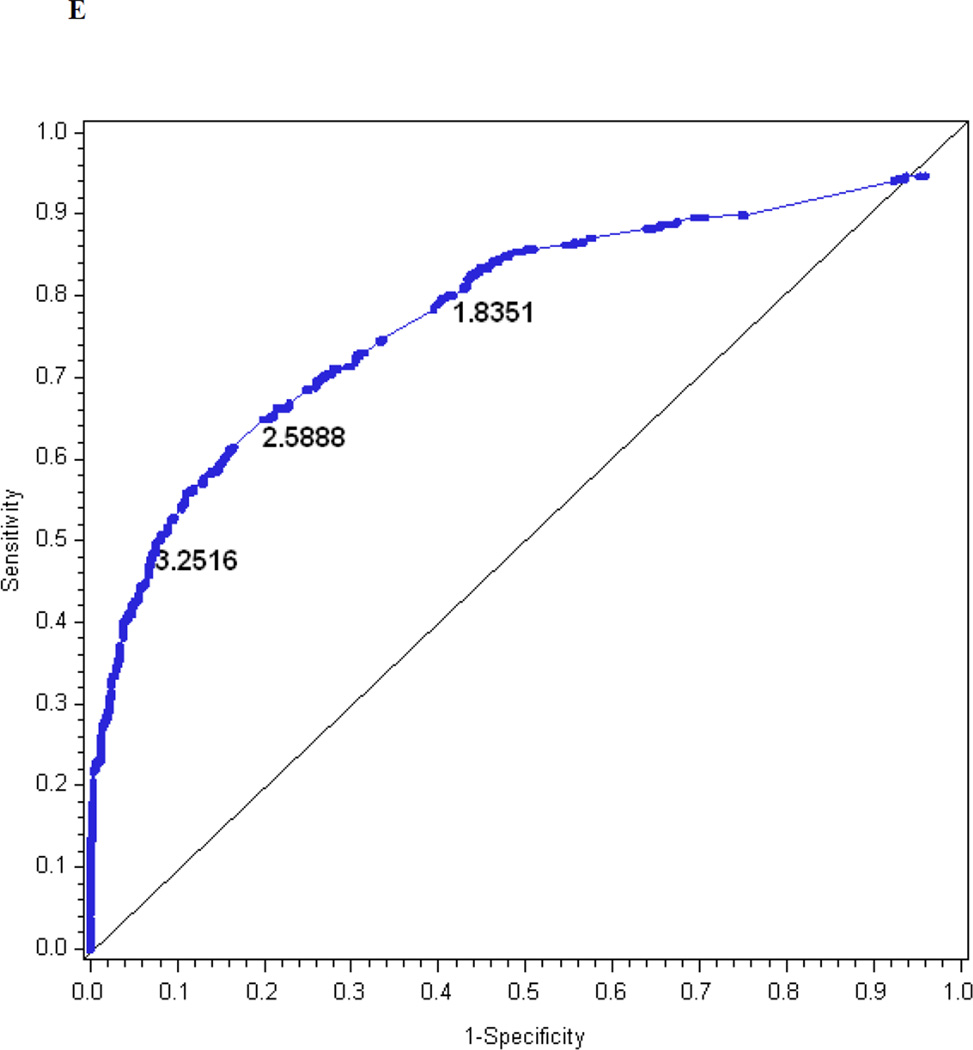
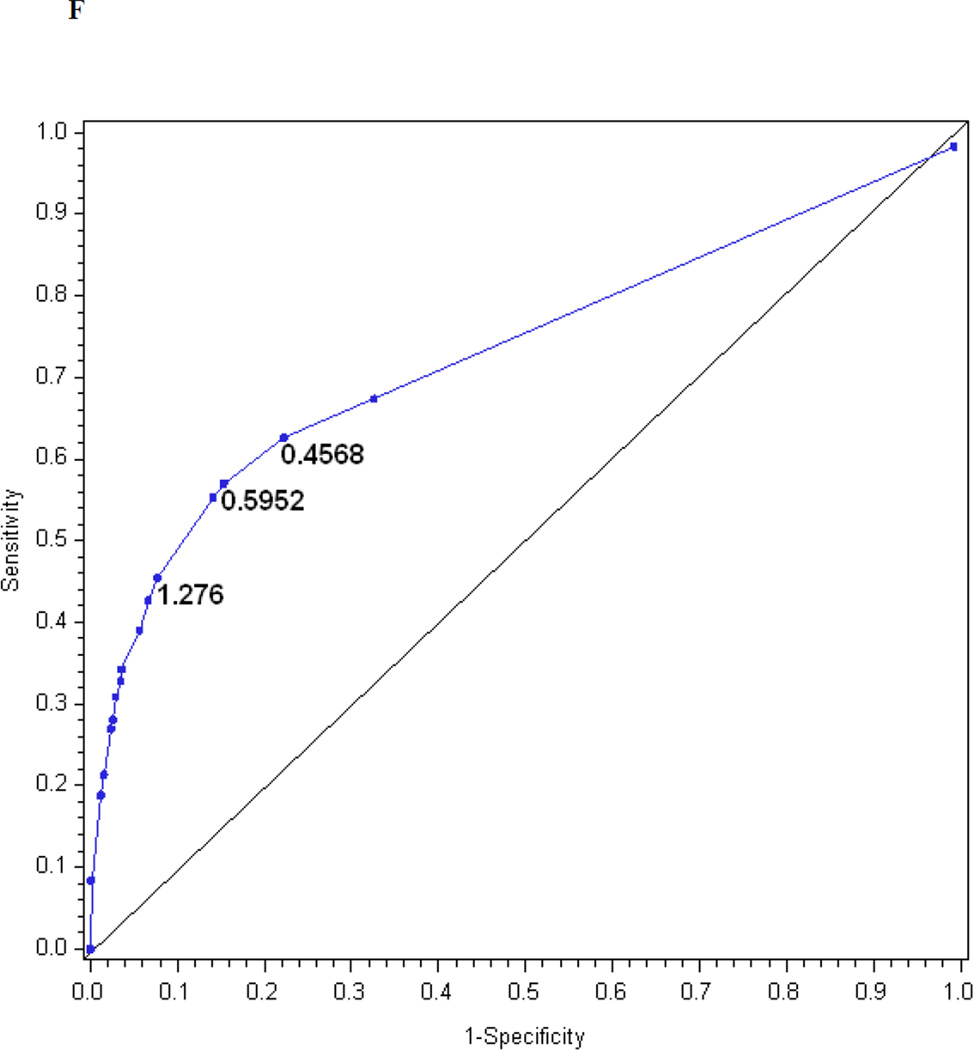
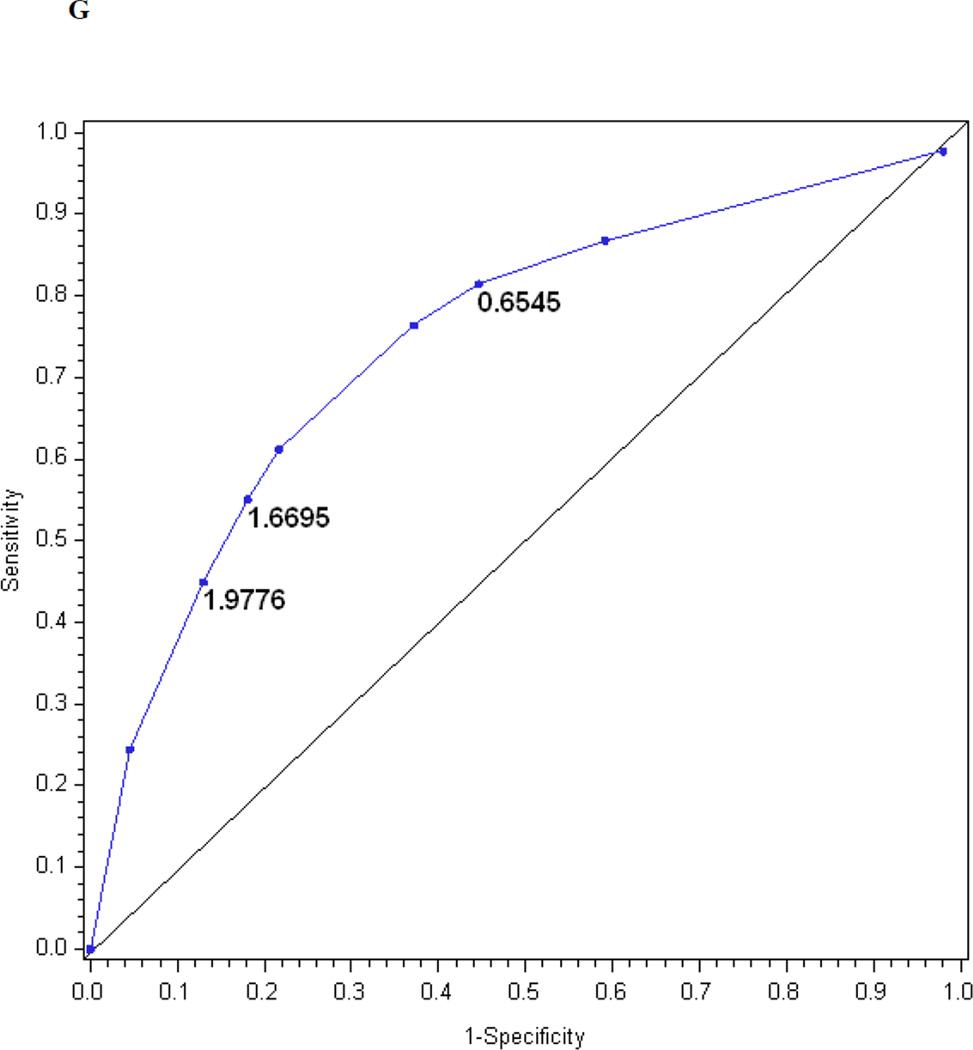
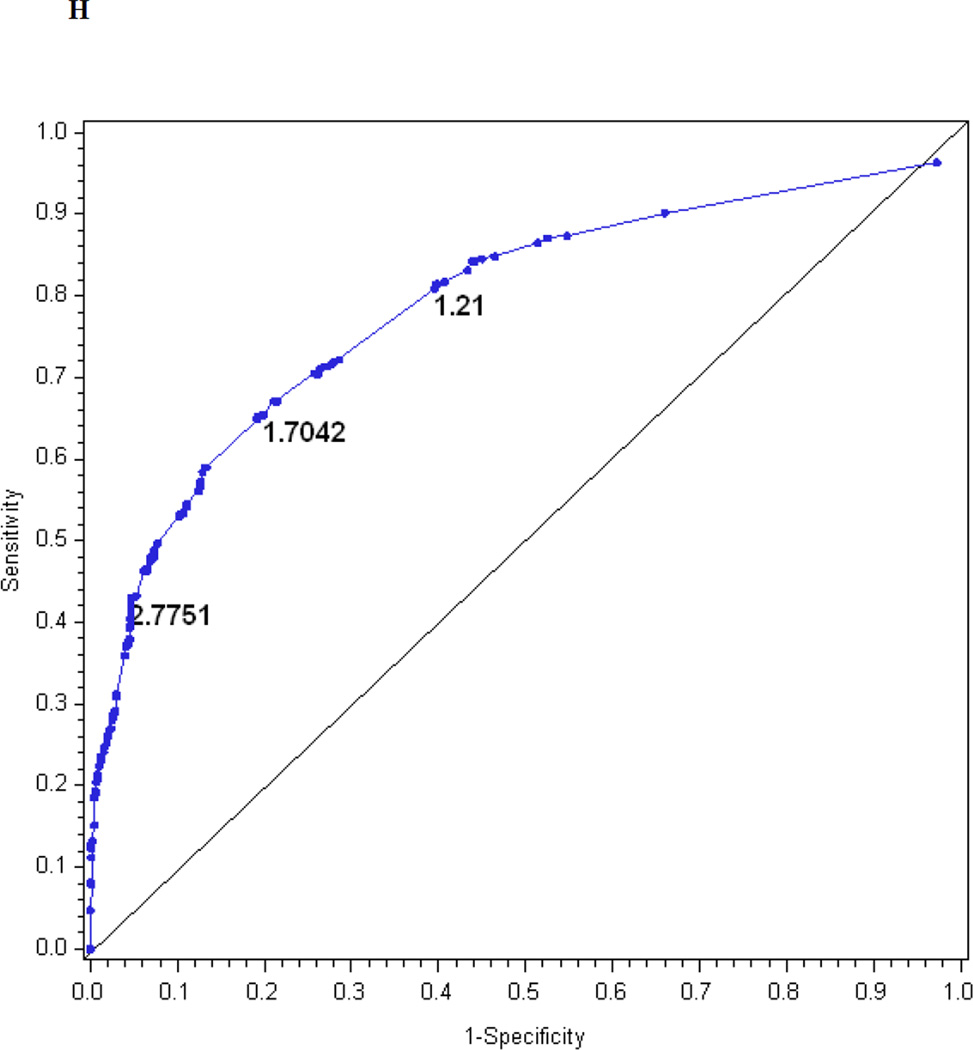
The performance of the Michigan Neuropathy Screening Instrument (MNSI) components for prediction of confirmed clinical neuropathy: (a) the MNSI questionnaire using the clinical scoring algorithm; (b) the MNSI examination using the clinical scoring algorithm; (c) the MNSI questionnaire index; (d) the MNSI examination index; (e) the MNSI combined questionnaire and examination index; (f) the MNSI questionnaire index; (g) the MNSI examination index; (h) the MNSI combined questionnaire and examination index. Graphs (c) to (h) identify the optimal cut-offs from Table 2 such that the proportion correctly classified is maximized. Additionally, cut-offs with approximately 80% sensitivity and 80% specificity are labelled.
Table 1 provides the sensitivity and specificity of each item in predicting confirmed clinical neuropathy. Sensitivities for the questionnaire items ranged from 4 to 49%, and specificities from 74 to 100%. The two questions with the best sensitivity were question 9 (Ever had diabetic neuropathy?) and question 4 (Get muscle cramps in your legs/feet?). The two questions with the best specificity, were question 15 (Have you ever had an amputation?) and question 6 (Hurt when bedcovers touch your skin?). The sensitivities for the examination items ranged from 2 to 72% and the specificities from 66 to 100% (Table 1). The reduction or absence of an ankle reflex was the most sensitive item and the presence of an ulcer the most specific. Items 1 and 9 from the questionnaire and the reduction or absence of an ankle reflex from the examination also had the highest χ2-values, indicating maximum discriminatory capability.
Table 1.
Performance of the individual components of the Michigan Neuropathy Screening Instrument (MNSI) questionnaire and examination in predicting confirmed clinical neuropathy
| Confirmed clinical neuropathy |
Sensitivity | Specificity | χ2-test* P-value |
|||
|---|---|---|---|---|---|---|
| Questionnaire | No | Yes | ||||
| 1. Are your legs and/or feet numb? | No | 790 | 231 | 34.6% | 95.5% | 192.1 |
| Yes | 37 | 122 | < 0.01 | |||
| 2. Do you ever have any burning pain in your legs and/or feet? | No | 765 | 267 | 25.0% | 92.7% | 70.9 |
| Yes | 60 | 89 | < 0.01 | |||
| 3. Are your feet too sensitive to touch? | No | 812 | 326 | 8.4% | 98.2% | 29.7 |
| Yes | 15 | 30 | < 0.01 | |||
| 4. Do you get muscle cramps in your legs and/or feet? | No | 613 | 203 | 42.8% | 74.1% | 33.3 |
| Yes | 214 | 152 | < 0.01 | |||
| 5. Do you ever have any prickling feelings in your legs or feet? | No | 724 | 221 | 37.6% | 87.6% | 97.8 |
| Yes | 103 | 133 | < 0.01 | |||
| 6. Does it hurt when the bedcovers touch your skin? | No | 812 | 332 | 6.7% | 98.3% | 20.4 |
| Yes | 14 | 24 | < 0.01 | |||
| 7. When you get into the bath or shower, are you able to tell the hot water from the cold water?† | No | 46 | 52 | 14.6% | 94.4% | 26.8 |
| Yes | 781 | 304 | < 0.01 | |||
| 8. Have you ever had an open sore on your foot? | No | 712 | 258 | 27.3% | 86.2% | 30.9 |
| Yes | 114 | 97 | < 0.01 | |||
| 9. Has your doctor ever told you that you have diabetic neuropathy? | No | 724 | 180 | 49.2% | 87.9% | 190.1 |
| Yes | 100 | 174 | < 0.01 | |||
| 10. Do you feel weak all over most of the time? | No | 809 | 324 | 9.0% | 97.7% | 27.1 |
| Yes | 19 | 32 | < 0.01 | |||
| 11. Are your symptoms worse at night? | No | 750 | 276 | 22.0% | 91.1% | 38.4 |
| Yes | 73 | 78 | < 0.01 | |||
| 12. Do your legs hurt when you walk? | No | 782 | 299 | 15.8% | 94.6% | 33.9 |
| Yes | 45 | 56 | < 0.01 | |||
| 13. Are you able to sense your feet when you walk?† | No | 75 | 39 | 11.0% | 90.9% | 1.0 |
| Yes | 752 | 317 | 0.31 | |||
| 14. Is the skin on your feet so dry that it cracks open? | No | 732 | 292 | 18.0% | 88.6% | 9.4 |
| Yes | 94 | 64 | < 0.01 | |||
| 15. Have you ever had an amputation? | No | 822 | 340 | 4.2% | 99.5% | 22.0 |
| Yes | 4 | 15 | < 0.01 | |||
| Examination | ||||||
| 1. Is the appearance of at least one foot abnormal? | No | 586 | 186 | 47.3% | 71.5% | 38.7 |
| Yes | 234 | 167 | < 0.01 | |||
| 2. Is ulceration present in at least one foot? | No | 817 | 348 | 2.0% | 99.5% | 5.9 |
| Yes | 4 | 7 | 0.02 | |||
| 3. Are the ankle reflexes reduced in both feet or absent in at least one foot? | No | 538 | 99 | 71.8% | 65.8% | 139.8 |
| Yes | 280 | 252 | < 0.01 | |||
| 4. Is vibration perception reduced in both feet or absent in at least one foot? | No | 617 | 149 | 58.2% | 75.2% | 121.2 |
| Yes | 204 | 207 | < 0.01 | |||
Testing that the proportion ‘yes’ on the MNSI questionnaire or examination is the same for those with and without confirmed clinical neuropathy.
Questions 7 and 13 were reversed scored so that ’no‘ responses indicated an abnormality and counted as one point in the scoring algorithms. The sensitivities and specificities are based on the reversed 2 × 2 tables.
Table 2 presents the β-coefficients for each of the items, as well as the mean, median, standard deviation and range of calculated MNSI index scores based on the questionnaire alone, examination alone and a combination of both. The logistic regression model for the MNSI questionnaire index explains R2 = 21% of the variance in confirmed clinical neuropathy. Figure 1c is the receiver operating characteristic curve showing the performance of the MNSI questionnaire index in predicting confirmed clinical neuropathy. The AUC is 0.75. The cut-off with the highest probability of correctly classifying (proportion correctly classified 0.79) confirmed clinical neuropathy is > 2.0318, with a sensitivity of 38%, specificity of 96%, positive predictive value of 82% and negative predictive value of 78%.
Table 2.
Performance of the Michigan Neuropathy Screening Instrument (MNSI) index equations in predicting confirmed clinical neuropathy (β-coefficients ± standard errors)
| MNSI index* | MSNI questionnaire index |
MSNI examination index |
MNSI combined index |
Reduced questionnaire index |
Reduced examination index |
Reduced combined index |
|---|---|---|---|---|---|---|
| Questionnaire | Beta-coefficients ± standard errors † | |||||
| 1. Are your legs and/or feet numb? | 1.55 (0.25) | 1.25 (0.27) | 1.55 (0.23) | 1.21 (0.25) | ||
| 2. Ever have any burning pain in your legs and/or feet? | 0.26 (0.25) | 0.65 (0.27) | 0.56 (0.26) | |||
| 1. Are your feet too sensitive to touch? | 0.69 (0.43) | 0.56 (0.46) | ||||
| 2. Do you get muscle cramps in your legs and/or feet? | 0.15 (0.18) | 0.03 (0.19) | ||||
| 3. Do you ever have any prickling feelings in your legs or feet? | 0.54 (0.20) | 0.51 (0.22) | 0.60 (0.18) | 0.49 (0.21) | ||
| 4. Does it hurt when the bedcovers touch your skin? | −0.63 (0.49) | −0.71 (0.52) | ||||
| 5. When you get into the bath or shower, are you able to tell the hot water from the cold water?§ | 0.61 (0.29) | 0.65 (0.31) | ||||
| 6. Have you ever had an open sore on your foot? | 0.39 (0.19) | 0.20 (0.21) | 0.46 (0.18) | |||
| 7. | 1.21 (0.18) | 0.83 (0.20) | 1.28 (0.17) | 0.89 (0.19) | ||
| 8. Do you feel weak all over most of the time? | 0.27 (0.40) | 0.32 (0.44) | ||||
| 9. Are your symptoms worse at night? | −0.53 (0.26) | −0.45 (0.27) | ||||
| 10. Do your legs hurt when you walk? | 0.18 (0.29) | 0.15 (0.31) | ||||
| 11. Are you able to sense your feet when you walk?§ | –0.22 (0.27) | –0.21 (0.29) | ||||
| 12. Is the skin on your feet so dry that it cracks open? | 0.10 (0.22) | –0.01 (0.23) | ||||
| 13. Have you ever had an amputation? | 0.92 (0.77) | 0.65 (0.85) | ||||
| Examination | ||||||
| 1. Is the appearance of at least one foot abnormal? | 0.63 (0.15) | 0.44 (0.17) | 0.65 (0.15) | 0.47 (0.16) | ||
| 2. Is ulceration present in at least one foot? | 1.27 (0.77) | 0.79 (0.92) | ||||
| 3. Are the ankle reflexes reduced or absent in at least one foot? | 1.32 (0.15) | 1.21 (0.17) | 1.32 (0.15) | 1.22(0.16) | ||
| 4. Is vibration perception reduced or absent in at least one foot? | 1.02 (0.15) | 0.60 (0.17) | 1.02 (0.15) | 0.61 (0.16) | ||
| Model R2 | 21% | 17% | 27% | 20% | 17% | 26% |
| Descriptive statistics for indices | ||||||
| n | 1157 | 1158 | 1132 | 1172 | 1160 | 1149 |
| Mean (SD) | 0.77 (1.16) | 1.18 (1.03) | 2.30 (1.46) | 0.70 (1.09) | 1.17 (1.02) | 1.45 (1.39) |
| Median | 0.16 | 1.32 | 2.00 | 0.00 | 1.17 | 1.22 |
| Range | (−0.85, 5.58) | (0.00, 4.24) | (−0.14, 8.38) | (0.00, 3.88) | (0.00, 2.99) | (0.00, 5.43) |
| Area under the receiver operating characteristic curve | 0.75 | 0.76 | 0.81 | 0.74 | 0.76 | 0.81 |
| Optimal cut-off‡ | 2.0318 | 1.9503 | 3.2516 | 1.2760 | 1.9776 | 2.7751 |
Each index was computed as the sum of the β-coefficients for all items for which there was a positive response possibly indicating distal symmetrical peripheral neuropathy.
Data are β-coefficients ± standard errors from multiple logistic regression models. Coefficients were used to derive MNSI indices for predicting confirmed clinical neuropathy.
The cut-off such that the proportion correctly classified is maximized.
Questions 7 and 13 were reversed scored so that ’no‘ responses indicated an abnormality and counted as one point in the scoring algorithms.
The logistic regression model for the MNSI examination index explains R2 = 17% of the variance in confirmed clinical neuropathy. Figure 1d is the receiver operating characteristic curve showing the performance of the MNSI examination index in predicting confirmed clinical neuropathy. The AUC is 0.76. The cut-off with the highest probability of correctly classifying (proportion correctly classified 0.74) confirmed clinical neuropathy is > 1.9503, with a sensitivity of 45%, specificity of 87%, positive predictive value of 60% and negative predictive value of 79%.
The logistic regression model for the combined questionnaire and examination index explains R2 = 27% of the variance in confirmed clinical neuropathy. Figure 1e is the receiver operating characteristic curve showing the performance of the MNSI combined index. The AUC is 0.81. The cut-off with the highest probability of correctly classifying (proportion correctly classified 0.80) confirmed clinical neuropathy is > 3.2516, with a sensitivity of 50%, specificity of 92%, positive predictive value of 74% and negative predictive value of 81%.
Table 2 also presents the reduced models based on stepwise regression. Only four questions entered the reduced index derived from the questionnaire, three items entered the reduced index derived from the examination and seven items entered the reduced index derived from the questionnaire and examination. Figures 1f, 1g, and 1h show the performance of the reduced MNSI questionnaire index, examination index and combined questionnaire and examination index in predicting confirmed clinical neuropathy. The logistic regression models explained R2 = 20, 17 and 26% of the variance, respectively. AUCs were 0.74, 0.76 and 0.81, respectively. The optimal cut-offs for achieving the highest probability of correctly classifying confirmed clinical neuropathy are > 1.2760 for the reduced MNSI questionnaire (proportion correctly classified 0.78, sensitivity 46%, specificity 92%, positive predictive value 72% and negative predictive value 80%), > 1.9776 for the reduced MNSI examination (proportion correctly classified 0.74, sensitivity 45%, specificity 87%, positive predictive value 60% and negative predictive value 79%) and > 2.7751 for the combined MNSI index (proportion correctly classified 0.80, sensitivity 43%, specificity 95%, positive predictive value 80% and negative predictive value 80%). These reduced models perform nearly as well as the more extensive models.
Discussion
Distal symmetrical peripheral neuropathy is a frequent complication of diabetes [10]. Historically, the diagnosis of distal symmetrical peripheral neuropathy has been based upon symptoms, signs and electrophysiological testing. More recent consensus guidelines have also recommended quantitative sensory testing and consideration of intraepidermal nerve fibre density as diagnostic tests [11–13]. Unfortunately, clinical examinations require specially trained and experienced personnel, nerve conduction studies and quantitative sensory testing require special equipment and skin biopsies are invasive. Therefore, simple non-invasive clinical tests that assess symptoms and signs have been developed and used, especially in clinical trials [14,15]. The MNSI is one such test. The MNSI was first proposed in 1994 [1]. Since then, it has been widely used to assess distal symmetrical peripheral neuropathy in clinical practice and in large clinical trials, including the DCCT/EDIC, the Action to Control Cardiovascular Disease in Diabetes (ACCORD) (16) and the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) [17]. During years 13–14 of EDIC, the DCCT/EDIC cohort with Type 1 diabetes had the MNSI performed and underwent neurologic examinations and electrodiagnostic testing [5]. This provided an opportunity to reassess the performance of the MNSI in detecting distal symmetrical peripheral neuropathy and to develop new scoring algorithms. When used separately, the MNSI questionnaire (AUC 0.73) and examination (AUC 0.76) performed similarly in predicting confirmed clinical neuropathy. We found, however, that the published cut point to define a positive test for the questionnaire (≥ 7) was very insensitive, missing many patients with confirmed clinical neuropathy. Changing the cut point to define a positive test for the questionnaire to ≥ 4 harmonized the sensitivity and specificity of the MNSI questionnaire and the MNSI examination.
We then examined the performance of each item in the MNSI questionnaire and examination in predicting confirmed clinical neuropathy. The two questions with the highest sensitivity were question 9 (ever had diabetic neuropathy?) and question 4 (get muscle cramps in your legs/feet?). The two questions with the highest specificity were question 15 (have you ever had an amputation?) and question 6 (hurt when bedcovers touch your skin?). The reduction or absence of an ankle reflex was the most sensitive examination item and the presence of an ulcer the most specific. Our finding that question 4 (get muscle cramps in your legs/feet?) was the second most sensitive for confirmed clinical neuropathy was of interest because it had previously been excluded from the published scoring algorithm as it was deemed to be a measure of impaired circulation and not neuropathy. We suspect that question 4 was a sensitive question because it is a common symptom associated with increased activity in poorly conditioned subjects, medications including diuretics and statins, vascular insufficiency and muscle denervation and reinnervation. The fact that question 4 was the least specific question for distal symmetrical peripheral neuropathy supports this hypothesis.
We next used multivariate logistic regression to derive an MNSI questionnaire index, an MNSI examination index and a combined index to predict confirmed clinical neuropathy. The AUCs were 0.75, 0.76 and 0.81, respectively. Finally, using stepwise logistic regression, we developed parsimonious models from the questionnaire, examination and combined questionnaire and examination. Only seven items entered the reduced index derived from both the questionnaire and examination and the reduced model performed nearly as well as the more extensive models.
The strength of our study was its large size, the uniform assessment of distal symmetrical peripheral neuropathy at all 28 EDIC sites and the rigorous methods used to test the sensitivity and specificity of the MNSI. The major limitation is that our study involved relatively young patients with Type 1 diabetes of long duration and with a moderate prevalence (30%) of distal symmetrical peripheral neuropathy. The performance of the MNSI may be different in older populations with Type 2 diabetes and additional comorbidities. In addition, the positive predictive value of the MNSI will not be as robust in populations with lower prevalences of distal symmetrical peripheral neuropathy. Finally, DCCT/EDIC patients had been followed for many years and were usually aware of their neuropathy status. While this might have biased their responses to question 9 (Has your doctor ever told you that you have diabetic neuropathy?), analyses performed including and excluding responses to question 9 did not substantially impact our results (see also Supporting Information, Appendix S1).
Our analyses confirm that the MNSI is a simple, non-invasive and valid measure of distal symmetrical peripheral neuropathy when compared with gold standard diagnostic testing that includes neurological examinations performed by board-certified neurologists and standardized electrophysiology examinations. The MNSI can be used in clinical practice and in large clinical trials to assess distal symmetrical peripheral neuropathy. Altering the cut point to define an abnormal questionnaire from ≥ 7 abnormal to ≥ 4 abnormal items improves the performance of the MNSI questionnaire relative to the examination, and defining a combined MNSI index further increases the sensitivity and specificity of the instrument. Assigning scores based on weights derived from multivariate regression models further improves the performance of the questionnaire, examination and the combination of the two.
Supplementary Material
Acknowledgements
Contributors of free or discounted supplies and/or equipment were: Abbott, Animas, Aventis, B-D, Bayer, Can-AM, Eli Lilly, Lifescan, Medtronic Minimed, Omron and Roche. The DCCT/EDIC is supported by contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, National Eye Institute, National Institute of Neurological Disorders and Stroke, the General Clinical Research Centers Program and the Clinical and Translational Science Awards Program, National Center for Research Resources, and by Genentech through a Cooperative Research and Development Agreement with the National Institute of Diabetes and Digestive and Kidney Diseases. A complete list of participants in the DCCT/EDIC research group can be found in Archives of Ophthalmology 2008; 126: 1713.
Abbreviations
- AUC
area under the receiver operating characteristic curve
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- MNSI
Michigan Neuropathy Screening Instrument
Footnotes
Competing interests
Nothing to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Performance of the MNSI index equations in predicting confirmed clinical neuropathy eliminating question 9.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than for missing material) should be directed to the corresponding author for the article.
References
- 1.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122:561–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial (DCCT) Research Group. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 5.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. for the DCCT Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabetes Care. 2010;33:1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox DR. The Analysis of Binary Data. 2nd edn. London: Chapman and Hall; 1970.. [Google Scholar]
- 7.Madalla GS. Limited-Dependent and Qualitative Variables in Econometrics. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- 8.Zweig M, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 9.SAS Institute Inc. Logistic Regression Examples Using the SAS System. 1st edn. Cary, NC: AS Institute Inc.; 1995. version 6. [Google Scholar]
- 10.Boulton AJM, Vinik AI, Arezzo JC, Bril V, Feldman EV, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 11.Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. on the behalf of the Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research. Report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- 13.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, et al. Practice parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review): Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72:177–184. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 14.Dyck PJ, Davies JL, Litchy WJ, O’Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997;49:229–239. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJ. Gries FA, Cameron NE, Low PA, Ziegler D. Textbook of Diabetic Neuropathy. New York, NY: Thieme New York; 2003. Severity and staging of diabetic polyneuropathy; pp. 170–174. [Google Scholar]
- 16.Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33:1688–1690. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pop-Busui R, Lu J, Lopes N, Jones TL. BARI 2D Investigators. Prevalence of diabetic peripheral neuropathy and relation to glycemic control therapies at baseline in the BARI 2D cohort. J Peripher Nerv Syst. 2009;14:1–13. doi: 10.1111/j.1529-8027.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.