Abstract
Proper integration and execution of the physiological stress response is essential for maintaining homeostasis. Stress responses are controlled in large part by the paraventricular nucleus of the hypothalamus, which contains three functionally distinct neural populations that modulate multiple stress effectors: 1) hypophysiotrophic PVN neurons that directly control the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis; 2) magnocellular neurons and their secreted neurohypophysial peptides; and 3) brainstem and spinal cord projecting neurons that regulate autonomic function. Evidence for activation of PVN neurons during acute stress exposure demonstrates extensive involvement of all three effector systems. In addition, all PVN regions appear to participate in chronic stress responses. Within the hypophysiotrophic neurons, chronic stress leads to enhanced expression of secreted products, reduced expression of glucocorticoid receptor and GABA receptor subunits, and enhanced glutamate receptor expression. In addition, there is evidence for chronic stress-induced morphological plasticity in these neurons, with chronic drive causing changes in cell size and altered GABAergic and glutamatergic innervation. The response of the magnocellular system varies with different chronic exposure paradigms, with changes in neurohypophysial peptide gene expression, peptide secretion and morphology seen primarily after intense stress exposure. The preautonomic cell groups are less well studied, but are likely to be associated with chronic stress-induced changes in cardiovascular function. Overall, the PVN is uniquely situated to coordinate responses of multiple stress effector systems in the face of prolonged stimulation, and likely plays a role in both adaptation and pathology associated with chronic stress.
Keywords: vasopressin, corticotropin releasing hormone, magnocellular, parvocellular, preautonomic
The paraventricular nucleus of the hypothalamus is a critical node for regulation and orchestration of physiological stress responses. Neurons resident in the PVN control three major physiological effector pathways: the hypothalamo-pituitary-adrenocortical (HPA) axis, through neurons in the dorsomedial parvocellular (PVNmpd) and anterior parvocellular (PVNap) divisions of the nucleus; neurohypophysial peptide signals, via magnocellular vasopressin (AVP) and oxytocin (OXT) neurons in the anterior (PVNam), medial (PVNmm) and posterior magnocellular (PVNpm) cell groups; and autonomic regulation, using brainstem and spinal cord projecting neurons in the lateral parvocellular (PVNlp), dorsal parvocellular (PVNdp) and ventromedial parvocellular (PVNmpv) divisions (Swanson and Sawchenko, 1983) (see Fig. 1). The intermingling of three physiologically distinct signaling systems places the PVN at the cross-roads for integration of diverse outputs in a coordinated and inter-regulated fashion.
Figure 1.
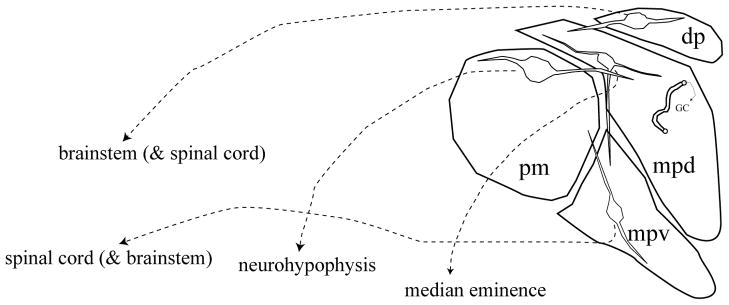
Schematic of the organization of the hypothalamic paraventricular nucleus. Neurons in the dorsal parvocellular (dp) and ventral division of the medial parvocellular regions (mpv) project primarily to brainstem and spinal cord regions associated with autonomic control. Neurons present in the dorsal division of the medial parvocellular zone (mpd) project primarily to the median eminence and control ACTH release. The posterior magnocellular (pm) subdivision sends projections to the neurohypophysis. Note that the dendrites of neurons localized in all three regions ramify across subdivisions, allowing for intranuclear communication.
Regulation of the HPA axis is perhaps the most clear-cut stress integrative role of the PVN. Neurons in the PVNmpd express corticotropin releasing hormone (CRH), which is required for activation of pituitary ACTH release (Antoni, 1986). Parvocellular CRH neurons also express numerous co-secreted peptides as well as glutamate (Swanson, 1991; Ziegler et al., 2002), indicating that multiple factors may be involved in parvocellular signaling. Importantly, AVP is among the peptides co-localized with CRH in PVNmpd neurons, and synergizes with CRH to enhance ACTH release at the level of corticotrope (Gillies et al., 1982).
The magnocellular neurosecretory system also plays a role in stress regulation. There is a substantial body of evidence to suggest that OXT can be released into the systemic circulation by acute stressors (Jezova et al., 1995; Wojtak et al, 1998). Peripheral release of AVP is more controversial, and does not occur following many stressors (Husain et al. 1979; Jezova et al., 1995; Wojtak et al, 1998). However, both peptides are released from dendrites in the SON following stress (Wojtak et al, 1998) and appear to diffuse to limbic brain sites (Ludwig and Leng, 2006), where they have the capacity to influence behavioral stress responses.
Vasopressin is implicated in amplification of pituitary ACTH secretion (Gillies et al., 1982). There is also some evidence for an ACTH-potentiating action of OXT (Gibbs et al., 1984). Both peptides are released into the hypophysial portal blood, supporting the notion that they have access to the anterior pituitary (Gibbs, 1984; Plotsky and Sawchenko, 1987). There are data to suggest release of peptide from magnocellular axons of passage in the median eminence (Holmes et al., 1986), which may account for the portal blood OXT and perhaps AVP. Thus, the magnocellular system is positioned to play a role in control of HPA axis responsivity (Engelmann et al, 2004).
The magnocellular system may also participate in immune responses to stress. Cytokine-like effects have been ascribed to both AVP and OXT (Kovacs, 2002), and neurointermediate pituitary lobectomy alters humoral and cell mediated immune responses (Quintanar-Stephano et al., 2004). Both AVP and OXT are capable of replacing the IL-2 requirement for the facilitation of interferon-γ from immune cells (Johnson et al., 1982). In addition to the ‘cytokine-like’ effects of the classic neurohypophysial peptides, several cytokines (i.e., IL-1β (Watt and Hobbs, 2000), IL-1ra (van Dam et al., 1998) and IL-6 (Ghorbel et al., 2003) are expressed in the magnocellular neurons of the PVN and SON. Both IL-6 and IL-1β are found in the posterior pituitary and median eminence (Watt and Hobbs, 2000; Jankord et al., 2007) and can be released in response to dehydration (Ghorbel et al., 2003; Summy-Long et al., 2006). The ability to store and secrete cytokines and other immune-modulating factors allows the magnocellular system to influence the organismal response to immune challenge. In combination with the output from the HPA axis and sympathetic nervous system, the secreted cytokine and cytokine-like products of the neurohypophyseal neurons may affect responses to immune challenge and thus participate in a ‘neuroimmune’ stress response.
Brainstem and spinal cord-projecting neurons represent the final major cellular subpopulation in the PVN. These neurons express a number of neuropeptides, with a substantial population being oxytocinergic (Hallbeck et al., 2001). The projection pathways suggest a role in autonomic regulation, and there is evidence to support a role for these PVN neurons in sympathetic activation consequent to stress. Viral tracing studies confirm that populations of neurons within the PVN have oligosynaptic connections with both sympathetic and parasympathetic targets (Kalsbeek et al., 2004), suggesting that the role of the PVN in autonomic integration may involve regulation of both systems.
It is important to note that there is substantial opportunity for intranuclear communication within the PVN. There is strong evidence for dendritic release in the PVN and magnocellular supraoptic nucleus (SON) (Landgraf and Neumann, 2004; Ludwig and Leng, 2006), implying that activation of one compartment or neuronal cell type can have a marked influence on adjoining, functionally distinct cell populations.
Activation of PVN Neurons by Acute Stressors
The central role of the PVNmpd in HPA activation is underscored by numerous studies documenting induction of immediate early gene expression (Fos, NGFI-B) following exposure to stressors, consistent with stimulation-induced transcriptional activation (Kovacs and Sawchenko, 1996). This conclusion is further supported by studies documenting rapid phosphorylation of cyclic AMP response element binding protein (CREB) and mitogen activated protein (MAP) kinase following stressors (Kovacs and Sawchenko, 1995; Khan and Watts, 2004). Stressor exposure induces transcription of CRH as well as AVP genes in the parvocellular PVN, and at later time points, increased CRH and AVP mRNA pools (Herman et al., 1992; Herman 1995; Kovacs and Sawchenko, 1995). Moreover, acute stress exposure results in decreased CRH content in the median eminence (Chappell et al., 1986; Feldman and Weidenfeld, 1998), consistent with release, and directly increases portal blood levels of CRH and AVP (as well as OXT) (Gibbs, 1984; Plotsky and Sawchenko, 1987; Romero et al., 1993).
Activation of magnocellular neurons, as reflected by immediate early gene expression, is also induced by acute stress. In general, the threshold for activation of OXT neurons appears to be lower than that of AVP cells. For example, exposure to immobilization causes release of OXT, but not AVP into the peripheral circulation (Jezova et al., 1995), and greater numbers of OXT neurons are activated by stressors such as lipopolysaccharide (Matsunaga et al., 2000), interleukin 1-beta (Ericsson et al., 1994) or immobilization (Jezova et al., 1995). Fos activation of magnocellular AVP neurons is observed following hypertonic saline exposure, hemorrhage and hypoxia (Sharp et al., 1991; Thrivikraman et al., 2000; Figueiredo et al., 2003), suggesting that these neurons are responding to disruption of fluid and electrolyte balance, rather than stress per se. Fos expression is observed in both AVP and OXT cells under these conditions (Giovannelli et al., 1992; Ding et al., 1994).
Other stressors, such as restraint (a considerably milder stimulus than aforementionned immobilization), swim, and novelty, have less clear-cut actions on the magnocellular system (Cullinan et al., 1995; Emmert and Herman, 1999; Figueiredo et al., 2003). Notably, time course studies suggest that induction of c-fos mRNA occurs at relatively long post-stress intervals (Cullinan et al., 1995), suggesting that under these conditions, magnocellular neurons are responding to the physiologic consequences of the stress response, rather than the stressor per se.
Fos activation in PVN preautonomic cell groups is also seen in response to acute stressors. Several combined tract-tracing and Fos mapping studies indicate that brainstem- and spinal cord-projecting PVN cell groups are activated by water deprivation, insulin, lipopolysaccharide and acute restraint (Zhang et al., 2000; Carrasco et al., 2001; Stocker et al., 2004; Radley et al., 2006), suggesting that like the parvocellular CRH system, the preautonomic PVN is responsive to a wide range of stressors. The response of the preautonomic system to interleukin 1-beta is prolonged relative to that of the PVNmpd (Ericsson et al., 1994), suggesting that this system may respond to some stimuli with an extended time course.
Chronic Stress: Cellular Responses of PVN neurons
Chronic stress exposure produces episodic and cumulative increases in circulating corticosteroids and catecholamines. Given the impact of single stressors on PVN neurons, it is not surprising that chronic exposure results in pronounced changes in gene expression, peptide levels, and releasable peptide pools. Within the PVNmpd, a variety of chronic stress paradigms, such as footshock, immobilization, chronic unpredictable stress, social subordination and social defeat, cause a reliable increase in CRH mRNA expression (Imaki et al., 1991; Herman et al., 1995; Makino et al., 1995; Albeck et al., 1997; Keeney et al., 2006). Importantly, CRH mRNA induction by repeated immobilization is observed across multiple rat strains (Gomez et al., 1996), indicating that this is a consistent result of chronic stress. In addition to CRH, chronic stress exposure increases expression of AVP mRNA in parvocellular neurons, both in terms of number of detectable cells and cellular grain density (Herman et al., 1995; Makino et al., 1995; Albeck et al., 1997). In addition, AVP peptide co-localization with CRH is increased in the median eminence following repeated immobilization (de Goeij et al., 1991). The latter observation predicts increased AVP activity at the level of the anterior pituitary, although this has yet to be definitively proven.
The changes discussed above are observed in most, but not all stress paradigms. Notably, adjuvant arthritis decreases CRH mRNA levels, despite the presence of elevated corticosterone (Harbuz et al., 1992). However, parvocellular AVP levels are markedly increased in arthritic rats, suggesting that this peptide may take on a larger role in mediating ACTH release during the inflammatory process (Shanks et al., 1998).
Activation of magnocellular neurons is observed in some, but not all chronc stress paradigms. Chronic stressors that involve modulation of fluid and electrolyte balance (e.g., hypertonic saline) reliably increase expression of both AVP and OXT in magnocellular neurons (Kiss and Aguilera, 1993; Glasgow et al., 2000). Neural activation is accompanied by increased secretion of peptide into the systemic circulation (Kiss and Aguilera, 1993). Activation of AVP neurons may be related to altered fluid and electrolyte homeostasis in this model. Other models, including chronic variable stress (Herman et al., 1995), chronic social stress (Albeck et al., 1997), do not result in increased AVP mRNA expression, consistent with the latter interpretation. The significance of elevated magnocellular OXT following chronic hypertonic saline is unclear, and surprisingly little is know about regulation of OXT in other chronic stress pardigms.
Magnocellular neurons may play an important role in behavioral responses to chronic stress. Recent studies from Landgraf and colleagues indicate that elevations in paraventricular AVP are correlated with high anxiety behavior in both rat and mouse strains bred for low or high anxiety (Landgraf et al., 2007). The effects of elevated AVP on behavior appear to be related to dendritic release (Ludwig and Leng, 2006), raising the possibility that magnocellular AVP may affect multiple domains of PVN action.
In addition to affecting production of PVN effector molecules, chronic stress also causes marked changes in expression of PVN neural signaling molecules. Notably, chronic unpredictable stress and repeated immobilization both elicit down-regulation of glucocorticoid receptor (GR) mRNA expression in the medial parvocellular PVN (Herman et al., 1995; Makino et al., 1995). The PVN GR is known to be involved in inhibition of the HPA axis (Kovacs et al., 1986; Kovacs and Makara, 1988). By virtue of its role as a ligand gated transcription factor, decrements in GR expression may precipitate broad changes in gene expression in the parvocellular PVN. Indeed, the loss of GR may be connected with up-regulation of both CRH and AVP mRNAs in the PVNmpd (Herman et al., 1995).
Expression of co-localized neuropeptide species are also modulated by chronic stress. Chronic hypertonic saline administration or morphine withdrawal lead to elevated proenkephalin expression in both parvocellular and magnocellular PVN neurons, as well as magnocellular neurons in the SON (Harbuz et al., 1991; Young and Lightman, 1992).
Chronic stress also causes marked changes in expression of a number of PVN neurotransmitter receptors. Notably, the beta-1 and beta-2 subunits of the GABA-A receptor are down-regulated in the PVNmpd (but not PVNpm) by chronic unpredictable stress (Cullinan, 2000), consistent with reduced efficacy of GABA signaling (and hence, inhibition) specifically in parvocellular PVN neurons. These data are supported by electrophysiological studies documenting reduced GABA signaling in PVN slices from chronically-stressed rats (Verkuyl et al., 2004).
In contrast, excitatory processes appear to be enhanced in PVNmpd neurons. Chronic variable stress causes marked increases in expression of the GluR5 subunit of the kainate-preferring glutamate receptor in the PVNmpd, consistent with enhanced capacity for glutamate signaling through non-NMDA channels (Fig. 2). Expression of NMDA-R1 and NMDA-R2A receptor subunits are not altered by chronic stress. However, the NMDA-R2B receptor is significantly down-regulated (Ziegler et al., 2005). Receptors containing the NMDA-R2B subunit are less permeable to calcium then those containing NMDA-R2A (c.f., (Loftis and Janowsky, 2003)), suggesting that decreased expression of this receptor subunit may enhance NMDA-mediated calcium currents, also consistent with enhanced glutamate signaling. In addition, there is evidence that the alpha-1B adrenergic receptor is up-regulated under conditions of high HPA axis drive (adrenalectomy) (Day et al., 1999). Coupled with studies showing enhancement of acute stress-induced PVN norepinephrine release following chronic cold exposure (Ma and Morilak, 2005), these data suggest the chronic stress enhances stress-excitatory norepinephrine signaling.
Figure 2.
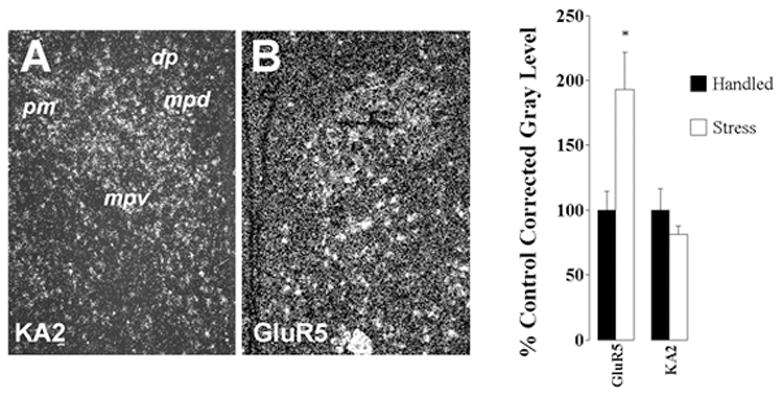
Distribution and regulation of KA2 and GluR5 receptor subunits in the PVN. As noted in A, KA2 mRNA is expressed in all subregions of the PVN (see Fig. 1 for abbreviations) GluR5 mRNA expression is particularly enriched in the PVNmpd. Semi-quantitative analysis of KA2 and GluR5 subunit mRNA expression in the PVN. Exposure to CVS causes a marked up-regulation of GluR5 expression in the PVN, whereas KA2 mRNA levels are unchanged.
The influence of chronic stress on regulation of preautonomic PVN neurons remains to be clearly established. There is evidence for alterations in preautonomic PVN regions in experimental models of chronic heart failure (Patel and Zhang, 1996; Vahid-Ansari and Leenen, 1998). However, in this model it is difficult to conclude if the activation pattern is due to stress or reflex compensation for autonomic dysfunction. Nonetheless, chronic stress exposure has deleterious effects on cardiovascular regulation (Grippo et al., 2002), and further study is clearly needed to determine the contribution of preautonomic PVN neurons to this process.
Chronic Stress: Morphological Plasticity in PVN
There is evidence that structural changes in magnocellular neurons are a consequence of prolonged neuronal activation. Electron micrographic studies present evidence for cellular hypertrophy and glial retraction in the supraoptic nucleus of rats during prolonged stimulation (in the case of AVP neurons, salt loading/dehydration; in the case of OXT neurons, lactation) (Hatton, 1997). Lactation increases excitatory and inhibitory synaptic contacts onto both AVP and OXT neurons (Theodosis and Poulain, 1993; Theodosis, 2002), accompanied by increases in norepinephrine-containing terminals onto OXT neurons (Michaloudi et al., 1997). In the vasopressinergic system, salt loading causes increased numbers of GABA and glutamate terminal appositions on magnocellular neurons of the SON (Mueller et al., 2005). However, there was a pronounced decrease in norepinephrine innervation of AVP neurons, suggesting that regulation of synaptic plasticity may be stressor-specific (Mueller et al., 2005). Finally, altered dendritic branching is also observed during lactation, with oxytocinergic neurons exhibiting retraction and vasopressinergic dendrites showing extension (Stern and Armstrong, 1998). The dendritic changes are consistent with activity-dependent remodeling in the magnocellular system, and further indicate that magnocellular neuronal phenotypes have distinctive growth responses to stimulation.
The studies cited above involve stimulation at extreme levels of physiologic drive. However, even milder stressors, i.e., repeated restraint, can increase cell size and incidence of multiple synapses on magnocellular SON neurons (Miyata et al., 1994), suggesting that chronic stress may also drive changes in magnocellular synaptology. For this reason, the effect of chronic stress on hypophysiotrophic and preautonomic cell populations is currently a topic of considerable interest. Recently, our group tested the ability of chronic stimulation to alter the innervation of parvocellular PVN neurons. To test this hypothesis, we assessed glutamate innervation of parvocellular PVN neurons following adrenalectomy, which produces pronounced hyperactivation of PVN CRH neurons (Sawchenko, 1987). Our data are summarized in Fig. 3. Using three-dimensional reconstructions of confocal stacks double-stained for CRH and vesicular glutamate transporter 2 (a glutamate marker), we were able to demonstrate enhanced glutamate innervation of CRH neurons following chronic central stimulation of the PVNmpd. Subsequent studies in chronically-stressed animals suggest that the same reorganization occurs in response to chronic variable stress (Flak, Ostrander, Mueller and Herman, unpublished observations).
Figure 3.
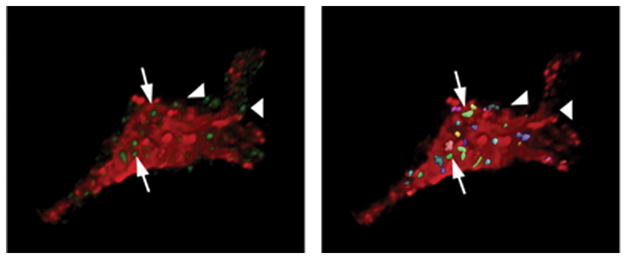
3-D Rendering of Confocal Images: CRH and VGLUT2. Left: cropped, 3-D rendering of a single CRH cell (red) amid green VGlut2 puncta, indicative of glutamatergic terminals. Right: processed image of the same cell, with multi-colored boutons indicating those showing significant overlap between red and green fluorescence signal. Arrows indicate examples of boutons that ‘contact’ the CRH neuron, as determined by the ‘overlap’ algorithm in Volocity 2; boutons that do not contact (arrowheads) do not register as overlapping entities. The volumes of positive elements in the cell and bouton channels were measured independently, using the classification features; overlap was determined using the co-localization feature, which determines incidence and volume of boutons contacting the immunolabeled cell. Preliminary analysis of ADX data using 6-cells/group (2 each from 3 animals/group) revealed a 56% increase in VGlut2 bouton counts/cell surface area using this method. In addition, ADX animals showed an increase in contact bouton overlap area and in overall bouton size.
Notably, administration of IL-1 or amphetamine produces long-lasting sensitization of HPA axis responses that correlates with a reduction of dopamine-beta-hydroxylase (DBH) immunoreactivity in the dorsal and medial parvocellular subdivisions of the PVN three weeks after presentation of the stressor (Jansen et al., 2003). Thus, it is possible that even short-term stress exposure can result in substantial alterations in PVN innervation.
Neuroplasticity and PVN Responses to Chronic Stress
The data reviewed above provides ample evidence for multifaceted neuroplastic responses in the PVN following chronic stress. Chronic stress-induced changes in PVN function are summarized in Fig. 4. First, chronic stress changes the cocktail of secretagogues synthesized and released by the PVNmpd. Enhancement of parvocellular CRH and AVP are likely to lead to enhanced pituitary responsiveness to stress, and may account for the sustenance of corticosterone secretion during chronic stress and perhaps in stress related pathologies. Second, the loss of local glucocorticoid receptors stands to decrease local glucocorticoid feedback and enhance cellular responsivity. Chronic stress-induced increases in parvocellular CRH and AVP may be fueled in part by the loss of glucocorticoid feedback in the PVN. Third, chronic stress causes changes in the receptor configuration in the PVNmpd, suggestive of reduced numbers of functional GABA-A receptors and increased glutamatergic and perhaps noradrenergic signaling. The aggregate response to receptor changes would be predicted to once again enhance excitability and increase stress responsiveness. Finally, recent data suggest that drive of the parvocellular system by adrenalectomy or chronic stress enhances glutamate innervation to CRH neurons. This change also predicts enhanced activational capacity of the HPA axis. Overall, it appears that chronic drive of the parvocellular system leads to a multiplicity of neuroplastic changes that, in toto, result in an HPA axis that is poised to hyperrespond to incoming stimuli.
Figure 4.
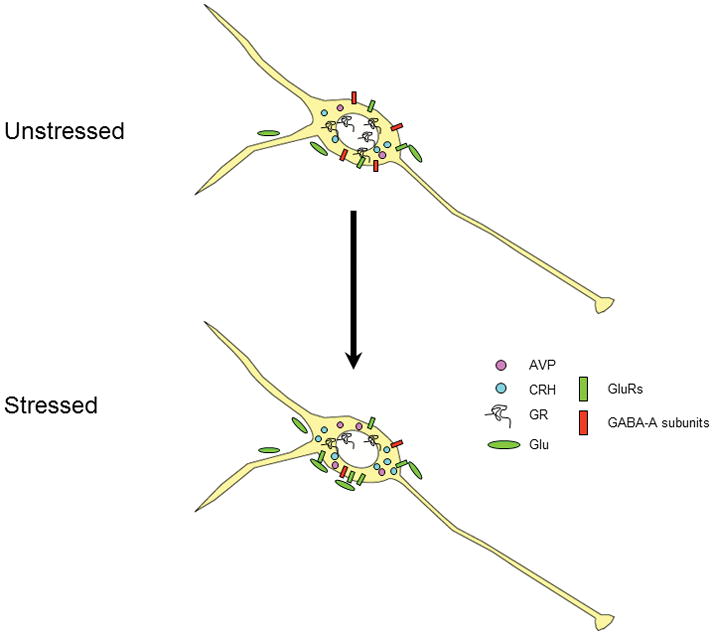
Schematic diagram of multiple neuroplastic changes in the PVNmpd under conditions of chronic drive. Following chronic stress, there is increased expression of ACTH secretagogs CRH and AVP. At the same time, expression of the glucocorticoid receptor (GR) is down-regulated, suggesting loss of feedback capacity. Expression of the GABA-A receptor subunits beta-1 and beta-2 are decreased, consistent with reduced GABAergic inhibition. In contrast, expression of the kainate preferring GluR5 subunit mRNA is increased, suggesting increased glutamate signaling via non-NMDA receptors. Finally, glutamatergic innervation of CRH neurons is markedly increased following chronic drive by adrenalectomy, suggesting the potential for stress-induced synaptic plasticity.
The impact of chronic stress on magnocellular and preautonomic components are less well understood. Certainly, stress can enhance magnocellular production of neurohypophysial peptides and increase cell size (see above), but the overall significance of these changes with respect to stress signaling are only now coming under study. One notable factor that emerges from the literature is an apparent connection between stressor modality and intensity. Stress activation of magnocellular neurons occurs under conditions of homeostatic stimuli (hypoxia, hemorrhage, salt loading), suggesting that the magnocellular system may be preferentially targeted by homeostatic response pathways. Notably, the magnocellular PVN and SON receive preferential input from the A1 and C1 catecholamine groups in the ventrolateral medulla (Cunningham and Sawchenko, 1988; Cunningham et al., 1990), which are thought to be primary effectors of sympathetic outflow. Thus, engagement of the magnocellular system may be keyed to ascending input signaling disruptions of the internal milieu. In addition, homeostatic stressors are typically quite intense, in terms of their ability to activate other stress pathways (such as the HPA axis). In this regard, it is important to note that the magnocellular system can also be activated by prolonged or intense stressors that are not of homeostatic origin (e.g., prolonged restraint). Thus, it is possible that the magnocellular system is recruited at high threshold stimulation.
The nature of the signal provided by the magnocellular system is also worthy of consideration. Vasopressin has the capacity to interact with CRH to stimulate the HPA axis and has direct pressor actions that can assist in stress activation. However, OXT released within the PVN has marked anti-stress properties (Neumann et al., 2000). Moreover, numerous studies indicate that stress exposure disproportionately activates magnocellular OXT neurons (Jezova et al., 1995; Ericsson et al., 1997; Matsunaga et al., 2000), and that some acute regimens activate the magnocellular system at long post-stimulus time frames (Cullinan et al., 1995). Thus, magnocellular OXT and perhaps AVP may play a role in stress recovery, serving to dampen rather than promote stress responses.
Finally, stress plasticity in the preautonomic division is largely unexplored. Given clear influences of stress and stress-related affective states (i.e., depression) on cardiovascular disease, this is clearly an area deserving of additional attention.
In summary, it is evident that the PVN is a critical coordinator of the organismal stress response. The interconnections within the nucleus and the diversity of outputs suggest considerable potential for orchestration and sculpting of the physiological response to adversity. Long-term functional changes in excitability following chronic stress are consistent with an adaptation of the animal to periods of challenge (i.e., maintenance of response capacity in the face of chronic drive). Naturally, inappropriate drive of the PVN by aggregated psychogenic stimuli (perhaps those encountered by many in daily life) may underlie stress-related disease in vulnerable individuals, wherein stress responses are initiated and perpetuated in the absence of truly life-threatening stimuli. Thus, the PVN (and perhaps SON) may be a major part of the effector pathways responsible for stress-related disease, and hence a potential target for intervention or amelioration of these disorders.
Acknowledgments
Supported by NIH grants MH049698, MH069725, and MH069680.
References
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17:4895–903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA. Hypothalamic control of adrenocorticotropin secretion: Advances since the discovery of 41-residue corticotropin-releasing factor. Endocrine Rev. 1986;7:351–378. doi: 10.1210/edrv-7-4-351. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Portillo F, Larsen PJ, Vallo JJ. Insulin and glucose administration stimulates Fos expression in neurones of the paraventricular nucleus that project to autonomic preganglionic structures. J Neuroendocrinol. 2001;13:339–46. doi: 10.1046/j.1365-2826.2001.00631.x. [DOI] [PubMed] [Google Scholar]
- Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, Nemeroff CB. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–51. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Bohn MC, Sawchenko PE. Organization of adrenergic inputs to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1990;292:651–67. doi: 10.1002/cne.902920413. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Expression of alpha(1b) adrenoceptor mRNA in corticotropin-releasing hormone-containing cells of the rat hypothalamus and its regulation by corticosterone. J Neurosci. 1999;19:10098–106. doi: 10.1523/JNEUROSCI.19-22-10098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeij DC, Kvetnansky R, Whitnall MH, Jezova D, Berkenbosch F, Tilders FJ. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin-releasing factor in the median eminence of rats. Neuroendocrinology. 1991;53:150–9. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- Ding JM, Carver WC, Terracio L, Buggy J. Proto-oncogene c-fos and the regulation of vasopressin gene expression during dehydration. Brain Res Mol Brain Res. 1994;21:247–55. doi: 10.1016/0169-328x(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: evidence for distinctive stress pathways. Brain Res. 1999;845:60–7. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. Interaction between the hypothalamic-neurohypophysial system (HNS) and the hypothalamic-pituitary-adrenal (HPA) axis under stress - an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Ericsson A, Arias C, Sawchenko PE. Evidence for an intramedullary prostaglandin-dependent mechanism in the activation of stress-related neuroendocrine circuitry by intravenous interleukin-1. J Neurosci. 1997;17:7166–79. doi: 10.1523/JNEUROSCI.17-18-07166.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. The excitatory effects of the amygdala on hypothalamo-pituitary-adrenocortical responses are mediated by hypothalamic norepinephrine, serotonin, and CRF-41. Brain Res Bull. 1998;45:389–93. doi: 10.1016/s0361-9230(97)00384-5. [DOI] [PubMed] [Google Scholar]
- Figueiredo HF, Bodie BL, Tauchi M, Dolgas CM, Herman JP. Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology. 2003;144:5249–58. doi: 10.1210/en.2003-0713. [DOI] [PubMed] [Google Scholar]
- Ghorbel MT, Sharman G, Leroux M, Barrett T, Donovan DM, Becker KG, Murphy D. Microarray analysis reveals interleukin-6 as a novel secretory product of the hypothalamo-neurohypophyseal system. J Biol Chem. 2003;278:19280–5. doi: 10.1074/jbc.M209902200. [DOI] [PubMed] [Google Scholar]
- Gibbs DM, Vale W, Rivier J, Yen SS. Oxytocin potentiates the ACTH-releasing activity of CRF(41) but not vasopressin. Life Sci. 1984;34:2245–9. doi: 10.1016/0024-3205(84)90212-1. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Linton EA, Lowry PJ. Corticotropin-releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982;299:355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Giovannelli L, Shiromani PJ, Jirikowski GF, Bloom FE. Expression of c-fos protein by immunohistochemically identified oxytocin neurons in the rat hypothalamus upon osmotic stimulation. Brain Res. 1992;588:41–8. doi: 10.1016/0006-8993(92)91342-c. [DOI] [PubMed] [Google Scholar]
- Glasgow E, Murase T, Zhang B, Verbalis JG, Gainer H. Gene expression in the rat supraoptic nucleus induced by chronic hyperosmolality versus hyposmolality. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1239–50. doi: 10.1152/ajpregu.2000.279.4.R1239. [DOI] [PubMed] [Google Scholar]
- Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–37. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–41. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–38. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Harbuz M, Russell JA, Sumner BE, Kawata M, Lightman SL. Rapid changes in the content of proenkephalin A and corticotrophin releasing hormone mRNAs in the paraventricular nucleus during morphine withdrawal in urethane-anaesthetized rats. Brain Res Mol Brain Res. 1991;9:285–91. doi: 10.1016/0169-328x(91)90074-8. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Rees RG, Eckland D, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic corticotropin-releasing factor (CRF) messenger ribonucleic acid (mRNA) and CRF-41 peptide and adenohypophysial proopiomelanocortin mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annu Rev Neurosci. 1997;20:375–97. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Herman JP. In situ hybridization analysis of vasopressin gene transcription in the paraventricular and supraoptic nuclei of the rat: Regulation by stress and glucocorticoids. J Comp Neurol. 1995;363:15–27. doi: 10.1002/cne.903630103. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt CM. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK-H, Thompson RC, Watson SJ. Rapid regulation of CRH gene expression in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Holmes MC, Antoni FA, Aguilera G, Catt KJ. Magnocellular axons in passage through the median eminence release vasopressin. Nature. 1986;319:326–329. doi: 10.1038/319326a0. [DOI] [PubMed] [Google Scholar]
- Husain MK, Manger WM, Rock TW, Weiss RJ, Frantz AG. Vasopressin release due to manual restraint in the rat: role of body compression and comparison with other stressful stimuli. Endocrinology. 1979;104:641–4. doi: 10.1210/endo-104-3-641. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–99. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Turk JR, Schadt JC, Casati J, Ganjam VK, Price EM, Keisler DH, Laughlin MH. Sex difference in link between interleukin-6 and stress. Endocrinology. 2007;148:3758–64. doi: 10.1210/en.2006-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen AS, Schmidt ED, Voorn P, Tilders FJ. Substance induced plasticity in noradrenergic innervation of the paraventricular hypothalamic nucleus. Eur J Neurosci. 2003;17:298–306. doi: 10.1046/j.1460-9568.2003.02453.x. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Johnson HM, Farrar WL, Torres BA. Vasopressin replacement of interleukin 2 requirement in gamma interferon production: lymphokine activity of a neuroendocrine hormone. J Immunol. 1982;129:983–6. [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–13. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. J Neuroendocrinol. 2006;18:330–8. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Khan AM, Watts AG. Intravenous 2-deoxy-D-glucose injection rapidly elevates levels of the phosphorylated forms of p44/42 mitogen-activated protein kinases (extracellularly regulated kinases 1/2) in rat hypothalamic parvicellular paraventricular neurons. Endocrinology. 2004;145:351–9. doi: 10.1210/en.2003-0539. [DOI] [PubMed] [Google Scholar]
- Kiss A, Aguilera G. Regulation of the hypothalamic pituitary adrenal axis during chronic stress: responses to repeated intraperitoneal hypertonic saline injection. Brain Res. 1993;630:262–70. doi: 10.1016/0006-8993(93)90665-a. [DOI] [PubMed] [Google Scholar]
- Kovacs K, Kiss JZ, Makara GB. Glucocorticoid implants around the hypothalamic paraventricular nucleus prevent the increase of corticotropin-releasing factor and arginine vasopressin immunostaining induced by adrenalectomy. Neuroendocrinology. 1986;44:229–34. doi: 10.1159/000124650. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Neurohypophyseal hormones in the integration of physiological responses to immune challenges. Prog Brain Res. 2002;139:127–46. doi: 10.1016/s0079-6123(02)39013-7. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Makara GB. Corticosterone and dexamethasone act at different brain sites to inhibit adrenalectomy-induced adrenocorticotropin hypersecretion. Brain Res. 1988;474:205–10. doi: 10.1016/0006-8993(88)90435-0. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1995;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–33. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Kessler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M, Nussbaumer M, Czibere L, Turck CW, Singewald N, Rujescu D, Frank E. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev. 2007;31:89–102. doi: 10.1016/j.neubiorev.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–76. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–9. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Matsunaga W, Miyata S, Takamata A, Bun H, Nakashima T, Kiyohara T. LPS-induced Fos expression in oxytocin and vasopressin neurons of the rat hypothalamus. Brain Res. 2000;858:9–18. doi: 10.1016/s0006-8993(99)02418-x. [DOI] [PubMed] [Google Scholar]
- Michaloudi HC, el Majdoubi M, Poulain DA, Papadopoulos GC, Theodosis DT. The noradrenergic innervation of identified hypothalamic magnocellular somata and its contribution to lactation-induced synaptic plasticity. J Neuroendocrinol. 1997;9:17–23. doi: 10.1046/j.1365-2826.1997.00583.x. [DOI] [PubMed] [Google Scholar]
- Miyata S, Itoh T, Matsushima O, Nakashima T, Kiyohara T. Not only osmotic stress but also repeated restraint stress causes structural plasticity in the supraoptic nucleus of the rat hypothalamus. Brain Res Bull. 1994;33:669–75. doi: 10.1016/0361-9230(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Di S, Paden CM, Herman JP. Activity-dependent modulation of neurotransmitter innervation to vasopressin neurons of the supraoptic nucleus. Endocrinology. 2005;146:348–54. doi: 10.1210/en.2004-0539. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol. 2000;12:235–43. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang K. Neurohumoral activation in heart failure: role of paraventricular nucleus. Clin Exp Pharmacol Physiol. 1996;23:722–6. doi: 10.1111/j.1440-1681.1996.tb01765.x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Sawchenko PE. Hypophysial-portal plasma levels, median eminence content, and immunohistochemical staining of corticotropin-releasing factor, arginine vasopressin, and oxytocin after pharmacological adrenalectomy. Endocrinology. 1987;120:1361–9. doi: 10.1210/endo-120-4-1361. [DOI] [PubMed] [Google Scholar]
- Quintanar-Stephano A, Kovacs K, Berczi I. Effects of neurointermediate pituitary lobectomy on humoral and cell-mediated immune responses in the rat. Neuroimmunomodulation. 2004;11:233–40. doi: 10.1159/000078441. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–76. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero LM, Plotsky PM, Sapolsky RM. Patterns of adrenocorticotropin secretagog release with hypoglycemia, novelty, and restraint after colchicine blockade of axonal transport. Endocrinology. 1993;132:199–204. doi: 10.1210/endo.132.1.7678213. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci. 1987;7:1093–106. doi: 10.1523/JNEUROSCI.07-04-01093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Harbuz MS, Jessop DS, Perks P, Moore PM, Lightman SL. Inflammatory disease as chronic stress. Ann N Y Acad Sci. 1998;840:599–607. doi: 10.1111/j.1749-6632.1998.tb09599.x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Sagar SM, Hicks K, Lowenstein D, Hisanaga K. c-fos mRNA, Fos, and Fos-related antigen induction by hypertonic saline and stress. J Neurosci. 1991;11:2321–31. doi: 10.1523/JNEUROSCI.11-08-02321.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. J Neurosci. 1998;18:841–53. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–83. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Summy-Long JY, Hu S, Pruss A, Chen X, Phillips TM. Response of interleukin-1beta in the magnocellular system to salt-loading. J Neuroendocrinol. 2006;18:926–37. doi: 10.1111/j.1365-2826.2006.01490.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Biochemical switching in hypothalamic circuits mediating responses to stress. Prog Brain Res. 1991;87:181–200. doi: 10.1016/s0079-6123(08)63052-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Theodosis DT. Oxytocin-secreting neurons: A physiological model of morphological neuronal and glial plasticity in the adult hypothalamus. Front Neuroendocrinol. 2002;23:101–35. doi: 10.1006/frne.2001.0226. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA. Activity-dependent neuronal-glial and synaptic plasticity in the adult mammalian hypothalamus. Neuroscience. 1993;57:501–35. doi: 10.1016/0306-4522(93)90002-w. [DOI] [PubMed] [Google Scholar]
- Thrivikraman KV, Nemeroff CB, Plotsky PM. Sensitivity to glucocorticoid-mediated fast-feedback regulation of the hypothalamic-pituitary-adrenal axis is dependent upon stressor specific neurocircuitry. Brain Res. 2000;870:87–101. doi: 10.1016/s0006-8993(00)02405-7. [DOI] [PubMed] [Google Scholar]
- Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol. 1998;275:H2140–6. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- van Dam AM, Poole S, Schultzberg M, Zavala F, Tilders FJ. Effects of peripheral administration of LPS on the expression of immunoreactive interleukin-1 alpha, beta, and receptor antagonist in rat brain. Ann N Y Acad Sci. 1998;840:128–38. doi: 10.1111/j.1749-6632.1998.tb09557.x. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–73. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- Watt JA, Hobbs NK. Interleukin-1beta immunoreactivity in identified neurons of the rat magnocellular neurosecretory system: evidence for activity-dependent release. J Neurosci Res. 2000;60:478–89. doi: 10.1002/(SICI)1097-4547(20000515)60:4<478::AID-JNR6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Lightman SL. Chronic stress elevates enkephalin expression in the rat paraventricular and supraoptic nuclei. Brain Res Mol Brain Res. 1992;13:111–7. doi: 10.1016/0169-328x(92)90050-l. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Lu J, Elmquist JK, Saper CB. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J Neurosci. 2000;20:6578–86. doi: 10.1523/JNEUROSCI.20-17-06578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Distribution of vesicular glutamate transporter mRNA in rat hypothalamus. J Comp Neurol. 2002;448:217–29. doi: 10.1002/cne.10257. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol. 2005;484:43–56. doi: 10.1002/cne.20445. [DOI] [PubMed] [Google Scholar]


